Effects of the Chromium Content in (TiVNb)100−xCrx Body-Centered Cubic High Entropy Alloys Designed for Hydrogen Storage Applications
Abstract
1. Introduction
2. Materials and Methods
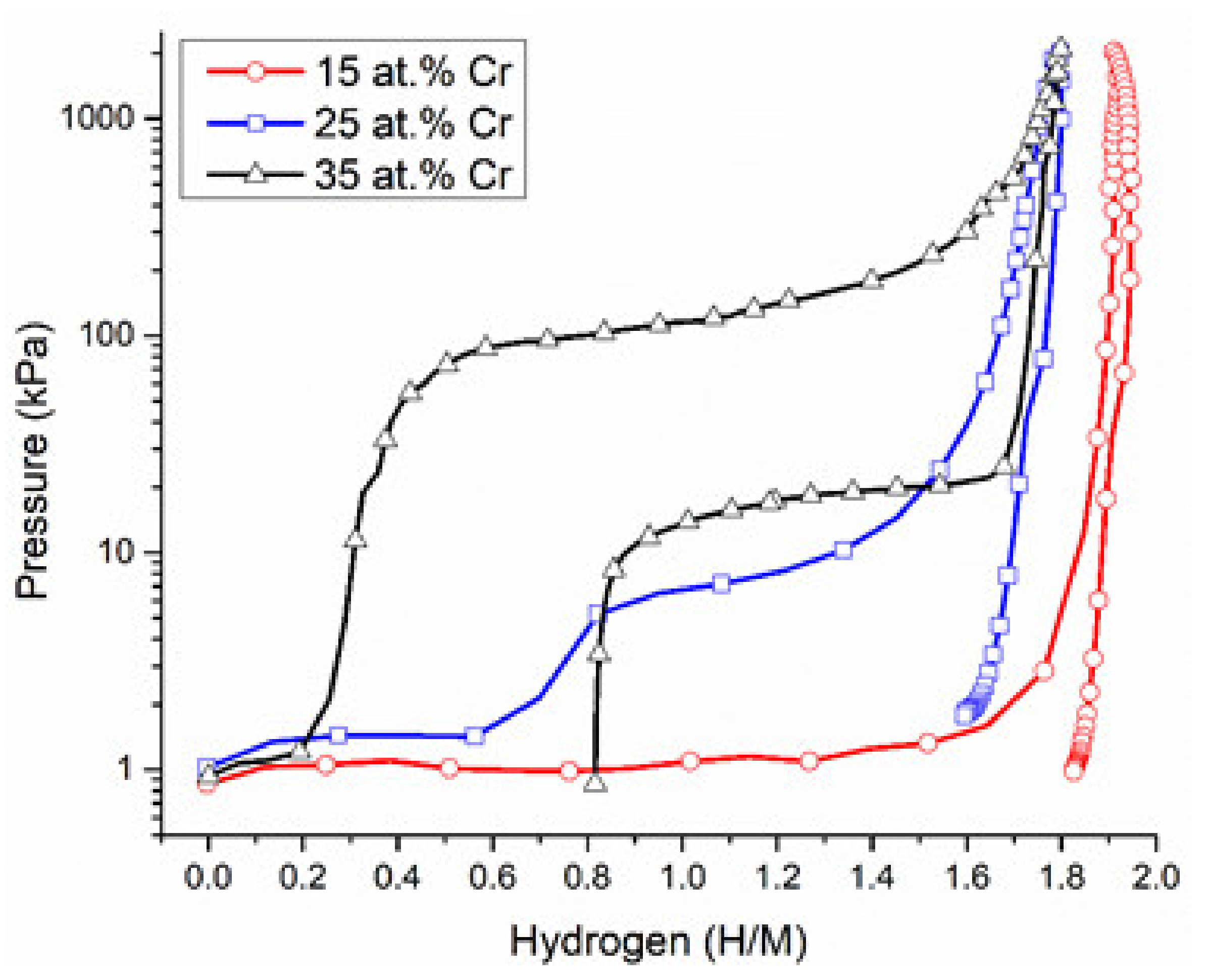
3. Results and Discussion
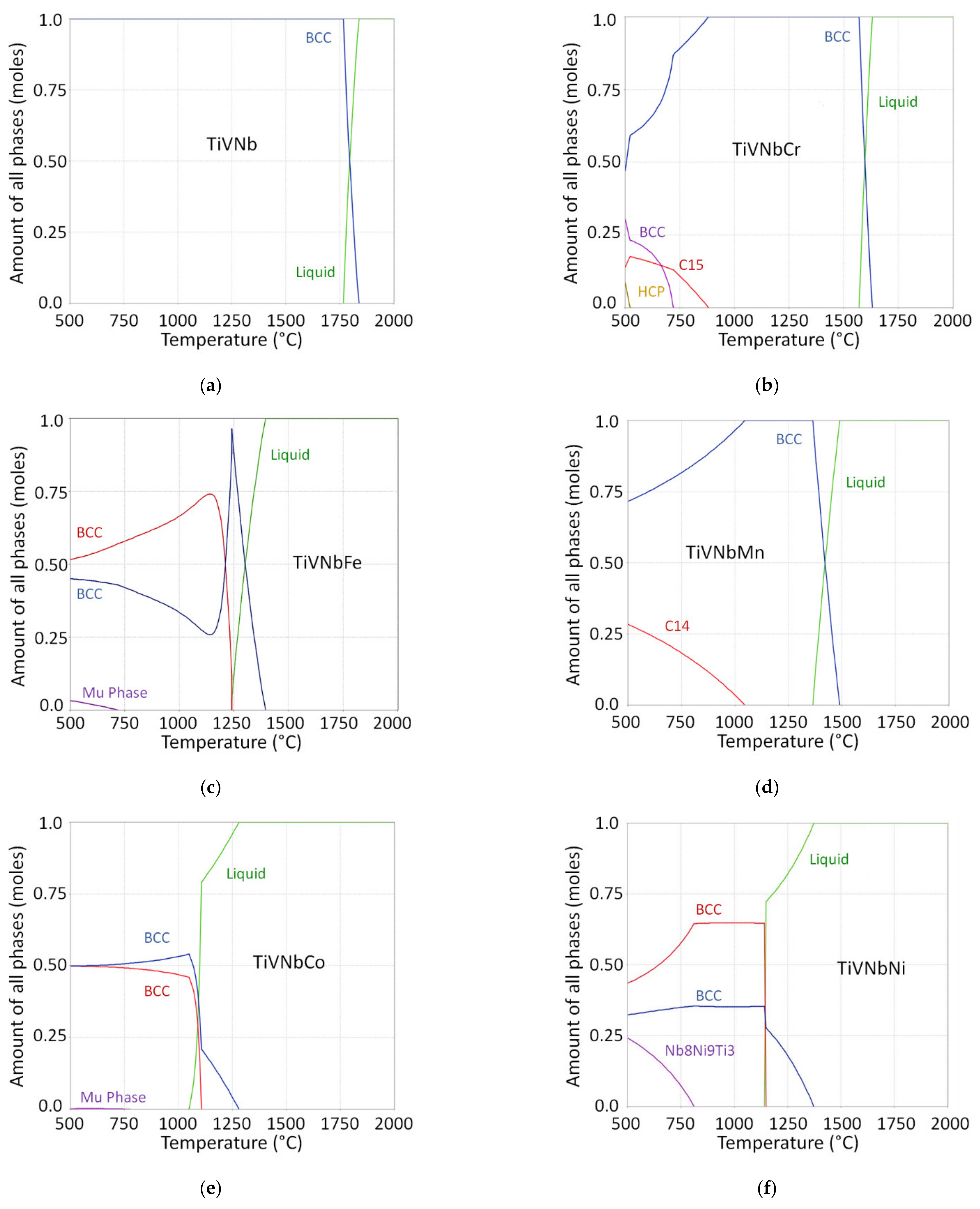
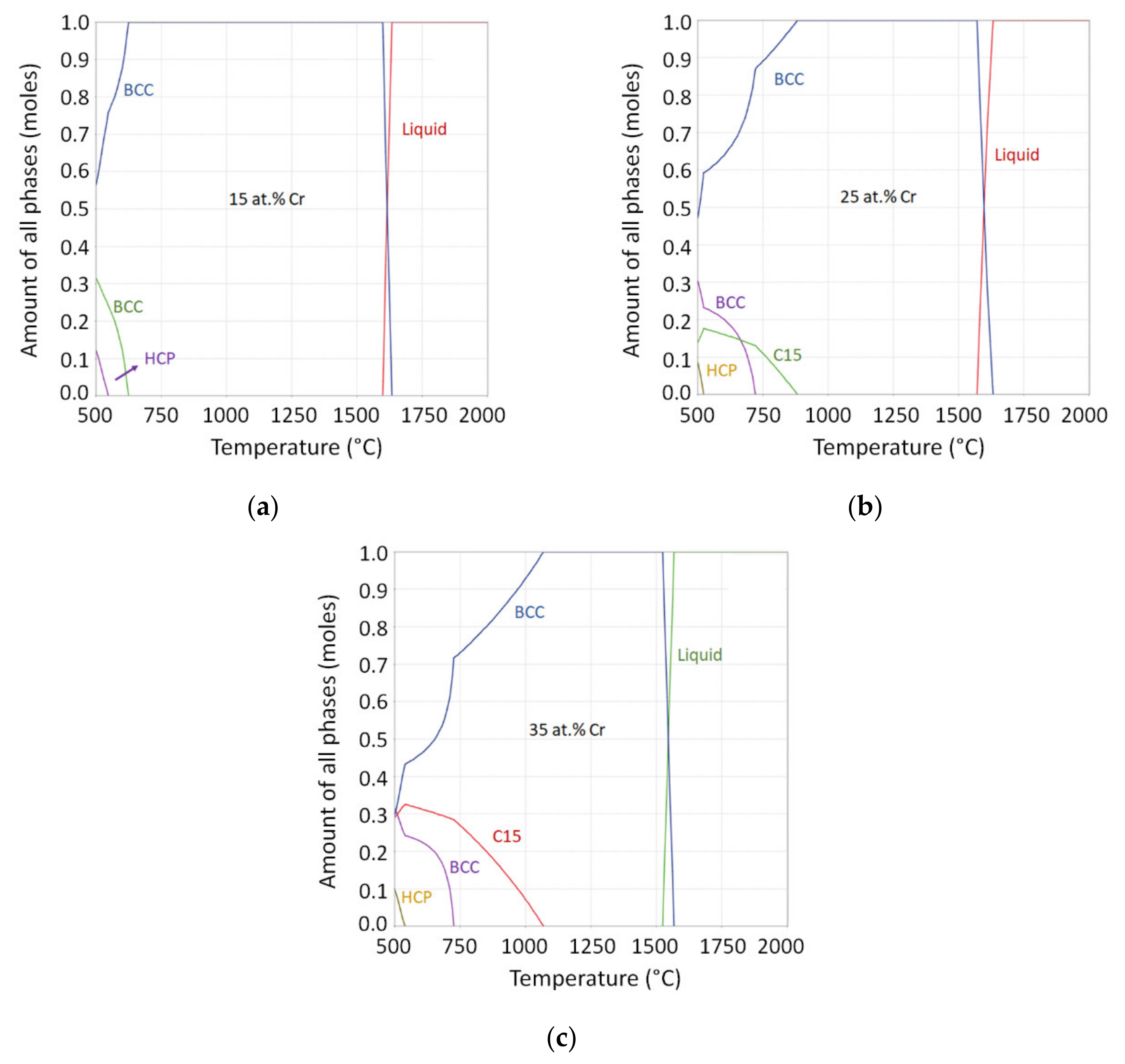
3.1. Alloy Design
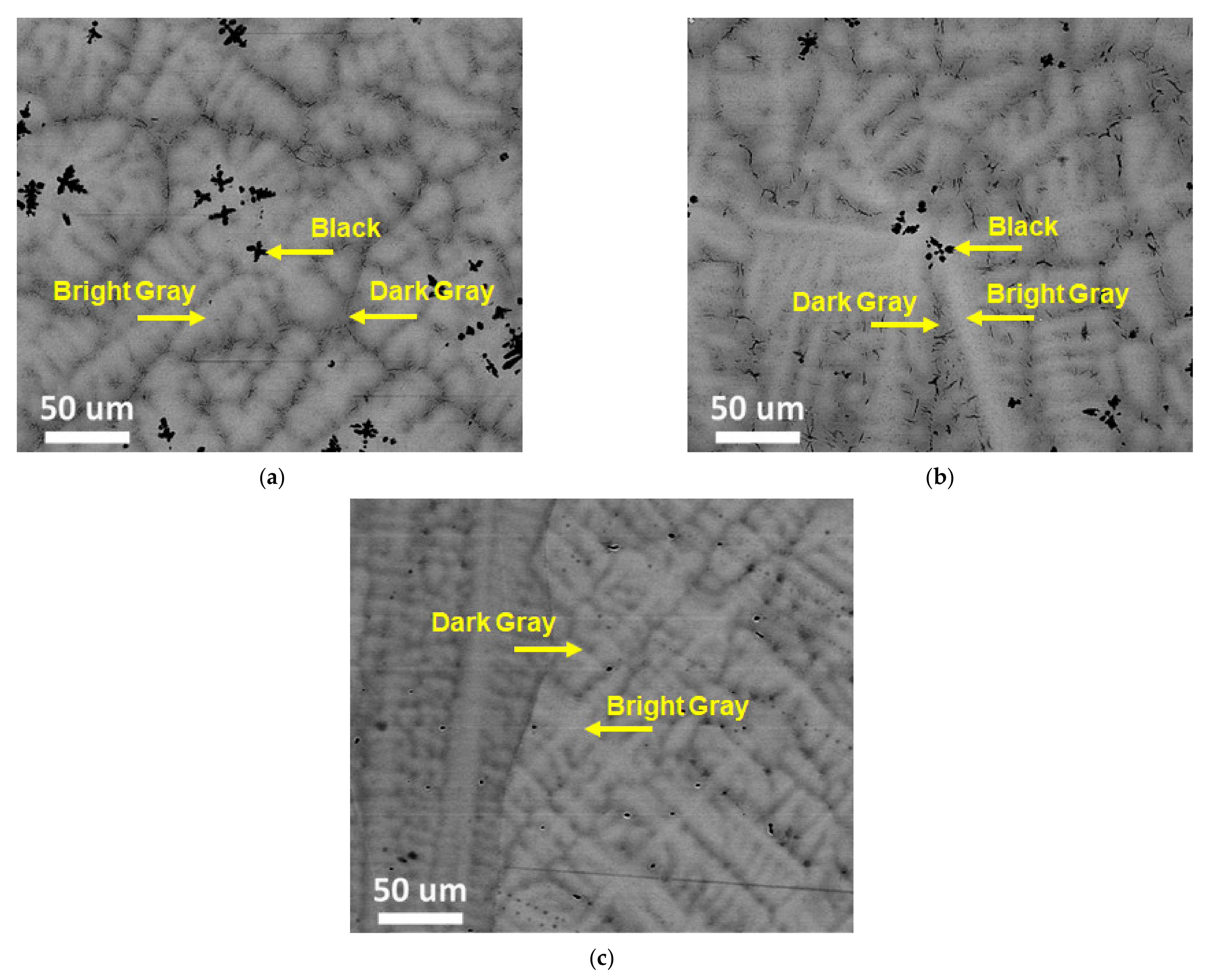
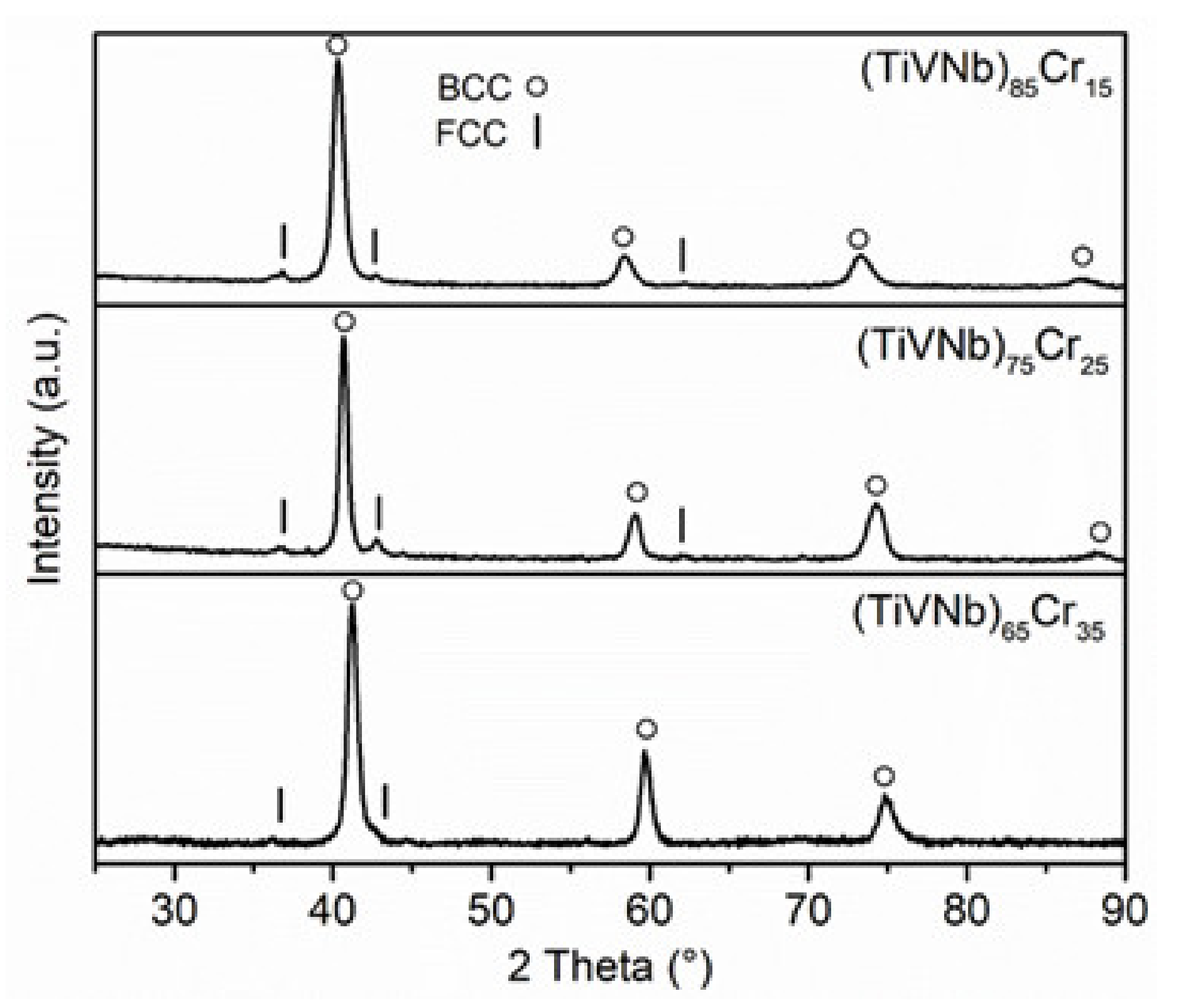
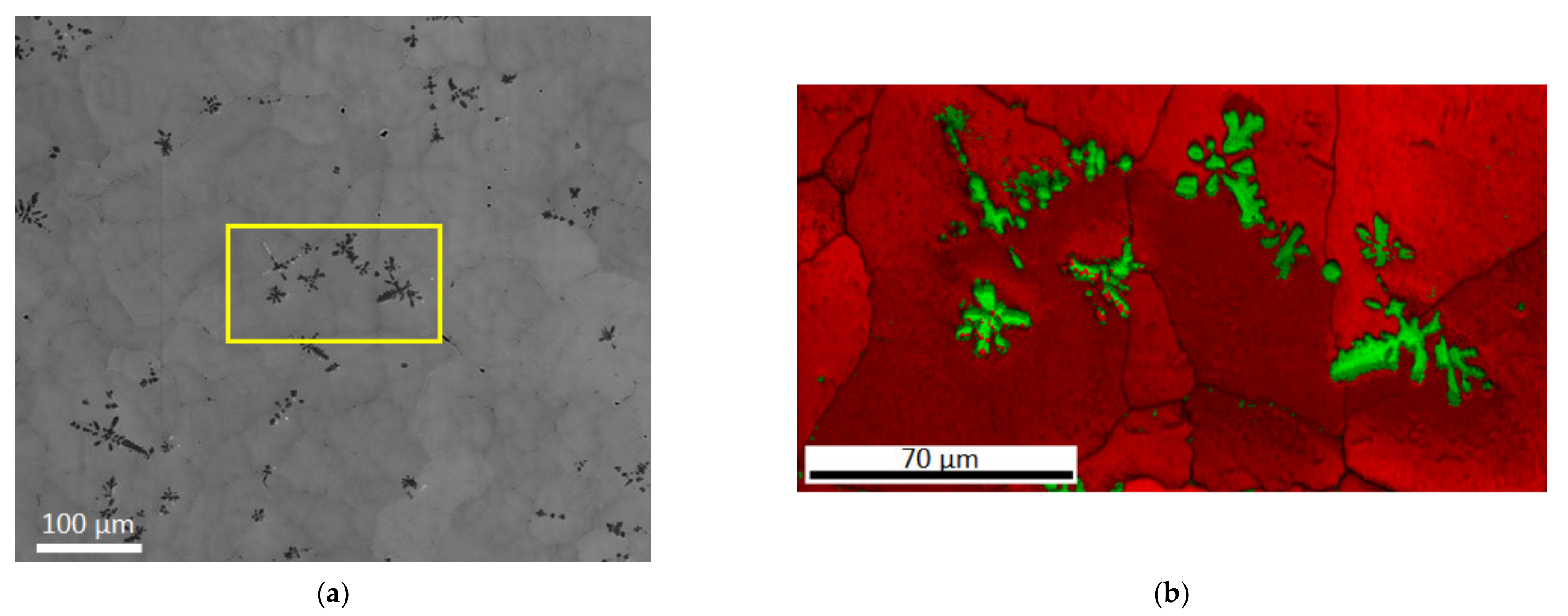
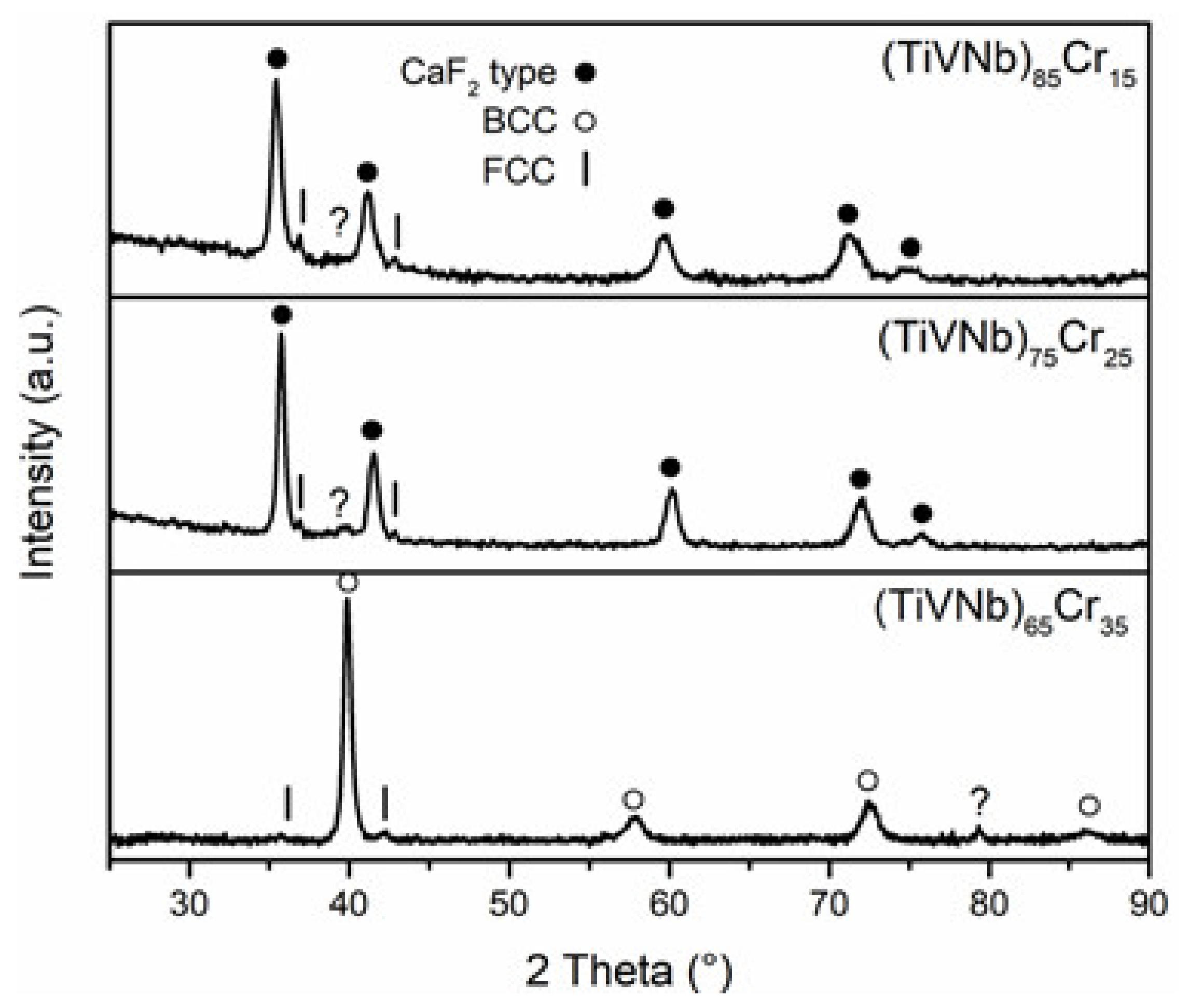
3.2. Structural Characterization
3.3. Structural Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, T.Y.; Lim, K.L.; Tseng, Y.S.; Chan, S.L.I. A review on the characterization of hydrogen in hydrogen storage materials. Renew. Sustain. Energy Rev. 2017, 79, 1122–1133. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.; Ares, J.R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Yartys, V.A.; Pollet, B.G.; Bowman, R.C.B., Jr. Metal hydride hydrogen compressors: A review. Int. J. Hydrogen Energy 2014, 39, 5818–5851. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, H.; Gao, M.; Wang, Q. Advanced hydrogen storage alloys for Ni/MH rechargeable batteries. J. Mater. Chem. 2011, 21, 4743–4755. [Google Scholar] [CrossRef]
- Na, T.W.; Park, K.B.; Lee, S.Y.; Yang, S.M.; Kang, J.W.; Lee, T.W.; Park, J.M.; Park, K.; Park, H.K. Preparation of spherical TaNbHfZrTi high-entropy alloy powders by a hydrogenation–dehydrogenation reaction and thermal plasma treatment. J. Alloys Compd. 2020, 817, 152757. [Google Scholar] [CrossRef]
- Manickam, K.; Mistry, P.; Walker, G.; Grant, D.; Buckley, C.E.; Humphries, T.D.; Paskevicius, M.; Jensen, T.; Albert, R.; Peinecke, K.; et al. Future perspectives of thermal energy storage with metal hydrides. Int. J. Hydrogen Energy 2019, 44, 7738–7745. [Google Scholar] [CrossRef]
- Huot, J. Metal Hydrides. In Handbook of Hydrogen Storage: New Materials for Future Energy Storage; Hirscher, M., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 81–116. ISBN 9783527629800. [Google Scholar]
- Zhang, C.; Song, A.; Yuan, Y.; Wu, Y.; Zhang, P.; Lu, Z.; Song, X. Study on the hydrogen storage properties of a TiZrNbTa high entropy alloy. Int. J. Hydrogen Energy 2019. [Google Scholar] [CrossRef]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior hydrogen storage in high entropy alloys. Sci. Rep. 2016, 6, 36770. [Google Scholar] [CrossRef] [PubMed]
- Zepon, G.; Leiva, D.R.; Strozi, R.B.; Bedoch, A.; Figueroa, S.J.A.; Ishikawa, T.T.; Botta, W.J. Hydrogen-induced phase transition of MgZrTiFe0.5Co0.5Ni0.5 high entropy alloy. Int. J. Hydrogen Energy 2018, 43, 1702–1708. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Bystrzycki, J. Microstructure and hydrogen storage properties of a TiZrNbMoV high entropy alloy synthesized using Laser Engineered Net Shaping (LENS). Int. J. Hydrogen Energy 2014, 39, 9904–9910. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303+274. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Chen, S.-K.; Sheu, J.-H.; Lin, J.-T.; Lin, W.-E.; Yeh, J.-W.; Lin, S.-J.; Liou, T.-H.; Wang, C.-W. Hydrogen storage properties of multi-principal-component CoFeMnTixVyZrz alloys. Int. J. Hydrogen Energy 2010, 35, 9046–9059. [Google Scholar] [CrossRef]
- Young, K.H.; Nei, J.; Wan, C.; Denys, R.V.; Yartys, V.A. Comparison of C14-and C15-predomiated AB 2 metal hydride alloys for electrochemical applications. Batteries 2017, 3, 22. [Google Scholar] [CrossRef]
- Edalati, P.; Floriano, R.; Mohammadi, A.; Li, Y.; Zepon, G.; Li, H.W.; Edalati, K. Reversible room temperature hydrogen storage in high-entropy alloy TiZrCrMnFeNi. Scr. Mater. 2020, 178, 387–390. [Google Scholar] [CrossRef]
- Karlsson, D.; Ek, G.; Cedervall, J.; Zlotea, C.; Møller, K.T.; Hansen, T.C.; Bednarčík, J.; Paskevicius, M.; Sørby, M.H.; Jensen, T.R.; et al. Structure and Hydrogenation Properties of a HfNbTiVZr High-Entropy Alloy. Inorg. Chem. 2018, 57, 2103–2110. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Sharma, V.K.; Sahu, P.; Kumar, V. Synthesis and characterization of hydrogenated novel AlCrFeMnNiW high entropy alloy. Int. J. Hydrogen Energy 2019. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sahlberg, M.; Sørby, M.H.; Hauback, B.C. Hydrogen storage in high-entropy alloys with varying degree of local lattice strain. Int. J. Hydrogen Energy 2019. [Google Scholar] [CrossRef]
- Montero, J.; Zlotea, C.; Ek, G.; Crivello, J.; Laversenne, L.; Sahlberg, M. TiVZrNb Multi-Principal-Element Alloy: Synthesis Optimization, Structural, and Hydrogen Sorption Properties. Molecules 2019, 24, 2799. [Google Scholar] [CrossRef]
- Zlotea, C.; Sow, M.A.; Ek, G.; Couzinié, J.P.; Perrière, L.; Guillot, I.; Bourgon, J.; Møller, K.T.; Jensen, T.R.; Akiba, E.; et al. Hydrogen sorption in TiZrNbHfTa high entropy alloy. J. Alloys Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Strozi, R.B.; Leiva, D.R.; Huot, J.; Botta, W.J.; Zepon, G. Synthesis and hydrogen storage behavior of Mg–V–Al–Cr–Ni high entropy alloys. Int. J. Hydrogen Energy 2020, 46, 2351–2361. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sørby, M.H.; Sahlberg, M.; Hauback, B.C. Counting electrons—A new approach to tailor the hydrogen sorption properties of high-entropy alloys. Acta Mater. 2019. [Google Scholar] [CrossRef]
- Silva, B.H.; Zlotea, C.; Champion, Y.; Botta, W.J.; Zepon, G. valence electron concentration for hydrogen storage. J. Alloys Compd. 2021, 865, 158767. [Google Scholar] [CrossRef]
- Akiba, E.; Iba, H. Hydrogen absorption by Laves phase related BCC solid solution. Intermetallics 1998, 9795, 461–470. [Google Scholar] [CrossRef]
- Bau, R.; Drabnis, M.H. Structures of transition metal hydrides determined by neutron diffraction. Inorg. Chim. Acta 1997, 259, 27–50. [Google Scholar] [CrossRef]
- Peisl, H. Lattice strains due to hydrogen in metals. In Hydrogen in Metals I. Topics in Applied Physics; Alefeld, G., Völkl, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1978; Volume 28, pp. 53–74. ISBN 978-3-540-35892-3. [Google Scholar]
- Sandrock, G. Panoramic overview of hydrogen storage alloys from a gas reaction point of view. J. Alloys Compd. 1999, 293, 877–888. [Google Scholar] [CrossRef]

| Composition for Each Alloy in the As-Cast Condition (at.%) | |||||
|---|---|---|---|---|---|
| Alloy | Contrast | Ti | V | Nb | Cr |
| (TiVNb)85Cr15 | Bright Gray | 28.0 (3) | 30.6 (3) | 27.6 (7) | 13.8 (5) |
| Dark Gray | 29.5 (8) | 30.8 (1) | 21.7 (2) | 17.2 (9) | |
| Black | 88.5 (4) | 5.0 (7) | 5.9 (7) | 0.7 (4) | |
| (TiVNb)75Cr25 | Bright Gray | 22.9 (3) | 29.0 (6) | 28.4 (6) | 23.2 (8) |
| Dark Gray | 24.9 (4) | 27.8 (4) | 20.3 (6) | 27.0 (8) | |
| Black | 89.0 (2) | 4.1 (5) | 5.1 (1) | 1.7 (9) | |
| (TiVNb)65Cr35 | Bright Gray | 22.8 (5) | 21.7 (4) | 22.0 (5) | 33.4 (3) |
| Dark Gray | 25 (1) | 19.2 (8) | 20.1 (7) | 35.2 (4) | |
| Alloy | Lattice Parameter (Å) | Average Atomic Radius (Å) | Ratio |
|---|---|---|---|
| (TiVNb)85Cr15 | 3.158 (1) | 1.380 | 2.2884 (7) |
| (TiVNb)75Cr25 | 3.122 (1) | 1.365 | 2.2872 (7) |
| (TiVNb)65Cr35 | 3.105 (6) | 1.350 | 2.300 (4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strozi, R.B.; Leiva, D.R.; Zepon, G.; Botta, W.J.; Huot, J. Effects of the Chromium Content in (TiVNb)100−xCrx Body-Centered Cubic High Entropy Alloys Designed for Hydrogen Storage Applications. Energies 2021, 14, 3068. https://doi.org/10.3390/en14113068
Strozi RB, Leiva DR, Zepon G, Botta WJ, Huot J. Effects of the Chromium Content in (TiVNb)100−xCrx Body-Centered Cubic High Entropy Alloys Designed for Hydrogen Storage Applications. Energies. 2021; 14(11):3068. https://doi.org/10.3390/en14113068
Chicago/Turabian StyleStrozi, Renato Belli, Daniel Rodrigo Leiva, Guilherme Zepon, Walter José Botta, and Jacques Huot. 2021. "Effects of the Chromium Content in (TiVNb)100−xCrx Body-Centered Cubic High Entropy Alloys Designed for Hydrogen Storage Applications" Energies 14, no. 11: 3068. https://doi.org/10.3390/en14113068
APA StyleStrozi, R. B., Leiva, D. R., Zepon, G., Botta, W. J., & Huot, J. (2021). Effects of the Chromium Content in (TiVNb)100−xCrx Body-Centered Cubic High Entropy Alloys Designed for Hydrogen Storage Applications. Energies, 14(11), 3068. https://doi.org/10.3390/en14113068








