Simulation of Coupled Power and Gas Systems with Hydrogen-Enriched Natural Gas
Abstract
1. Introduction
1.1. The Role of Natural Gas (NG) in Future Energy Systems
1.2. Blending Hydrogen into Existing NG Infrastructure
1.3. Simulation of Hydrogen-Enriched NG Network
2. Modeling of Gas Pipeline Systems
2.1. Modeling of Gas Pipelines
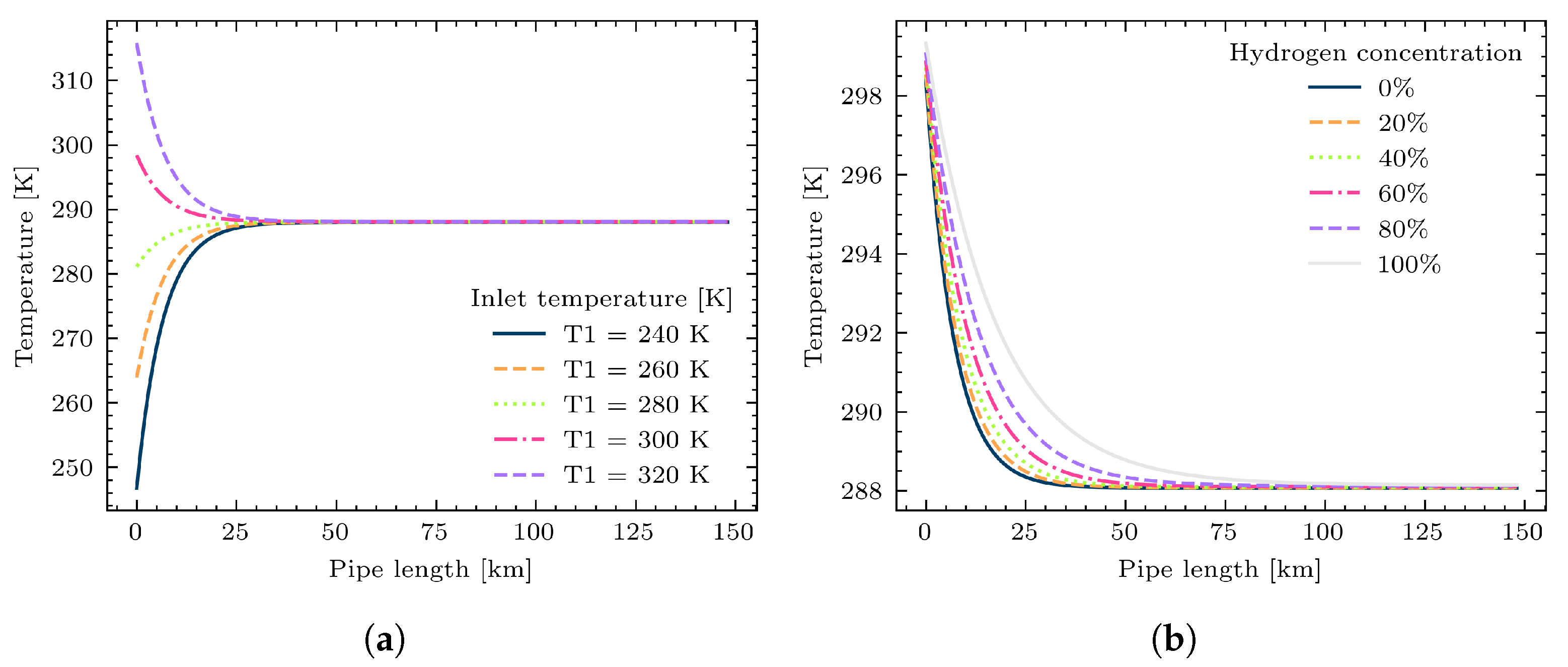
2.2. Thermal Formulation
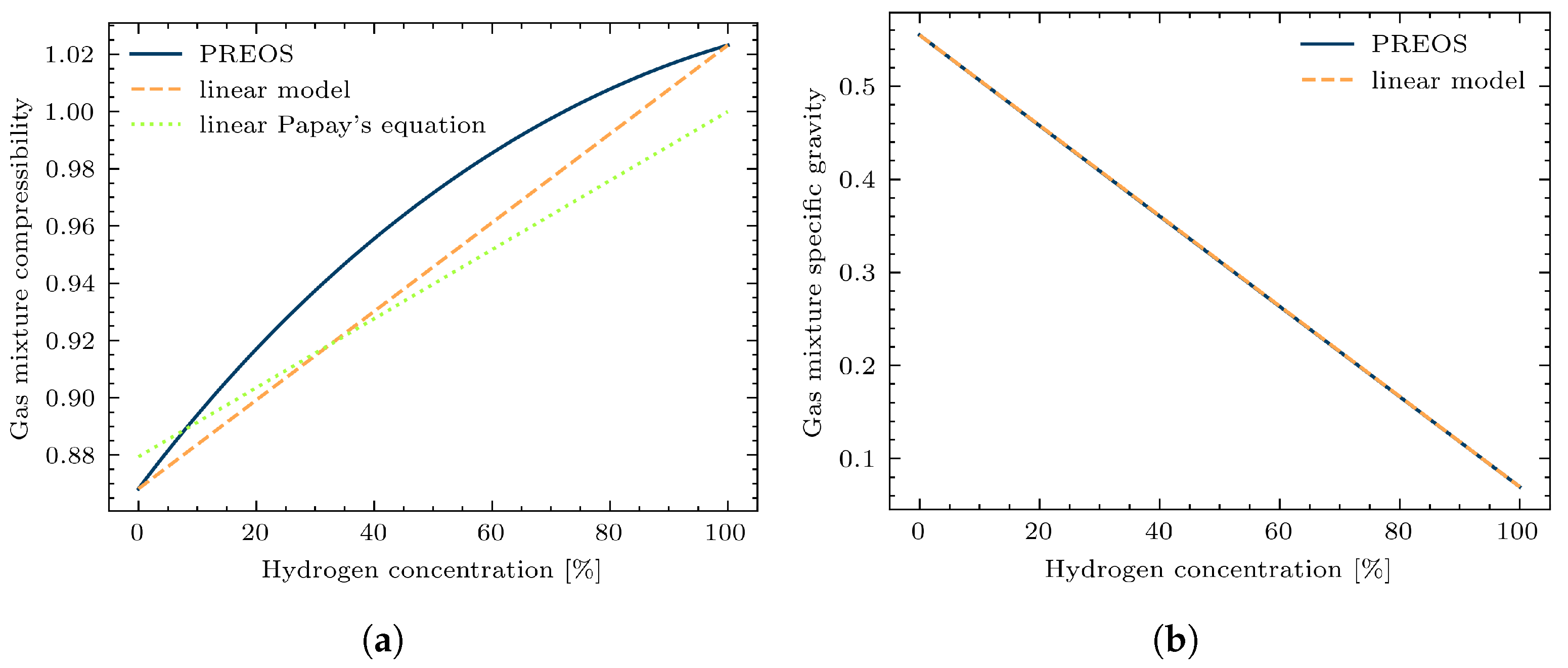
2.3. Calculation of Gas Mixture Properties
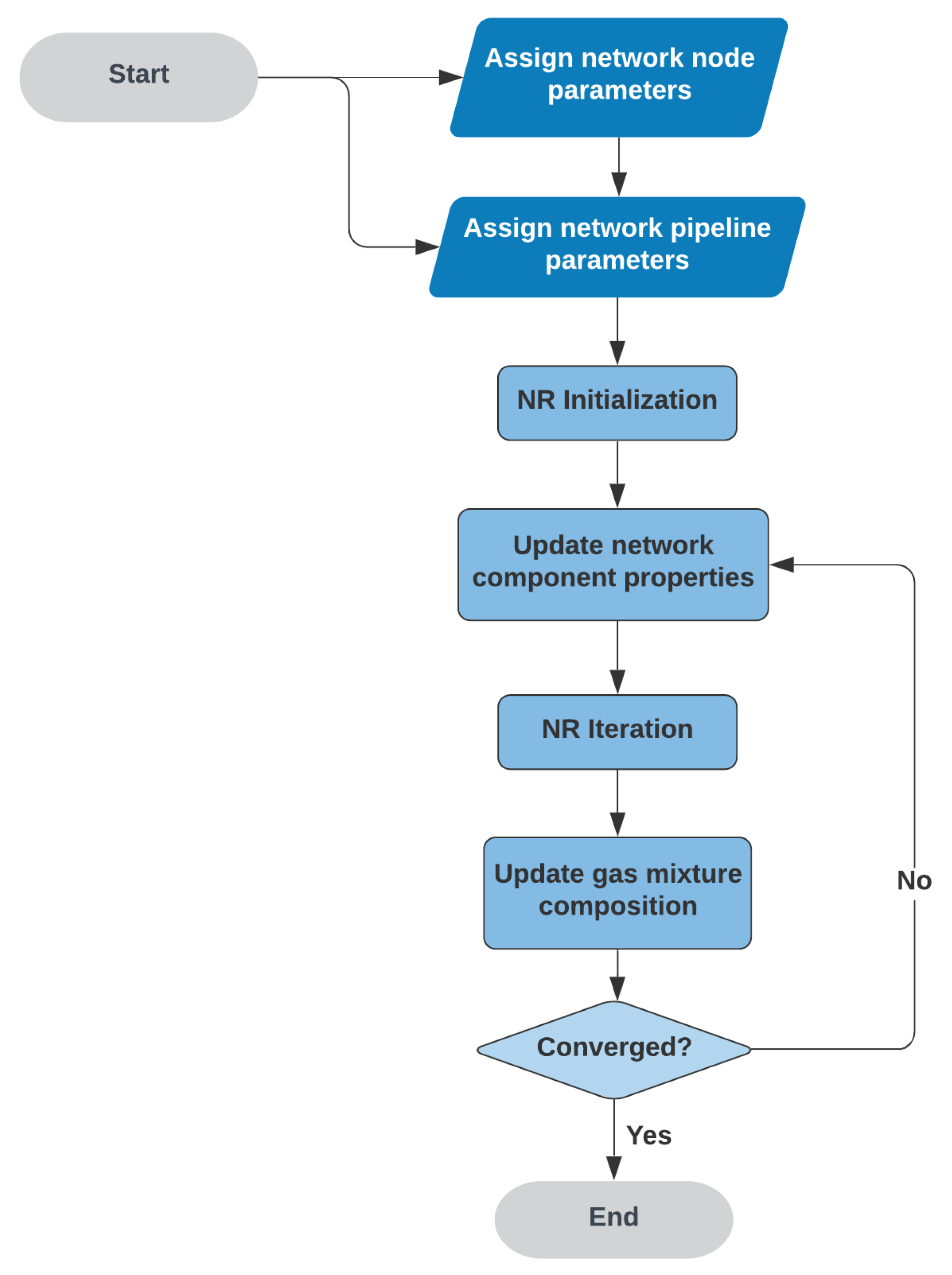
2.4. Solution Flow
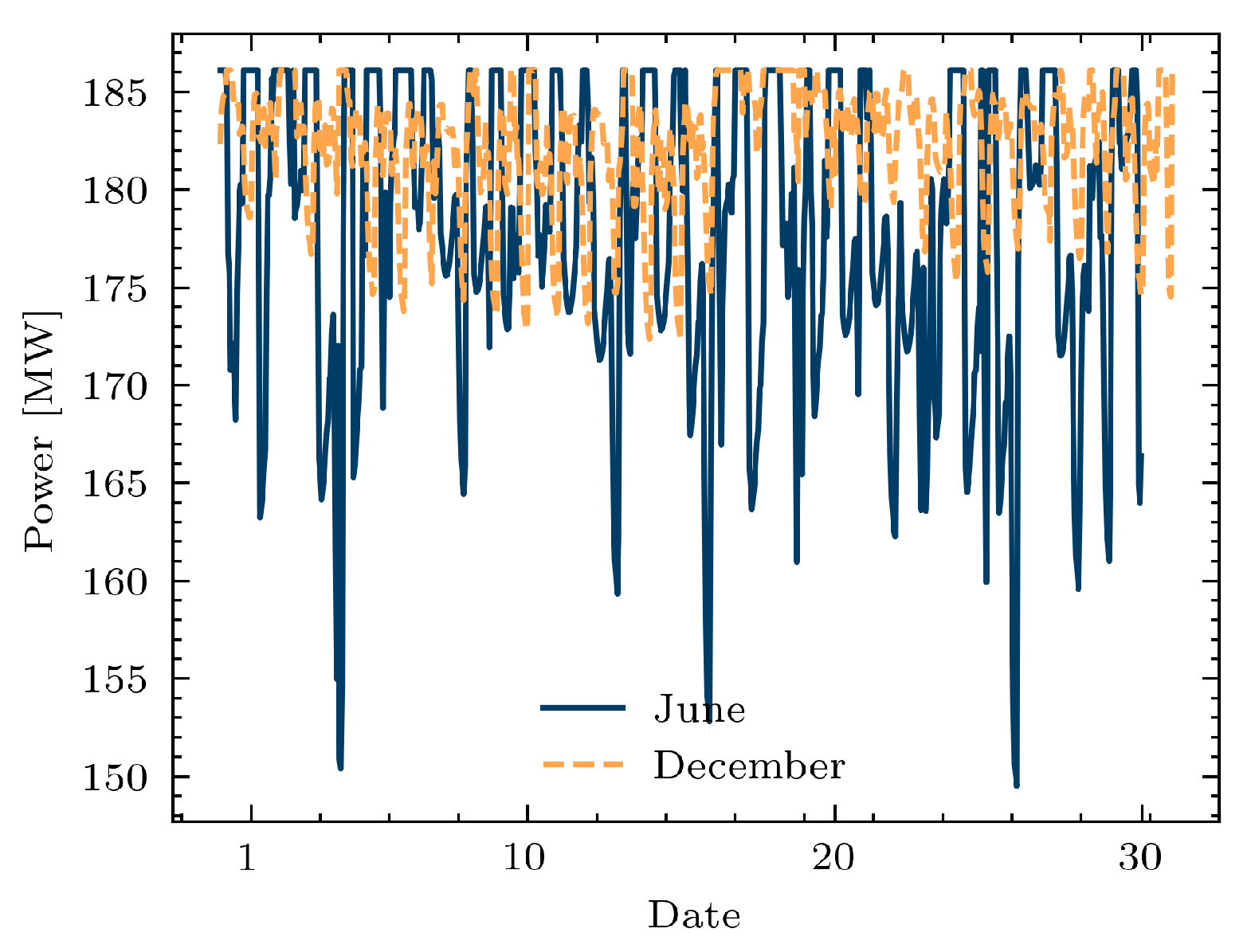
3. Simulation and Results
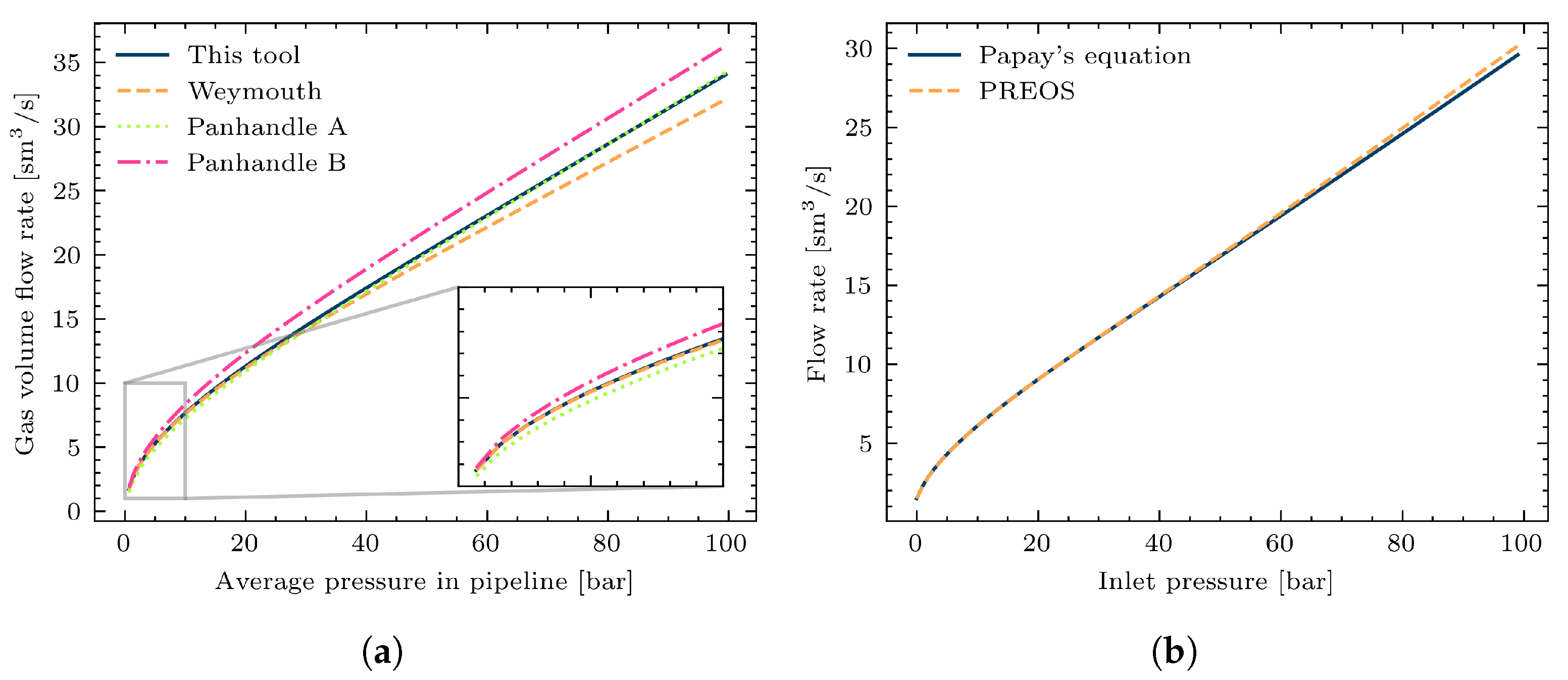
3.1. Study Case 1: Impacts of Different Calculation Methods on a Single Pipeline with Variable Gas Mixture Composition
3.2. Study Case 2: A Simple Gas Network with Hydrogen Injection
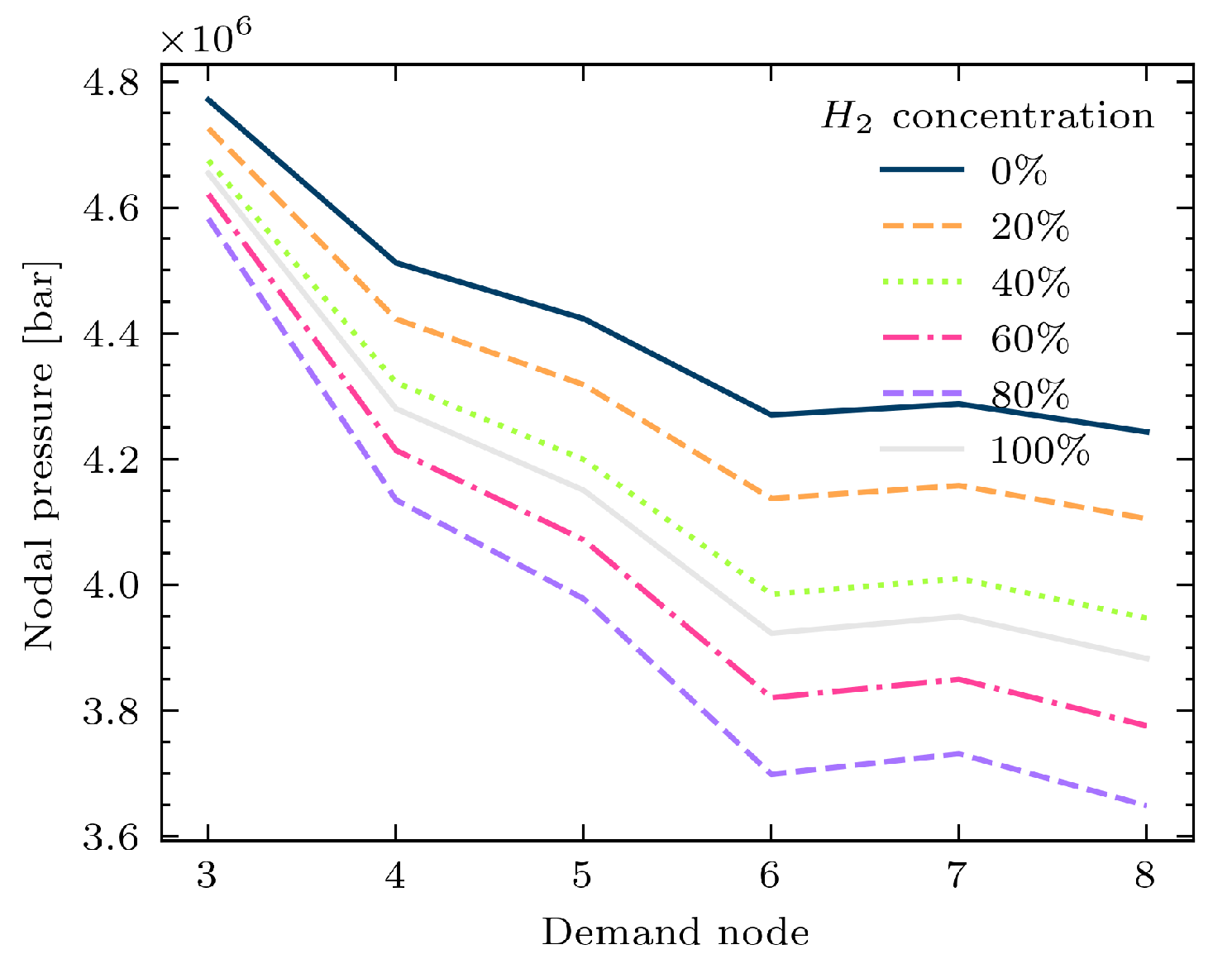
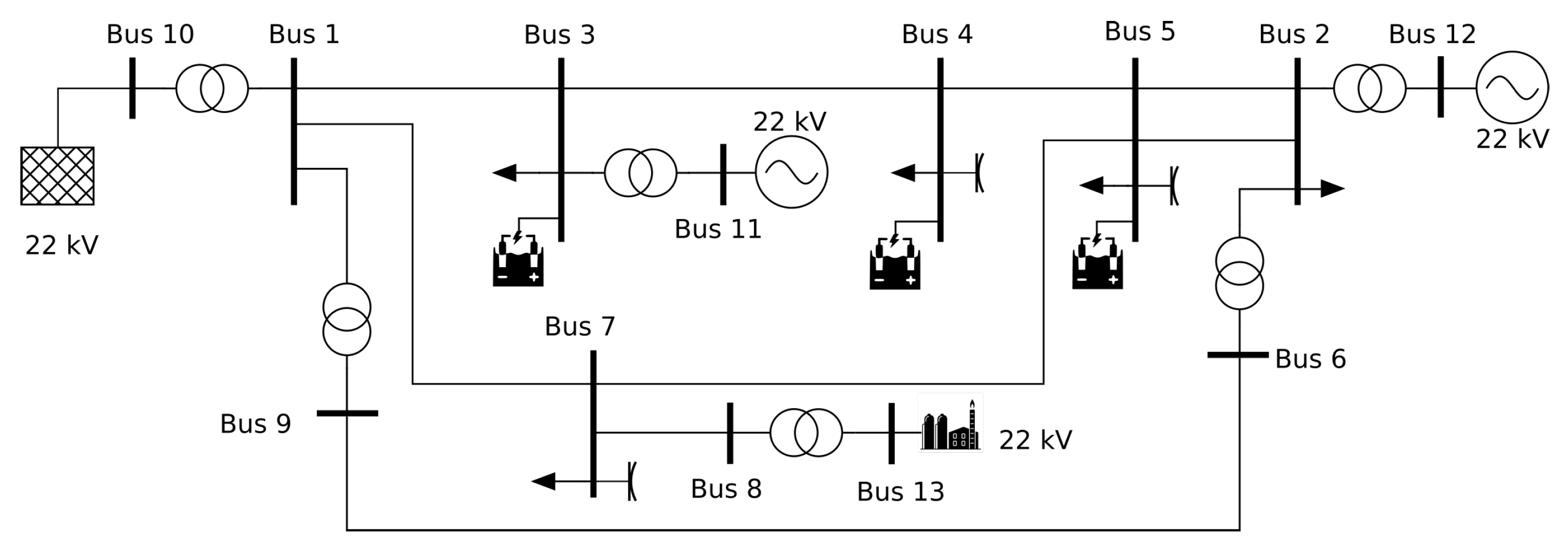
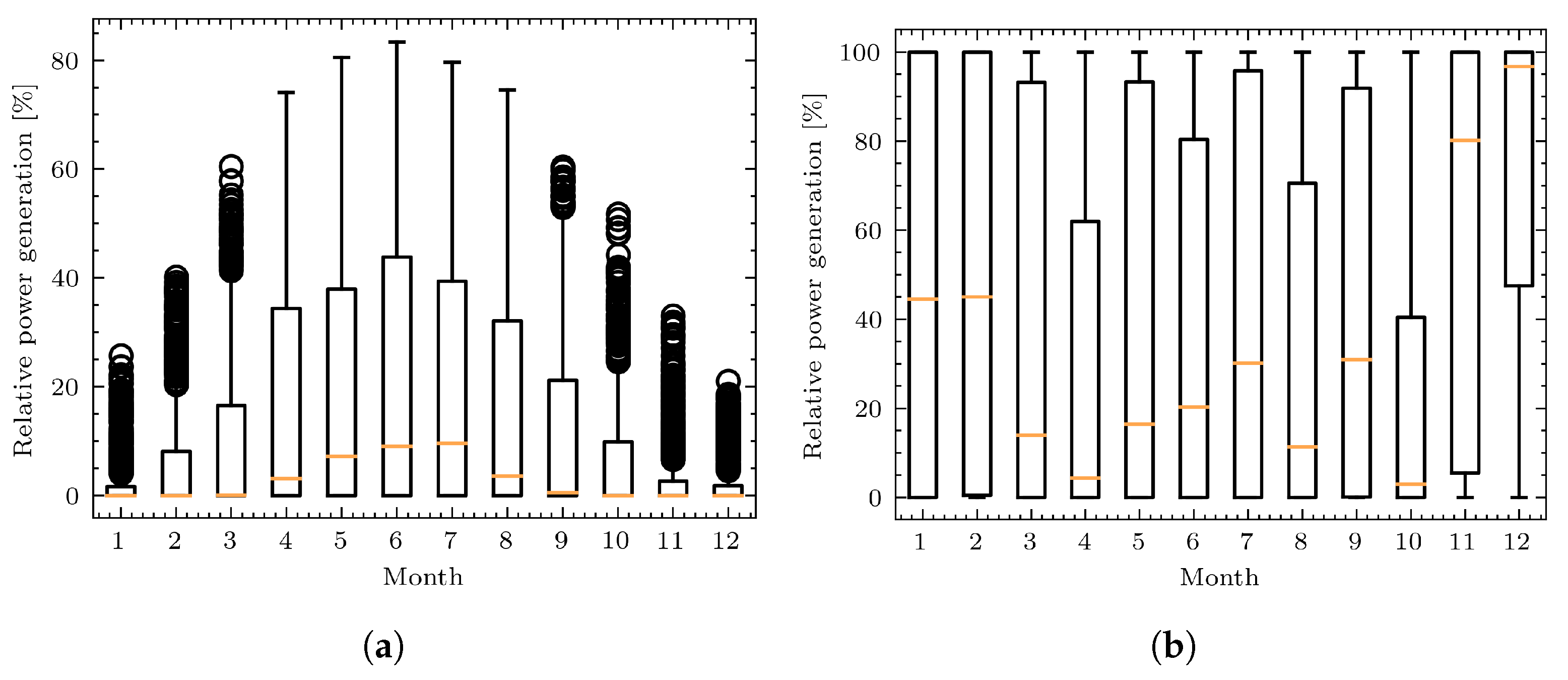
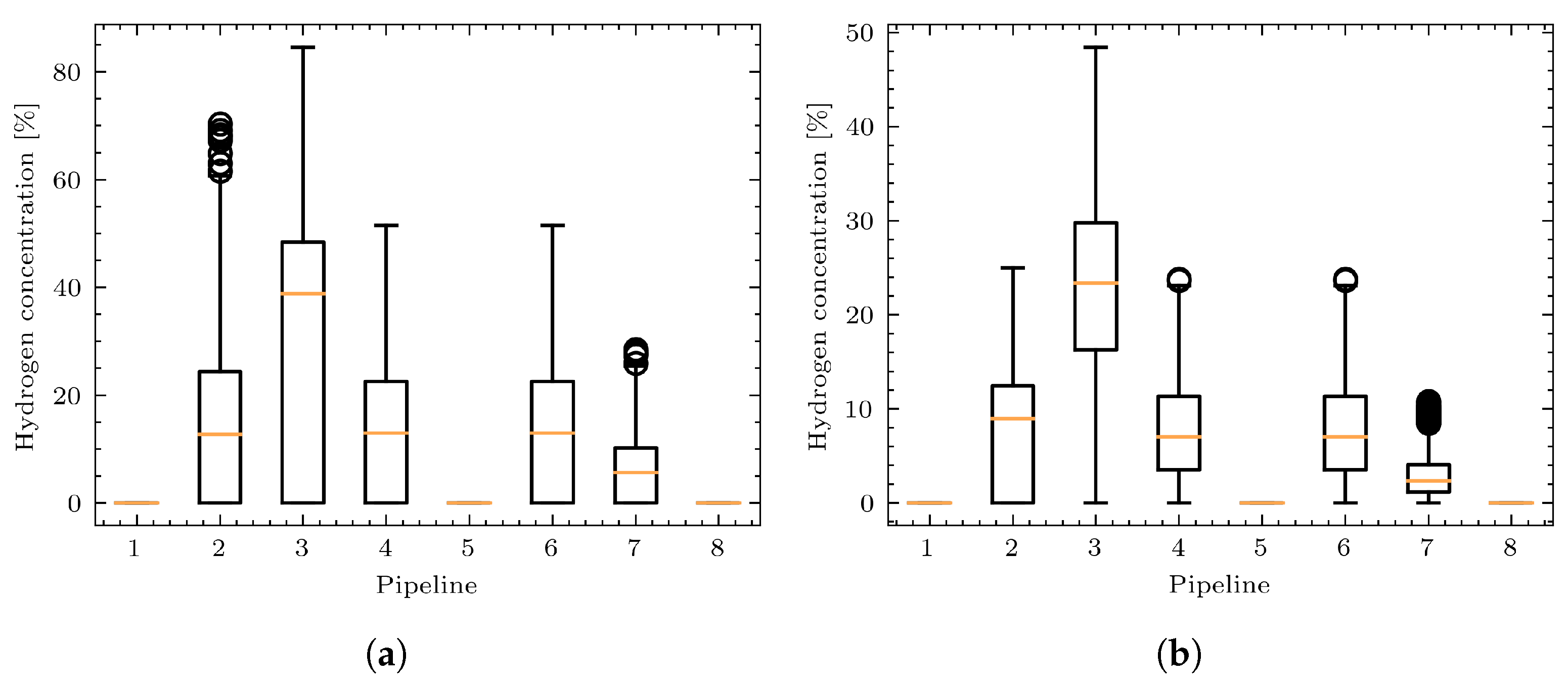
3.3. Study Case 3: A Simple Example of a Simulation of Coupled Power and Gas Networks
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DVGW | German Technical and Scientific Association for Gas and Water |
| EOS | Equation of state |
| HV | Heating value |
| HHV | High heating value |
| NG | Natural gas |
| NR | Newton–Raphson |
| PREOS | Peng–Robinson equation of state |
| PtG | Power-to-gas |
| PV | Photovoltaics |
Nomenclature
| C | constant, , which is around 13.29, |
| D | pipe diameter (m), |
| pipe efficiency (dimensionless), | |
| f | friction factor (dimensionless), |
| G | gas specific gravity (dimensionless), |
| g | gravitational acceleration (), |
| inlet height (m), | |
| outlet height (m), | |
| heating value (), | |
| higher heating value (), | |
| h | specific enthalpy (J/kg), |
| L | pipe length (m), |
| reference pressure (Pa), | |
| inlet pressure (Pa), | |
| outlet pressure (Pa), | |
| critical pressure (K), | |
| pseudo-reduced pressure (dimensionless), | |
| Q | volumetric flow rate (), |
| mass flow rate (kg/s) | |
| average temperature (K), | |
| critical pressure (K), | |
| pseudo-reduced pressure (dimensionless), | |
| ambient temperature (K), | |
| reference temperature (K), | |
| heat transfer coefficient (), | |
| v | gas flow velocity (m/s), |
| Z | compressibility factor (dimensionless), |
| slope angle (dimensionless) |
Appendix A
| Gas Species | Typical Analysis (Mole %) | Range (Mole %) |
|---|---|---|
| Methane | 94.7 | 87.0–98.0 |
| Ethane | 4.2 | 1.5–9.0 |
| Propane | 0.2 | 0.1–1.5 |
| Iso-butane | 0.02 | trace–0.3 |
| Butane | 0.02 | trace–0.3 |
| Iso-pentane | 0.01 | trace–0.04 |
| Pentane | 0.01 | trace–0.04 |
| Hexanes plus | 0.01 | trace–0.06 |
| Nitrogen | 0.5 | 0.2–5.5 |
| Carbon dioxide | 0.3 | 0.05–1.0 |
| Oxygen | 0.01 | trace–0.1 |
| Hydrogen | 0.02 | trace–0.05 |
References
- Rockström, J.; Gaffney, O.; Rogelj, J.; Meinshausen, M.; Nakicenovic, N.; Schellnhuber, H.J. A roadmap for rapid decarbonization. Science 2017, 355, 1269–1271. [Google Scholar] [CrossRef]
- Wiser, R.; Jenni, K.; Seel, J.; Baker, E.; Hand, M.; Lantz, E.; Smith, A. Expert elicitation survey on future wind energy costs. Nat. Energy 2016, 1, 16135. [Google Scholar] [CrossRef]
- Mancarella, P. MES (multi-energy systems): An overview of concepts and evaluation models. Energy 2014, 65, 1–17. [Google Scholar] [CrossRef]
- Pesch, T.C. Multiskalare Modellierung Integrierter Energie- und Elektrizitätssysteme; Schriften des Forschungszentrums Jülich. Energie & Umwelt/Energy & Environment; Verlag des Forschungszentrums Jülich: Jülich, Germany, 2019; Volume 485. [Google Scholar]
- Fraunhofer-Gesellschaft. Energy-Charts. Available online: https://energy-charts.info/index.html?l=en&c=DE (accessed on 9 November 2020).
- Energie Informationsdienst. Underground Gas Storage in Germany; DVV Media Group GmbH: Hamburg, Germany, 2020. [Google Scholar]
- Thema, M.; Sterner, M.; Lenck, T.; Götz, P. Necessity and Impact of Power-to-gas on Energy Transition in Germany. Energy Procedia 2016, 99, 392–400. [Google Scholar] [CrossRef]
- Clegg, S.; Mancarella, P. Storing renewables in the gas network: Modelling of power–to–gas seasonal storage flexibility in low–carbon power systems. IET Gener. Transm. Distrib. 2016, 10, 566–575. [Google Scholar] [CrossRef]
- Schiebahn, S.; Grube, T.; Robinius, M.; Tietze, V.; Kumar, B.; Stolten, D. Power to gas: Technological overview, systems analysis and economic assessment for a case study in Germany. Int. J. Hydrog. Energy 2015, 40, 4285–4294. [Google Scholar] [CrossRef]
- Dolci, F. Green Hydrogen Opportunities in Selected Industrial Processes. JRC Tech. Rep. 2018. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC114766 (accessed on 20 September 2021).
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; National Renewable Energy Laboratory: Golden, CO, USA, 2013.
- Fuel Cells and Hydrogen Joint Undertaking. Development of Business Cases for Fuel Cells and Hydrogen Applications for Regions and Cities: Hydrogen Injection into the Natural Gas Grid. Available online: https://www.fch.europa.eu/sites/default/files/FCH%20Docs/171121_FCH2JU_Application-Package_WG5_P2H_Hydrogen%20into%20gas%20grid%20%28ID%202910558%29%20%28ID%202911642%29.pdf (accessed on 20 September 2021).
- German Bundestag. Critical Values for Hydrogen (H2) in the Natural Gas Infrastructure. Available online: https://www.bundestag.de/resource/blob/646488/a89bbd41acf3b90f8a5fbfbcb8616df4/WD-8-066-19-pdf-data.pdf (accessed on 8 November 2020).
- Leicher, J.; Nowakowski, T.; Giese, A.; Görner, K. Power-to-gas and the consequences: Impact of higher hydrogen concentrations in natural gas on industrial combustion processes. Energy Procedia 2017, 120, 96–103. [Google Scholar] [CrossRef]
- Leicher, J.; Giese, A.; Görner, K.; Werschy, M.; Krause, H.; Dörr, H. Natural gas quality fluctuations–surveys and statistics on the situation in Germany. Energy Procedia 2017, 120, 165–172. [Google Scholar] [CrossRef]
- Martinez-Mares, A.; Fuerte-Esquivel, C.R. A Unified Gas and Power Flow Analysis in Natural Gas and Electricity Coupled Networks. IEEE Trans. Power Syst. 2012, 27, 2156–2166. [Google Scholar] [CrossRef]
- Ekhtiari, A.; Dassios, I.; Liu, M.; Syron, E. A Novel Approach to Model a Gas Network. Appl. Sci. 2019, 9, 1047. [Google Scholar] [CrossRef]
- Abdolahi, F.; Mesbah, A.; Boozarjomehry, R.B.; Svrcek, W.Y. The effect of major parameters on simulation results of gas pipelines. Int. J. Mech. Sci. 2007, 49, 989–1000. [Google Scholar] [CrossRef]
- Guandalini, G.; Colbertaldo, P.; Campanari, S. Dynamic modeling of natural gas quality within transport pipelines in presence of hydrogen injections. Appl. Energy 2017, 185, 1712–1723. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill: New York, NY, USA; London, UK, 2000. [Google Scholar]
- Pellegrino, S.; Lanzini, A.; Leone, P. Greening the gas network – The need for modelling the distributed injection of alternative fuels. Renew. Sustain. Energy Rev. 2017, 70, 266–286. [Google Scholar] [CrossRef]
- Siemens. PSS®SINCAL—Simulation Software for Analysis and Planning of Electric and Pipe Networks. Available online: https://assets.new.siemens.com/siemens/assets/public.1537970929.31ece3a2-e9cc-4528-b9f9-6bf61b613de2.ref-no-69-ps-c-pss-sincal-brochure-hires-intl-sept2018.pdf (accessed on 9 November 2020).
- Pambour, K.A.; Cakir Erdener, B.; Bolado-Lavin, R.; Dijkema, G.P. SAInt–A novel quasi-dynamic model for assessing security of supply in coupled gas and electricity transmission networks. Appl. Energy 2017, 203, 829–857. [Google Scholar] [CrossRef]
- Merkuryev, Y. (Ed.) SIMULTECH 2016: Proceedings of the 6th International Conference on Simulation and Modeling Methodologies, Technologies and Applications; IEEE: Piscataway, NJ, USA, 2016. [Google Scholar]
- Andresen, L.; Dubucq, P.; Peniche Garcia, R.; Ackermann, G.; Kather, A.; Schmitz, G. Status of the TransiEnt Library: Transient Simulation of Coupled Energy Networks with High Share of Renewable Energy. In Proceedings of the 11th International Modelica Conference, Linköping Electronic Conference Proceedings, Versailles, France, 21–23 September 2015; Linköping University Electronic Press: Linköping, Sweden, 2015; pp. 695–705. [Google Scholar]
- Lohmeier, D.; Cronbach, D.; Drauz, S.R.; Braun, M.; Kneiske, T.M. Pandapipes: An Open Source Piping Grid Calculation Package for the Application in Coupled Multi-Energy Grid Simulations. Sustainability 2020, 12, 9899. [Google Scholar] [CrossRef]
- Chaczykowski, M.; Osiadacz, A.J. Comparative assesment of steady-state pipeline gas flow models / Analiza porównawcza modeli przepływu gazu w rurociągu w stanach ustalonych. Arch. Min. Sci. 2012, 57, 23–38. [Google Scholar] [CrossRef]
- Bales, P.; Kolb, O.; Lang, J. Hierarchical Modelling and Model Adaptivity for Gas Flow on Networks. In Computational science-ICCS 2009; Allen, G., Ed.; Lecture Notes in Computer Science; Springer: Berlin, Germany, 2009; Volume 5544, pp. 337–346. [Google Scholar]
- Coelho, P.M.; Pinho, C. Considerations about equations for steady state flow in natural gas pipelines. J. Braz. Soc. Mech. Sci. Eng. 2007, 29, 262–273. [Google Scholar] [CrossRef]
- German Technical and Scientific Association for Gas and Water. Gas Infrastructure-Quality of Gas-Group H, German version EN 16726:2015+A1:2018; Beuth Verlag GmbH: Berlin, Germany, 2019. [Google Scholar]
- Schroeder, D.W., Jr. A tutorial on pipe flow equations. In Proceedings of the PSIG Annual Meeting, Bonita Springs, FL, USA, 11–14 May 2010. [Google Scholar]
- Osiadacz, A.J. Simulation and Analysis of Gas Networks; Gulf Publishing Company: Houston, TX, USA, 1987. [Google Scholar]
- Mohitpour, M.; Thompson, W.; Asante, B. The Importance of Dynamic Simulation on the Design and Optimization of Pipeline Transmission Systems. In Proceedings of the First International Pipeline Conference, Calgary, AB, Canada, 9–13 June 1996; Yoon, M., Mensik, M., Mohitpour, M., Eds.; American Society of Mechanical Engineers: New York, NY, USA, 1996; pp. 1183–1188. [Google Scholar]
- Farzaneh-Gord, M.; Khamforoush, A.; Hashemi, S.; Namin, H. Computing Thermal Properties of Natural Gas by Utilizing AGA8 Equation of State. Int. J. Chem. Eng. Appl. 2010, 1, 20–24. [Google Scholar] [CrossRef][Green Version]
- German Technical and Scientific Association for Gas and Water. DVGW G 260-Gas Quality; Wirtschafts- und Verlagsgesellschaft Gas und Wasser mbH: Bonn, Germany, 2013. [Google Scholar]
- Caleb Bell and Contributors (2016–2021) Thermo: Chemical Properties Component of Chemical Engineering Design Library (ChEDL). GitHub Repository. Available online: https://github.com/CalebBell/thermo (accessed on 12 September 2021).
- Peng, D.Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Robinson, D.B.; Peng, D.Y.; Chung, S.Y.K. The development of the Peng—Robinson equation and its application to phase equilibrium in a system containing methanol. Fluid Phase Equilibria 1985, 24, 25–41. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Speth, R.L.; Moffat, H.K.; Weber, B.W. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes, 2018. GitHub Repository. Available online: https://github.com/Cantera/cantera (accessed on 12 September 2021).
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr.; et al. GRI-MECH 3.0. Available online: http://combustion.berkeley.edu/gri-mech/version30/text30.html (accessed on 12 September 2021).
- Klein, S.A.; Nellis, G. Thermodynamics; Cambridge University Press: New York, NY, USA, 2012. [Google Scholar]
- Enbridge Gas. Chemical Composition of Natural Gas. Available online: https://www.enbridgegas.com/about-enbridge-gas/learn-about-natural-gas (accessed on 2 October 2021).
- Moiseeva, E.F.; Malyshev, V.L. Compressibility factor of natural gas determination by means of molecular dynamics simulations. AIP Adv. 2019, 9, 055108. [Google Scholar] [CrossRef]
- Bundesnetzagentur. Definitionen der Daten für den Gasbereich. Available online: https://www.bundesnetzagentur.de/SharedDocs/Downloads/DE/Sachgebiete/Energie/Unternehmen_Institutionen/DatenaustauschUndMonitoring/MaStR/DefinitionenDatenGasbereich.pdf?__blob=publicationFile&v=3 (accessed on 15 October 2020).
- Obuba, J.; Ikiesnkimama, S.S.; Ubani, C. Natural Gas Compressibility Factor Correlation Evaluation for Niger Delta Gas Fields. IOSR J. Electr. Electron. Eng. 2013, 6, 01–10. [Google Scholar] [CrossRef]
- Strunz, K.; Abbasi, E.; Fletcher, R.; Hatziargyriou, N.; Iravani, R.; Joos, G. TF C6.04.02: TB 575–Benchmark Systems for Network Integration of Renewable and Distributed Energy Resources; CIGRE: Paris, France, 2014. [Google Scholar]
- Davis, W.; Martín, M. Optimal year-round operation for methane production from CO2 and water using wind and/or solar energy. J. Clean. Prod. 2014, 80, 252–261. [Google Scholar] [CrossRef]
- de Boer, H.S.; Grond, L.; Moll, H.; Benders, R. The application of power-to-gas, pumped hydro storage and compressed air energy storage in an electricity system at different wind power penetration levels. Energy 2014, 72, 360–370. [Google Scholar] [CrossRef]
- Bundesverband der Energie- und Wasserwirtschaft. Standardlastprofile Strom. Available online: https://www.bdew.de/energie/standardlastprofile-strom/ (accessed on 16 September 2021).
- Bundesverband der Energie- und Wasserwirtschaft. Standardlastprofile Gas. Available online: https://www.bdew.de/energie/standardlastprofile-gas/ (accessed on 16 September 2021).
- Pfenninger, S.; Staffell, I. Long-term patterns of European PV output using 30 years of validated hourly reanalysis and satellite data. Energy 2016, 114, 1251–1265. [Google Scholar] [CrossRef]
- Quaschning, V. Understanding Renewable Energy Systems, revised edition ed.; Routledge Taylor & Francis Group Earthscan from Routledge: London, UK; New York, NY, USA, 2016. [Google Scholar]
- Zhou, W.; Yang, H.; Fang, Z. A novel model for photovoltaic array performance prediction. Appl. Energy 2007, 84, 1187–1198. [Google Scholar] [CrossRef]
- McGill, R.; Tukey, J.W.; Larsen, W.A. Variations of box plots. Am. Stat. 1978, 32, 12–16. [Google Scholar]



| Gas Properties | Units | L-Gas | H-Gas |
|---|---|---|---|
| Heating value | MJ/sm3 | 30.24–40.5 | 36–47.16 |
| Specific gravity | - | 0.55–0.75 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Pesch, T.; Benigni, A. Simulation of Coupled Power and Gas Systems with Hydrogen-Enriched Natural Gas. Energies 2021, 14, 7680. https://doi.org/10.3390/en14227680
Lu Y, Pesch T, Benigni A. Simulation of Coupled Power and Gas Systems with Hydrogen-Enriched Natural Gas. Energies. 2021; 14(22):7680. https://doi.org/10.3390/en14227680
Chicago/Turabian StyleLu, Yifei, Thiemo Pesch, and Andrea Benigni. 2021. "Simulation of Coupled Power and Gas Systems with Hydrogen-Enriched Natural Gas" Energies 14, no. 22: 7680. https://doi.org/10.3390/en14227680
APA StyleLu, Y., Pesch, T., & Benigni, A. (2021). Simulation of Coupled Power and Gas Systems with Hydrogen-Enriched Natural Gas. Energies, 14(22), 7680. https://doi.org/10.3390/en14227680






