Abstract
The content of pharmaceuticals in natural waters is steadily increasing. Especially nonsteroidal anti-inflammatory drugs (NSAIDs) are often detected in natural waters due to their widespread use. This group of compounds includes commonly used representatives, such as paracetamol and ketoprofen. The quality of natural waters determines the processes applied for the treatment of drinking water. The methods used in order to remove pharmaceuticals from treated water include adsorption and biologically active filtration. Both processes also occur during artificial infiltration (forced flow of intake surface water through the ground to the collecting wells) at surface water intakes. The processes, which occur in the soil, change the water quality characteristics to a great extent. The goal of the study was to evaluate the removal efficiency of paracetamol and ketoprofen in the process of artificial infiltration used as a pre-treatment of surface water. The studies were conducted at a field experimental installation located at the technical artificial infiltration intake. The experimental installation consisted of three metering wells (piezometers) which were located on the way between the bank of the infiltration pond and the collecting well. The collected water samples allowed to evaluate the change of selected NSAIDs concentrations during the passage of water through the ground. The analysis procedure included solid phase extraction (SPE) and high-performance liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Removal of the studied NSAIDs in the infiltration process occurred with variable effectiveness throughout the year. Paracetamol was removed with annual efficiency equal to 42%, although no significant removal of ketoprofen was observed.
1. Introduction
The pharmaceutical industry is among the most prosperous and rapidly developing sectors [1]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are a group of pharmaceuticals used in very high quantities, most of which are available without a prescription. NSAIDs include diclofenac, naproxen, ketoprofen and paracetamol. These drugs are used for treatment of degenerative diseases and rheumatic syndromes.
As a result of increased uptake, a significant increase in the level of pharmaceuticals present in surface waters has been observed during recent years [2,3,4,5,6,7,8]. Studies conducted in many countries have indicated the occurrence of NSAIDs in sewage, surface waters as well as tap water [9]. Municipal and hospital wastewater as well as improper disposal of out-of-date drugs are an important source of NSAIDs in the environment [10].
It should be highlighted that the occurrence of NSAIDs in natural waters is a serious threat to the environment. Their presence in water is dangerous for aquatic organisms, including fish, phytoplankton and zooplankton [11]. Furthermore, contamination of drinking water with pharmaceuticals is dangerous for infants, young children, the elderly as well as people with liver or kidney failure [12,13,14,15].
The European law has not yet set a limit for surface water contamination by drugs; however, on the 12 August 2013, the European Commission proposed the addition of 15 chemical compounds to the list of 33 pollutants that should be monitored in EU waters. These compounds include diclofenac [16] as the first NSAID pharmaceutical.
The natural surface waters are the source for production of drinking water. Removal of pharmaceuticals from surface waters often requires the use of advanced technological processes such as adsorption, membrane processes, oxidation or photodegradation process in the presence of semiconductors, typically metallic oxides [17].
The natural process of artificial infiltration, often applied at surface water intakes, can be a promising alternative. This solution is highly effective in water quality improvement and requires no additional reagents. The process is based on feeding groundwater with surface water through ponds and forcing the flow through the bottom of the pond and further through the ground to the wells receiving water after infiltration [8,18,19,20].
The infiltration time ranges from several to several dozen days. Such a long residence time of water in the ground allows to compare infiltration to slow filtration or a multifunctional reactor with an extremely large volume [21,22]. Various physical, chemical, physico-chemical and biochemical processes occur during the slow flow of water through the ground [18,19,20,23].
The composition of surface water after infiltration resembles groundwater in terms of quality. Implementation of the infiltration ponds into the treatment system allows to notably reduce the color, turbidity, content of organic compounds and microbiological parameters; however, the concentrations of iron and manganese may increase [24,25]. Artificial infiltration allows to notably simplify the treatment of surface water [5,26].
The infiltration process is commonly used in every region of the world, for example, in Hungary [19], Germany (Zurich, Dusseldorf and Berlin) [23], Poland (Warszawa, Legnica, Poznań, Bydgoszcz) [22], Australia [20] and Malaysia [18].
Many of these intakes have been operated since the beginning of the 20th century, while others are new objects (e.g., Bydgoszcz, Poland). Years of operation and technological observations confirmed the high efficiency of these techniques in terms of removal of organic compounds measured by the reduction in TOC, COD and color [18,19,22,23,24]. Scientists are now interested in the ability of the infiltration process to remove micropollutants, including pharmaceuticals [5,6,8,19,25]. This interest comes from understanding the complexity of physical, chemical and biological processes in the soil. However, it should be borne in mind that, despite the trials and proven rules of a general nature, each intake has its own specificity, which is manifested in the variability of the quality of surface water collected, hydrogeological parameters of the intake and its exploitation. For this reason, it is important to determine the individual’s capacity to improve water quality in terms of removing micropollutants. Research aimed at determining the effects of artificial infiltration should be carried out according to a known and confirmed research methodology, implementing a sensitive and precise analytical technique.
The aim of the study was to determine the effects of the artificial infiltration process on the removal of selected nonsteroidal anti-inflammatory and analgesic drugs (NSAIDs) from treated water.
The novelty of the presented research comes from the combination of a hydrological experiment, using a field research installation, that allows to track changes in water quality during water flow through the ground as well as from the use of the experimental method of determining the water retention time in the soil and a sensitive analytical method (based on solid state extraction and LC-MS/MS technique), that allow to elucidate the actual (considering the flow of the water) changes in the amount of analyzed compounds during infiltration. The removal efficiency of these contaminants was determined in relation to raw river and infiltration water.
2. Materials and Methods
2.1. Study Area and Experimental Installation
During the study, eleven research series were carried out, which were focused on the measurement of changes in the concentration of paracetamol and ketoprofen during the process of artificial infiltration, by collecting water samples at 5 points along its flow from the pond to the well.
The studies presented in this manuscript were conducted at the artificial infiltration intake for the city of 500,000 inhabitants in central Europe. The river water is pumped with no treatment to the infiltration ponds at the intake. The experimental installation consisted of three metering wells (piezometers)—marked as PP-1, PP-2 and PP-3—which were located on the way between the bank of the infiltration pond (one of 27 operating ponds at the intake) and one of the intake wells included in the water collecting system—levar II. The levar II collects water from 92 wells with average capacity of 1200 m3/h. The infiltration pond is 750 m long and approximately 20 m wide at the bottom. The depth of the metering wells is equal to 5.5, 7.5 and 9.5 m, respectively. The diameter of the metering wells is equal to 10 cm. The depth of piezometers is correlated with the infiltration water level in the ground. The cross section of the infiltration path is shown in Figure 1.
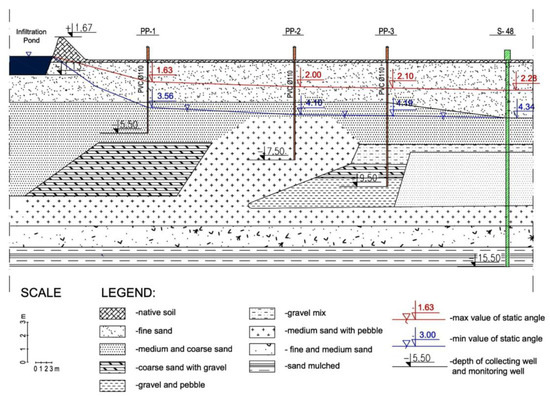
Figure 1.
The cross section of the infiltration path [22].
The passage starts at the bottom of the pond as the infiltration water enters the ground (aeration and saturation zones), with three piezometers on its way, and ends with the well S-48. The passage direction is perpendicular to the line of the pond bank and the line of collecting wells.
The retention time of infiltrated water in the ground was evaluated based on temperature measurements. The temperature of water was measured in the infiltration pond, in the piezometers and in the collecting well S-48, approximately two meters below the water level, every 2 weeks.
2.2. Analytical Methods
The NSAID analysis procedure included the separation and concentration of analytes from water samples by means of solid phase extraction (SPE) and determination based on high-performance liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) using an UltiMate 3000 RSLC liquid chromatograph (Dionex, Sunnyvale, CA, USA) coupled with an API 4000 QTRAP mass spectrometer (Biosystems MDS Sciex, Foster City, CA, USA). A C18 cartridge (3 mL, 500 mg, J.T. Baker) was used for SPE. A Baker vacuum system (J. T. Baker, The Netherlands) was used for the preconcentration step. Firstly, the SPE cartridge was conditioned with 5 mL of methanol followed by 5 mL of deionized water, at a flow rate of 1 mL/min. After the conditioning step, a 200 mL water sample was percolated through the cartridge at a flow rate of 10 mL/min. The cartridge was dried under vacuum for 20 min in order to remove excess water. Elution of the analytes from the cartridges was performed by using two 2.5-mL aliquots of methanol. The extracts were evaporated under a gentle stream of nitrogen and reconstituted in the mobile phase to a final volume of 0.2 mL for further LC-MS/MS analysis.
LC analysis was performed using the UltiMate 3000 RSLC chromatographic system. Five µL samples were injected into a 100 × 2.1 mm I.D. analytical column packed with 1.9 µm Hypersil GOLD C18 RP from Thermo Scientific (Waltham, MA, USA). In the experiments, the column was kept at 35 °C and the mobile phase consisted of 5 mM ammonium acetate in water (A) and methanol (B), at a flow rate of 0.2 mL/min. The following gradient was used: 0 min 30% B, 10 min 67% B, 12 min 100% B.
The LC system was connected with the API 4000 QTRAP triple quadrupole mass spectrometer. The Turbo Ion Spray source operated in negative ion mode. The dwell time for mass transition detected in the MS/MS multiple reaction monitoring mode (MRM) was set at 70 ms. All the studied NSAIDs were detected using the following settings for the ion source and mass spectrometer: curtain gas 10 psi, nebulizer gas 45 psi, auxiliary gas 45 psi, temperature 350 °C, ion spray voltage −4500 V and collision gas set to medium. The MS/MS parameters used for quantitative determination of NSAIDs are presented in Table 1. [M-H]- complexes of tested NSAIDs were used as precursor ions.

Table 1.
MS/MS parameters for the acquisition of NSAIDs.
The concentrations of NSAIDs were determined using the standard curve technique.
3. Results
Analysis of changes of water temperature in soil throughout the infiltration route allowed to determine the infiltration time between the measuring points. Water which infiltrated in the soil flows in time determined by waves with the same temperature. The temperature was measured during the experiment at measuring points every 1–2 weeks. The graph that presents the temperature of the water measured at the measuring points is shown in Figure 2. The graphs of temperature changes during the measurement year at each measurement point are sinusoidal. The distances between the curves’ maxima (summer) and minima (winter) correspond to the times of travel of the wave of the same temperature and the same times of water flow between the measuring points. The distances of the measuring points from the bank of the pond and the average time when the water overcomes this distance are presented in Table 2.
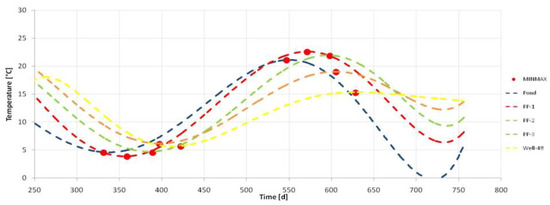
Figure 2.
Temperature changes in the sampling points during the research period from October 2018 to March 2020.

Table 2.
The distance of measuring points from the bank of the pond and retention time.
The concentrations of selected NSAIDs in water collected from the measuring points were determined every month. Eleven research series were carried out during the entire considered period. Values of paracetamol and ketoprofen concentrations measured at measuring points throughout the test period are presented in Figure 3. The relationships presented in Figure 3 were used to determine the approximate concentrations of the measured NSAID compounds at the given piezometer after the retention time corresponding to the time of water flow to this metering point.
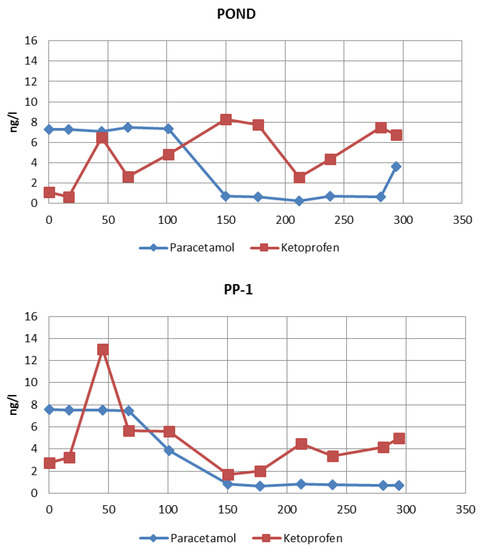
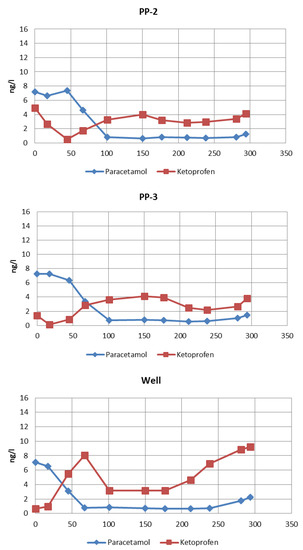
Figure 3.
Concentrations of the studied pharmaceuticals in water collected from measuring points during the experiments.
The concentration of pharmaceuticals in the infiltration pond was subjected to significant fluctuations during the study period. The occurrence of NSAIDs (especially paracetamol) in pond water is periodic and fluctuations result from increased use of anti-inflammatory drugs during periods of colds. In order to determine the actual effect of pharmaceuticals removal during filtration in the soil, it is necessary to consider the time which is required for water to travel the distance from one measuring point to another. Based on the data presented in those graphs, the concentration values of the tested pharmaceuticals were approximated for a given initial concentration in the pond at subsequent measuring points after the time corresponding to the retention of water in the soil (Table 2). The approximated concentrations are presented in Table 3. The water characteristics at measuring points during the study are presented in Table 4.

Table 3.
Approximated concentrations of tested NSAID in water samples after the time of retention in the ground.

Table 4.
The average season values of water quality parameters measured at measuring points during the research period.
4. Interpretation and Discussion of Results
The concentrations of paracetamol and ketoprofen measured during the year, which were presented above (Figure 3, pond), show that the content of paracetamol in water collected from the pond ranged from 0.27 to 7.46 ng/L. It can be stated that the paracetamol concentration fluctuated, giving higher values in winter and spring, which corresponds with the higher NSAID consumption in these seasons. The content of ketoprofen in water samples from the pond ranged from 0.65 to 8.2 ng/L. It can be observed that the concentration of ketoprofen in surface pond water does not depend on the season, as in the case of paracetamol (Figure 3, pond).
Graphs presented in Figure 4 were prepared after considering the retention for the determined approximate concentrations of pharmaceuticals at the measuring points (pond, piezometers and well). Values presented in Figure 4 are the average season values. In the case of paracetamol, there is a clear decrease in concentration during infiltration in summer when the temperature of water is higher (Figure 2). In the case of ketoprofen, a significant decrease in concentration during infiltration was observed in fall. The air temperature and pond water temperature in this particular fall were high, comparable with summer temperatures (Table 4). In this season, the average concentration of ketoprofen decreased from 6.84 to 3.63 ng. It can be observed that an increase in water temperature favors the processes of removing paracetamol and ketoprofen from infiltrating water.
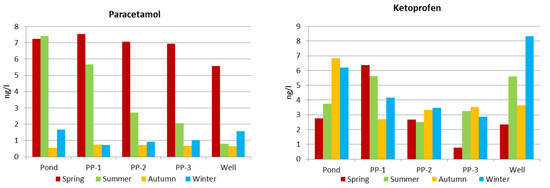
Figure 4.
Concentrations of paracetamol and ketoprofen throughout the infiltration route in subsequent measurement series.
Figure 5 shows the average annual concentrations of the tested pharmaceuticals at measurement points after the retention time in soil. During the assessment of the annual average concentration of paracetamol, it should be noted that it decreases gradually and steadily during the flow of infiltrating water. The annual ketoprofen concentration decreases on the way from the pond to the PP3 piezometer. Then, an increase in ketoprofen in the water taken from the well occurs. The greatest increase in ketoprofen in the well occurred in winter (Figure 4). This resulted in a low average annual ketoprofen removal efficiency. The detected increase in ketoprofen in the well may be the effect of the desorption phenomenon. This phenomenon has been described in the literature devoted to the migration of pharmaceuticals in the ground [12].
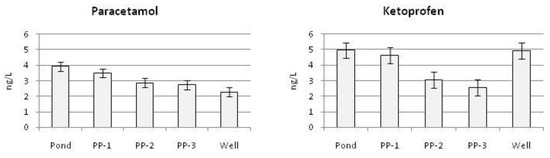
Figure 5.
Annual average concentrations of paracetamol and ketoprofen throughout the infiltration route in subsequent measurement series.
The approximate values of paracetamol and ketoprofen concentrations (Figure 5) were used to determine the average annual removal efficiency of these pharmaceuticals from infiltration water. The average annual removal efficiency was calculated according to the formula:
where Cpond is the average concentration of the studied pharmaceutical in water from the infiltration pond during the research period and CS48 is the average concentration of the studied pharmaceutical in water from well S-48 during the research period.
The removal efficiency of the studied pharmaceuticals in the infiltration process during the research period, along with their specific characteristic, is presented in Table 5. The parameters presented in Table 5 are helpful for analysis of the obtained results and evaluation of the ability of tested drugs to undergo biodegradation and adsorption. Many contaminants, including NSAIDs present in the water, undergo biodegradation and/or adsorption during the ground passage of infiltration [22].

Table 5.
Paracetamol and ketoprofen characteristics and annual average removal efficiency in the infiltration process during the research period.
The logP value, shown in Table 5, is a log10 of partition coefficient—P. The compound is more hydrophilic (which means it has a higher affinity for the aqueous phase) when the logP has negative value. Log10 value (logP) is the partition coefficient between octanol and water (P). Hydrophilicity is a measure of the tendency of chemical molecules to associate with water. Compounds with logP > 3 are characterized by low hydrophilicity. Among the two analyzed NSAID substances, paracetamol (logP = 0.46) is more hydrophilic, which may suggest its higher biodegradability in comparison to ketoprofen. In turn, ketoprofen, characterized by low hydrophilicity (more lipophilic) (logP = 3.12), has a higher bioaccumulation potential and adsorbs better in sediments [7].
According to literature data, the acidic environment increases the susceptibility of the studied pharmaceuticals to the sorption processes, which reduces the effectiveness of microbiological decomposition [27]. In the infiltration process, the pH value decreased along the ground passage (Table 4), which results in a gradual advantage of adsorption at the expense of biodegradation as water flows. Adsorption may occur on soil grains, especially on iron oxide coated soil grains near the well. The iron oxides may be considered as a mesoporous adsorbent [28,29] with the ability to remove organics, including NSAIDs.
The strength of an acid, pKa, is a parameter which characterizes how weak or strong an acid is. The pKa is defined as the negative base-10 logarithm of the acid dissociation constant (Ka) of a solution. The lower the pKa value, the stronger the acid. Pharmaceuticals characterized by an acidic nature are poorly adsorbed and circulate in the water phase [30]. Ketoprofen is a derivative of propionic acid and is acidic in nature. The pKa value for ketoprofen is 4.8; therefore, it is poorly adsorbed and remains in the water phase [7]. In turn, paracetamol is a very weak acid and is characterized by a much higher value, pKa = 9.7, which determines its better absorption ability [31]. This means that, apart from biodegradation, paracetamol can also undergo a sorption process, thus increasing the removal efficiency compared to ketoprofen.
The literature states that heterotrophic bacteria are present at the first stage of infiltration in the water rich of organics. The heterotrophic bacteria, which include Zoogleara migera, Pseudomonas fluorescens, Pseudomonas putida, Achromobacter sp., Flavobacterium sp. (gram negative bacteria), Micrococcus sp. as well as Bacillus sp. (gram positive bacteria), are primarily aerobic (except for Micrococcus sp., which is an anaerobic, and Bacillus sp. which is facultative anaerobic), mesophilic bacteria, for which the optimal growth temperature is 30–40 °C, while the optimal pH of the environment is approximately 7. The analysis of data presented in Table 4 indicates that the water in the pond is well oxygenated. At the first section of infiltration, from the pond to PP1, aerobic conditions prevail, and the organic compounds are present, as shown in Table 4 by the TOC parameter. The concentration of organic compounds, measured with the TOC value, decreased from (9.02 ÷ 4.66) to (5.64 ÷ 0.79) on the way from the pond to PP1. The first part of the infiltration path is characterized by the highest removal efficiency of organics. In the next section, from PP1 to PP2, the oxygen level drops to a value lower than 2.0 mg/L. Consequently, there are good conditions for the development of aerobic heterotrophic bacteria in the first section, while in the second section, facultative and anaerobic heterotrophic bacteria may be responsible for the removal of organics. In the first section of the infiltration route, paracetamol is removed in summer from an average value of 7.41 to 5.67 ng/L. The overall efficiency of paracetamol removal in summer is high, reaching 89% (decrease in concentration from 7.41 in the pond to 0.81 ng/L in the well, Figure 4). These high effects were favored by the high water temperature in the pond, equal to 21.4 °C. The highest efficiency of ketoprofen removal, equal to 46.86%, was observed in fall, with the average water temperature at this time of year equal to 16.3 °C (in the pond).
As the water flows, the environment becomes depleted in organic matter, which contributes to the death of heterotrophic bacteria.
The molar mass of paracetamol (Table 5) is much lower than that of ketoprofen. It is an additional factor that increases the effects of paracetamol biodegradation compared to ketoprofen.
Nonsteroidal anti-inflammatory drugs which do not belong to salicylate derivatives (naproxen, ketoprofen, paracetamol and ibuprofen) are degraded to a high extent by fungi using lignolytic enzymes and the cytochrome P-450 system [32]. In the process of pharmaceutical transformation and degradation, the fungi belonging to the Cunninghamella elegans, Beauveria bassiana and Cunninghamella echinulate species take action [33,34]. The optimal temperature for the growth of these species is 25–30 °C and the pH is 6–8. The analysis of data presented in Table 4 indicates that, in the first section, from the pond to PP1, in summer and fall (with higher air and water temperatures in the pond), there are good conditions for the development of these organisms, which increases the removal efficiency of the tested NSAID compounds.
5. Conclusions
The concentration of both tested NSAIDs changed in river water during the research year. The concentration of paracetamol ranged from 0.27 to 7.46 ng/L and clearly depends on the seasons, giving higher values in winter and spring and showing a sinusoidal trend. In turn, ketoprofen content changes in a similar concentration range from 0.65 to 8.2 ng/L; however, the changes do not correlate with the seasons.
Temperature is a particularly important parameter in biodegradation processes, which significantly affects the removal of pharmaceuticals. For paracetamol, there is a clear decrease in concentration in summer when the temperature of water is higher. For ketoprofen, a significant decrease in concentration during infiltration was observed in fall, which was relatively warm. In this season, the average concentration of ketoprofen decreased from 6.84 to 3.63 ng. It can be observed that an increase in water temperature favors the processes of removing paracetamol and ketoprofen from infiltrating water.
Paracetamol decreases gradually and steadily during the flow of infiltrating water. The annual ketoprofen concentration decreases on the way from the pond to the PP3 piezometer. Then, an increase in ketoprofen in the water taken from the well occurs especially in winter. This resulted in a very low average annual ketoprofen removal efficiency. Desorption is a phenomenon that may affect the final removal effect of NSAIDs during infiltration, which was observed in the case of ketoprofen.
Paracetamol was removed during infiltration with the annual average efficiency equal to 42%, but ketoprofen was not removed efficiently.
The characteristic chemical parameters of pharmaceuticals are helpful for predicting the ability of tested drugs to undergo biodegradation and adsorption. Taking into account the hydrophilic properties and logP criterion, paracetamol shows greater biodegradability than ketoprofen. The lower molecular weight of paracetamol also contributes to its better biodegradation compared to ketoprofen.
Taking into account the pKa parameter, paracetamol can also undergo a sorption process (to a greater extent than ketoprofen), thus increasing its removal.
Further research should focus on the analysis based on molecular biology. The results of such an analysis would allow to check the presence of microorganisms, including bacteria and fungi, responsible for the biodegradation of pharmaceuticals present in the infiltrating water. This, in turn, would allow for a more precise determination and experimentation of the conditions for optimal biodegradation of pharmaceuticals.
Author Contributions
Conceptualization, A.M.; data curation, A.M., J.J.-W. and A.S.; formal analysis, A.M., Z.D., J.J.-W., A.S. and J.Z.; funding acquisition, Z.D. and J.Z.; investigation, A.M., Z.D., J.J.-W., A.S. and J.Z.; methodology, A.M., J.J.-W. and J.Z.; project administration, Z.D. and J.Z.; resources, Z.D., J.J.-W. and J.Z.; supervision, Z.D., J.J.-W. and J.Z.; validation, Z.D., J.J.-W. and J.Z.; visualization, A.M. and A.S.; writing—original draft, A.M. and J.J.-W.; writing—review and editing, Z.D., J.J.-W., A.S. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Poznan University of Technology, grant numbers 504101/0713/SBAD/0948 and 0911/SBAD/2105.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taylor, D. The Pharmaceutical Industry and the Future of Drug Development. In Pharmaceuticals in the Environment; Royal Society of Chemistry: London, UK, 2015; pp. 1–33. [Google Scholar]
- Kot-Wasik, A.; Dębska, J.; Wasik, A.; Namieśnik, J. Detremination of nonsteroidal antiinflammatory drugs in natural waters using off-line and on-line SPE followed by LC coupled with DAD-MS. Chromatographia 2006, 64, 13–21. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [Green Version]
- Rocca, L.M.; Gentili, A.; Caretti, F.; Curini, R.; Pérez-Fernández, V. Occurrence of non-steroidal antiinflammatory drugs in surface waters of Central Italy by liquid chromatography–tandem mass spectrometry. Int. J. Environ. Anal. Chem. 2015, 95, 685–697. [Google Scholar] [CrossRef]
- Hamman, E.; Stuyfzanw, P.J.; Greskowiak, J.; Timmer, H.; Massmann, G. The fate of organic micropollutants during long- term/ long- distance river bank filtration. Sci. Total Environ. 2016, 545/546, 629–640. [Google Scholar] [CrossRef]
- Kovačević, S.; Radišić, M.; Laušević, M.; Dimkić, M. Occurrence and behavior of selected pharmaceutical during riverbank filtration in The Republic of Serbia. Environ. Sci. Pollut. Res. 2017, 24, 2075–2088. [Google Scholar] [CrossRef]
- Szymonik, A.; Lach, J. Zagrożenia środowiska wodnego obecnością środków farmaceutycznych. Inżynieria i Ochrona Środowiska 2012, 15, 249–263. [Google Scholar]
- Kruć, R.; Dragon, K.; Górski, J. Pharmaceuticals in River and bank filtrate water in Krajkowo (Poland). Biul. PIG 2019, 475, 109–116. [Google Scholar] [CrossRef]
- Caban, M.; Lis, E.; Kumirska, J.; Stepnowski, P. Determination of pharmaceutical residues in drinking water in Poland using a new SPE-GC-MS (SIM) method based on Speedisk extraction disks and DIMETRIS derivatization. Sci. Total Environ. 2015, 538, 402–411. [Google Scholar] [CrossRef]
- Zając, A.; Zembrzuska, J.; Kruszelnicka, I.; Ginter-Kramarczyk, D. Methods for removing pharmaceuticals and their metabolites from water and wastewater. Przem. Chem. 2015, 94, 76–80. [Google Scholar]
- Fent, K. Effects of pharmaceuticals on aquatic organisms. In Pharmaceuticals in the Environment; Springer: Berlin/Heidelberg, Germany, 2008; pp. 175–203. [Google Scholar]
- Okońska, M.; Marciniak, M.; Zembrzuska, J.; Kaczmarek, M. Laboratory investigations of diclofenac migration in saturated porous media—A case study. Geologos 2019, 25, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, L.; Fiorentino, A.; Grassi, M.; Attanasio, D.; Guida, M. Advanced treatment of urban wastewater by sand filtration and graphene adsorption for wastewater reuse: Effect on a mixture of pharmaceuticals and toxicity. J. Environ. Chem. Eng. 2015, 3, 122–128. [Google Scholar] [CrossRef]
- Soubrier, M.; Rosenbaum, D.; Tatar, Z.; Lahayea, C.; Dubost, J.-J.; Mathieu, S. Vascular effects of nonsteroidal antiinflammatory drugs. Jt. Bone Spine 2013, 80, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Arfe, A.; Scotti, L.; Varas-Lorenzo, C.; Nicotra, F.; Zambon, A.; Kollhorst, B.; Schink, T.; Garbe, E.; Herings, R.; Straatman, H.; et al. Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: Nested case-control study. Br. Med. J. 2016, 354, 4857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EU. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy; EU: Maastricht, The Netherland, 2013. [Google Scholar]
- Bohdziewicz, J.; Kudlek, E.; Dudziak, M. Influence of the catalyst type (TiO2 and ZnO) on the photocatalytic oxidation of pharmaceuticals in the aquatic environment. Desalination Water Treat. 2016, 57, 1552–1563. [Google Scholar] [CrossRef]
- Ibrahim, N.; Aziz, H.A.; Suffian, Y.M. River Bank Filtration: Study of Langat River Water and Borehole Water Quality. Mech. Mater. 2015, 773–774, 1194–1198. [Google Scholar] [CrossRef] [Green Version]
- Nagy-Kovács, Z.; László, B.; Fleit, E.; Czihat-Mártonné, K.; Till, G.; Börnick, H.; Adomat, Y.; Grischek, T. Behavior of Organic Micropollutants during River Bank Filtration in Budapest, Hungary. Water 2018, 10, 1861. [Google Scholar] [CrossRef] [Green Version]
- Rose, A.K.; Fabbro, L.; Kinnear, S. Cyanobacteria breakthrough: Effects of Limnothrix redekei contamination in an artificial bank filtration on a regional water supply. Harmful Algae 2018, 76, 1–10. [Google Scholar] [CrossRef]
- Dillon, P.J.; Miller, M.; Fallowfield, H.; Hutson, J. The potential of riverbank filtration for drinking water supplies in relation to microsystin removal in brackish aquifers. J. Hydrol. 2002, 266, 209–221. [Google Scholar] [CrossRef]
- Cierniak, D.; Dymaczewski, Z.; Jeż-Walkowiak, J.; Makała, A.; Wyrwas, B. Impact of artificial infiltration on removal of surfactants in surface water treatment process. Desalination Water Treat. 2020, 199, 241–251. [Google Scholar] [CrossRef]
- Grünheida, S.; Amy, G.; Jekel, M. Removal of bulk dissolved organic carbon (DOC) and trace organic compounds by bank filtration and artificial recharge. Water Res. 2005, 39, 3219–3228. [Google Scholar] [CrossRef]
- Weiss, W.J.; Bouwer, E.J.; Aboytes, R.; LeChevallier, M.W.; O’melia, C.R.; Le, B.T.; Schwab, K.J. Riverbank filtration for control of microorganisms: Results from field monitoring. Water Res. 2005, 39, 1990–2001. [Google Scholar] [CrossRef]
- Maeng, S.K.; Ameda, E.; Sharma, S.K.; Grutzmacher, G.; Amy, G.L. Organic micropollutant removal from wastewater effluent- impacted drinking water sources during bank filtration and artificial recharge. Water Res. 2010, 44, 4003–4014. [Google Scholar] [CrossRef]
- Lasagna, M.; Deluca, D.A.; Franchino, E. Nitrates contamination of groundwater in the western Po Plain (Italy): The effects of groundwater and surface water interactions. Environ. Earth Sci. 2016, 75, 240. [Google Scholar] [CrossRef]
- Zając, A. Skuteczność Usuwania Wybranych Niesteroidowych Leków Przeciwzapalnych ze Ścieków Metodą Osadu Czynnego. Ph.D. Thesis, Poznan University of Technology, Poznan, Poland, 2017; pp. 1–189. [Google Scholar]
- Jeż-Walkowiak, J.; Dymaczewski, Z. Effectiveness of oxidative filter materials for manganese removal from groundwater. J. Water Supply Res. Technol. AQUA 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Dymaczewski, Z.; Falkowska, J.; Frąckowiak, A.; Jeż-Walkowiak, J.; Nawrot, J.; Dudek, L.; Topór, T. The impact of microstructure of filtration materials on its auto-activation for manganese removal from groundwater. Minerals 2020, 10, 502. [Google Scholar] [CrossRef]
- Wandzik, I. Materiały Pomocnicze do Przedmiotu “Związki Biologicznie Aktywne” dla Studentów Specjalności Chemia Bioorganiczna; Silesian Univeristy of Technology Publishing House: Gliwice, Poland, 2013. [Google Scholar]
- Lin, A.Y.; Lin, C.A.; Tung, H.H.; Chary, N.S. Potential for biodegradation and sorption of acetaminophen, caffeine, propanololacebutolol in lab-scale aqueous environments. J. Hazard. Mater. 2010, 183, 242–250. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Mazur, A.; Wojcieszyńska, D. Biotransformacja wybranych niesteroidowych leków przeciwzapalnych w środowisku. Bromatol. Chem. Toksykol. 2013, 1, 105–112. [Google Scholar]
- Domagała, M.; Wanot, B. Zanieczyszczenie wody hormonami i innymi farmaceutykami oraz ich degradacja. Technol. Wod. 2019, 4, 30–34. [Google Scholar]
- Rezka, P.; Balcerzak, W. Occurrence of antibiotics in the environment. Tech. Trans. 2016, 1-Ś, 133–143. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).