Biomass Potential for Producing Power via Green Hydrogen
Abstract
:1. Introduction
2. Materials and Methods
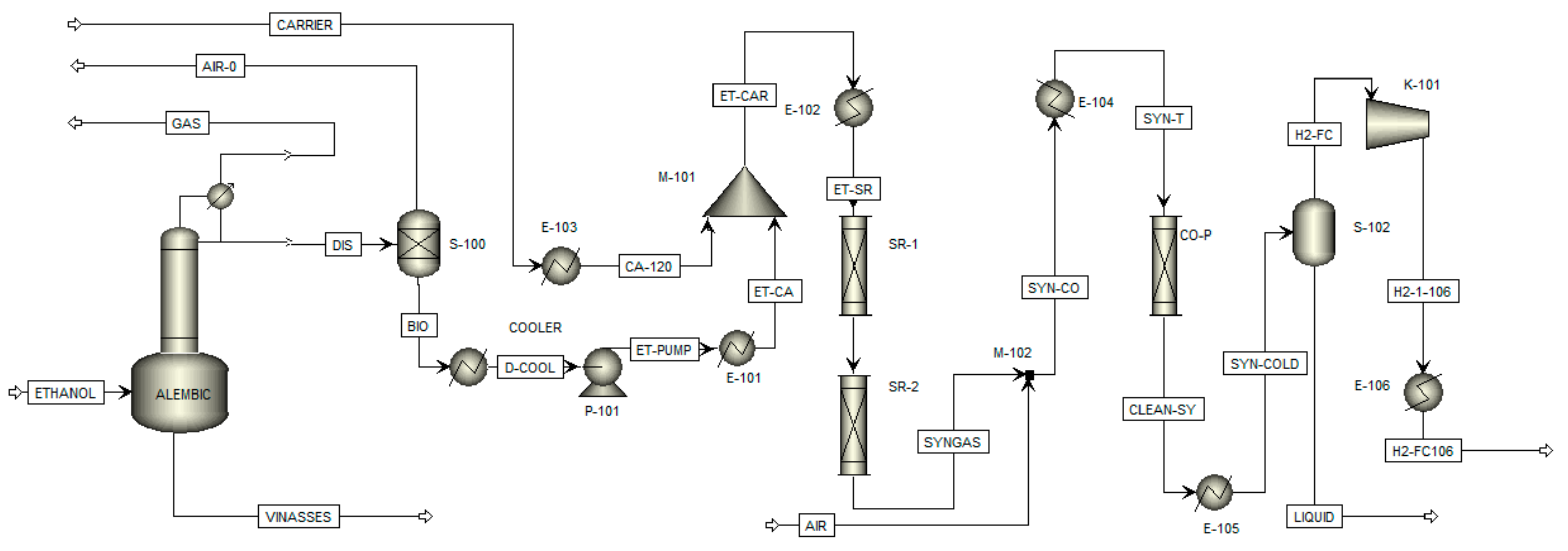
2.1. Aspen Plus® Simulation
2.1.1. Alembic
2.1.2. Ethanol Steam Reforming
2.1.3. Carbon Monoxide Removal
2.2. Matlab
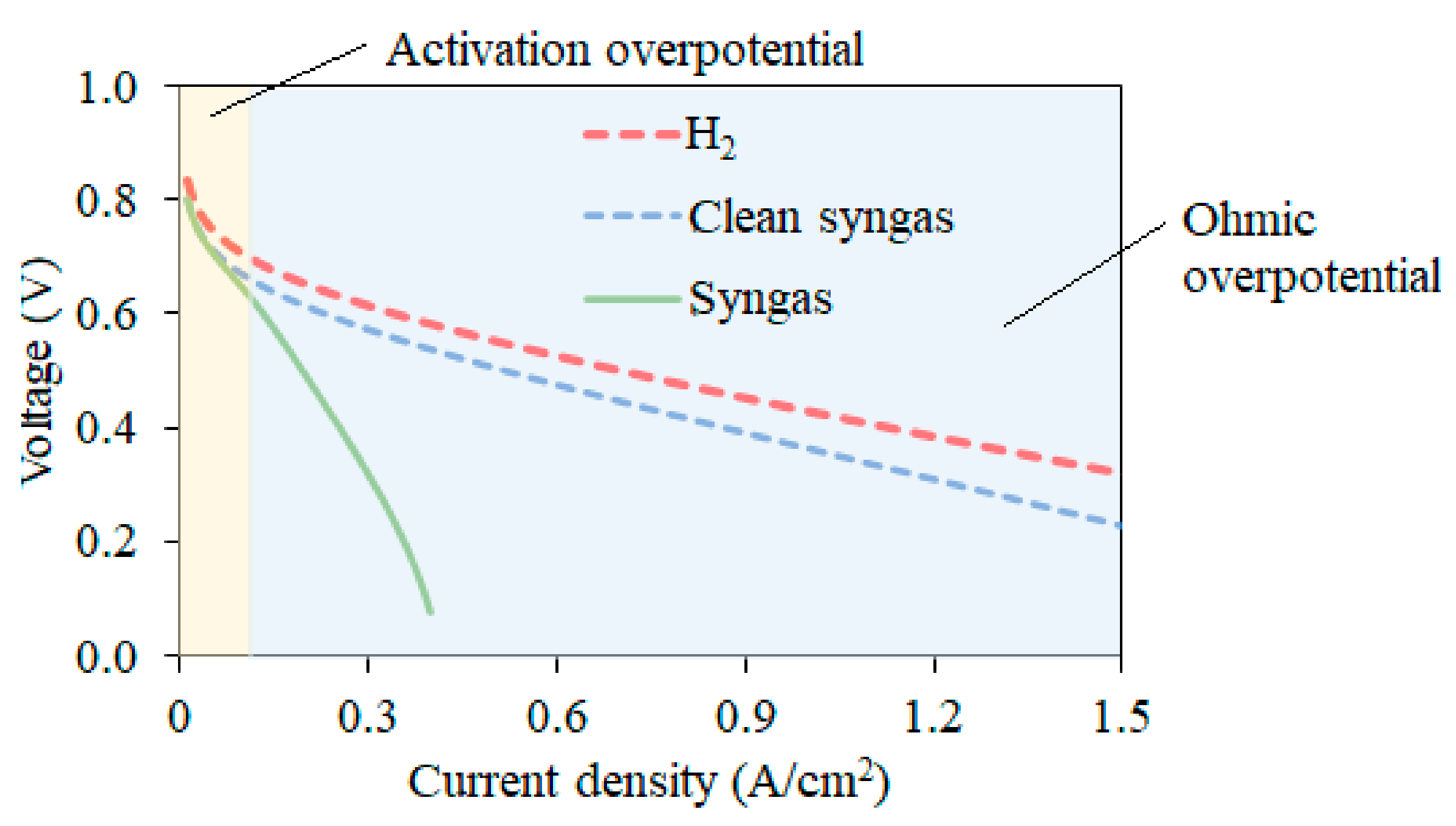
High Temperature Proton Exchange Membrane Fuel Cell (HT-PEMFC)
2.3. Response Surface Methodology (RSM)
3. Results
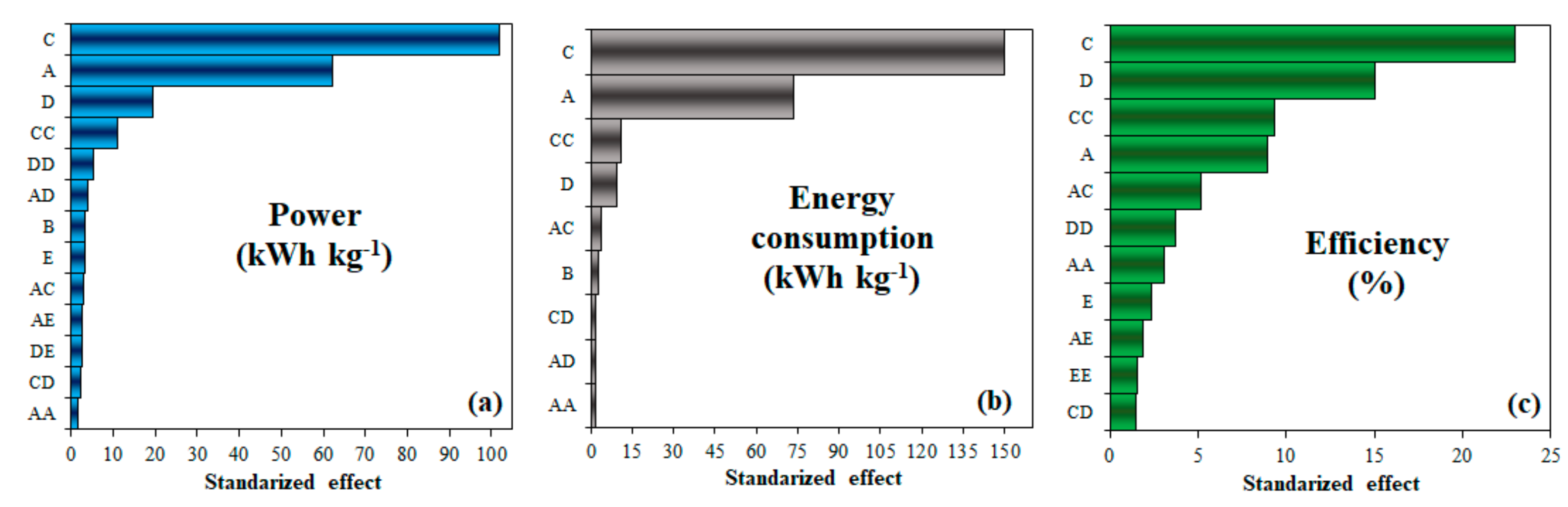
3.1. Box–Behkhen Design
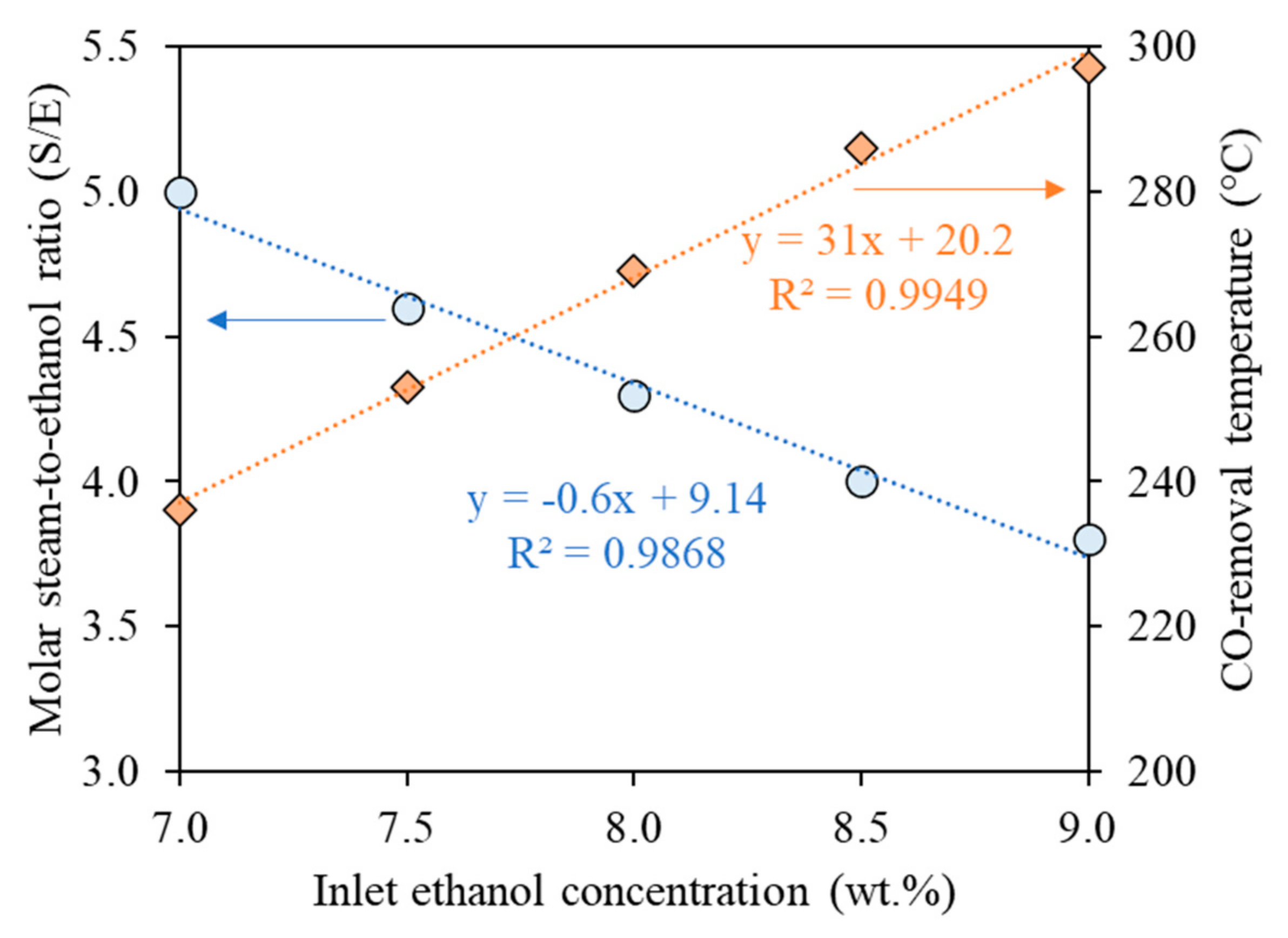
3.1.1. Steam-to-Ethanol Ratio (S/E)
3.1.2. Ethanol Concentration
3.1.3. Steam Reforming Temperature
3.1.4. CO-Removal Temperature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sachs, J.D.; Schmidt-Traub, G.; Mazzucato, M.; Messner, D.; Nakicenovic, N.; Rockström, J. Six Transformations to achieve the Sustainable Development Goals. Nat. Sustain. 2019, 2, 805–814. [Google Scholar] [CrossRef]
- McKinsey & Company. Hydrogen Insights: A Perspective on Hydrogen Investment, Market Development and Cost Competitiveness. Available online: https://hydrogencouncil.com/en/hydrogen-insights-2021/ (accessed on 19 November 2021).
- Hajjaji, N.; Pons, M.; Renaudin, V.; Houas, A. Comparative life cycle assessment of eight alternatives for hydrogen production from renewable and fossil feedstock. J. Clean. Prod. 2013, 44, 177–189. [Google Scholar] [CrossRef]
- Sanchez, N.; Ruiz, R.; Hacker, V.; Cobo, M. Impact of bioethanol impurities on steam reforming for hydrogen production: A review. Int. J. Hydrogen Energy 2020, 45, 11923–11942. [Google Scholar] [CrossRef]
- Sanchez, N.; Ruiz, R.; Rödl, A.; Cobo, M. Technical and environmental analysis on the power production from residual biomass using hydrogen as energy vector. Renew. Energy 2021, 175, 104743. [Google Scholar] [CrossRef]
- Authayanun, S.; Hacker, V. Energy and exergy analyses of a stand-alone HT-PEMFC based trigeneration system for residential applications. Energy Convers. Manag. 2018, 160, 230–242. [Google Scholar] [CrossRef]
- Rossetti, I.; Compagnoni, M.; Torli, M. Process simulation and optimization of H2 production from ethanol steam reforming and its use in fuel cells. 2. Process analysis and optimization. Chem. Eng. J. 2015, 281, 1036–1044. [Google Scholar] [CrossRef]
- Cobo, M.; Pieruccini, D.; Abello, R.; Ariza, L.; Córdoba, L.F.; Conesa, J.A. Steam reforming of ethanol over bimetallic RhPt/La2O3: Long-term stability under favorable reaction conditions. Int. J. Hydrogen Energy 2013, 38, 5580–5593. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Kumar, A.; Prasad, R.; Upadhyay, S.N. Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renew. Sustain. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Rabenstein, G.; Hacker, V. Hydrogen for fuel cells from ethanol by steam-reforming, partial-oxidation and combined auto-thermal reforming: A thermodynamic analysis. J. Power Sources 2008, 185, 1293–1304. [Google Scholar] [CrossRef]
- Angili, T.S.; Grzesik, K.; Rödl, A.; Kaltschmitt, M. Life cycle assessment of bioethanol production: A review of feedstock, technology and methodology. Energies 2021, 14, 2939. [Google Scholar] [CrossRef]
- Sanchez, N.; Cobo, M.; Rodriguez-fontalvo, D.; Uribe-laverde, M.Á.; Ruiz-pardo, R.Y. Bioethanol Production from Sugarcane Press-Mud: Assessment of the Fermentation Conditions to Reduce Fusel Alcohol. Fermentation 2021, 7, 194. [Google Scholar] [CrossRef]
- Coelho, T.C.; Souza, O.; Sellin, N.; Medeiros, S.H.W.; Marangoni, C. Analysis of the reflux ratio on the batch distillation of bioethanol obtained from lignocellulosic residue. Procedia Eng. 2012, 42, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Ramírez Gil, J.G. Characterization of traditional production systems of sugarcane for panela and some prospects for improving their sustainability. Rev. Fac. Nac. Agron. 2016, 70, 8045–8055. [Google Scholar] [CrossRef]
- Banerjee, S.; Mudliar, S.; Sen, R.; Giri, B.; Saptute, D.; Tapan, C.; Pandey, R. Commercializing lignocellulosic bioethanol: Technology bottlenecks and possible remedies. Biofuels Bioprod. Biorefining 2010, 4, 77–93. [Google Scholar] [CrossRef]
- Luna, R.; López, F.; Pérez-Correa, J.R. Minimizing methanol content in experimental charentais alembic distillations. J. Ind. Eng. Chem. 2018, 57, 160–170. [Google Scholar] [CrossRef]
- Cifuentes, B.; Valero, M.F.; Conesa, J.A.; Cobo, M. Hydrogen production by steam reforming of ethanol on Rh-Pt catalysts: Influence of CeO2, ZrO2 and La2O3 as supports. Catalysts 2015, 5, 1872. [Google Scholar] [CrossRef] [Green Version]
- Cifuentes, B.; Gómez, J.; Sánchez, N.; Proaño, L.; Bustamante, F.; Cobo, M. Bioethanol steam reforming over monoliths washcoated with RhPt/CeO2–SiO2: The use of residual biomass to stably produce syngas. Int. J. Hydrogen Energy 2021, 46, 4007–4018. [Google Scholar] [CrossRef]
- Sanchez, N.; Ruiz, R.Y.; Cifuentes, B.; Cobo, M. Controlling Sugarcane Press-Mud Fermentation to Increase Bioethanol Steam Reforming for Hydrogen Production. Available online: https://data.mendeley.com/datasets/g4jrbkk7v9/1 (accessed on 15 April 2019).
- Cifuentes, B.; Figueredo, M.; Cobo, M. Response Surface Methodology and Aspen Plus Integration for the Simulation of the Catalytic Steam Reforming of Ethanol. Catalysts 2017, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Cifuentes, B.; Bustamante, F.; Araiza, D.G.; Diaz, G.; Cobo, M. Hydrogen purification of actual syngas streams for energy applications: Au-Cu supported over nano-shaped CeO2 as stable catalysts for the carbon monoxide removal. Appl. Catal. A Gen. 2020, 598, 117568. [Google Scholar] [CrossRef]
- Cifuentes, A.; Torres, R.; Llorca, J. Modelling of the ethanol steam reforming over Rh-Pd/CeO2 catalytic wall reactors. Int. J. Hydrogen Energy 2019, 45, 26265–26273. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.K.; Tirkey, J.V. Modeling and multi-objective optimization of variable air gasification performance parameters using Syzygium cumini biomass by integrating ASPEN Plus with Response surface methodology. Int. J. Hydrogen Energy 2021, 46, 18816–18831. [Google Scholar] [CrossRef]
- Ozcan, O.; Akin, A.N. Thermodynamic analysis of methanol steam reforming to produce hydrogen for HT-PEMFC: An optimization study. Int. J. Hydrogen Energy 2019, 44, 14117–14126. [Google Scholar] [CrossRef]
- Kayathi, A.; Prasad, P.; Bonfim-rocha, L.; Cardozo-filho, L.; Bollampalli, A.; Jegatheesan, V. Extraction of γ-Oryzanol from defatted rice bran using supercritical carbon dioxide (SC-CO2): Process optimisation of extract yield, scale-up and economic analysis. Process Saf. Environ. Prot. 2021, 148, 179–188. [Google Scholar] [CrossRef]
- Zaman, S.A.; Roy, D.; Ghosh, S. Process modeling and optimization for biomass steam-gasification employing response surface methodology. Biomass Bioenergy 2020, 143, 105847. [Google Scholar] [CrossRef]
- Long, N.V.D.; Lee, M. Dividing wall column structure design using response surface methodology. Comput. Chem. Eng. 2012, 37, 119–124. [Google Scholar] [CrossRef]
- Hemmati, A.; Rashidi, H.; Hemmati, A.; Kazemi, A. Using rate based simulation, sensitivity analysis and response surface methodology for optimization of an industrial CO2 capture plant. J. Nat. Gas Sci. Eng. 2019, 62, 101–112. [Google Scholar] [CrossRef]
- Nalbant, Y.; Colpan, C.O.; Devrim, Y. Development of a one-dimensional and semi-empirical model for a high temperature proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2017, 43, 5939–5950. [Google Scholar] [CrossRef]
- Gwak, G.; Kim, M.; Kim, D.; Faizan, M.; Oh, K.; Lee, J.; Choi, J.; Lee, N.; Lim, K.; Ju, H. Performance and efficiency analysis of an HT-PEMFC system with an absorption chiller for tri-generation applications. Energies 2019, 12, 905. [Google Scholar] [CrossRef] [Green Version]
- Cifuentes, B.; Cifuentes, A.; Bustamante, F.; Soler, L.; Llorca, J.; Cobo, M. Monoliths washcoated with AuCu catalysts for CO removal in an ethanol fuel processor: Effect of CeO 2 e SiO 2 dual support on the catalytic performance and reactor cost. Int. J. Hydrogen Energy 2020, 46, 2166–2181. [Google Scholar] [CrossRef]
- Veljković, V.B. Comment on “study of fuel properties of rubber seed oil based biodiesel” [Energy Convers. Manage. 2014;78:266-275] by Ahmad et al. Energy Convers. Manag. 2014, 86, 1186–1188. [Google Scholar] [CrossRef]
- Sacher, J.; García-Llobodanin, L.; López, F.; Segura, H.; Pérez-Correa, J.R. Dynamic modeling and simulation of an alembic pear wine distillation. Food Bioprod. Process. 2013, 91, 447–456. [Google Scholar] [CrossRef]
- Sanchez, N.; Ruiz, R.; Plazas, A.; Vasquez, J.; Cobo, M. Effect of pretreatment on the ethanol and fusel alcohol production during fermentation of sugarcane press-mud. Biochem. Eng. J. 2020, 161, 107668. [Google Scholar] [CrossRef]
- Hajar, S.; Azhar, M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Azlan, J.; Azifa, A.; Faik, M.; Francis, K. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef]
- Roukas, T.; Kotzekidou, P. Rotary biofilm reactor: A new tool for long-term bioethanol production from non-sterilized beet molasses by Saccharomyces cerevisiae in repeated-batch fermentation. J. Clean. Prod. 2020, 257, 120519. [Google Scholar] [CrossRef]
- Malik, K.; Salama, E.S.; El-Dalatony, M.M.; Jalalah, M.; Harraz, F.A.; Al-Assiri, M.S.; Zheng, Y.; Sharma, P.; Li, X. Co-fermentation of immobilized yeasts boosted bioethanol production from pretreated cotton stalk lignocellulosic biomass: Long-term investigation. Ind. Crops Prod. 2021, 159, 113122. [Google Scholar] [CrossRef]
- Ben Atitallah, I.; Arous, F.; Louati, I.; Zouari-Mechichi, H.; Brysch-Herzberg, M.; Woodward, S.; Mechichi, T. Efficient bioethanol production from date palm (Phoenix dactylifera L.) sap by a newly isolated Saccharomyces cerevisiae X19G2. Process Biochem. 2021, 105, 102–112. [Google Scholar] [CrossRef]
- Han, W.; Liu, Y.; Xu, X.; Huang, J.; He, H.; Chen, L.; Qiu, S.; Tang, J.; Hou, P. Bioethanol production from waste hamburger by enzymatic hydrolysis and fermentation. J. Clean. Prod. 2020, 264, 121658. [Google Scholar] [CrossRef]
- Jin, X.; Ma, J.; Song, J.; Liu, G.Q. Promoted bioethanol production through fed-batch semisimultaneous saccharification and fermentation at a high biomass load of sodium carbonate-pretreated rice straw. Energy 2021, 226, 120353. [Google Scholar] [CrossRef]
- Thi, L.; Trinh, P.; Lee, J.; Lee, H. Acidified glycerol pretreatment for enhanced ethanol production from rice straw. Biomass Bioenergy 2016, 94, 39–45. [Google Scholar] [CrossRef]
- Lu, J.; Song, F.; Liu, H.; Chang, C.; Cheng, Y.; Wang, H. Production of high concentration bioethanol from reed by combined liquid hot water and sodium carbonate-oxygen pretreatment. Energy 2021, 217, 119332. [Google Scholar] [CrossRef]
- Ho, S.; Huang, S.; Chen, C.; Hasunuma, T.; Kondo, A. Biore source Technology Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Onay, M. Bioethanol production via different saccharification strategies from H. tetrachotoma ME03 grown at various concentrations of municipal wastewater in a flat-photobioreactor. Fuel 2019, 239, 1315–1323. [Google Scholar] [CrossRef]
- Min, K.J.; Oh, D.Y.; Park, K.Y. Pilot-scale cultivation of water-net in secondary effluent using an open pond raceway for nutrient removal and bioethanol production. Chemosphere 2021, 277, 130129. [Google Scholar] [CrossRef] [PubMed]
- Oakley, J.H.; Hoadley, A.F.A. Industrial scale steam reforming of bioethanol: A conceptual study. Int. J. Hydrogen Energy 2010, 35, 8472–8485. [Google Scholar] [CrossRef]
- Cifuentes, B.; Bustamante, F.; Conesa, J.A.; Córdoba, L.F.; Cobo, M. Fuel-cell grade hydrogen production by coupling steam reforming of ethanol and carbon monoxide removal. Int. J. Hydrogen Energy 2018, 3, 17216–17229. [Google Scholar] [CrossRef] [Green Version]
- Mamlouk, M.; Sousa, T.; Scott, K. A High Temperature Polymer Electrolyte Membrane Fuel Cell Model for Reformate Gas. Int. J. Electrochem. 2011, 2011, 520473. [Google Scholar] [CrossRef] [Green Version]
- Fatsikostas, A.N.; Kondarides, D.I.; Verykios, X.E. Steam reforming of biomass-derived ethanol for the production of hydrogen for fuel cell applications. Chem. Commun. 2001, 1, 851–852. [Google Scholar] [CrossRef]
- Montero, C.; Remiro, A.; Benito, P.L.; Bilbao, J.; Gayubo, A.G. Optimum operating conditions in ethanol steam reforming over a Ni/La2O3-αAl2O3 catalyst in a fluidized bed reactor. Fuel Process. Technol. 2018, 169, 207–216. [Google Scholar] [CrossRef]
- Llorca, J.; Corberán, V.C.; Divins, N.J.; Fraile, R.O.; Taboada, E. Hydrogen from Bioethanol. Renew. Hydrog. Technol. Prod. Purif. Storage Appl. Saf. 2013, 135–169. [Google Scholar] [CrossRef]
- Nejat, T.; Jalalinezhad, P.; Hormozi, F.; Bahrami, Z. Hydrogen production from steam reforming of ethanol over Ni-Co bimetallic catalysts and MCM-41 as support. J. Taiwan Inst. Chem. Eng. 2019, 97, 216–226. [Google Scholar] [CrossRef]
- López, E.; Divins, N.J.; Anzola, A.; Schbib, S.; Borio, D.; Llorca, J. Ethanol steam reforming for hydrogen generation over structured catalysts. Int. J. Hydrogen Energy 2013, 38, 4418–4428. [Google Scholar] [CrossRef]
- Hernández, L.; Kafarov, V. Thermodynamic evaluation of hydrogen production for fuel cells by using bio-ethanol steam reforming: Effect of carrier gas addition. J. Power Sources 2009, 192, 195–199. [Google Scholar] [CrossRef]
- Arévalo, J.D.; Martinez-hernández, Á.; Vargas, J.C.; Córdoba, L.F. Hydrogen Production and Purification by Bioethanol Steam Reforming and Preferential Oxidation of CO. Tecciencia 2018, 13, 55–64. [Google Scholar]
- Francesconi, J.A.; Mussati, M.C.; Aguirre, P.A. Effects of PEMFC operating parameters on the performance of an integrated ethanol processor. Int. J. Hydrogen Energy 2010, 35, 5940–5946. [Google Scholar] [CrossRef]
- Suwanmanee, U.; Saebea, D.; Hacker, V.; Assabumrungrat, S.; Arpornwichanop, A.; Authayanun, S. Conceptual design and life cycle assessment of decentralized power generation by HT-PEMFC system with sorption enhanced water gas shift loop. Energy Convers. Manag. 2018, 171, 20–30. [Google Scholar] [CrossRef]
- Moradi Bilondi, A.; Abdollahzadeh, M.; Kermani, M.J.; Heidary, H.; Havaej, P. Numerical study of anode side CO contamination effects on PEM fuel cell performance; and mitigation methods. Energy Convers. Manag. 2018, 177, 519–534. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Ricca, A. Ceramic foams coated with Pt-Ni/CeO2-ZrO2 for bioethanol steam reforming. Int. J. Hydrogen Energy 2016, 41, 11526–11536. [Google Scholar] [CrossRef]
- Authayanun, S.; Suwanmanee, U.; Arpornwichanop, A. Enhancement of dilute bio-ethanol steam reforming for a proton exchange membrane fuel cell system by using methane as co-reactant: Performance and life cycle assessment. Int. J. Hydrogen Energy 2015, 40, 12144–12153. [Google Scholar] [CrossRef]
- George, D.; Suresh, P.V. Detailed analysis of Integrated Steam Ethanol Reformer and High Temperature Polymer Electrolyte Membrane Fuel Cell. Int. J. Hydrogen Energy 2015, 41, 1248–1258. [Google Scholar] [CrossRef]
- Authayanun, S.; Wiyaratn, W.; Assabumrungrat, S.; Arpornwichanop, A. Theoretical analysis of a glycerol reforming and high-temperature PEMFC integrated system: Hydrogen production and system efficiency. Fuel 2013, 105, 345–352. [Google Scholar] [CrossRef]
- Authayanun, S.; Arpornwichanop, A.; Patcharavorachot, Y.; Wiyaratn, W.; Assabumrungrat, S. Hydrogen production from glycerol steam reforming for low- and high-temperature PEMFCs. Int. J. Hydrogen Energy 2011, 36, 267–275. [Google Scholar] [CrossRef]




| Feedstock | Generation | Fermentation Conditions | Ethanol (g L−1) | Ethanol (wt.%) | Ref. |
|---|---|---|---|---|---|
| Sugarcane press-mud | First | T = 30 °C, 200 rpm, pH = 5.5, 24 h | 61.0 | 6.21 | [34] |
| Sugar molasses | First | T = 30 °C, 115 rpm, 12 h | 78.6 | 8.00 | [35] |
| Cane molasses | First | T = 33 °C, 150 rpm, 48 h | 92.0 | 9.37 | [35] |
| Beet molasses | First | T = 30 °C, pH = 5.0, 40 rpm, 36 h | 52.3 | 5.33 | [36] |
| Cassava mash | First | T = 33 °C, 100 rpm, 42 h | 86.1 | 8.77 | [35] |
| Sorghum juice | First | T = 30 °C, 72 h | 98.5 | 10.03 | [35] |
| Waste wood | Second | T = 30 °C, 150 rpm, pH = 5.5, 16 h | 18.5 | 1.88 | [35] |
| Wheat straw | Second | T = 42 °C, pH = 5.5, 72 h | 36.2 | 3.69 | [35] |
| Cotton stalk | Second | T = 30 °C, 150 rpm, 96 h | 9.81 | 0.99 | [37] |
| Date palm sap | Second | T = 30 °C, 150 rpm, 72 h | 86.8 | 8.91 | [38] |
| Waste hamburger | Second | T = 30 °C, 400 rpm, 50 h | 27.4 | 2.77 | [39] |
| Rice straw | Second | T = 37 °C, 48 h | 112.3 | 11.61 | [40] |
| Rice straw | Second | T = 30 °C, 150 rpm, 72 h | 9.65 | 0.95 | [41] |
| Reed | Second | T= 36 °C, 48 h | 66.5 | 6.79 | [42] |
| C. vulgaris | Third | T = 30 °C, pH= 5.0–6.0, 60 h | 11.7 | 1.18 | [43] |
| H. tetrachotoma ME03 | Third | T = 30 °C, 150 rpm, 36 h | 11.2 | 1.13 | [44] |
| Hydrodictyon reticulatum | Third | T = 30 °C, 120 rpm, 48 h | 26.2 | 2.65 | [45] |
| Parameter | Unit | Value | ||||
|---|---|---|---|---|---|---|
| EtOH content | wt.% | 7.0 | 7.5 | 8.0 | 8.5 | 9.0 |
| Alembic load | kg | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| S/E | 5.0 | 4.6 | 4.3 | 4.0 | 3.8 | |
| ESR temperature | °C | 700 | 700 | 700 | 700 | 700 |
| CO-removal temperature | °C | 236 | 253 | 269 | 286 | 297 |
| Power | kWh kg−1 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| Efficiency | % | 17.8 | 17.6 | 17.4 | 17.4 | 17.2 |
| Energy consumption | kW kg−1 | 0.37 | 0.37 | 0.37 | 0.36 | 0.36 |
| Number of cells | 13 | 13 | 13 | 13 | 13 | |
| H2 flow rate | mol s−1 kg−1 | 2.68 | 2.73 | 2.77 | 2.77 | 2.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, N.; Rodríguez-Fontalvo, D.; Cifuentes, B.; Cantillo, N.M.; Uribe Laverde, M.Á.; Cobo, M. Biomass Potential for Producing Power via Green Hydrogen. Energies 2021, 14, 8366. https://doi.org/10.3390/en14248366
Sanchez N, Rodríguez-Fontalvo D, Cifuentes B, Cantillo NM, Uribe Laverde MÁ, Cobo M. Biomass Potential for Producing Power via Green Hydrogen. Energies. 2021; 14(24):8366. https://doi.org/10.3390/en14248366
Chicago/Turabian StyleSanchez, Nestor, David Rodríguez-Fontalvo, Bernay Cifuentes, Nelly M. Cantillo, Miguel Ángel Uribe Laverde, and Martha Cobo. 2021. "Biomass Potential for Producing Power via Green Hydrogen" Energies 14, no. 24: 8366. https://doi.org/10.3390/en14248366
APA StyleSanchez, N., Rodríguez-Fontalvo, D., Cifuentes, B., Cantillo, N. M., Uribe Laverde, M. Á., & Cobo, M. (2021). Biomass Potential for Producing Power via Green Hydrogen. Energies, 14(24), 8366. https://doi.org/10.3390/en14248366






