Generating Electricity from Natural Evaporation Using PVDF Thin Films Incorporating Nanocomposite Materials
Abstract
1. Introduction
2. Results
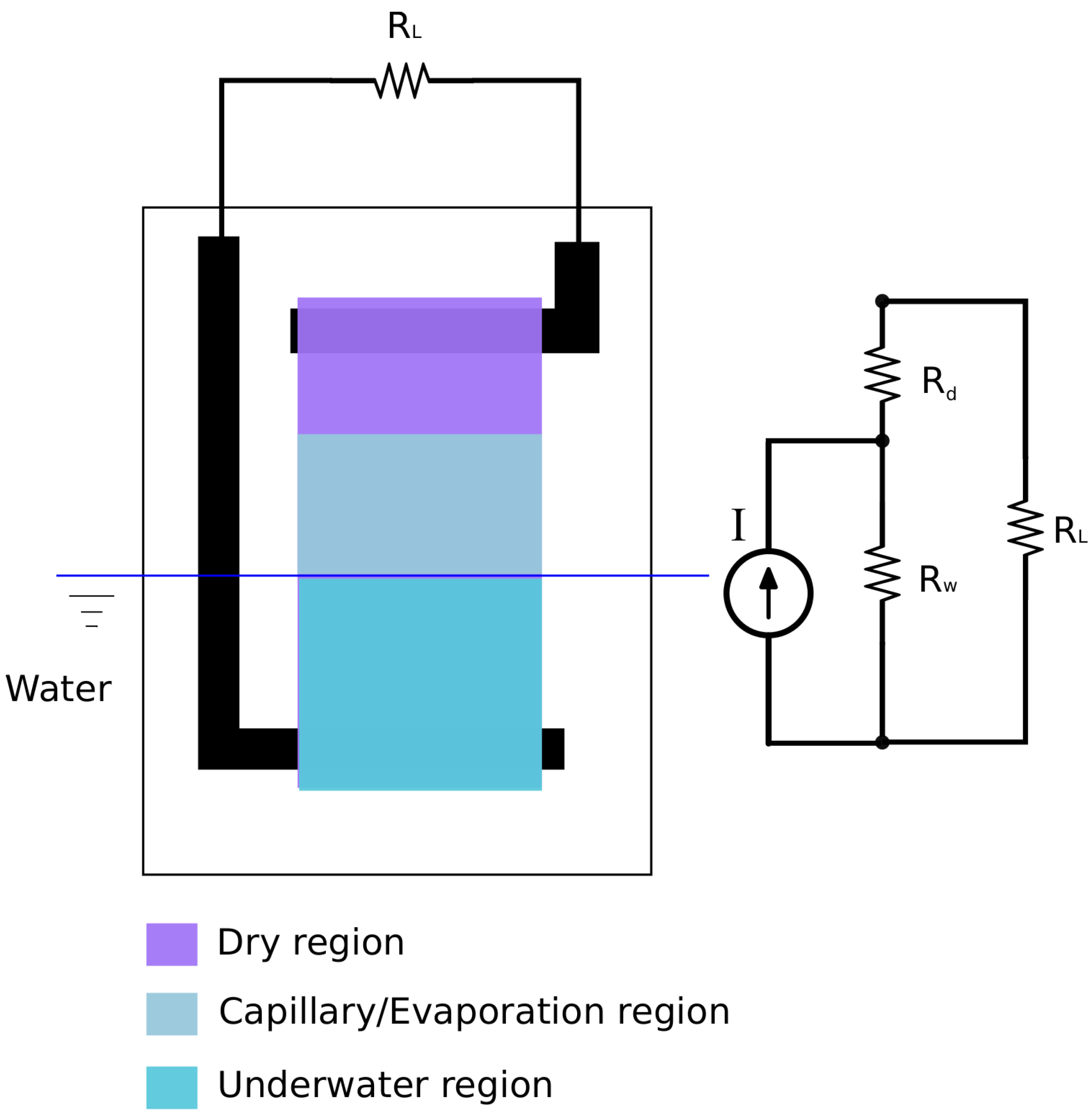
2.1. Device Design and Working Mechanism
2.2. Performance Evaluation of the Device
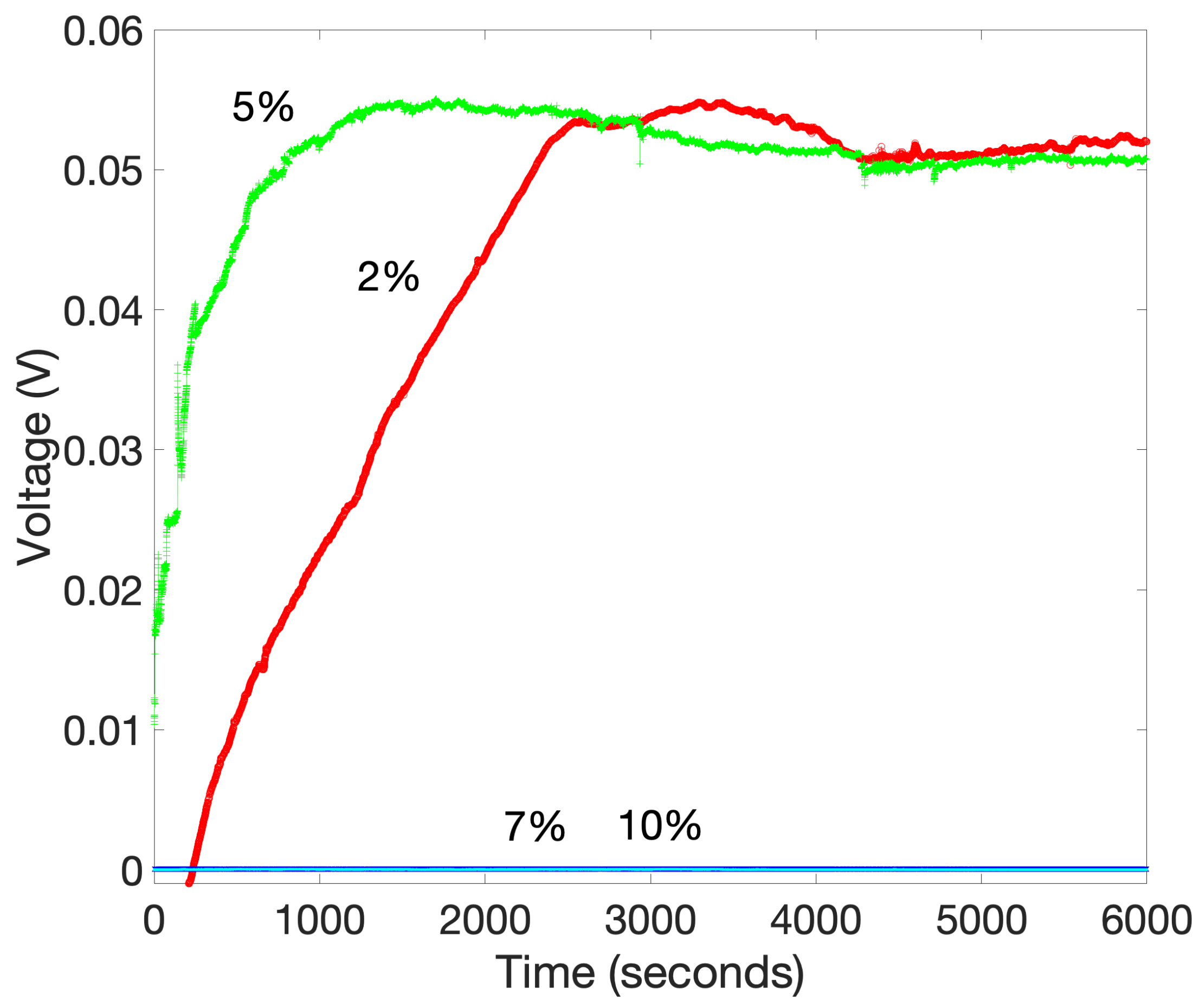
2.2.1. Carbon Tubes
2.2.2. Mesoporous Silica (SBA-15)
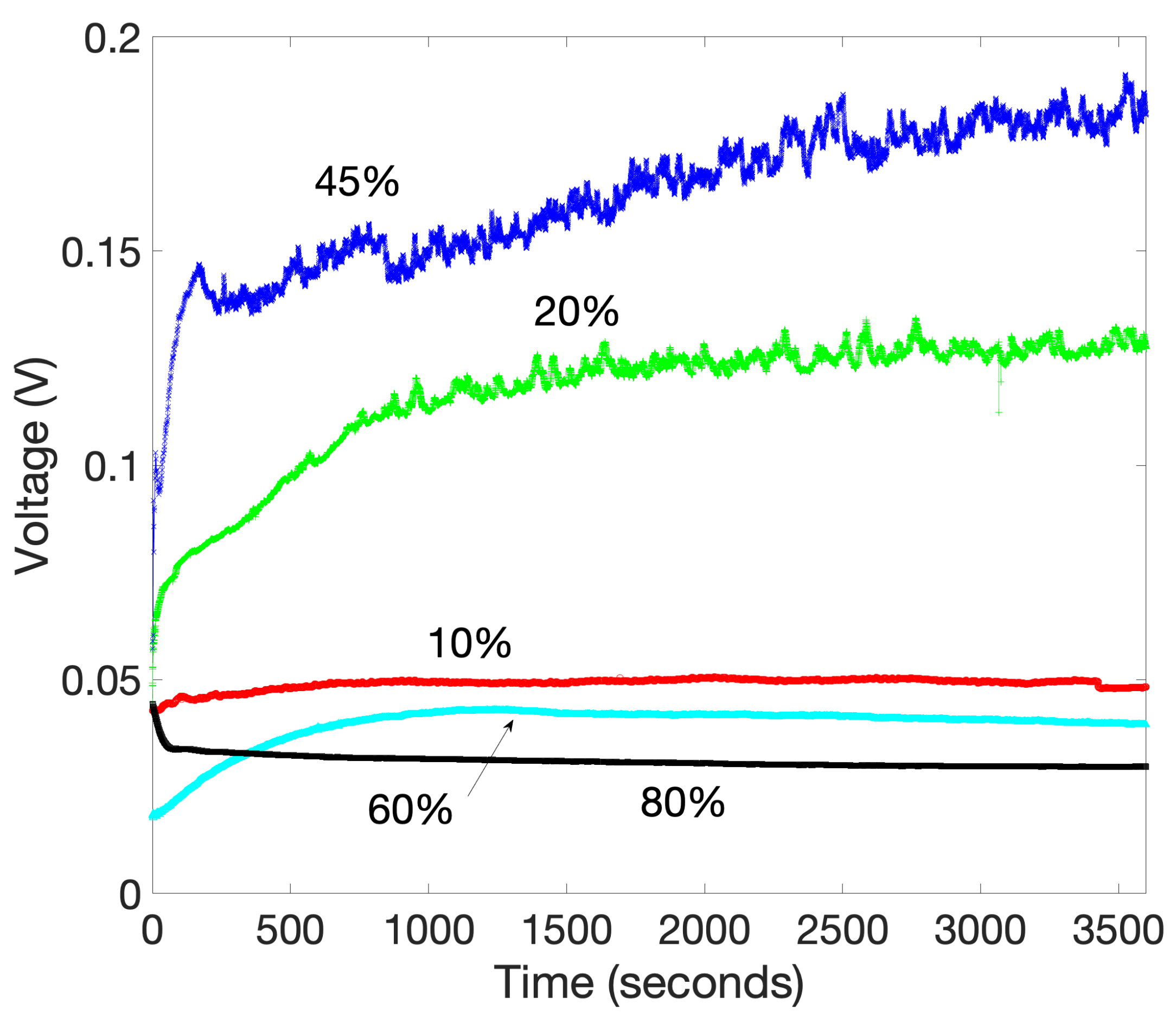
2.2.3. Graphene Oxide
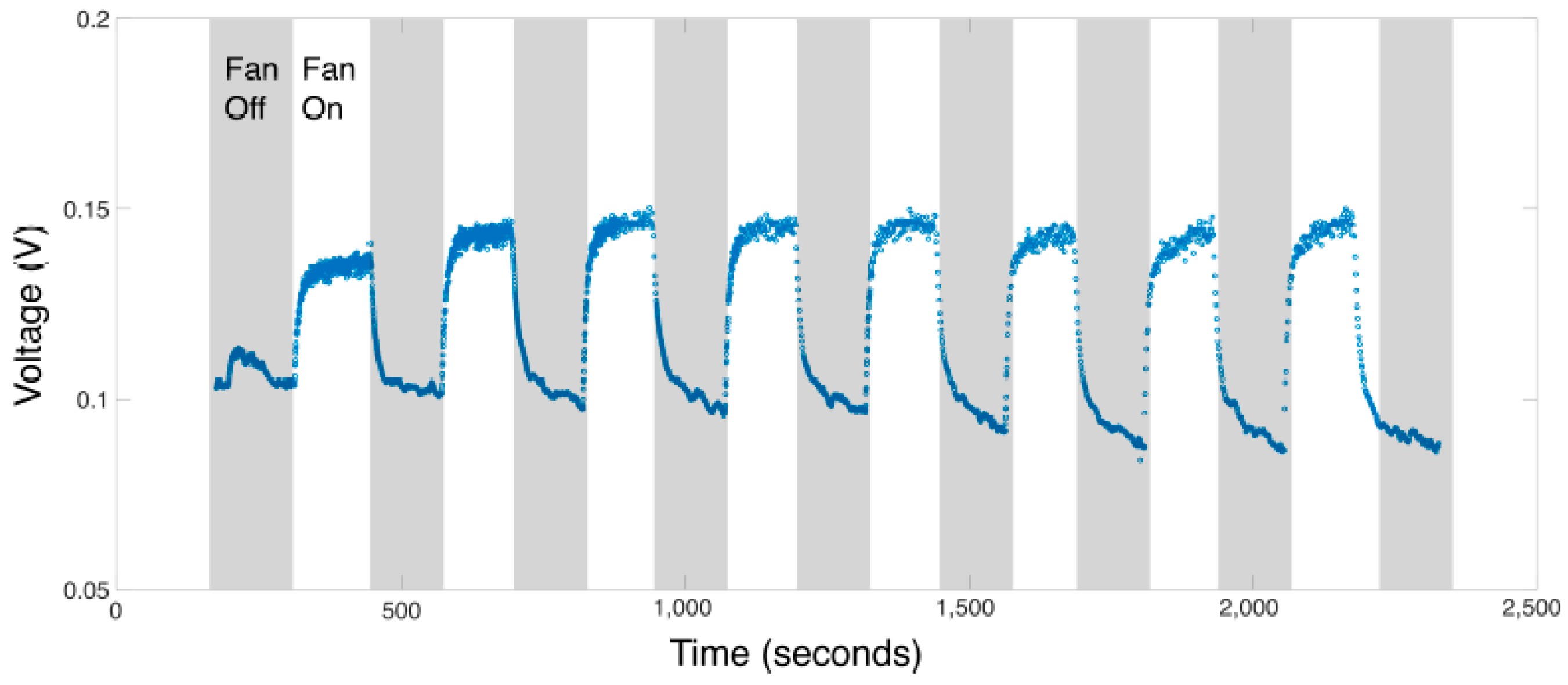
2.3. Performance of the Device under Different Ambient Conditions
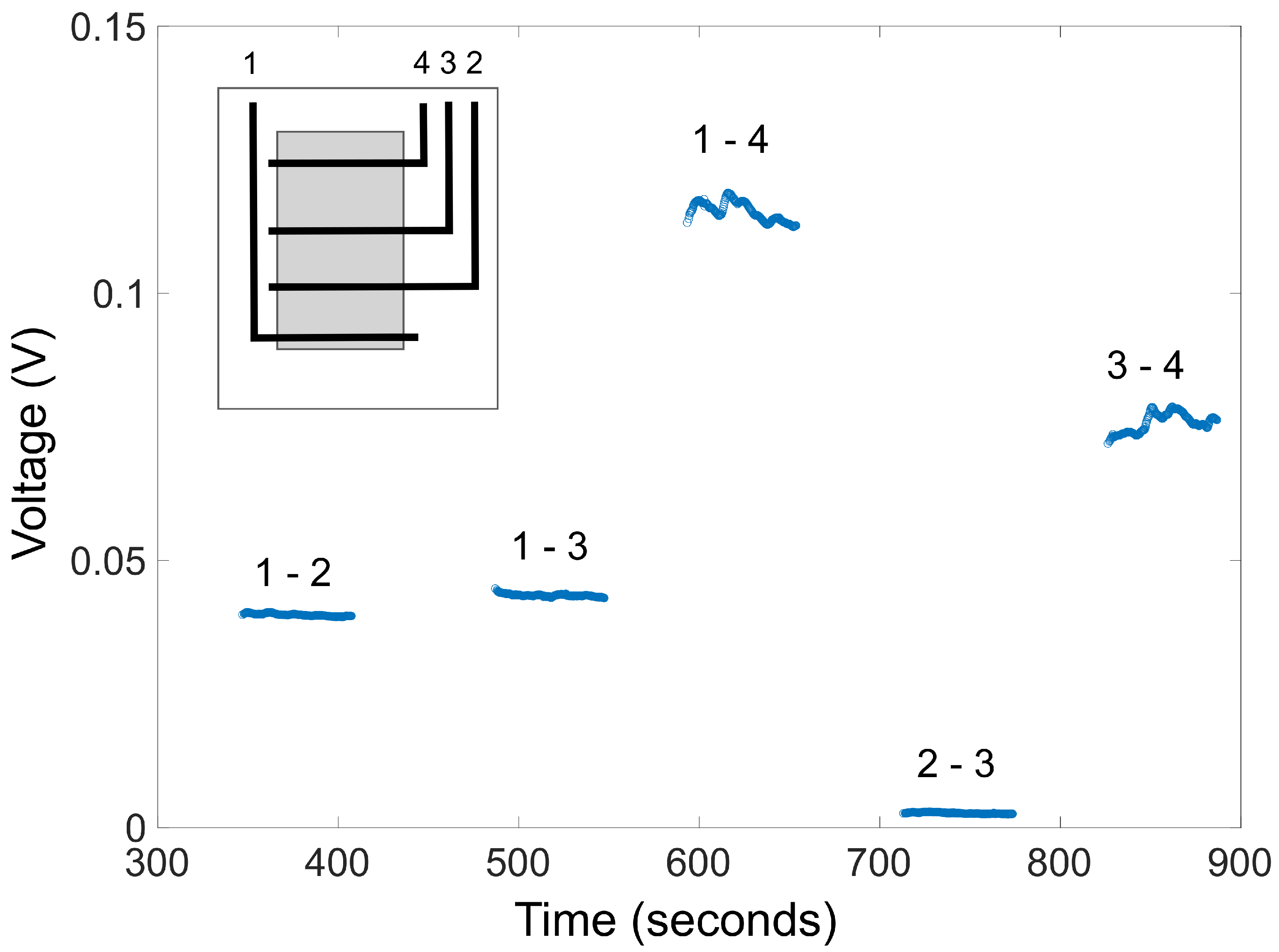
2.4. Voltage Profile within the Working Area
2.5. Electrical Outputs
3. Discussion
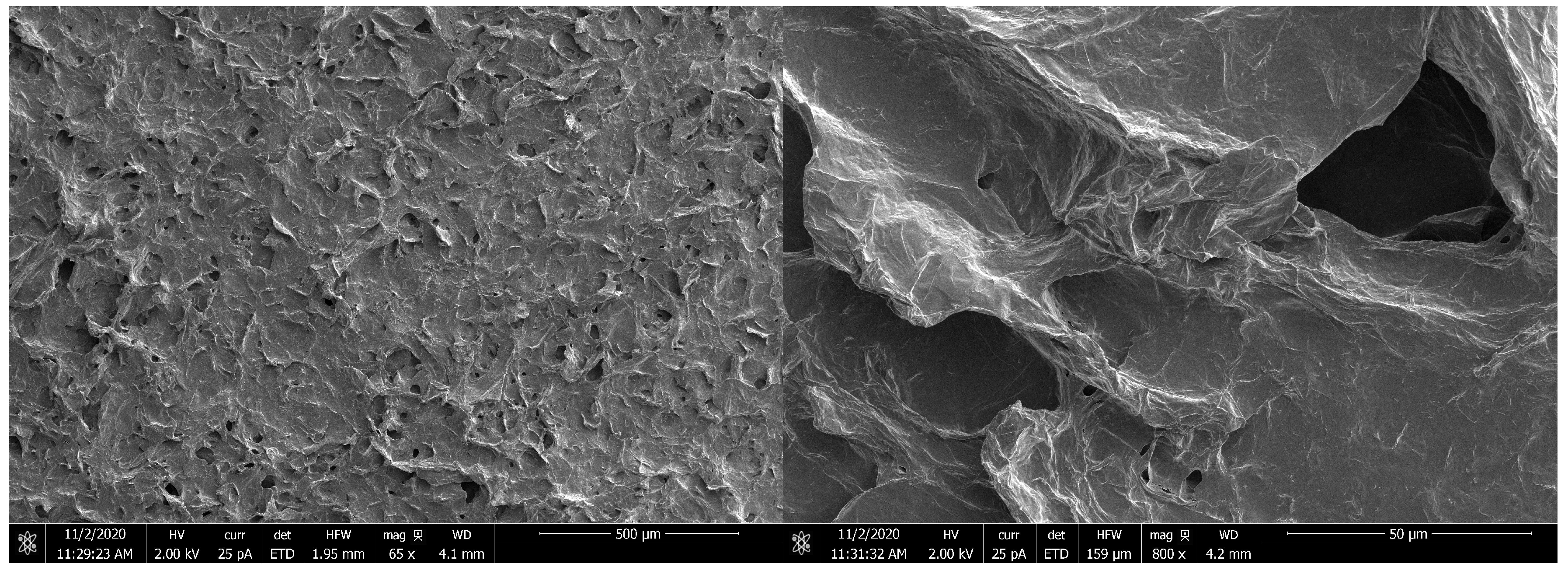
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wild, M.; Folini, D.; Schär, C.; Loeb, N.; Dutton, E.G.; König-Langlo, G. The global energy balance from a surface perspective. Clim. Dyn. 2013, 40, 3107–3134. [Google Scholar] [CrossRef]
- Cavusoglu, A.H.; Chen, X.; Gentine, P.; Sahin, O. Potential for natural evaporation as a reliable renewable energy resource. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Worden, J.; Noone, D.; Bowman, K. Importance of rain evaporation and continental convection in the tropical water cycle. Nature 2007, 445, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, F.; Zika, J.; Mudryk, L.; Kushner, P.; Kjellsson, J.; Döös, K. Constrained work output of the moist atmospheric heat engine in a warming climate. Science 2015, 347, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Beeby, S.P.; Tudor, M.J.; White, N.M. Energy harvesting vibration sources for microsystems applications. Meas. Sci. Technol. 2006, 17, R175. [Google Scholar] [CrossRef]
- Caliò, R.; Rongala, U.B.; Camboni, D.; Milazzo, M.; Stefanini, C.; De Petris, G.; Oddo, C.M. Piezoelectric energy harvesting solutions. Sensors 2014, 14, 4755–4790. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Chen, J.; Lin, L. Progress in triboelectric nanogenerators as a new energy technology and self-powered sensors. Energy Environ. Sci. 2015, 8, 2250–2282. [Google Scholar] [CrossRef]
- Yu, J.; Ma, E.; Ma, T. Exponential energy harvesting through repetitive reconfigurations of a system of capacitors. Commun. Phys. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, H.; Liu, Y.; Zhou, X.; Zhang, C.; Song, Y.; Deng, X.; Leung, M.; Yang, Z.; Xu, R.X.; et al. A droplet-based electricity generator with high instantaneous power density. Nature 2020, 578, 392–396. [Google Scholar] [CrossRef]
- Bell, L.E. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and old concepts in thermoelectric materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar]
- Post, J.W.; Veerman, J.; Hamelers, H.V.; Euverink, G.J.; Metz, S.J.; Nymeijer, K.; Buisman, C.J. Salinity-gradient power: Evaluation of pressure-retarded osmosis and reverse electrodialysis. J. Membr. Sci. 2007, 288, 218–230. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Power generation with pressure retarded osmosis: An experimental and theoretical investigation. J. Membr. Sci. 2009, 343, 42–52. [Google Scholar] [CrossRef]
- Rica, R.A.; Ziano, R.; Salerno, D.; Mantegazza, F.; Van Roij, R.; Brogioli, D. Capacitive mixing for harvesting the free energy of solutions at different concentrations. Entropy 2013, 15, 1388–1407. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. In Materials For Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 26–32. [Google Scholar]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Guo, L.; Anderson, D.G.; Langer, R. Bio-inspired polymer composite actuator and generator driven by water gradients. Science 2013, 339, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mahadevan, L.; Driks, A.; Sahin, O. Bacillus spores as building blocks for stimuli-responsive materials and nanogenerators. Nat. Nanotechnol. 2014, 9, 137–141. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, H.; Jacob, J.; Naumov, P. Photogated humidity-driven motility. Nat. Commun. 2015, 6, 1–12. [Google Scholar]
- Chen, X.; Goodnight, D.; Gao, Z.; Cavusoglu, A.H.; Sabharwal, N.; DeLay, M.; Driks, A.; Sahin, O. Scaling up nanoscale water-driven energy conversion into evaporation-driven engines and generators. Nat. Commun. 2015, 6, 1–7. [Google Scholar]
- Arazoe, H.; Miyajima, D.; Akaike, K.; Araoka, F.; Sato, E.; Hikima, T.; Kawamoto, M.; Aida, T. An autonomous actuator driven by fluctuations in ambient humidity. Nat. Mater. 2016, 15, 1084–1089. [Google Scholar] [CrossRef]
- Yin, J.; Li, X.; Yu, J.; Zhang, Z.; Zhou, J.; Guo, W. Generating electricity by moving a droplet of ionic liquid along graphene. Nat. Nanotechnol. 2014, 9, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Xu, Y.; Ding, T.; Li, J.; Yin, J.; Fei, W.; Cao, Y.; Yu, J.; Yuan, L.; Gong, L.; et al. Water-evaporation-induced electricity with nanostructured carbon materials. Nat. Nanotechnol. 2017, 12, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Liu, K.; Li, J.; Xue, G.; Chen, Q.; Huang, L.; Hu, B.; Zhou, J. All-printed porous carbon film for electricity generation from evaporation-driven water flow. Adv. Funct. Mater. 2017, 27, 1700551. [Google Scholar] [CrossRef]
- Ma, Q.; He, Q.; Yin, P.; Cheng, H.; Cui, X.; Yun, Q.; Zhang, H. Rational Design of MOF-Based Hybrid Nanomaterials for Directly Harvesting Electric Energy from Water Evaporation. Adv. Mater. 2020, 32, 2003720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Duan, Z.; Qi, X.; Xu, Y.; Li, L.; Ma, W.; Zhang, H.; Liu, C.; Yao, W. Harvesting environment energy from water-evaporation over free-standing graphene oxide sponges. Carbon 2019, 148, 1–8. [Google Scholar] [CrossRef]
- He, L.; Tjong, S.C. A graphene oxide–polyvinylidene fluoride mixture as a precursor for fabricating thermally reduced graphene oxide–polyvinylidene fluoride composites. RSC Adv. 2013, 3, 22981–22987. [Google Scholar] [CrossRef]
- Fontananova, E.; Bahattab, M.A.; Aljlil, S.A.; Alowairdy, M.; Rinaldi, G.; Vuono, D.; Nagy, J.B.; Drioli, E.; Di Profio, G. From hydrophobic to hydrophilic polyvinylidenefluoride (PVDF) membranes by gaining new insight into material’s properties. RSC Adv. 2015, 5, 56219–56231. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Boo, C.; Lee, J.; Elimelech, M. Omniphobic Polyvinylidene Fluoride (PVDF) Membrane for Desalination of Shale Gas Produced Water by Membrane Distillation. Environ. Sci. Technol. 2016, 50, 12275–12282. [Google Scholar] [CrossRef] [PubMed]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions; Cambridge Monographs on Mechanics; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar] [CrossRef]
- Probstein, R.F. Physicochemical Hydrodynamics; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Mosthaf, K.; Helmig, R.; Or, D. Modeling and analysis of evaporation processes from porous media on the REV scale. Water Resour. Res. 2014, 50, 1059–1079. [Google Scholar] [CrossRef]
- Kang, A.K.; Zandi, M.H.; Gorji, N.E. Fabrication and Degradation Analysis of Perovskite Solar Cells with Graphene Reduced Oxide as Hole Transporting Layer. J. Electron. Mater. 2020, 49, 2289–2295. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, A.; Yu, J.; Uspal, W. Generating Electricity from Natural Evaporation Using PVDF Thin Films Incorporating Nanocomposite Materials. Energies 2021, 14, 585. https://doi.org/10.3390/en14030585
Ma A, Yu J, Uspal W. Generating Electricity from Natural Evaporation Using PVDF Thin Films Incorporating Nanocomposite Materials. Energies. 2021; 14(3):585. https://doi.org/10.3390/en14030585
Chicago/Turabian StyleMa, Ariel, Jian Yu, and William Uspal. 2021. "Generating Electricity from Natural Evaporation Using PVDF Thin Films Incorporating Nanocomposite Materials" Energies 14, no. 3: 585. https://doi.org/10.3390/en14030585
APA StyleMa, A., Yu, J., & Uspal, W. (2021). Generating Electricity from Natural Evaporation Using PVDF Thin Films Incorporating Nanocomposite Materials. Energies, 14(3), 585. https://doi.org/10.3390/en14030585





