Multiscale Molecular Dynamics Simulations of Fuel Cell Nanocatalyst Plasma Sputtering Growth and Deposition
Abstract
1. Introduction
2. Materials and Methods
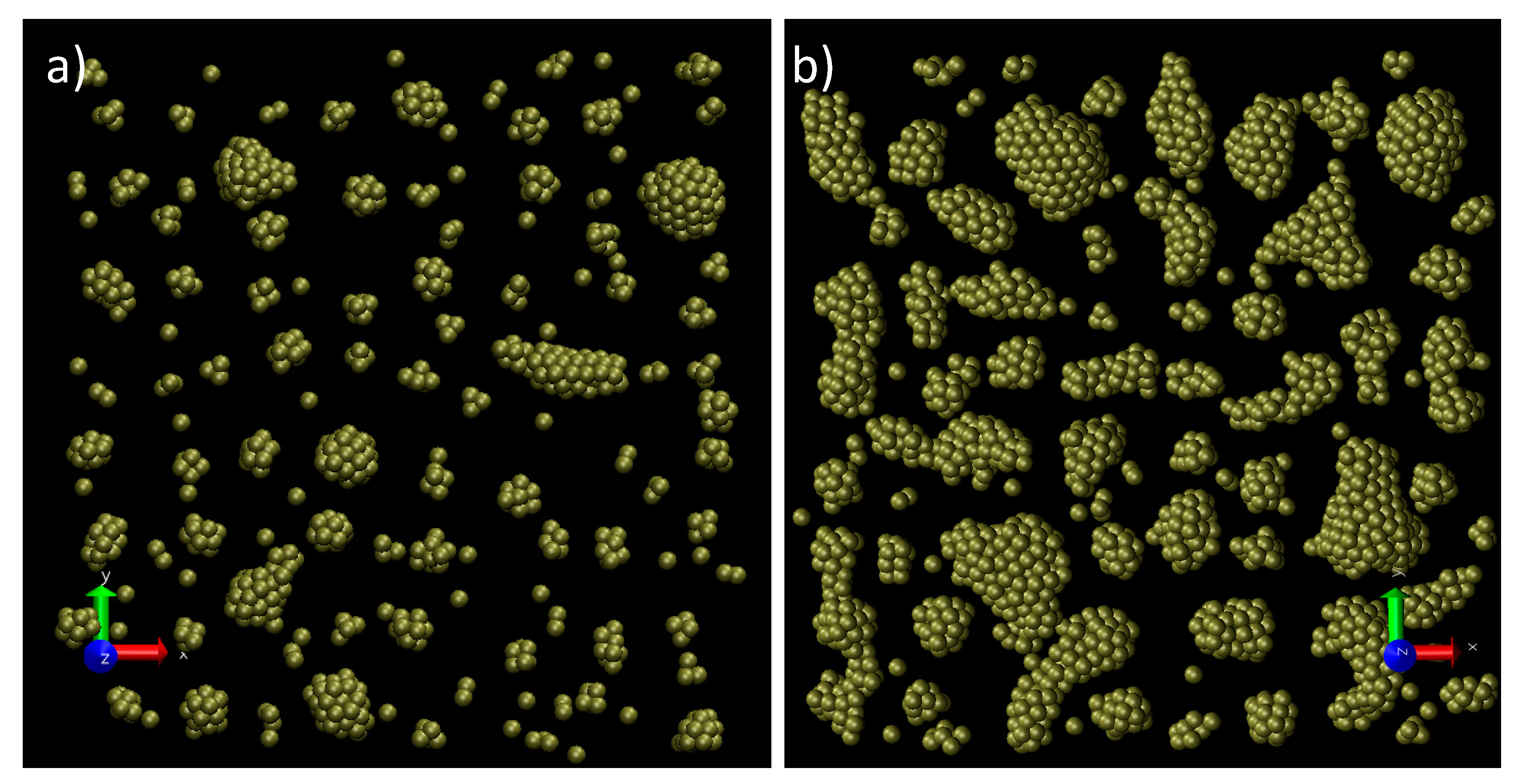
3. Results and Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Antolini, E. Recent Developments in Polymer Electrolyte Fuel Cell Electrodes. J. Appl. Electrochem. 2004, 34, 563–576. [Google Scholar] [CrossRef]
- Litster, S.; McLean, G. PEM fuel cell electrodes. J. Power Sources 2004, 130, 61. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygenreduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Brault, P.; Caillard, A.; Thomann, A.L. Polymer electrolyte fuel cell electrodes grown by vapor deposition techniques. Chem. Vap. Depos. 2011, 17, 296–304. [Google Scholar] [CrossRef]
- Cuynet, S.; Caillard, A.; Lecas, T.; Bigarre, J.; Buvat, P.; Brault, P. High Power Impulse Magnetron Sputtering deposition of Pt inside fuel cell electrodes. J. Phys. D Appl. Phys. 2014, 47, 272001. [Google Scholar] [CrossRef]
- Caillard, A.; Cuynet, S.; Lecas, T.; Andreazza, P.; Mikikian, M.; Thomann, A.L.; Brault, P. PdPt catalyst synthesized by gas aggregation source and magnetron sputtering for fuel cell electrodes. J. Phys. D Appl. Phys. 2015, 48, 475302. [Google Scholar] [CrossRef]
- Brault, P. Review of low pressure plasma processing of proton exchange membrane fuel cell electrocatalysts. Plasma Process. Polym. 2016, 13, 10–18. [Google Scholar] [CrossRef]
- Bauchire, J.-M.; Thomann, A.-L.; Bedra, L. Molecular Dynamics simulations of clusters and thin film growth in the context of plasma sputtering deposition. J. Phys. D Appl. Phys. 2014, 47, 224004. [Google Scholar]
- Xie, L.; Brault, P.; Coutanceau, C.; Caillard, A.; Berndt, J.; Neyts, E. Efficient amorphous platinum catalyst cluster growth on porous carbon: A combined Molecular Dynamics and experimental study. Appl. Cat. B 2015, 62, 21–26. [Google Scholar] [CrossRef]
- Brault, P.; Neyts, E.C. Molecular dynamics simulations of supported metal nanocatalyst formation by plasma sputtering. Catal. Today 2015, 256, 3–12. [Google Scholar] [CrossRef]
- Brault, P.; Chuon, S.; Bauchire, J.-M. Molecular Dynamics simulations of platinum plasma sputtering: A comparative case study. Front. Phys. 2016, 4, 20. [Google Scholar] [CrossRef]
- Brault, P.; Coutanceau, C.; Jennings, P.C.; Vegge, T.; Berndt, J.; Caillard, A.; Baranton, S.; Lankiang, S. Molecular dynamics simulations of ternary PtxPdyAuz fuel cell nanocatalyst growth. Int. J. Hydrog. Energy 2016, 41, 22589–22597. [Google Scholar] [CrossRef]
- Brault, P. Multiscale Molecular Dynamics Simulation of Plasma Processing: Application to Plasma Sputtering. Front. Phys. 2018, 6, 59. [Google Scholar] [CrossRef]
- Brault, P.; Chamorro-Coral, W.; Chuon, S.; Caillard, A.; Bauchire, J.-M.; Baranton, S.; Coutanceau, C.; Neyts, E.C. Molecular Dynamics simulations of initial Pd and PdO nanocluster growths in a magnetron gas aggregation source. Front. Chem. Sci. Eng. 2019, 13, 324–329. [Google Scholar] [CrossRef]
- Brault, P.; Coutanceau, C.; Caillard, A.; Baranton, S. Pt3MeAu (Me = Ni, Cu) fuel cell nanocatalyst growth, shapes and efficiency: A molecular dynamics simulation approach. J. Phys. Chem. C 2019, 123, 29656–29664. [Google Scholar] [CrossRef]
- Kouamé, B.S.R.; Baranton, S.; Brault, P.; Canaff, C.; Chamorro-Coral, W.; Caillard, A.; De Oliveira Vigier, K.; Coutanceau, C. Insights on the unique electro-catalytic behavior of PtBi/C materials. Electrochim. Acta 2020, 329, 135161. [Google Scholar] [CrossRef]
- Pikunic, J.; Clinard, C.; Cohaut, N.; Gubbins, K.E.; Guet, J.M.; Pellenq, R.J.M.; Rannou, I.; Rouzaud, J.-N. Structural modeling of porous carbons: Constrained reverse monte carlo method. Langmuir 2003, 19, 8565. [Google Scholar] [CrossRef]
- Brault, P.; Caillard, A.; Baranton, S.; Mougenot, M.; Cuynet, S.; Coutanceau, C. One-step synthesis and chemical characterization of Pt C nanowire composites by plasma sputtering. ChemSusChem 2013, 6, 1168–1171. [Google Scholar] [CrossRef]
- Foiles, S.; Baskes, M.; Daw, M. Embedded-atom-method functions for the fcc metals Cu, Ag, Au, Ni, Pd, Pt, and their alloys. Phys. Rev. B 1986, 33, 7983. [Google Scholar] [CrossRef]
- Tersoff, J. Modeling solid-state chemistry: Interatomic potentials for multicomponent systems. Phys. Rev. B 1989, 39, 5566. [Google Scholar] [CrossRef]
- Graves, D.B.; Brault, P. Molecular dynamics for low temperature plasma–surface interaction studies. J. Phys. D Appl. Phys. 2009, 42, 194011. [Google Scholar] [CrossRef]
- Morrow, B.H.; Striolo, A. Assessing how metal–carbon interactions affect the structure of supported platinum nanoparticles. Mol. Simul. 2009, 35, 795. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comp. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- LAMMPS. Molecular Dynamics Simulator. Available online: http://lammps.sandia.gov (accessed on 17 June 2020).
- Chamorro-Coral, W.; Caillard, A.; Brault, P.; Andreazza, P.; Coutanceau, C.; Baranton, S. The Role of Oxygen on the Growth of Palladium Clusters Synthesized by Gas Aggregation Source. Plasma Process. Polym. 2019, 16, e1900006. [Google Scholar] [CrossRef]


© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brault, P. Multiscale Molecular Dynamics Simulations of Fuel Cell Nanocatalyst Plasma Sputtering Growth and Deposition. Energies 2020, 13, 3584. https://doi.org/10.3390/en13143584
Brault P. Multiscale Molecular Dynamics Simulations of Fuel Cell Nanocatalyst Plasma Sputtering Growth and Deposition. Energies. 2020; 13(14):3584. https://doi.org/10.3390/en13143584
Chicago/Turabian StyleBrault, Pascal. 2020. "Multiscale Molecular Dynamics Simulations of Fuel Cell Nanocatalyst Plasma Sputtering Growth and Deposition" Energies 13, no. 14: 3584. https://doi.org/10.3390/en13143584
APA StyleBrault, P. (2020). Multiscale Molecular Dynamics Simulations of Fuel Cell Nanocatalyst Plasma Sputtering Growth and Deposition. Energies, 13(14), 3584. https://doi.org/10.3390/en13143584





