Managing Non-Sewered Human Waste Using Thermochemical Waste Treatment Technologies: A Review
Abstract
:1. Introduction
2. Sewage Waste/Biomass Treatment Technologies
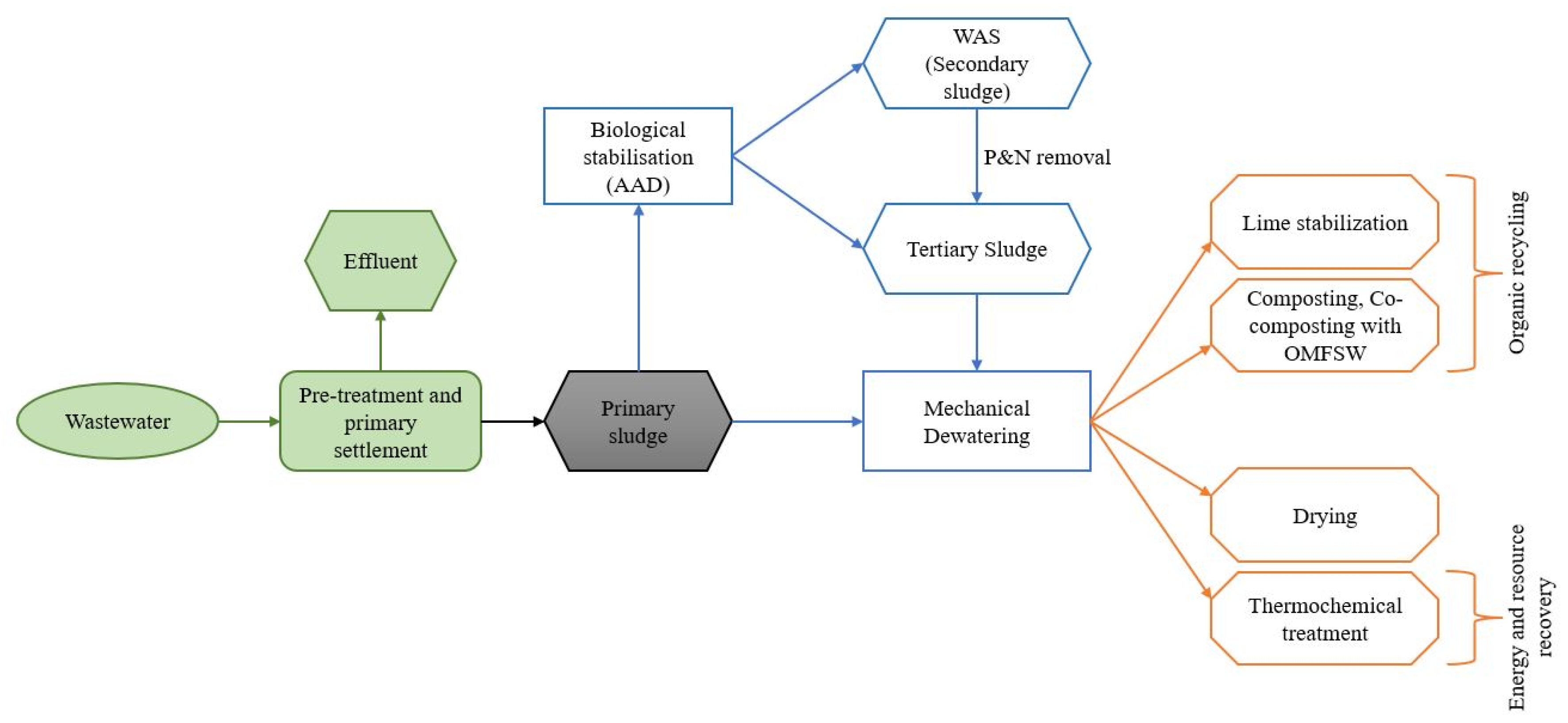
2.1. Anaerobic Digestion
2.2. Conventional Combustion
2.3. Emerging Methods
2.3.1. Co-Combustion
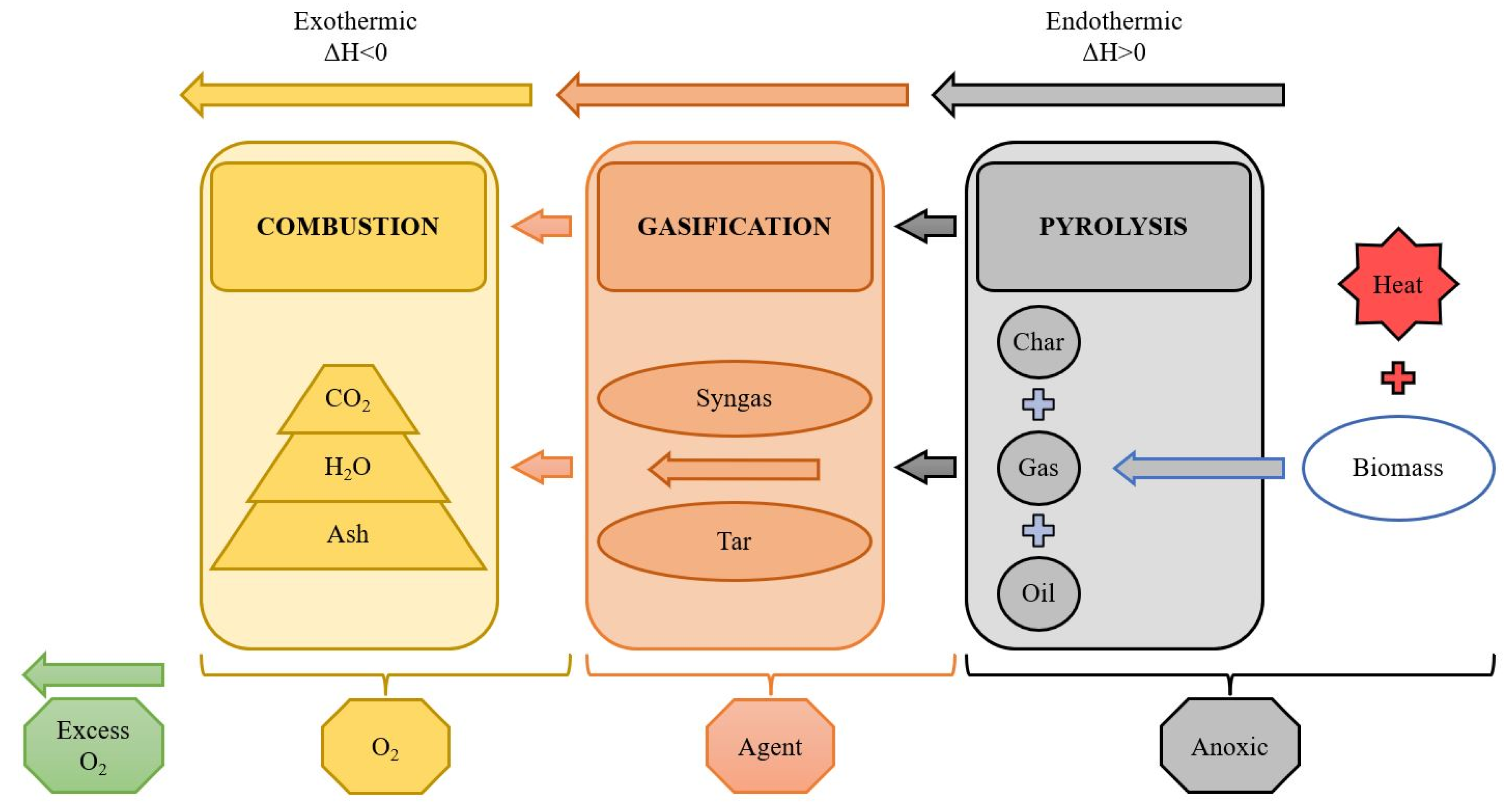
2.3.2. Advanced Thermochemical Treatment (ATT)
Pyrolysis
Gasification
2.4. Review of Comparative Assessment Studies
2.5. Review of Micro-Scale Human Faecal Sludge/Faeces Treatment Studies
3. Discussion
3.1. Energy Consumption
3.2. Technology Development
- (1)
- Limiting energy consumption as much as possible;
- (2)
- Lesser chances of fatigue, particularly when the least maintenance is feasible;
- (3)
- Accommodating any incoming feedstock volumes with different physical characteristics;
- (4)
- Delivering emissions below regulating limits for both solid and gaseous by-products, and;
- (5)
- Reducing the solid waste volumes in considerable amounts.
3.3. Syngas Yield and Energy Content—A Trade-Off with Combustion Performance, Emissions and Bio-Oil Properties
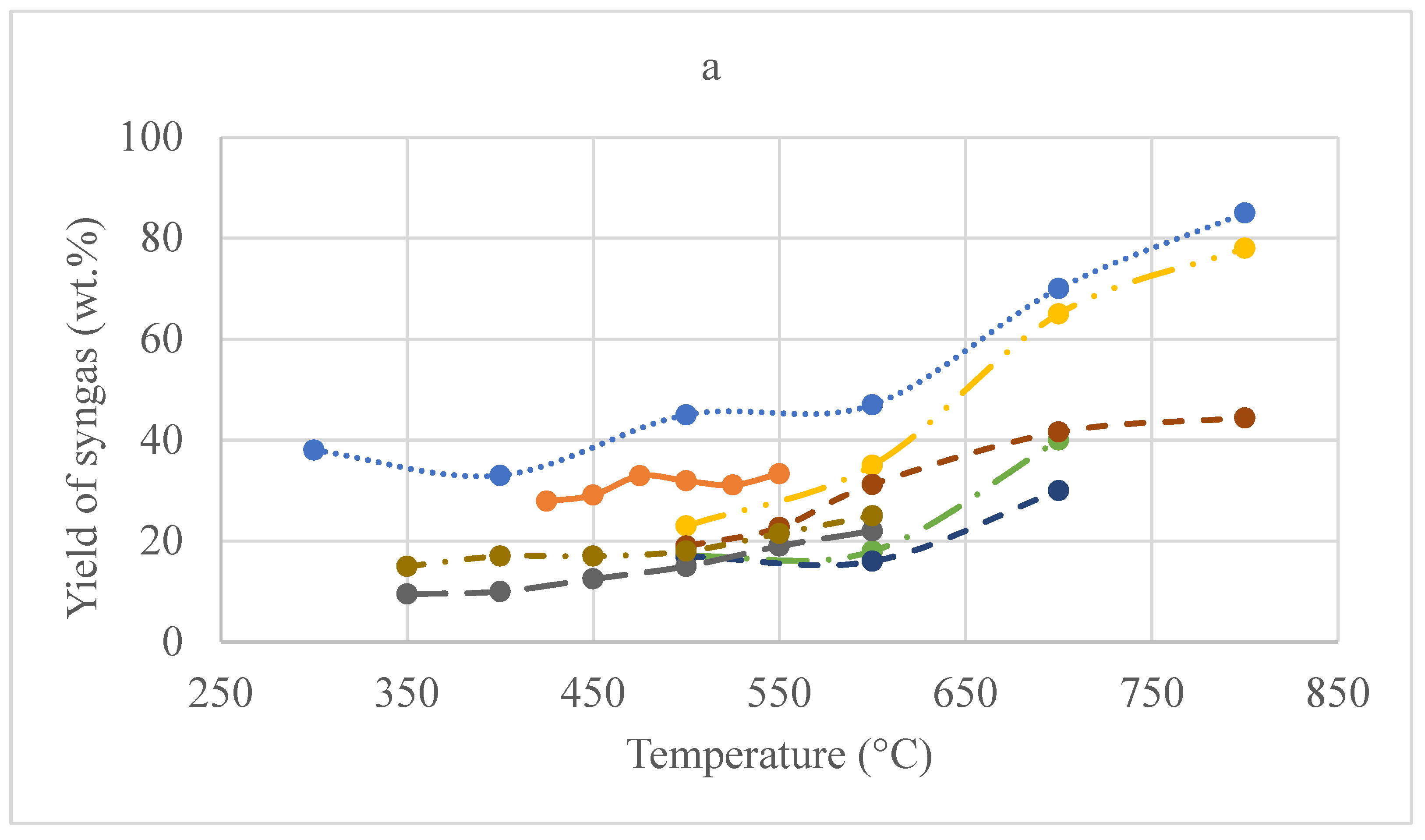
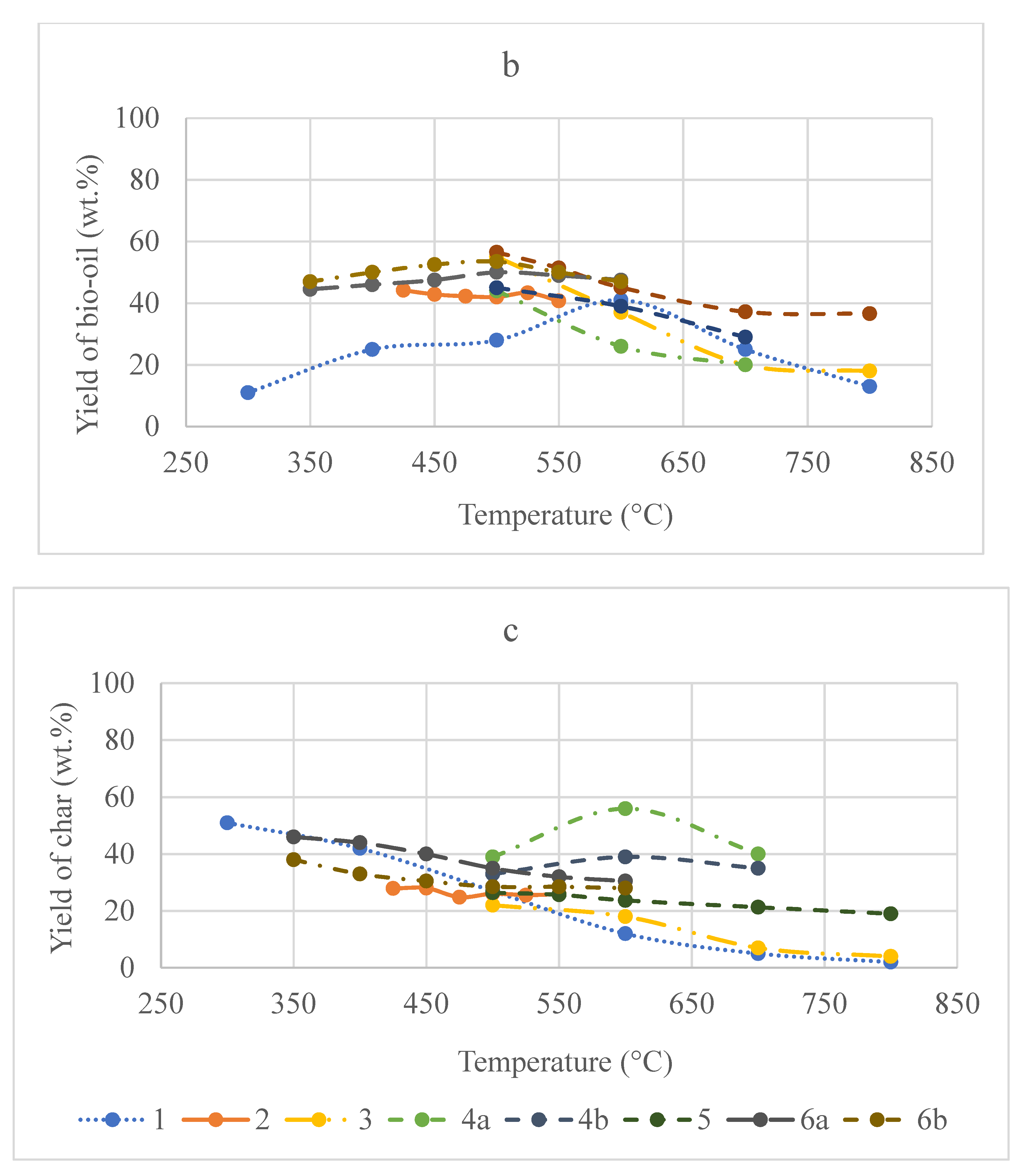
3.3.1. Temperature vs. Yield
3.3.2. Heating Rate vs. Yield
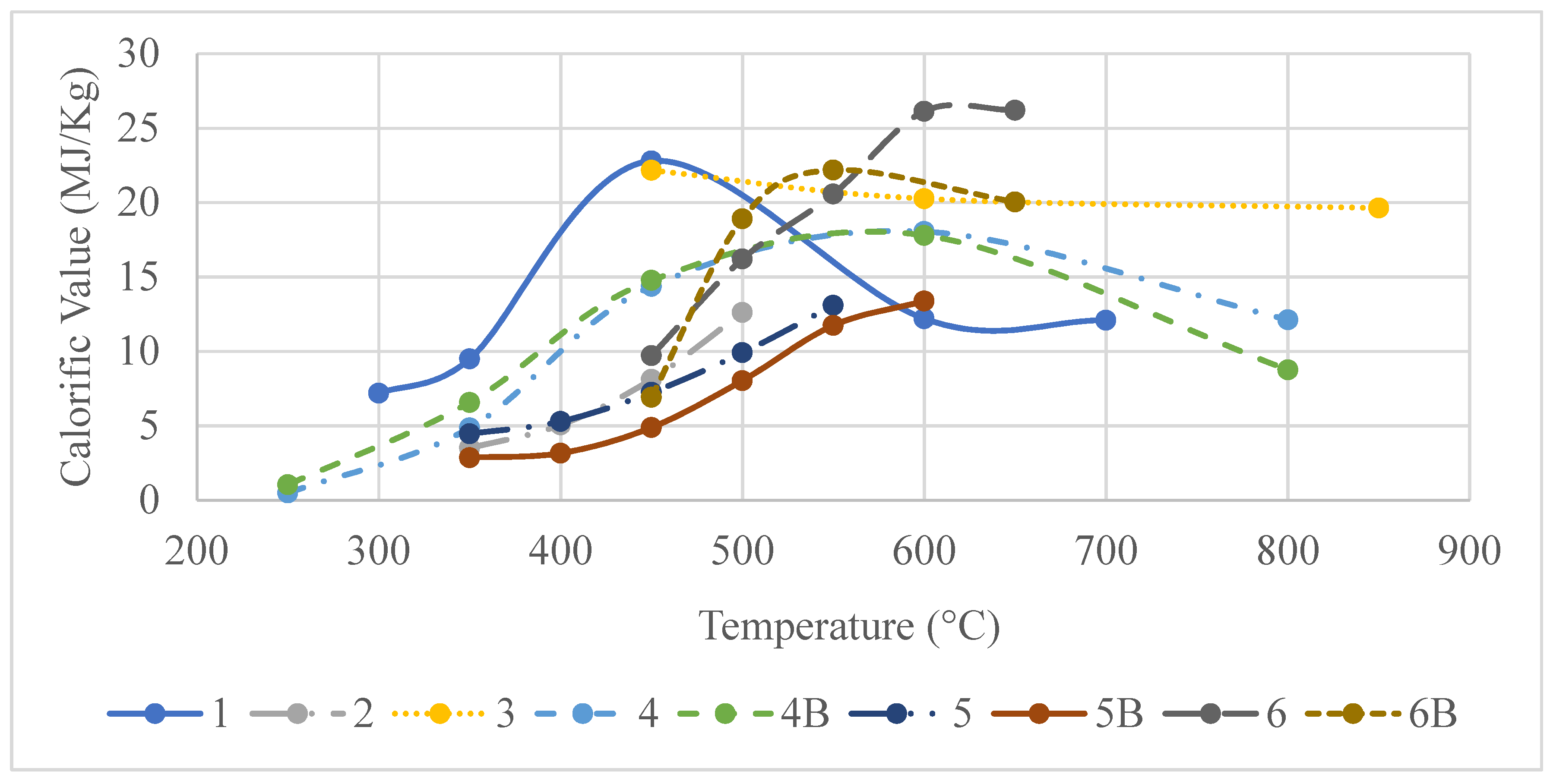
3.3.3. Temperature vs. Syngas Calorific Value
3.3.4. Heating Rate vs. Syngas Calorific Value
3.3.5. Effects of Residence Time (RT)
3.3.6. Effects of Feedstock Flow Rate
3.3.7. Effects of Initial Moisture Content
4. Conclusions
- To provide an overview on the existing solid waste management/stabilisation methods and their associated implementation challenges in commercial/pilot, laboratory and small scales;
- To select the most promising method and further address the drawbacks.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zseni, A. Human Excreta Management: Human Excreta as an Important Base of Sustainable Agriculture. In Proceedings of the 4th Multidisciplinary Academic Conference (4th MAC), Prague, Czech, 20 February 2015. [Google Scholar]
- Strande, L.; Ronteltap, M.; Brdjanovic, D. Faecal Sludge Management, System Approach for Implementation and Operation; IWA Publishing: London, UK, 2014. [Google Scholar] [CrossRef] [Green Version]
- Peal, A.; Evans, B.; Blackett, I.; Hawkins, P.; Heymans, C. Fecal Sludge Management: Analytical tools for assessing FSM in cities. Water Sanit. Hyg. Dev. 2014, 4, 371–383. [Google Scholar] [CrossRef] [Green Version]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2015, 17, 1827–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kone, D. Fecal Sludge Management—Sandec Training Tool 1.0—Module 5; Department of Water and Sanitation in Developing Countries, Swiss Federal institute of Aquatic Science and Technology (Eawag/SANDEC): Duebendorf, Switzerland, 2008. [Google Scholar]
- Cantinho, P.; Matos, M.; Trancoso, M.A.; Correia dos Santos, M.M. Behaviour and fate of metals in urban wastewater treatment plants: A review. Int. J. Environ. Sci. Technol. 2016, 13, 359–386. [Google Scholar] [CrossRef] [Green Version]
- EUROSTAT. Sewage Sludge Production and Disposal. Available online: http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=env_ww_spd&lang=en (accessed on 1 November 2021).
- Chan, W.P.; Wang, J.W. Comprehensive characterisation of sewage sludge for thermochemical conversion Processes—Based on singapore survey. Waste Manag. 2016, 54, 131–142. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijalkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almas, A.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- OFWAT. Water 2020: Regulatory Framework for Wholesale Markets and the 2019 Price Review—Appendix 1: Sludge Treatment, Transport and Disposal—Supporting Evidence and Design Options; OFWAT: Birmingham, UK, 2015. [Google Scholar]
- Harvey, F. Nearly 30000 Tonnes of Sewage Sludge Containing Human Waste to Enter UK. 2020. Available online: https://www.theguardian.com/environment/2020/sep/02/sewage-sludge-containing-human-waste-uk (accessed on 9 November 2021).
- Dowler, C.; Boren, Z. Revealed: Salmonella, Revealed: Salmonella, Toxic Chemicals and Plastic Found in Sewage Spread on Farmland. Available online: https://unearthed.greenpeace.org/2020/02/04/sewage-sludge-landspreading-environment-agency-report/ (accessed on 1 November 2021).
- Andreoli, C.V.; Sperling, M.V.; Fernandes, F. Sludge Treatment and Disposal; IWA Publishing: London, UK, 2007. [Google Scholar] [CrossRef]
- Raheem, A.; Sikarwar, S.V.; He, J.; Datyar, W.; Dionysiou, D.D.; Wang, W.; Zhao, M. Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: A review. Chem. Eng. J. 2018, 616–641. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Sludge: A waste or renewable source for energy and resource recovery? Renew. Sustain. Energy Rev. 2013, 25, 708–728. [Google Scholar] [CrossRef]
- Winkler, M.K.; Bennenbroek, M.; Horstink, F.; Loosdrecht, M.V.; Pol, G.J. The biodrying concept: An innovation technology creating energy from sewage sludge. Bioresour. Technol. 2013, 147, 124–129. [Google Scholar] [CrossRef]
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A Review of Sludge-to-Energy Recovery Methods. Energies 2019, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Kosov, V.; Umnova, O.; Zaichenko, V. The Pyrolysis Process of Sewage Sludge. [Online]. 2019. Available online: https://iopscience.iop.org/article/10.1088/1742-6596/653/1/012032/meta (accessed on 9 November 2021).
- Cao, Y.; Pawlowski, A. Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: Brief overview and energy efficiency assessment. Renew. Sustain. Energy Rev. 2012, 16, 1657–1665. [Google Scholar] [CrossRef]
- Batistella, L.; Silva, V.; Suzin, R.C.; Virmond, E.; Althoff, C.A.; Moreira, R.F.; Jose, H.J. Gaseous emissions from sewage sludge combustion in a moving bed combustor. Waste Manag. 2015, 46, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, B.M.; Namiesnik, J.; Konieczka, P. Review of sewage sludge management: Standards, regulations and analytical methods. J. Clean. Prod. 2014, 90, 1–15. [Google Scholar] [CrossRef]
- Syed-Hassan, A.S.S.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Han, X.; Niu, M.; Jiang, X.; Liu, J. Combustion characteristics of sewage sludge in a fluidized bed. Ind. Eng. Chem. Res. 2012, 51, 10565–10570. [Google Scholar] [CrossRef]
- Werther, J.; Ogada, T. Sewage sludge combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- Chen, J.; He, Y.; Liu, J.; Liu, C.; Xie, W.; Kuo, J.; Zhang, X.; Li, J.; Sun, S.; Buyukada, M.; et al. The mixture of sewage sludge and biomass waste as solid biofuels: Process characteristic and environmental implication. Renew. Energy 2019, 139, 707–717. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Y.; Zhu, J.; Zuo, W.; Yin, L. Characterization of nitrogen transformation during microwave induced pyrolysis of sewage sludge. J. Anal. Appl. Pyrolysis 2014, 105, 335–341. [Google Scholar] [CrossRef]
- Lu, S.; Yang, L.; Zhou, F.; Wang, F.; Yan, J.; Li, X.; Chi, Y.; Cen, K. Atmospheric emission characterization of a novel sludge drying and co-combustion system. J. Environ. Sci. 2013, 25, 2088–2092. [Google Scholar] [CrossRef]
- Velden, M.; Dewil, R.; Baeyens, J.; Josson, L.; Lanssens, P. The distribution of heavy metals during fluidized bed combustion of sludge (FBSC). J. Hazard. Mater. 2008, 151, 96–102. [Google Scholar] [CrossRef]
- Marani, D.; Braguglia, C.; Mininni, G.; Maccioni, F. Behaviour of Cd, Cr, Mn, Ni, Pb, and Zn in sewage sludge incineration by fluidised bed furnace. Waste Manag. 2003, 23, 117–124. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Jiang, X.; Li, F.; Lei, Y.; Lin, Q. The thermal behaviour and kinetics of co-combustion between sewage sludge and wheat straw. Fuel Process. Technol. 2019, 189, 1–14. [Google Scholar] [CrossRef]
- Rong, H.; Wang, T.; Zhou, M.; Wang, H.; Hou, H.; Xue, Y. Combustion Characteristics and slagging during Co-Combustion of RIce Husk and Sewage Sludge Blends. Energies 2017, 10, 438. [Google Scholar] [CrossRef] [Green Version]
- Samolada, M.; Zabaniotou, A. Comparative assessment of mucipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in greece. Waste Manag. 2014, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Lopez, G.; Amutio, M.; Artetxe, M.; Barbarias, I.; Arregi, A.; Bilbao, J.; Olazar, M. Charaterization of the bio-oil obtained by fast pyrolysis of sewage sludge in a conical spouted bed reactor. Fuel Process. Technol. 2016, 149, 169–175. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Peng, L.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Gil-Lalaguna, N.; Sanchez, J.; Murillo, M.; Ruiz, V.; Gea, G. Air-steam gasification of char derived from sewage sludge pyrolysis. Comaprison with the gasification of sewage sludge. Fuel 2014, 129, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Stolarek, P.; Malinowski, A.; Lepez, O. Thermochemical treatment of sewage sludge by integration of drying and pyrolysis/autogasification. Renew. Sustain. Energy Rev. 2019, 319–327. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martinez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Kundu, K.; Chatterjee, A.; Bhattacharyya, T.; Roy, M.; Kaur, A. Thermochemical conversion of biomass to bioenergy: A Review. In Prospects of Altenative Transportation Fuels; Springer: Singapore, 2018; pp. 235–268. [Google Scholar] [CrossRef]
- Park, H.J.; Heo, H.S.; Park, Y.K.; Yim, J.H.; Jeon, J.K.; Park, J.; Ryu, C.; Kim, S.S. Clean bio-oil production from fast pyrolysis of sewage sludge: Effects of reaction conditions and metal oxide catalysts. Bioresour. Technol. 2010, 101, S83–S85. [Google Scholar] [CrossRef]
- Bridgewater, T. Challenges and Oppurtunities in Fast Pyrolysis of Biomass: Part 1, Introduction to the technology, feedstocks and science behind a promisingsource of fuels and chemicals. Johns. Matthey Technol. Rev. 2018, 118–130. [Google Scholar] [CrossRef]
- Karaca, C.; Sozen, S.; Orhon, D.; Okutan, H. High temperature pyrolysis of sewage sludge as a sustainable process for energy recovery. Waste Manag. 2018, 217–226. [Google Scholar] [CrossRef]
- Funke, A.; Richter, D.; Niebel, A.; Dahmen, N.; Sauer, J. Fast Pyrolysis of Biomass Residues in a Twin-screw Mixing Reactor. J. Vis. Exp. 2016, 115. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.D.; Murillo, R.; Garcia, T.; Veses, A. Demonstration of the waste tire pyrolysis process on pilot scale in a continuous auger reactor. J. Hazard. Mater. 2013, 261, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kyto, M.; Chiaramonti, D. Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy 2014, 116, 178–190. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, S.; Xiao, B.; Yu, D.; Jia, X. Mechanism of wet sewage sludge pyrolysis in a tubular furnace. Int. J. Hydrogen Energy 2011, 36, 355–363. [Google Scholar] [CrossRef]
- Dominguez, A.; Menendez, J.; Pis, J. Hydrogen rich fuel gas production from the pyrolysis of wet sewage sludge at high temperature. J. Anal. Appl. Pyrolysis 2006, 77, 127–132. [Google Scholar] [CrossRef]
- Conesa, J.; Font, R.; Fullana, A.; Martin-Gullon, I.; Arcil, I.; Galvez, A.; Molto, J.; Gomez, M. Comparison between emissions from the pyrolysis and combustion of different wastes. J. Anal. Appl. Pyrolysis 2009, 84, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Vineet, S.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Mohammad, Z.M.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef] [Green Version]
- Adar, E.; Karatop, B.; Ince, M.; Bilgili, M.S. Comparison of methods for sustainable energy management with sewage sludge in Turkey based on SWOT-FAHP analysis. Renew. Sustain. Energy Rev. 2016, 62, 429–440. [Google Scholar] [CrossRef]
- Hanak, D.P.; Kolios, A.J.; Onabanjo, T.; Wagland, S.T.; Patchigolla, K.; Fidalgo, B.; Manovic, V.; McAdam, E.; Parker, A.; Williams, L.; et al. Conceptual energy and water recovery system for self-sustained nano-membrane toilet. Energy Convers. Manag. 2016, 126, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Yacob, T.W.; Fisher, R.C.; Linden, K.G.; Weimer, A. Pyrolysis of human feces: Gas yield analysis and kinetic modelling. Waste Manag. 2018, 214–222. [Google Scholar] [CrossRef]
- Jurado, N.; Somorin, T.; Kolios, A.J.; Wagland, S.; Patchigolla, K.; Fidalgo, B.; Parker, A.; McAdam, E.; Williams, L.; Tyrrel, S. Design and commisioning of a multi-mode prototype for thermochemical conversion of human faeces. Energy Convers. Manag. 2018, 507–524. [Google Scholar] [CrossRef]
- Onabanjo, T.; Kolios, A.J.; Patchigolla, K.; Wagland, S.T.; Fidalgo, B.; Jurado, N.; Hanak, D.P.; Manovic, V.; Parker, A.; McAdam, L.; et al. An experimental Investigation of the combustion performance of human faeces. Fuel 2016, 184, 780–791. [Google Scholar] [CrossRef] [Green Version]
- Septien, S.; Pocock, J.; Teba, L.; Velkushanova, K.; Buckley, C. Rheological characteristics of faecal sludge from VIP latrines and implications on pit emptying. J. Environ. Manag. 2018, 228, 149–157. [Google Scholar] [CrossRef]
- Onabanjo, T.; Patchigolla, K.; Wagland, S.; Fidalgo, B.; Kolios, A.; McAdam, E.; Parker, A.; Williams, L.; Tyrrel, S.; Cartmell, E. Energy recovery from human faeces via gasification: A thermodynamic equilibrium modelling approach. Energy Convers. Manag. 2016, 118, 364–376. [Google Scholar] [CrossRef] [Green Version]
- Monhol, F.A.F.; Martins, M.F. Co-current Combustion of Human Feces and Polyethylene Waste. Waste Biomass Valor 2015, 6, 425–432. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis; Elsevier Inc.: Oxford, UK, 2010. [Google Scholar] [CrossRef]
- McNamara, P.; Koch, J.; Liu, Z.; Zitomer, D. Pyrolysis of Dried Wastewater Biosolids can Be Energy Positive. Water Environ. Res. 2019, 91, 804–810. [Google Scholar] [CrossRef]
- Khiari, B.; Marias, F.; Zagrouba, F.; Vaxelaire, J. Analytical study of the pyrolysis process in a wastewater treatment pilot station. Desalination 2004, 167, 39–47. [Google Scholar] [CrossRef]
- Reed, T.; Gaur, S. The High Heat of Fast Pyrolysis for Large Particles. In Developments in Thermochemical Biomass Conversion; Bridgwater, A., Boocock, D., Eds.; Springer-Science+Business Media: Dordrecht, The Netherlands, 1997; pp. 97–103. [Google Scholar] [CrossRef]
- Daugaard, D.E.; Brown, R.C. Enthalpy for Pyrolysis for Several Types of Biomass. Energy Fuels 2003, 17, 934–939. [Google Scholar] [CrossRef] [Green Version]
- Roy, C.; Lemieux, R.; deCaumia, B.; Blanchette, D. Processing of Wood Chips in a Semicontinuous Multiple-Hearth Vacuum Pyrolysis Reactor. In Pyrolysis Oils from Biomass; Soltes, E., Ed.; American Chemical Society: Bryan, TX, USA, 1998; pp. 16–30. [Google Scholar] [CrossRef]
- Yang, H.; Kudo, S.; Kuo, H.; Norinaga, K.; Mori, A.; Masek, O.; Hayashi, J. Estimation of Enthalpy of Bio-Oil Vapor and Heat Required for Pyrolysis of Biomass. Energy Fuels 2013, 27, 2675–2686. [Google Scholar] [CrossRef]
- Gronnow, M.J.; Budarin, V.L.; Masek, O.; Crombie, K.N.; Brownsort, P.A.; Shuttleworth, P.S.; Hurst, P.R.; Clark, J.H. Torrefaction/biochar production by microwave and conventional slow pyrolysis—Comparison of energy properties. Bioenergy 2013, 5, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Crombie, K.; Masek, O. Investigation the potential for a self-sustaining slow pyrolysis system under varying operating conditions. Bioresour. Technol. 2014, 162, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.K.; Strezov, V.; Nelson, P.F. Thermal characterisation of the products of wastewater sludge pyrolysis. J. Anal. Appl. Pyrolysis 2009, 85, 442–446. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Oladejo, J.M. ‘Patented blunderings’, efficiency awareness, and self-sustainability claims in the pyrolysis energy from waste sector. Resour. Conserv. Recycl. 2019, 141, 233–242. [Google Scholar] [CrossRef]
- Ward, B.J.; Yacob, T.W.; Montoya, L.D. Evaluation of Solid Fuel Char Briquettes from Human Waste. Environ. Sci. Technol. 2014, 48, 9852–9858. [Google Scholar] [CrossRef]
- Oasmaa, A.; Kalli, A.; Lindfors, C.; Elliot, D.C.; Springer, D. Peacocke and D. Chiaramonti. Guidelines for Transportation, Handling, and Use of Fast Pyrolysis Bio-oil. 1. Flammability and Toxicity. Energy Fuels 2012, 26, 3864–3873. [Google Scholar] [CrossRef]
- Li, C.; Suzuki, K. Tar property, analysis, reforming mechanism and model for biomass gasification—An overview. Renew. Sustain. Energy Rev. 2009, 13, 594–604. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.; Wei, L.; Zhang, J.; Law, C.K. Experimental and modeling study on ignition delays of lean mixtures of methane, hydrogen, oxygen and argon at elevated pressures. Combust. Flame 2012, 159, 918–931. [Google Scholar] [CrossRef]
- Stylianidis, N.; Azimov, U.; Birkett, M. Investigation of the Effect of Hydrogen and Methane on Combustion of Multicomponent Syngas Mixtures using a Constructed Reduced Chemical Kinetics Mechansim. Energies 2019, 12, 2442. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, B.B.; Sahoo, N.S. Effect of H2:CO ratio in syngas on the performance of a dual fuel diesel engine operation. Appl. Therm. Eng. 2012, 49, 139–146. [Google Scholar] [CrossRef]
- Fonts, I.; Gea, G.; Azuara, M.; Abrego, J.; Arauzo, J. Sewage sludge pyrolysis for liquid production: A review. Renew. Sustain. Ebergy Rev. 2012, 16, 2781–2805. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 134–149. [Google Scholar] [CrossRef]
- Jaramillo-Arango, A.; Fonts, I.; Chjne, F.; Arauzo, J. Product compositions from sewage sludge pyrolysis in a fluidized bed and correlations with temperature. J. Anal. Appl. Pyrolysis 2016, 121, 287–296. [Google Scholar] [CrossRef]
- Kim, S.; Agblevor, F.A. Thermogravimetric analysis and fast pyrolysis of Milkweed. Bioresour. Technol. 2014, 169, 367–373. [Google Scholar] [CrossRef]
- Shiguang, L.; Shaoping, X.; Shuqin, L.; Chen, Y.; Qinghua, L. Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process. Technol. 2004, 85, 1201–1211. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A.; Antonakou, E.; Papazisi, K.; Lappas, A. Athanassiou. Investigating the potential for energy, fuel, materials and chemicals production from corn residues (cobs and stalks) by non-catalytic and catalytic pyrolysis in two reactor configurations. Renew. Sustain. Energy Rev. 2009, 13, 750–762. [Google Scholar] [CrossRef]
- Puy, N.; Murillo, R.; Navarro, M.; Lopez, J.; Rieradevall, J.; Fowler, G.; Aranguren, I.; Garcia, T.; Bartroli, J.; Mastral, A. Valorisation of forestry waste by pyrolysis in an auger reactor. Waste Manag. 2011, 31, 1339–1349. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.; Cheng, Z.; Blanco, P.; Liu, R.; Bridgwater, A.; Cai, J. Pyrolysis of Rice Husk and Corn Stalk in Auger Reactor. 1. Characterisation of Char and Gas at Various Temperatures. Energy Fuel 2016, 30, 10568–10574. [Google Scholar] [CrossRef] [Green Version]
- Kan, T.; Strezov, V.; Evans, T. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Weldekidan, H.; Strezov, V.; Li, R.; Kan, T.; Town, G.; Kumar, R.; He, J.; Flamant, G. Distribution of solar pyrolysis products and product gas composition produced from agricultural residues and animal wastes at different operating parameters. Renew. Energy 2020, 151, 1102–1109. [Google Scholar] [CrossRef]
- Li, R.; Zeng, K.; Soria, J.; Mazza, G.; Gauthier, D.; Rodriguez, R.; Flamant, G. Product distribution from solar pyrolysis of agricultural and forestry biomass residues. Renew. Energy 2016, 89, 27–35. [Google Scholar] [CrossRef]
- Morgano, M.; Leibold, H.; Richter, F.; Stapf, D.; Seifert, H. Screw pyrolysis technology for sewage sludge treatment. Waste Manag. 2018, 487–495. [Google Scholar] [CrossRef]
- Inguanzo, M.; Dominguez, A.; Menendez, J.; Blanco, C.; Pis, J. On the pyrolysis of sewage sludge: The influence of pyrolysis conditions on solid, liquid and gas fractions. J. Anal. Appl. Pyrolysis 2002, 63, 209–222. [Google Scholar] [CrossRef]
- Gao, N.; Li, J.; Qi, B.; Li, A.; Duan, Y.; Wang, Z. Thermal analysis and products distribution of dried sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2014, 105, 43–48. [Google Scholar] [CrossRef]
- Waldheim, L.; Nilsson, T. Heating Value of Gases from Biomass Gasification; TPS Termiska Processer AB: Nykoping, Sweden, 2001. [Google Scholar]
- Morf, P.; Hasler, P.; Nussbaumer, T. Mechanisms and kinetics of homogeneous secondary reactions of tar from continuous pyrolysis of woodchips. Fuel 2002, 81, 843–853. [Google Scholar] [CrossRef]
- Uddin, M.N.; Wan Daud, W.; Abbas, H.F. Potential hydrogen and non-condensable gases production from biomass pyrolysis: Insights into the process variables. Renew. Sustain. Energy Rev. 2013, 27, 204–224. [Google Scholar] [CrossRef]
- Muvhiiwa, R.; Sempuga, B.; Hildebrandt, D.; Van Der Walt, J. Study of the effects of temperature on syngas composition from pyrolysis of wood pellets using a nitrogen plasma torch reactor. J. Anal. Appl. Pyrolysis 2018, 159–168. [Google Scholar] [CrossRef]
- Hornung, U.; Schneider, D.; Hornung, A.; Tumiatti, V.; Seifert, H. Sequential pyrolysis and catalytic low temperature reforming of wheat straw. J. Anal. Appl. Pyrolysis 2009, 85, 145–150. [Google Scholar] [CrossRef]
- Xiong, S.; Zhang, B.X.; Xiao, B.; He, M. Feasibility Study on the Pyrolysis Production for Hydrogen-Riched Fuel Gas from the Wet Sewage Sludge; IEEE: Beijing, China, 2009; pp. 1–4. [Google Scholar] [CrossRef]
- Soria, J.; Zeng, K.; Asensio, D.; Gauthier, D.; Flamant, G.; Mazza, G. Comprehensive CFD modelling of solar fast pyrolysis of beech wood pellets. Fuel Process. Technol. 2017, 158, 226–237. [Google Scholar] [CrossRef]
- Gao, N.; Liu, B.; Li, A.; Li, J. Continuous pyrolysis of pine sawdust at different pyrolysis temperatures and solid residence times. J. Anal. Appl. Pyrolysis 2015, 114, 155–162. [Google Scholar] [CrossRef]
- Kelkar, S.; Saffron, C.M.; Chai, L.; Bovee, J.; Stuecken, T.R.; Garedew, M.; Li, Z.; Kriegel, R.M. Pyrolysis of spent coffee grounds using a screw-conveyor reactor. Fuel Process. Technol. 2015, 137, 170–178. [Google Scholar] [CrossRef]
- Ferreira, S.D.; Altafini, C.R.; Perondi, D.; Godinho, M. Pyrolysis of Medium Density Fiberboard (MDF) wastes in a screw reactor. Energy Convers. Manag. 2015, 92, 223–233. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K.; Helleur, R. Production and Characterization of Pyrolysis Oil from Sawmill Residues in an Auger Reactor. Ind. Eng. Chem. Res. 2017, 56, 1920–1925. [Google Scholar] [CrossRef]
- Soria-Verdugo, A.; Morato-Godino, A.; Garcia-Gutierrez, L.; Garcia-Hernando, N. Pyrolysis of sewage sludge in a fixed and bubbling fluidized bed—Estimation and experimental validation of the pyrolysis time. Energy Convers. Manag. 2017, 144, 235–242. [Google Scholar] [CrossRef]
- Mei, Z.; Chen, D.; Zhang, J.; Yin, L.; Huang, Z.; Xin, Q. Sewage sludge pyrolysis coupled with self-supplied steam reforming for high quality syngas production and the influence of initial moisture content. Waste Manag. 2020, 106, 77–87. [Google Scholar] [CrossRef]
| Pyrolysis Process | Temperature (°C) | Heating Rate (°C/min) | Solid Residence Time (s) | Particle Size (mm) | Product Yield (%) | ||
|---|---|---|---|---|---|---|---|
| Oil | Gas | Char | |||||
| Slow/int | 400–500 | 10 | ~500 | 5–50 | 30 | 35 | 35 |
| Fast | 400–650 | 100 | 0.5–10 | <1 | 50 | 20 | 30 |
| Flash | 700–1000 | >500 | <0.5 | <0.2 | 75 | 12 | 13 |
| Main Sewage Sludge Stabilisation Methods | ||||
|---|---|---|---|---|
| Biological | Thermochemical | |||
| Anaerobic Digestion (AAD) | Combustion | Gasification | Pyrolysis | |
| Advantages |
|
|
|
|
| Disadvantages |
|
|
|
|
| Waste Type | Enthalpy (MJ/kg) | Moisture Content (%) | Description | Reference |
|---|---|---|---|---|
| Oak Oat hulls Pine Corn stover | 1.46 ± 0.28 0.78 ± 0.20 1.64 ± 0.33 1.35 ± 0.28 | 8.0 10.2 7.5 8.8 | Pilot-scale fluidised bed pyrolysis reactor operating at about 500 °C. | [63] |
| Birch dowels | 2.9–3.5 8.1–8.6 | 0 55 | ‘Water tracer’ technique was applied for the determination of the time of pyrolysis for a dry and an identical moist particle. However, this non-conventional method is known to result in errors within calculation [63]. | [62] |
| Wood chips | 0.7 | 5.9 | The analysis of the enthalpy of pyrolysis was performed on a multiple-hearth reactor. An empirical method ‘P.D.U.’ was used to indicate the difference between electric energy consumption prior and through wood feeding. | [64] |
| Cedar, Pine, Willow, Bamboo, Sasa bamboo | 1.3 ± 0.2 1.5 1.5 1.5 1.6 | 0 | Enthalpy for the pyrolysis of five different types of biomass materials was calculated using a screw feeding reactor. The analysis aimed for the determination of the enthalpy of the formation of products based on their composition. However, the method used for the enthalpy of bio-oil included errors. | [65] |
| Willow chips | 1.17 | 9.1 | Analysis on conventional and MW pyrolysis (required electricity). | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beik, F.; Williams, L.; Brown, T.; Wagland, S.T. Managing Non-Sewered Human Waste Using Thermochemical Waste Treatment Technologies: A Review. Energies 2021, 14, 7689. https://doi.org/10.3390/en14227689
Beik F, Williams L, Brown T, Wagland ST. Managing Non-Sewered Human Waste Using Thermochemical Waste Treatment Technologies: A Review. Energies. 2021; 14(22):7689. https://doi.org/10.3390/en14227689
Chicago/Turabian StyleBeik, Farhad, Leon Williams, Tim Brown, and Stuart T. Wagland. 2021. "Managing Non-Sewered Human Waste Using Thermochemical Waste Treatment Technologies: A Review" Energies 14, no. 22: 7689. https://doi.org/10.3390/en14227689
APA StyleBeik, F., Williams, L., Brown, T., & Wagland, S. T. (2021). Managing Non-Sewered Human Waste Using Thermochemical Waste Treatment Technologies: A Review. Energies, 14(22), 7689. https://doi.org/10.3390/en14227689







