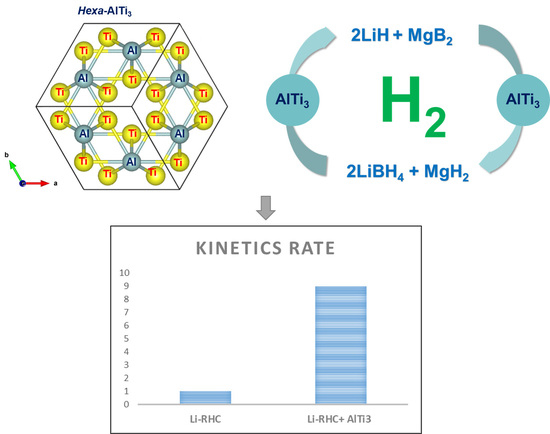
Enhanced Hydrogen Storage Properties of Li-RHC System with In-House Synthesized AlTi3 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
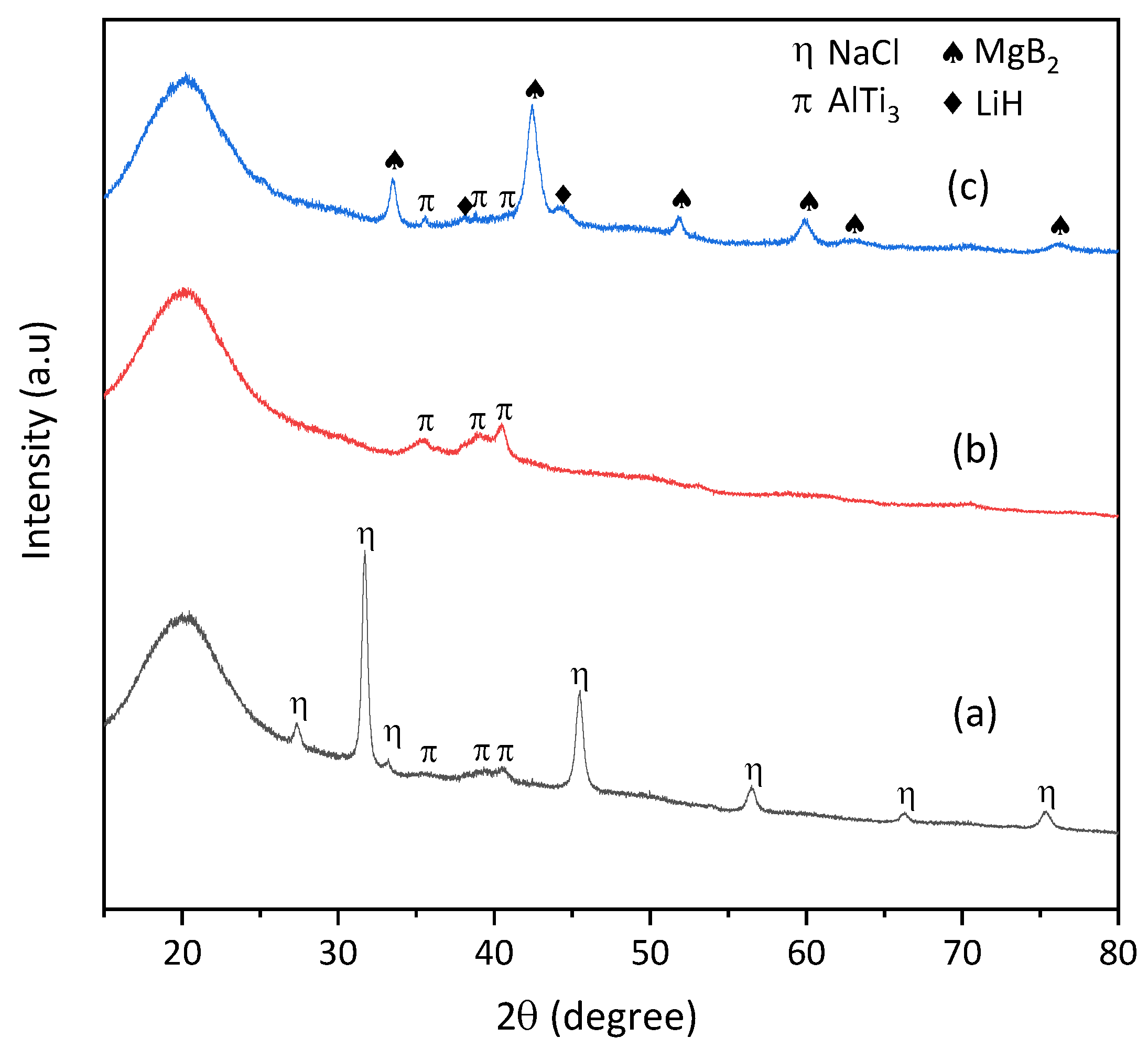
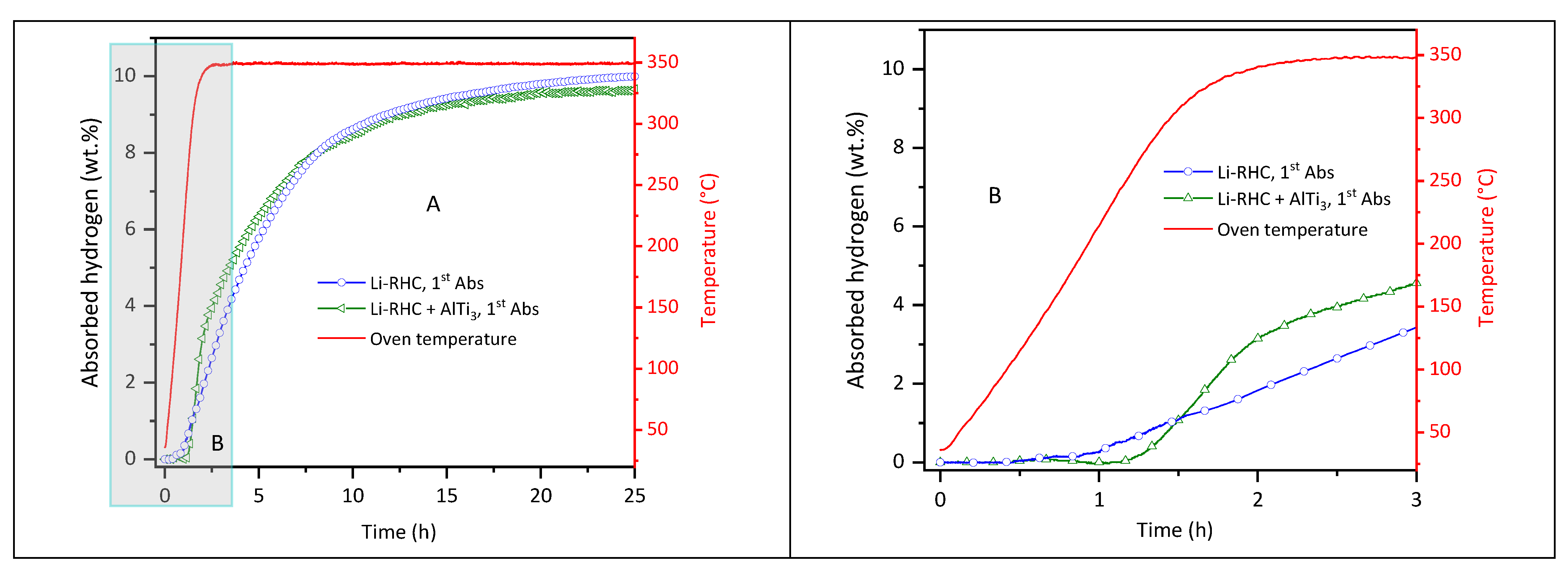
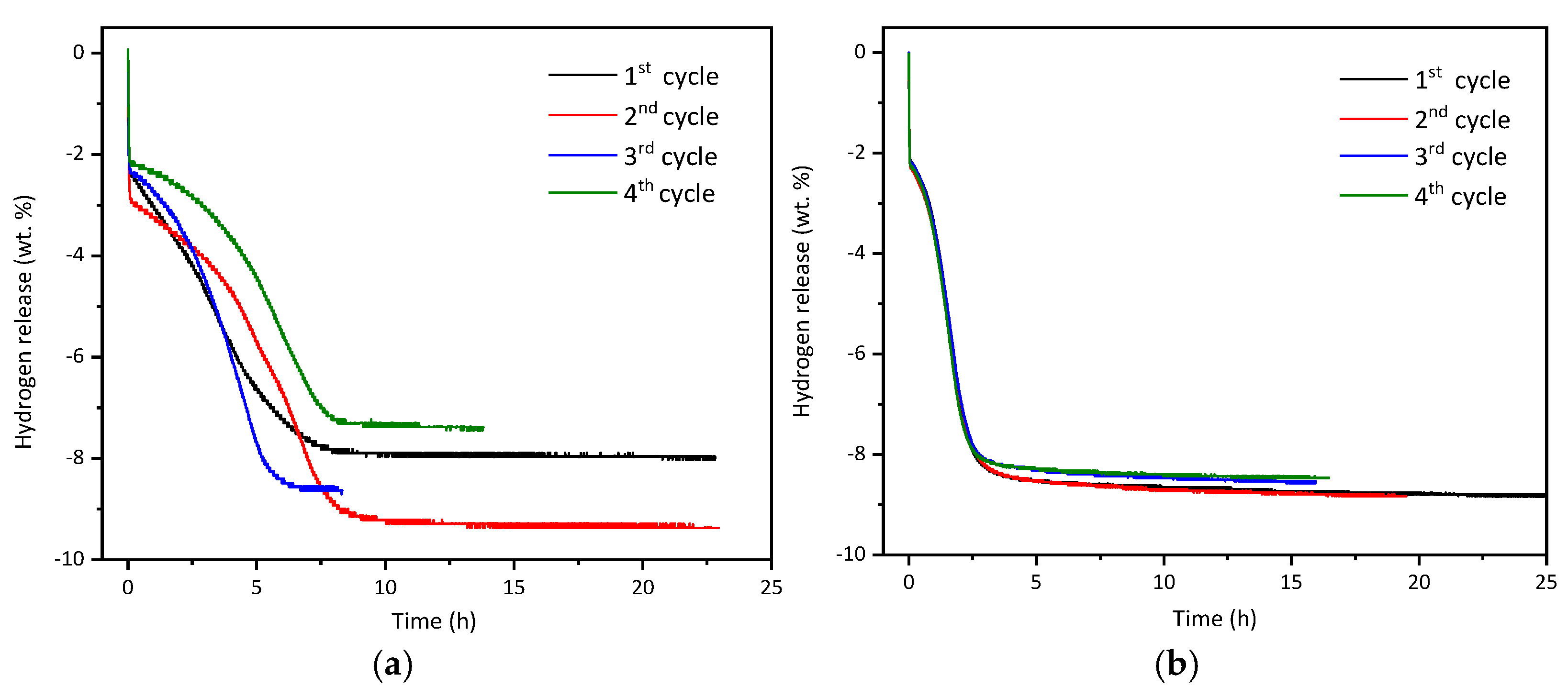
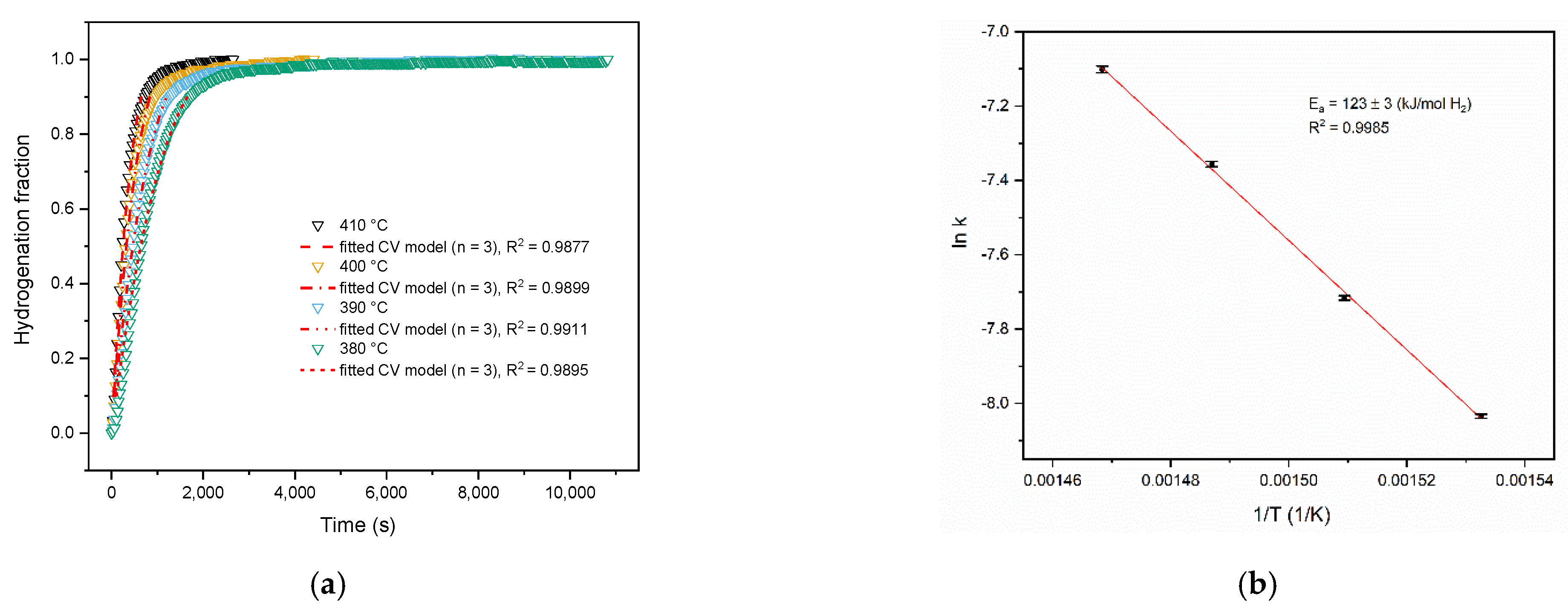
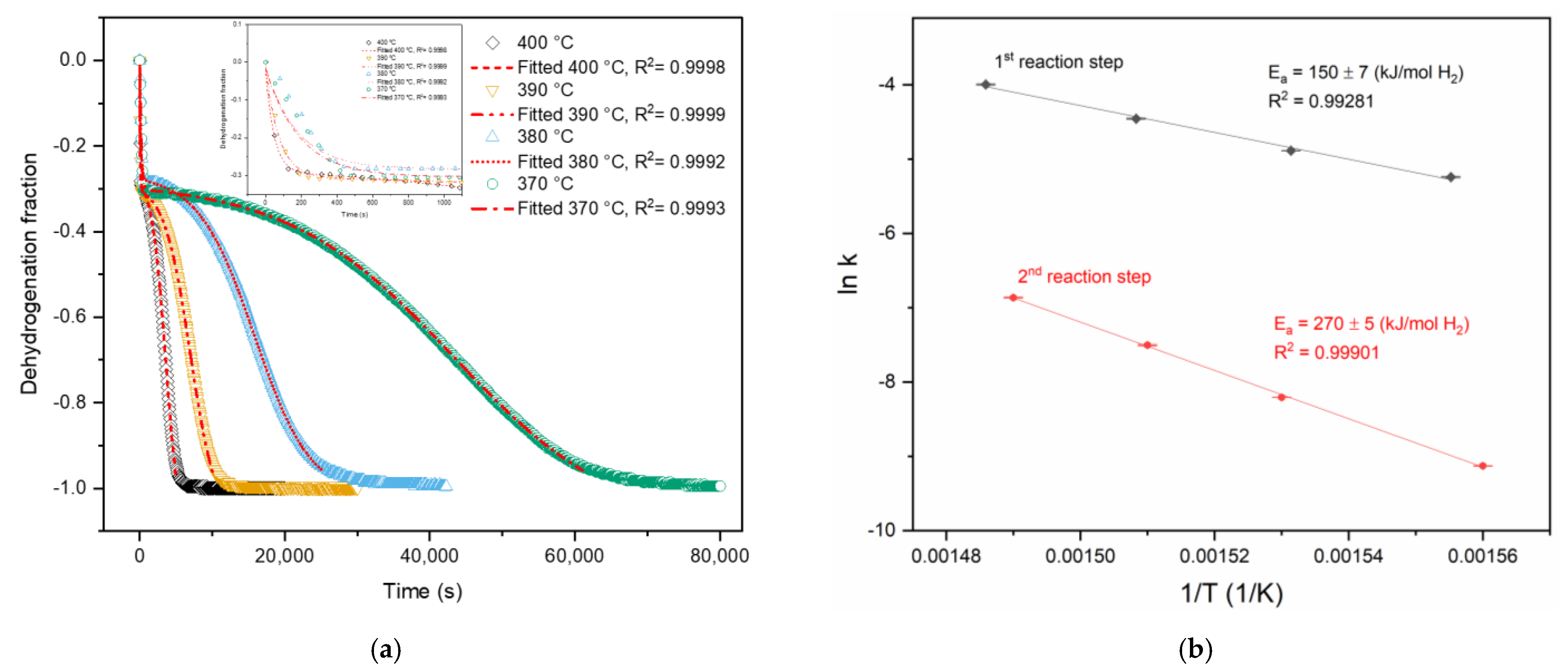
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A.; Borgschulte, A.; Schlapbach, L. Hydrogen as a Future Energy Carrier. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 368, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Global Warming of 1.5 °C. Summary for Policymakers. Available online: https://www.ipcc.ch/sr15/ (accessed on 30 October 2021).
- Hassanpouryouzband, A.; Joonaki, E.; Edlmann, K.; Haszeldine, R.S. Offshore Geological Storage of Hydrogen: Is This Our Best Option to Achieve Net-Zero? ACS Energy Lett. 2021, 6, 2181–2186. [Google Scholar] [CrossRef]
- Klebanoff, L. Hydrogen Storage Technology: Materials and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Hirscher, M. Handbook of Hydrogen Storage: New Material for Future Energy Storage; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Felderhoff, M.; Weidenthaler, C.; von Helmolt, R.; Eberle, U. Hydrogen storage: The remaining scientific and technological challenges. Phys. Chem. Chem. Phys. 2007, 9, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A. Materials for Hydrogen Storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Broom, D.P. Hydrogen Storage Materials: The Characterisation of Their Storage Properties. In Green Energy and Technology; Springer: London, UK, 2011. [Google Scholar]
- Broom, D.P.; Webb, C.J.; Fanourgakis, G.S.; Froudakis, G.E.; Trikalitis, P.N.; Hirscher, M. Concepts for improving hydrogen storage in nanoporous materials. Int. J. Hydrogen Energy 2019, 44, 7768–7779. [Google Scholar] [CrossRef]
- Broom, D.P.; Webb, C.J.; Hurst, K.E.; Parilla, P.A.; Gennett, T.; Brown, C.M.; Zacharia, R.; Tylianakis, E.; Klontzas, E.; Froudakis, G.E.; et al. Outlook and challenges for hydrogen storage in nanoporous materials. Appl. Phys. A 2016, 122, 151. [Google Scholar] [CrossRef]
- 2025 DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Available online: https://www.energy.gov/ (accessed on 30 October 2021).
- Lee, H.; Lee, J.-w.; Kim, D.Y.; Park, J.; Seo, Y.-T.; Zeng, H.; Moudrakovski, I.L.; Ratcliffe, C.I.; Ripmeester, J.A. Tuning clathrate hydrates for hydrogen storage. Nature 2005, 434, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Florusse Louw, J.; Peters Cor, J.; Schoonman, J.; Hester Keith, C.; Koh Carolyn, A.; Dec Steven, F.; Marsh Kenneth, N.; Sloan, E.D. Stable Low-Pressure Hydrogen Clusters Stored in a Binary Clathrate Hydrate. Science 2004, 306, 469–471. [Google Scholar] [CrossRef]
- Jepsen, L.H.; Ley, M.B.; Lee, Y.-S.; Cho, Y.W.; Dornheim, M.; Jensen, J.O.; Filinchuk, Y.; Jørgensen, J.E.; Besenbacher, F.; Jensen, T.R. Boron–nitrogen based hydrides and reactive composites for hydrogen storage. Mater. Today 2014, 17, 129–135. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Bellosta von Colbe, J.M.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Buchner, H.; Schmidt.-Ihn, E.; Lang, U. Stationäre Hydridspeicher als Ausgleichsspeicher elektrischer Spitzenenergien. In Kommission der Europäischen Gemeinschaften; European Commission: Luxembourg, 1981. [Google Scholar]
- Metallurgy, G.P. GKN Powder Metallurgy. Available online: https://www.gknpm.com/en/innovation/hydrogen-technology/hy2green/ (accessed on 30 October 2021).
- Psoma, A.; Sattler, G. Fuel cell systems for submarines: From the first idea to serial production. J. Power Sources 2002, 106, 381–383. [Google Scholar] [CrossRef]
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwicky, C.N.; Zuttel, A. Stability and reversibility of LiBH4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Kitahara, G.; Miwa, K.; Ohba, N.; Towata, S.; Züttel, A. Dehydriding and rehydriding reactions of LiBH4. J. Alloys Compd. 2005, 404–406, 427–430. [Google Scholar] [CrossRef]
- Orimo, S.-I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.-I.; Nakamori, Y.; Ohba, N.; Miwa, K.; Aoki, M.; Towata, S.-I.; Züttel, A. Experimental studies on intermediate compound of LiBH4. Appl. Phys. Lett. 2006, 89, 021920. [Google Scholar] [CrossRef]
- Puszkiel, J.; Garroni, S.; Milanese, C.; Gennari, F.; Klassen, T.; Dornheim, M.; Pistidda, C. Tetrahydroborates: Development and Potential as Hydrogen Storage Medium. Inorganics 2017, 5, 74. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356–357, 515–520. [Google Scholar] [CrossRef]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrogen Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Reaction of hydrogen with alloys of magnesium and copper. Inorg. Chem. 1967, 6, 2220–2223. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Dornheim, M.; Bormann, R. Unexpected kinetic effect of MgB2 in reactive hydride composites containing complex borohydrides. J. Alloys Compd. 2007, 440, L18–L21. [Google Scholar] [CrossRef]
- Vajo, J.J.; Skeith, S.L. Reversible Storage of Hydrogen in Destabilized LiBH4. Phys. Chem. B Lett. 2005, 109, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Garroni, S.; Pistidda, C.; Brunelli, M.; Vaughan, G.B.M.; Suriñach, S.; Baró, M.D. Hydrogen desorption mechanism of 2NaBH4+MgH2 composite prepared by high-energy ball milling. Scr. Mater. 2009, 60, 1129–1132. [Google Scholar] [CrossRef]
- Czujko, T.; Varin, R.A.; Wronski, Z.; Zaranski, Z.; Durejko, T. Synthesis and hydrogen desorption properties of nanocomposite magnesium hydride with sodium borohydride (MgH2+NaBH4). J. Alloys Compd. 2007, 427, 291–299. [Google Scholar] [CrossRef]
- Xiong, Z.; Hu, J.; Wu, G.; Chen, P.; Luo, W.; Gross, K.; Wang, J. Thermodynamic and kinetic investigations of the hydrogen storage in the Li–Mg–N–H system. J. Alloys Compd. 2005, 398, 235–239. [Google Scholar] [CrossRef]
- Nakamori, Y.; Kitahara, G.; Orimo, S. Synthesis and dehydriding studies of Mg–N–H systems. J. Power Sources 2004, 138, 309–312. [Google Scholar] [CrossRef]
- Gizer, G.; Puszkiel, J.; Cao, H.; Pistidda, C.; Le, T.T.; Dornheim, M.; Klassen, T. Tuning the reaction mechanism and hydrogenation/dehydrogenation properties of 6Mg(NH2)2-9LiH system by adding LiBH4. Int. J. Hydrogen Energy 2019, 44, 11920–11929. [Google Scholar] [CrossRef]
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Berlouis, L.E.A.; Cabrera, E.; Hall-Barientos, E.; Hall, P.J.; Dodd, S.; Morris, S.; Imam, M.A. A thermal analysis investigation of the hydriding properties of nanocrystalline Mg–Ni based alloys prepared by high energy ball milling. J. Alloys Compd. 2000, 305, 82–89. [Google Scholar] [CrossRef]
- Gertsman, V.Y.; Birringer, R. On the room-temperature grain growth in nanocrystalline copper. Scr. Metall. Mater. 1994, 30, 577–581. [Google Scholar] [CrossRef]
- Suzuki, Y.; Haraki, T.; Uchida, H. Effect of LaNi5H6 hydride particles size on desorption kinetics. J. Alloys Compd. 2002, 330–332, 488–491. [Google Scholar] [CrossRef]
- Vigeholm, B.; Kjøller, J.; Larsen, B.; Schrøder Pedersen, A. Hydrogen sorption performance of pure magnesium during continued cycling. Int. J. Hydrogen Energy 1983, 8, 809–817. [Google Scholar] [CrossRef]
- Vega, L.E.R.; Leiva, D.R.; Leal Neto, R.M.; Silva, W.B.; Silva, R.A.; Ishikawa, T.T.; Kiminami, C.S.; Botta, W.J. Mechanical activation of TiFe for hydrogen storage by cold rolling under inert atmosphere. Int. J. Hydrogen Energy 2018, 43, 2913–2918. [Google Scholar] [CrossRef]
- Strozi, R.B.; Ivanisenko, J.; Koudriachova, N.; Huot, J. Effect of HPT on the First Hydrogenation of LaNi5 Metal Hydride. Energies 2021, 14, 6710. [Google Scholar] [CrossRef]
- Huot, J. Enhancing Hydrogen Storage Properties of Metal Hybrides Enhancement by Mechanical Deformations. In Briefs in Applied Sciences and Technology; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Bösenberg, U.; Kim, J.W.; Gosslar, D.; Eigen, N.; Jensen, T.R.; von Colbe, J.M.B.; Zhou, Y.; Dahms, M.; Kim, D.H.; Günther, R. Role of additives in LiBH4–MgH2 reactive hydride composites for sorption kinetics. Acta Mater. 2010, 58, 3381–3389. [Google Scholar] [CrossRef]
- Bosenberg, U.; Vainio, U.; Pranzas, P.K.; von Colbe, J.M.; Goerigk, G.; Welter, E.; Dornheim, M.; Schreyer, A.; Bormann, R. On the chemical state and distribution of Zr- and V-based additives in reactive hydride composites. Nanotechnology 2009, 20, 204003. [Google Scholar] [CrossRef] [PubMed]
- Deprez, E.; Justo, A.; Rojas, T.C.; López-Cartés, C.; Bonatto Minella, C.; Bösenberg, U.; Dornheim, M.; Bormann, R.; Fernández, A. Microstructural study of the LiBH4–MgH2 reactive hydride composite with and without Ti-isopropoxide additive. Acta Mater. 2010, 58, 5683–5694. [Google Scholar] [CrossRef]
- Fan, M.; Sun, L.; Zhang, Y.; Xu, F.; Zhang, J.; Chu, H. The catalytic effect of additive Nb2O5 on the reversible hydrogen storage performances of LiBH4–MgH2 composite. Int. J. Hydrogen Energy 2008, 33, 74–80. [Google Scholar] [CrossRef]
- Gizer, G.; Puszkiel, J.; Riglos, M.V.C.; Pistidda, C.; Ramallo-López, J.M.; Mizrahi, M.; Santoru, A.; Gemming, T.; Tseng, J.-C.; Klassen, T.; et al. Improved kinetic behaviour of Mg(NH2)2-2LiH doped with nanostructured K-modified-LixTiyOz for hydrogen storage. Sci. Rep. 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-T.; Pistidda, C.; Puszkiel, J.; Castro Riglos, M.V.; Karimi, F.; Skibsted, J.; GharibDoust, S.P.; Richter, B.; Emmler, T.; Milanese, C.; et al. Design of a Nanometric AlTi Additive for MgB2-Based Reactive Hydride Composites with Superior Kinetic Properties. J. Phys. Chem. C 2018, 122, 7642–7655. [Google Scholar] [CrossRef]
- Liu, B.H.; Zhang, B.J.; Jiang, Y. Hydrogen storage performance of LiBH4+1/2MgH2 composites improved by Ce-based additives. Int. J. Hydrogen Energy 2011, 36, 5418–5424. [Google Scholar] [CrossRef]
- Santoru, A.; Garroni, S.; Pistidda, C.; Milanese, C.; Girella, A.; Marini, A.; Masolo, E.; Valentoni, A.; Bergemann, N.; Le, T.T.; et al. A new potassium-based intermediate and its role in the desorption properties of the K–Mg–N–H system. Phys. Chem. Chem. Phys. 2016, 18, 3910–3920. [Google Scholar] [CrossRef] [PubMed]
- Sridechprasat, P.; Suttisawat, Y.; Rangsunvigit, P.; Kitiyanan, B.; Kulprathipanja, S. Catalyzed LiBH4 and MgH2 mixture for hydrogen storage. Int. J. Hydrogen Energy 2011, 36, 1200–1205. [Google Scholar] [CrossRef]
- Wang, P.; Ma, L.; Fang, Z.; Kang, X.; Wang, P. Improved hydrogen storage property of Li–Mg–B–H system by milling with titanium trifluoride. Energy Environ. Sci. 2009, 2, 120–123. [Google Scholar]
- Bonatto Minella, C.; Garroni, S.; Pistidda, C.; Baró, M.D.; Gutfleisch, O.; Klassen, T.; Dornheim, M. Sorption properties and reversibility of Ti(IV) and Nb(V)-fluoride doped-Ca(BH4)2–MgH2 system. J. Alloys Compd. 2015, 622, 989–994. [Google Scholar] [CrossRef][Green Version]
- Karimi, F.; Pranzas, P.K.; Pistidda, C.; Puszkiel, J.A.; Milanese, C.; Vainio, U.; Paskevicius, M.; Emmler, T.; Santoru, A.; Utke, R.; et al. Structural and kinetic investigation of the hydride composite Ca(BH4)2 + MgH2 system doped with NbF5 for solid-state hydrogen storage. Phys. Chem. Chem. Phys. 2015, 17, 27328–27342. [Google Scholar] [CrossRef] [PubMed]
- Bonatto Minella, C.; Pellicer, E.; Rossinyol, E.; Karimi, F.; Pistidda, C.; Garroni, S.; Milanese, C.; Nolis, P.; Baro, M.D.; Gutfleisch, O.; et al. Chemical State, Distribution, and Role of Ti- and Nb-Based Additives on the Ca(BH4)2 System. J. Phys. Chem. C 2013, 117, 4394–4403. [Google Scholar] [CrossRef]
- Xian, K.; Gao, M.; Li, Z.; Gu, J.; Shen, Y.; Wang, S.; Yao, Z.; Liu, Y.; Pan, H. Superior Kinetic and Cyclic Performance of a 2D Titanium Carbide Incorporated 2LiH + MgB2 Composite toward Highly Reversible Hydrogen Storage. ACS Appl. Energy Mater. 2019, 2, 4853–4864. [Google Scholar] [CrossRef]
- Le, T.T.; Pistidda, C.; Abetz, C.; Georgopanos, P.; Garroni, S.; Capurso, G.; Milanese, C.; Puszkiel, J.; Dornheim, M.; Abetz, V.; et al. Enhanced Stability of Li-RHC Embedded in an Adaptive TPX™ Polymer Scaffold. Materials 2020, 13, 991. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Pranzas, P.K.; Puszkiel, J.A.; Castro Riglos, M.V.; Milanese, C.; Vainio, U.; Pistidda, C.; Gizer, G.; Klassen, T.; Schreyer, A.; et al. A comprehensive study on lithium-based reactive hydride composite (Li-RHC) as a reversible solid-state hydrogen storage system toward potential mobile applications. RSC Adv. 2021, 11, 23122–23135. [Google Scholar] [CrossRef]
- Karimi, F.; Börries, S.; Pranzas, P.K.; Metz, O.; Hoell, A.; Gizer, G.; Puszkiel, J.A.; Riglos, M.V.C.; Pistidda, C.; Dornheim, M.; et al. Characterization of LiBH4–MgH2 Reactive Hydride Composite System with Scattering and Imaging Methods Using Neutron and Synchrotron Radiation. Adv. Eng. Mater. 2021, 23, 2100294. [Google Scholar] [CrossRef]
- Karimi, F.; Riglos, M.V.C.; Santoru, A.; Hoell, A.; Raghuwanshi, V.S.; Milanese, C.; Bergemann, N.; Pistidda, C.; Nolis, P.; Baro, M.D.; et al. In Situ Formation of TiB2 Nanoparticles for Enhanced Dehydrogenation/Hydrogenation Reaction Kinetics of LiBH4–MgH2 as a Reversible Solid-State Hydrogen Storage Composite System. J. Phys. Chem. C 2018, 122, 11671–11681. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M. Edge-to-edge matching model for predicting orientation relationships and habit planes—the improvements. Scr. Mater. 2005, 52, 963–968. [Google Scholar] [CrossRef]
- Moon, K.I.; Lee, K.S. A study of the microstructure of nanocrystalline Al–Ti alloys synthesized by ball milling in a hydrogen atmosphere and hot extrusion. J. Alloys Compd. 1999, 291, 312–321. [Google Scholar] [CrossRef]
- HSC Chemistry. Available online: http://www.hsc-chemistry.net/index.html (accessed on 30 October 2021).
- Kattner, U.R.; Lin, J.C.; Chang, Y.A. Thermodynamic Assessment and Calculation of the Ti-Al System. Metall. Trans. A 1992, 23, 2081–2090. [Google Scholar] [CrossRef]
- Bösenberg, U.; Doppiu, S.; Mosegaard, L.; Barkhordarian, G.; Eigen, N.; Borgschulte, A.; Jensen, T.R.; Cerenius, Y.; Gutfleisch, O.; Klassen, T.; et al. Hydrogen sorption properties of MgH2–LiBH4 composites. Acta Mater. 2007, 55, 3951–3958. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Castro Riglos, M.V.; Ramallo-Lopez, J.M.; Mizrahi, M.; Karimi, F.; Santoru, A.; Hoell, A.; Gennari, F.C.; Larochette, P.A.; Pistidda, C.; et al. A novel catalytic route for hydrogenation-dehydrogenation of 2LiH + MgB2 via in situ formed core-shell LixTiO2 nanoparticles. J. Mater. Chem. A 2017, 5, 12922–12933. [Google Scholar] [CrossRef]
- Jones, L.F.; Dollimore, D.; Nicklin, T. Comparison of experimental kinetic decomposition data with master data using a linear plot method. Thermochim. Acta 1975, 13, 240–245. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-State Kinetic Models: Basics and Mathematical Fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.H.; Brindley, G.W.; Achar, B.N.N. Numerical Data for Some Commonly Used Solid State Reaction Equations. J. Am. Ceram. Soc. 1966, 49, 379–382. [Google Scholar] [CrossRef]
- Kelly, P.M.; Ren, H.P.; Qiu, D.; Zhang, M.X. Identifying close-packed planes in complex crystal structures. Acta Mater. 2010, 58, 3091–3095. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.L.; Wu, Y.M.; Wang, L.M.; Zhang, H.J. Extended application of edge-to-edge matching model to HCP/HCP (α-Mg/MgZn2) system in magnesium alloys. Mater. Sci. Eng. A 2007, 460–461, 296–300. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M. Edge-to-edge matching and its applications: Part I. Application to the simple HCP/BCC system. Acta Mater. 2005, 53, 1073–1084. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Easton, M.A.; Taylor, J.A. Crystallographic study of grain refinement in aluminum alloys using the edge-to-edge matching model. Acta Mater. 2005, 53, 1427–1438. [Google Scholar] [CrossRef]
- Lee, W.E.; Lagerlof, K.P.D. Structural and electron diffraction data for sapphire (α-Al2O3). J. Electron. Microsc. Tech. 1985, 2, 247–258. [Google Scholar] [CrossRef]
- Won Kyung, S.; Sangjun, O.; Won Nam, K. Perfect Domain-Lattice Matching between MgB2 and Al2O3: Single-Crystal MgB2 Thin Films Grown on Sapphire. Jpn. J. Appl. Phys. 2012, 51, 083101. [Google Scholar]
- Tian, W.; Pan, X.Q.; Bu, S.D.; Kim, D.M.; Choi, J.H.; Patnaik, S.; Eom, C.B. Interfacial structure of epitaxial MgB2 thin films grown on (0001) sapphire. Appl. Phys. Lett. 2002, 81, 685–687. [Google Scholar] [CrossRef]
- Hallstedt, B.; Liu, Z.-K.; Ågren, J. Reactions in Al2O3-Mg metal matrix composites during prolonged heat treatment at 400, 550 and 600 °C. Mater. Sci. Eng. A 1993, 169, 149–157. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.-T.; Pistidda, C.; Puszkiel, J.; Castro Riglos, M.V.; Dreistadt, D.M.; Klassen, T.; Dornheim, M. Enhanced Hydrogen Storage Properties of Li-RHC System with In-House Synthesized AlTi3 Nanoparticles. Energies 2021, 14, 7853. https://doi.org/10.3390/en14237853
Le T-T, Pistidda C, Puszkiel J, Castro Riglos MV, Dreistadt DM, Klassen T, Dornheim M. Enhanced Hydrogen Storage Properties of Li-RHC System with In-House Synthesized AlTi3 Nanoparticles. Energies. 2021; 14(23):7853. https://doi.org/10.3390/en14237853
Chicago/Turabian StyleLe, Thi-Thu, Claudio Pistidda, Julián Puszkiel, María Victoria Castro Riglos, David Michael Dreistadt, Thomas Klassen, and Martin Dornheim. 2021. "Enhanced Hydrogen Storage Properties of Li-RHC System with In-House Synthesized AlTi3 Nanoparticles" Energies 14, no. 23: 7853. https://doi.org/10.3390/en14237853
APA StyleLe, T.-T., Pistidda, C., Puszkiel, J., Castro Riglos, M. V., Dreistadt, D. M., Klassen, T., & Dornheim, M. (2021). Enhanced Hydrogen Storage Properties of Li-RHC System with In-House Synthesized AlTi3 Nanoparticles. Energies, 14(23), 7853. https://doi.org/10.3390/en14237853