3.1. Polyimide/Graphene Nanosheets-Poly(acrylic acid) Porous Composite
Figure 2a,b show the SEM images of the porous composites fabricated using 1 wt% and 5 wt% of poly(acrylic acid) resin. The addition of poly(acrylic acid) resins resulted in the production of pores ranging from 2.35–6.02 μm in diameter in the composite electrode. It is shown that the higher the amount of poly(acrylic acid) resin used, the bigger the pore size (
Figure 2a,b). The specific capacitance obtained from CV measurement of neat GR/PI composites at a scan rate of 5 mV/s was 49 F/g, which is 3.5 times lower than that obtained for the modified porous composite at the same scan rate of 170.50 F/g. The reason for this increase in specific capacitance of the porous composite electrode is believed to be due to the presence of pores in the electrode which enhanced both bulk and ion transport through the electrode. The CV curve of modified porous poly(acrylic acid) resin-GRP/PI composites performed at 5, 50 and 100 mV/s are shown in
Figure 3. These voltammograms are typical for a material showing double layer capacitance behavior attributed to the presence of graphene. The shape and symmetry of the voltammogram obtained at 5 mV/s is different from those obtained at higher scan rates of 50 and 100 mV/s. It shows a predominantly negative sweep current at all voltages. Studies show that the reason for negative current could be due to the presence of a large background current, as well as complex electrochemical reaction mechanisms. Additionally, it is believed that changes in the process variables may lead to changes in the structure and morphology of GR/PI composite electrode with a significant impact on the electrochemical properties of the composite. It is reported that incorporation of macro-sized pores and voids facilitates better electrolyte ion transport and improves embedded graphene surface utilization [
1].
The Nyquist plots for neat and porous GR/PI are shown in
Figure 4. The shape of the plot is typical for a system exhibiting multiple time constants. Multiple time constant distributions results from non-uniform mass transfer, geometry-induced non-uniform current/potential distributions, and electrode porosity [
21]. At higher frequencies, seen at the extreme left of the real axis, resistance and capacitance are low because little or no current flows deep down into the pores of the material [
22]. However, at low frequencies, observed to the extreme right, impedance tends to infinity due to distributed resistance and capacitance arising from surface heterogeneity along the pore length of the electrode. An equivalent electrical circuit element consisting of a capacitor and resistors in series was used to fit the plot. Moderate to high equivalent series resistance (ESR) values obtained from the fit are due to the cumulative distributed resistances as a result of material’s microstructure. Lower ESR values were obtained from the porous composite electrodes compared to that for the neat GR/PI electrode because of improved electrolyte/electrode interaction resulting from their porous morphology. The dense microstructure and morphology of the neat GR/PI composite electrode limits ion transport due to highly tortuous pathways caused by random orientation of dispersed rigid graphene fillers.
Table 1 shows the specific capacitance and bulk resistance of the modified porous graphene/polyimide electrode, Modified porous GR/PI and graphene/polyimide, GR/PI composite electrodes. The modified porous GR/PI composite electrode’s specific capacitance of 170.5 F/g is more than 3 times higher than that for the dense GR/PI composite electrode of 49.13 F/g as shown on
Table 1.
3.2. Polyimide/Graphene Nanosheet-Poly(acrylic acid)-NiO Hybrid Composite
The electrochemical behavior of the porous poly(acrylic acid) resin-GR/PI composite electrode was further modified by the insertion of NiO particles into the porous electrode by electrochemical deposition. The properties of NiO deposited into the electrode is dependent on its microstructure which is substantially influenced by deposition parameters. Studies show that many factors including the current density,
J, concentration of metal ions, pH of solution and deposition time influence the type of deposit obtained at the cathode [
23,
24,
25]. F. Ebrahimi et al. [
26], in their work, showed that increased current density resulted in the increased grain size of nickel deposits due to the enhanced evolution of hydrogen at high current densities, resulting in the formation of larger crystals. Increased current density also results in a high over potential, which increases nucleation rate. During deposition, the thickness of the electrodeposit was shown to increase with deposition time [
27,
28]. NiO deposition on the porous GR/PI working electrode was carried out using two different current densities at varying deposition times. NiO deposition onto the porous GR/PI electrode was carried out using two different current densities. The working electrode was immersed in a continuously stirred aqueous solution containing nickel sulphate (anhydrous), sodium acetate and sodium sulphate. The possible chemical reactions that resulted in the deposition of nickel hydroxide, Ni(OH)
2 are shown below:
Reaction of sodium acetate in water:
Reaction of sodium sulphate in water:
Nickel sulphate dissociation:
The redox potential for reduction of sodium ions and nickel ions to their respective metals are reported to be −2.71 and −0.23 V, respectively—i.e., (Na
+/Na
E° = −2.71 V and Ni
2+/Ni
E°\<= −0.23 V) [
29].
The acetate ion, CH
3COO
− being the conjugate base of acetic acid, accepts a proton from water. The presence of this extra OH
−(aq) makes the solution basic, resulting in the increased pH of the solution, allowing for deposition of Ni(OH)
2 onto porous GR/PI substrate to occur. Nickel ions (Ni
2+), having a lower electrode potential than sodium (Na
+), are reduced to nickel salt and deposits onto the working electrode composite substrate (cathode) as nickel hydroxide salt Ni(OH)
2. The deposited Ni(OH)
2 is then decomposed to NiO by thermal annealing at 330 °C for 60 min.
The presence of facile pores and voids in GR/PI composites provides multiple channels for trapping of nickel hydroxide during the deposition process forming a hybrid composite with multi-phase regions [
1]. Porosity is a crucial factor in determining the capacitive behavior of NiO. It is observed that the structure of NiO particles deposited on the porous composite is highly dependent on the (i) size of pores, (ii) deposition time and (iii) current density.
The pores created in the composite electrode were smaller and well distributed for composite electrodes prepared with a lower poly (acrylic acid) resin weight percent of 1wt% compared to those created in electrodes formed by using higher poly (acrylic acid) resin concentrations between 5 wt% and 20 wt%. The larger NiO aggregates formed in electrodes prepared with higher concentrations of poly (acrylic acid) resin decreases the effective surface area of NiO particles, there-by making them unavailable for charge transfer. It is important that ions to be transported rapidly through the film during high rate of charging and discharging. It has been reported that nickel oxide structure with particulate/aggregated morphology produce specific capacitance lower than the theoretical capacitance value [
10].
The effect of deposition time on structure of NiO formed was studied using the GR/PI composite electrode synthesized with 5 wt% of poly (acrylic acid) resin. Anodic currents of 1 and 100 mA (corresponding to current densities 25 and 250 mA/cm
2) were used and deposition time was varied from 5 to 60 min. Theoretically, the size of the NiO clusters and aggregates with radius
R (
t) varies with time, over potential, and exchange current density. A relationship between the size of the cluster [
d (
t) = 2
R (
t)] and the process parameters is given in Equation (5) below:
where
io (A/cm
2) is the exchange current density,
Vm (cm
3/mol) is the molar volume of the deposit,
z is the valence of the reduced (Ni
2+) ion, (As/mol) is the Faraday’s constant,
t(s) is the time of cluster growth and
f(
η) is the function of over potential according to Equation (6):
where
α is the cathodic charge transfer coefficient,
η is the over potential,
R is the molar gas constant and
T is the absolute temperature [
25,
27,
30]. Equation (5) shows a linear relationship between deposition time and size of the aggregates. The relationship between the growth of crystals and deposition time is confirmed experimentally both gravimetrically and spectroscopically, as will be discussed in subsequent paragraphs.
3.2.1. Weight Gain Due to Electrodeposition
The composite electrodes were analyzed before and after deposition and changes in electrode weight were attributed to the build-up of nickel salt. It is observed that the sample obtained after 60 min of deposition had the highest weight gain compared to those obtained at shorter deposition times. There is a consistent increase in weight gain as deposition time progressed, due to sufficient reaction time for deposition of nickel salt on the working electrode.
Figure 5 shows the trend analysis of sample weight gain at different current densities and deposition times, which is in agreement with Equation (5).
3.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra of the composite electrodes show changes in peak intensities as deposition time increased from 5 to 60 min (shown in
Figure 6). Characteristic NiO IR absorption peaks are seen at lower wavenumbers between 470–530 and 980 cm
−1. The duplex peak at 620 cm
−1 are attributed to the presence of Ni-OH. NiO peak intensities increased with deposition time, as seen from the spectra. The sample obtained at 60 min of deposition shows the most intense peaks compared to samples obtained at 5 min of deposition. Graphene/polyimide peaks are not visible from the spectrum due to the thick nickel deposit layer on the composite surface. However, for the sample deposited at 5 min, the polyimide carbonyl peak at 1720 cm
−1 is clearly shown and the intensity of this peak slowly decreased as deposition time increased. It is rarely seen in samples deposited between 45 and 60 min.
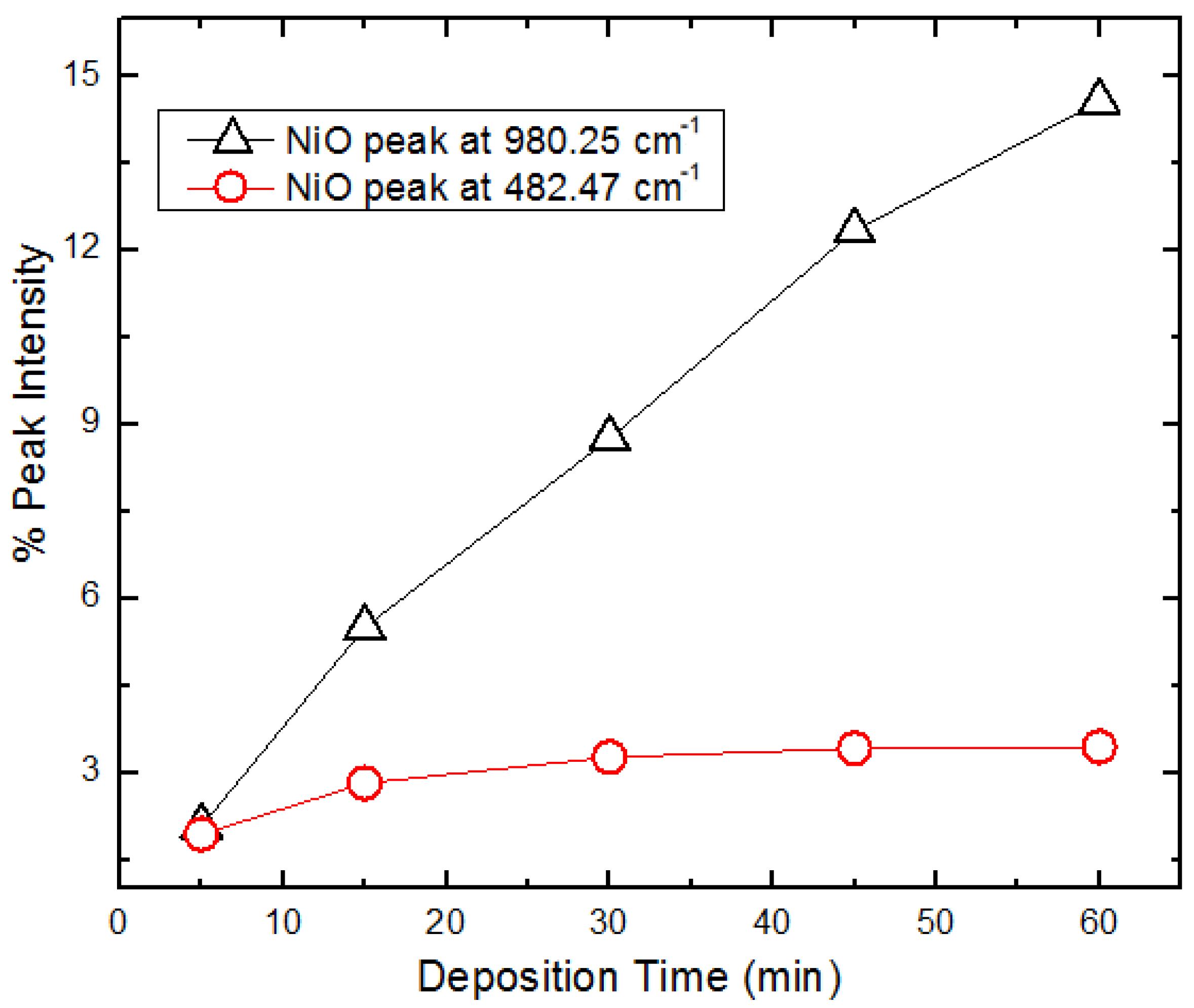
The FTIR peak intensities correlates with the gain in mass for the composite electrode as deposition time was increased due to increased layer of the deposit. A plot of peak intensity versus deposition times for the NiO peaks at 482 cm
−1 and 980 cm
−1 is shown in
Figure 7. There is a linear increase in peak intensity, suggesting the continuous growth of NiO particles with increasing deposition time.
3.2.3. Scanning Electron Microscopy, SEM
The SEM images taken after 5 min and 60 min of deposition are shown in
Figure 8. It is seen that at shorter deposition time of 5 min, just enough of the particles, are deposited and dispersed at various spots. However, at higher deposition times of 60 min, several spots show aggregated structures due to a larger build-up of NiO particles. The presence of facile pores and voids on the GR/PI composite electrode provide multiple channels for the trapping of nickel oxide during the deposition process, forming a hybrid composite with multi-phase regions [
1]. The better control of deposition time and deposition rate would yield nano-structured particles with improved structural and electrochemical properties. Studies show that nanoporous NiO particles possess better intrinsic properties compared to aggregated NiO particulates [
12]. It is believed that the nanostructures contribute to higher pseudo-capacitance due to increased surface area in contact with the electrolyte ions [
12]. The NiO particles deposited onto the composite electrodes after a long deposition time and current density of 250 mA/cm
−2 show an aggregated structure, which leads to lower capacitances than typically observed for porous NiO [
10,
11,
12].
3.2.4. Electrochemical Impedance Spectroscopy, EIS
Electrochemical measurements on the composite electrodes prepared after 5 to 60 min of deposition showed immense variations in the properties of NiO-GR/PI composites.
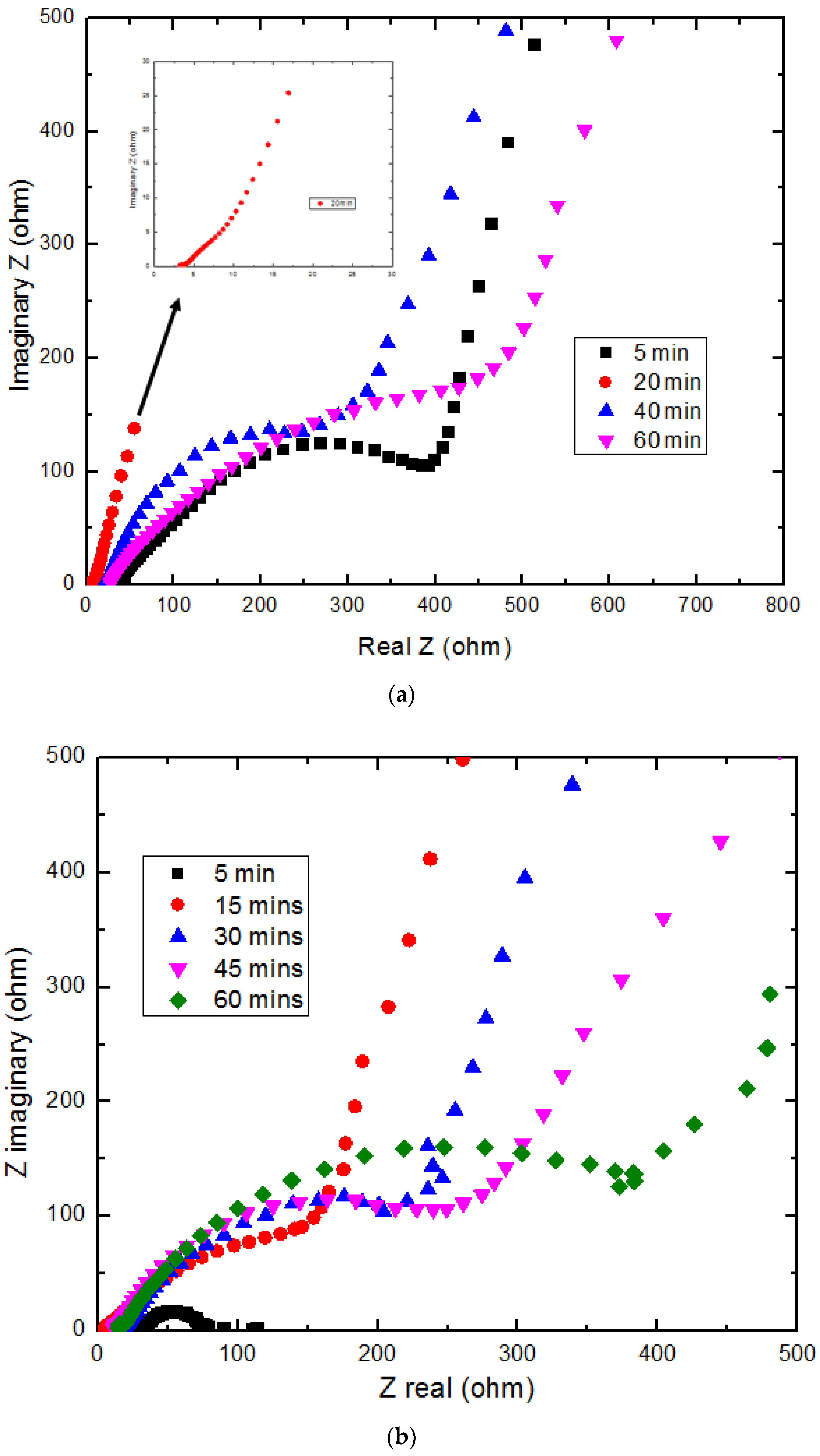
Figure 9a,b show the Nyquist plot of the sample deposited at different times at a current density of 25 and 250 mA/cm
2, respectively. It is observed that samples obtained at higher deposition times showed higher bulk resistance. This is due to a larger build-up of aggregated NiO particles, which decreased the total surface area available to electrolyte ions for charge transfer, thereby leading to very high charge transfer resistances. At shorter deposition times of 5 to 20 min, enough of the particles are deposited, thereby creating necessary pathways for electrolyte ions to interact with the NiO particles. In this study, the use of a higher current density ~250 mA/cm
2 during deposition caused a faster build-up rate of the particles and larger particle size compared to the samples obtained at lower current density ~25 mA/cm
2.
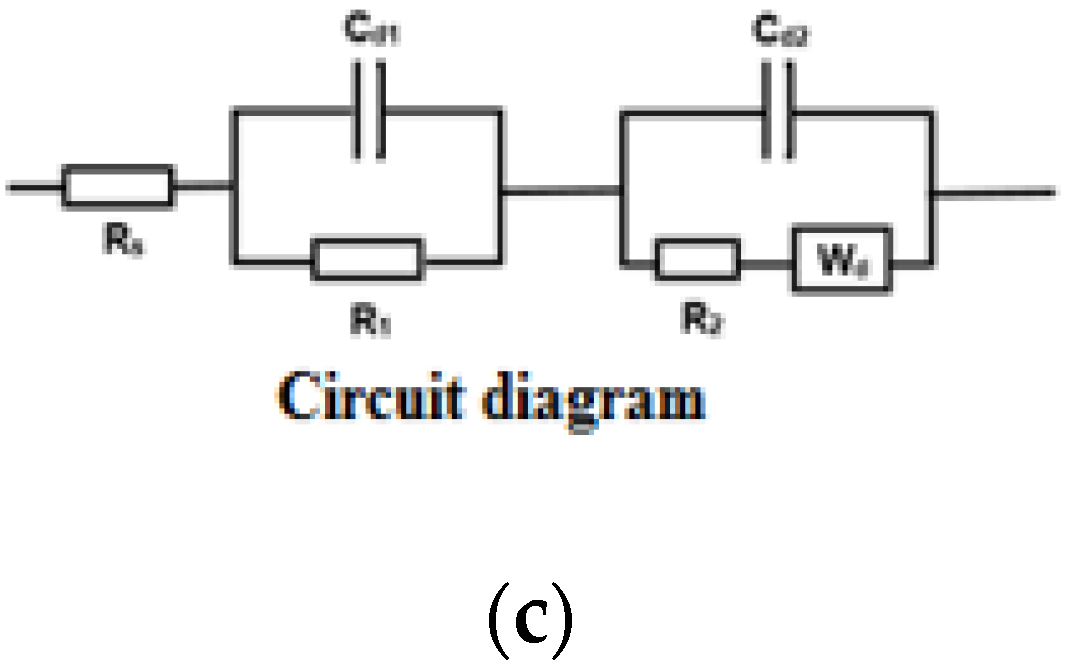
The equivalent circuit diagram shown in
Figure 9c was used to fit the Nyquist plot, and the bulk resistance values obtained are shown in
Figure 10. It is observed from the shapes of the Nyquist plots and bulk resistance values for both current densities that the sample deposited at a 20-min deposition time, using 25 mA/cm
2 current density, showed the lowest bulk resistance (~2.3 ohm). A linear increase in bulk resistance with deposition time is observed and this has been linked to the thickness of the NiO deposit/layer, which clogs up most of the pores, thereby limiting the electrolyte contact with the nickel oxide surfaces. This results in very high bulk resistances observed. Porosity is the key for achieving high performance and the aggregated NiO layer on the composite decreases the electrochemical properties. In summary, rate of deposition is affected by the current density and deposition time and it controls the NiO structure as well as the capacitive charging/discharging behavior of the composite electrode.
Figure 11a,b show the CV curves for NiO-GR/PI electrodes obtained at current densities of 25 and 250 mA/cm
2, respectively, at 5–60 min of deposition.
Figure 11a (CV run at 25 mA/cm
2) shows voltammograms with distinct redox peaks due to faradaic charge transfer process of nickel ions. NiO redox reaction in alkaline solution is given by Equation (7):
The CV curve for sample prepared at 20 min of deposition showed a large hysteresis loop with distinct cathodic and anodic peaks at ~0.2 and −0.16 V respectively, attributed to nickel oxide’s redox reaction shown (Equation (7)). The composites prepared at 5, 40 and 60 min showed similar redox peaks; however, these had smaller hysteresis loops compared to the composite formed at 20 min. CV curve for the composite prepared at 20 min of deposition shows a higher sweep current due to its low bulk resistances, the other composites showed low current sweep due to their higher bulk resistances. This behavior is linked to an increase in formation of aggregated structure of NiO at higher deposition times, resulting in lower charge storage ability.
Figure 11b (CV curves for 250 mA/cm
2) shows voltammograms that are stretched out and narrower with no visible redox peaks, except for the composite synthesized at 15 min of deposition time, which showed a distinct cathodic redox peak at ~0.23 V, due to the backward reaction process due to the reduction of Ni
3+ to Ni
2+ (Equation (7)). The anodic peak (oxidation process), though not visible, is present but occurs at a slower deposition rate than the latter because of other competing reactions. The CV curves for composites prepared at longer deposition times (30–60 min) showed smaller hysteresis loops confirming smaller current sweep due to their higher bulk resistances. In summary, similar characteristic CV curves were obtained at 15 and 20 min of deposition for both current densities, suggesting that 15–20 min time range to be the optimum deposition time for formation composite electrodes with better surface structures for charge storage. Longer deposition times yield highly aggregated non-porous NiO particles, which produce low specific capacitance.
A plot of specific capacitance as a function of deposition time for the composites is shown in
Figure 12. Higher capacitance values were obtained at shorter deposition times compared to the values obtained at higher deposition times. This is due to the non-porous and aggregated structure of the nickel oxide particles formed at longer electrodeposition times that tend to restrict electrolyte–electrode interaction. The NiO-GR/PI composite electrodes prepared at lower current density showed higher overall maximum specific capacitance of 513 F/g for the electrode prepared after 20 min of deposition time.
However, at higher deposition current density of 250 mA/cm2, the highest specific capacitance of about 330 F/g was obtained for the NiO-GR/PI composite, prepared at 5 min of electrodeposition. The further increase in the electrodeposition time resulted in a decreased specific capacitance.
The comparison of the electrochemical properties of the three composite electrodes, including the GR/PI composites, porous GR/PI composite and NiO-GR/PI composite, is shown in
Figure 13. The NiO-GR/PI composite resulted in significantly higher specific capacitance and lower bulk resistance than both the neat GR/PI and porous-GR/PI composites.
In fact, the specific capacitance of the hybrid NiO-GR/PI composite of about 640 F/g, which is about 3.7-times higher than that (170 F/g) produced by the porous-GR/PI composite and about 13 times higher than that produced by the neat GR/PI composite. It is demonstrated that, by systematically varying the composition and structure of the graphene/polyimide composite electrode, the electrochemical properties were remarkably improved. The combination of in situ condensation polymerization and electrochemical deposition with associated thermal treatment step, resulted in a hybrid composite electrode with remarkably improved morphology and performance.