Comparison of Flame Propagation Statistics Extracted from Direct Numerical Simulation Based on Simple and Detailed Chemistry—Part 1: Fundamental Flame Turbulence Interaction
Abstract
:1. Introduction
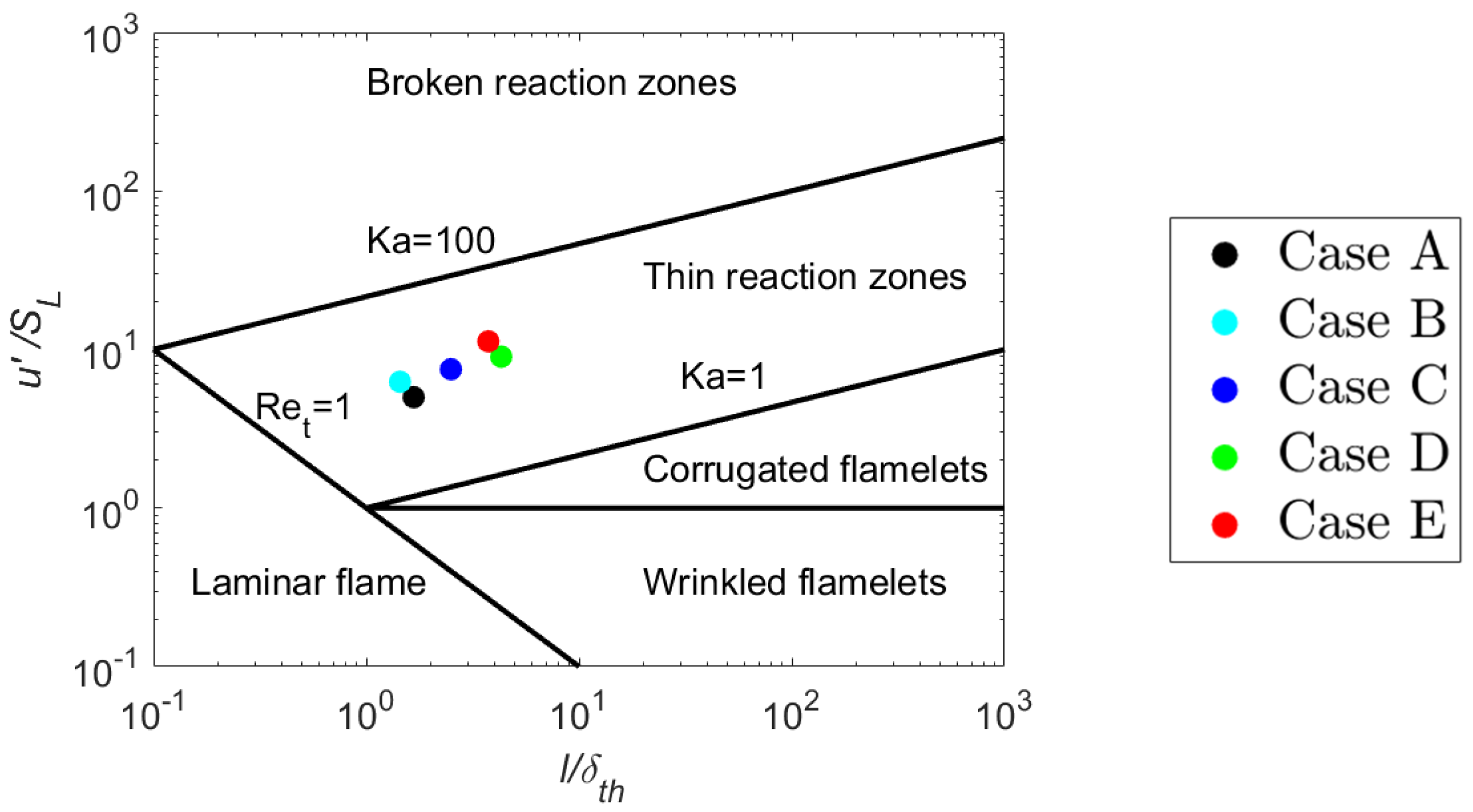
2. Direct Numerical Simulation Database
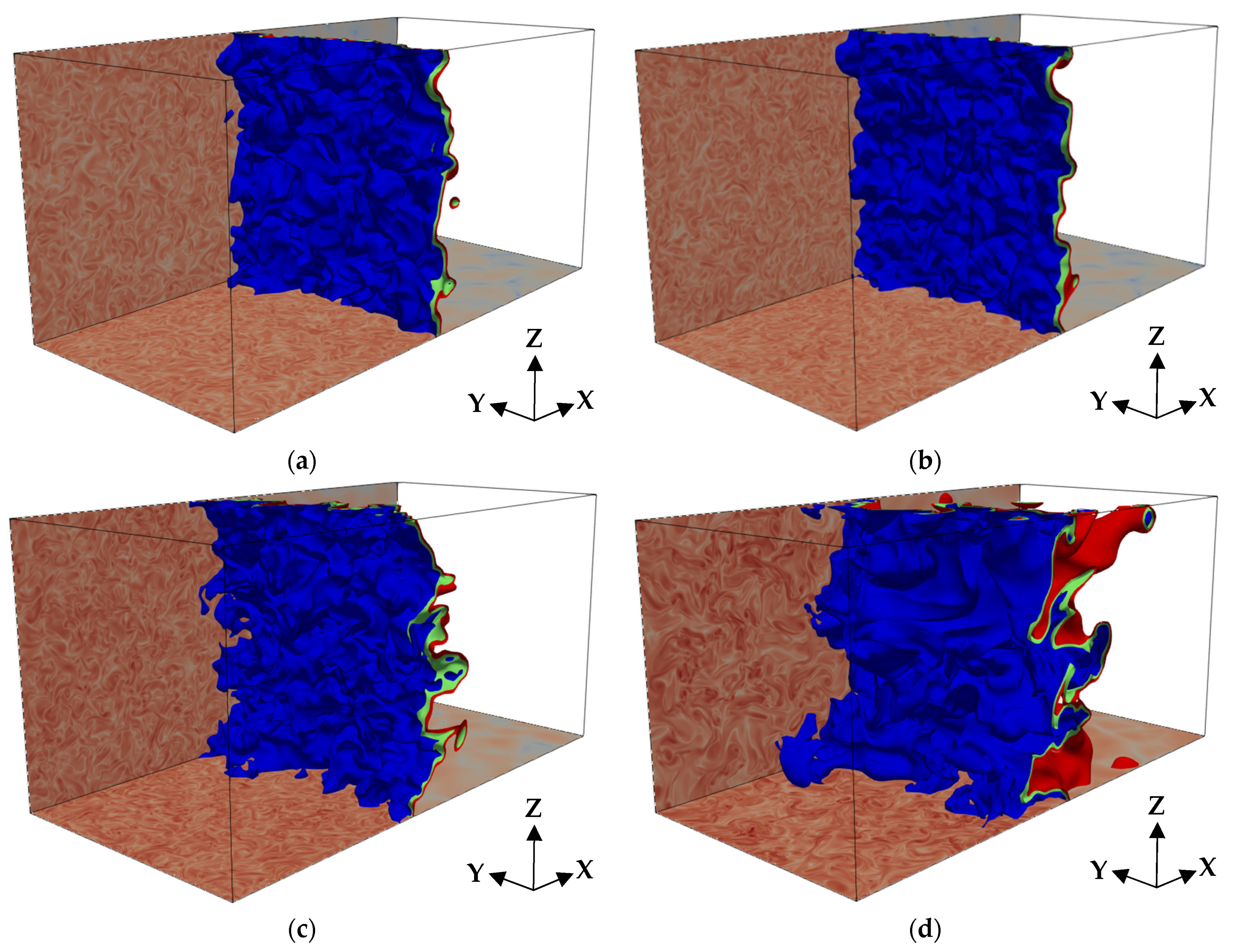
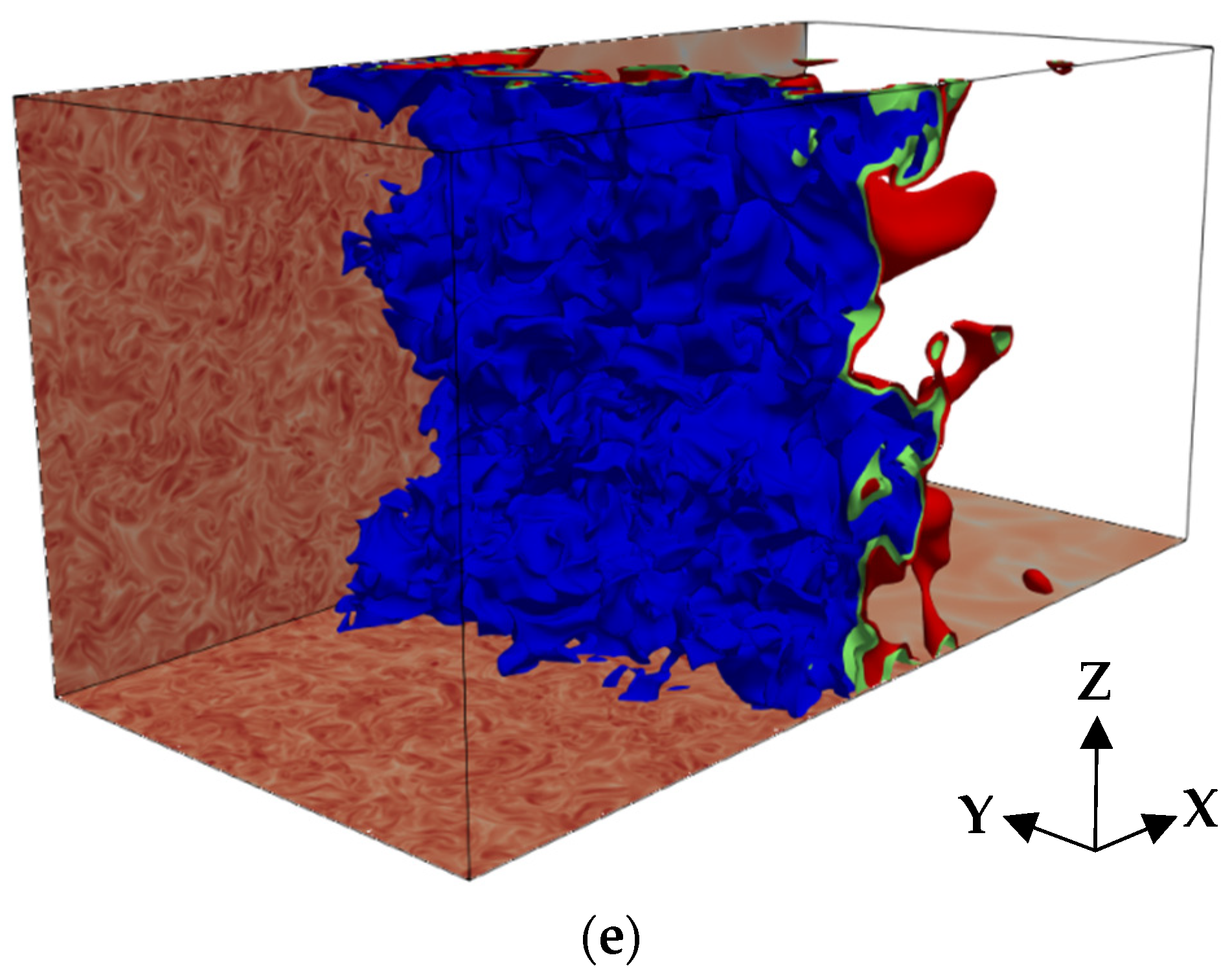
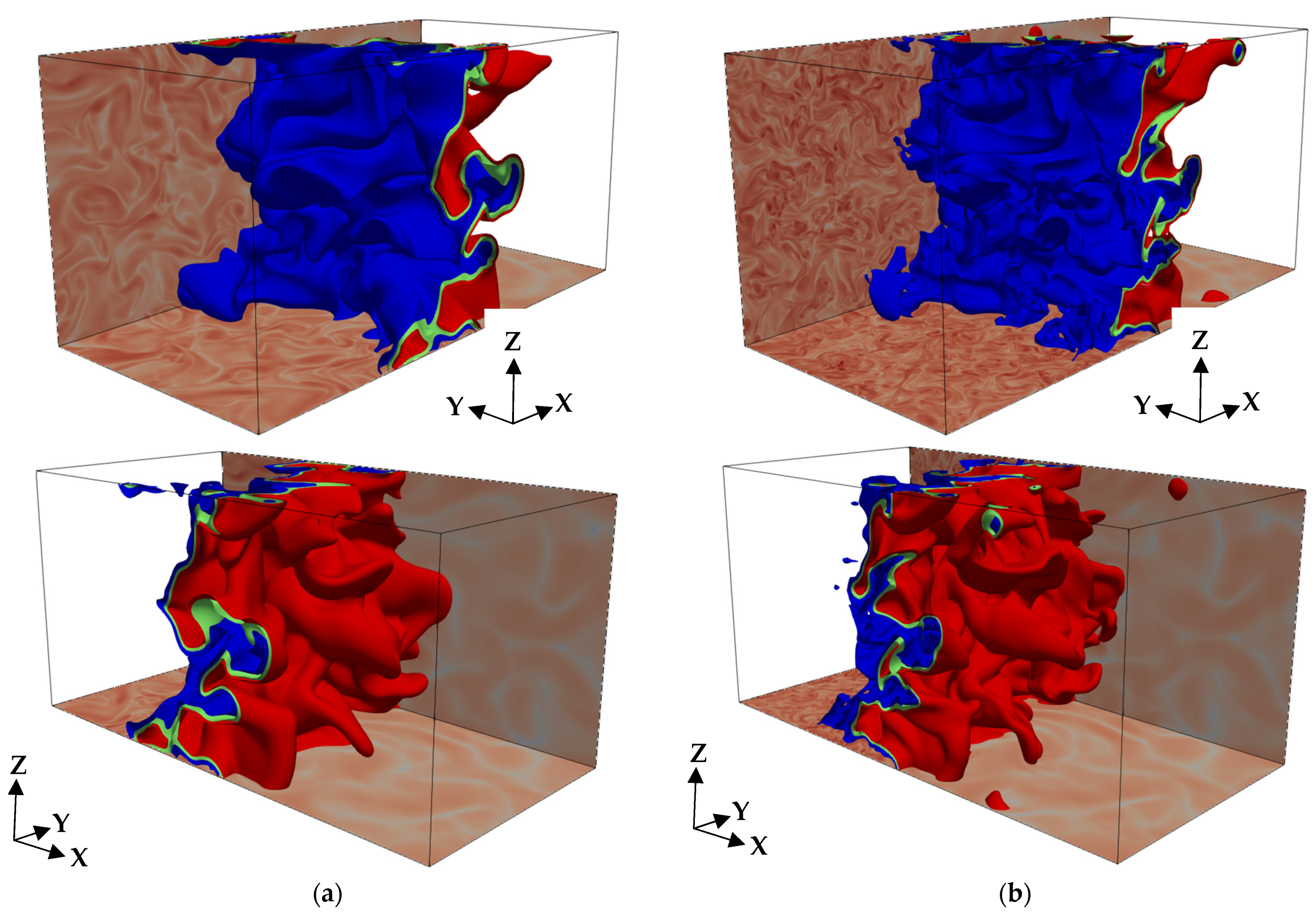
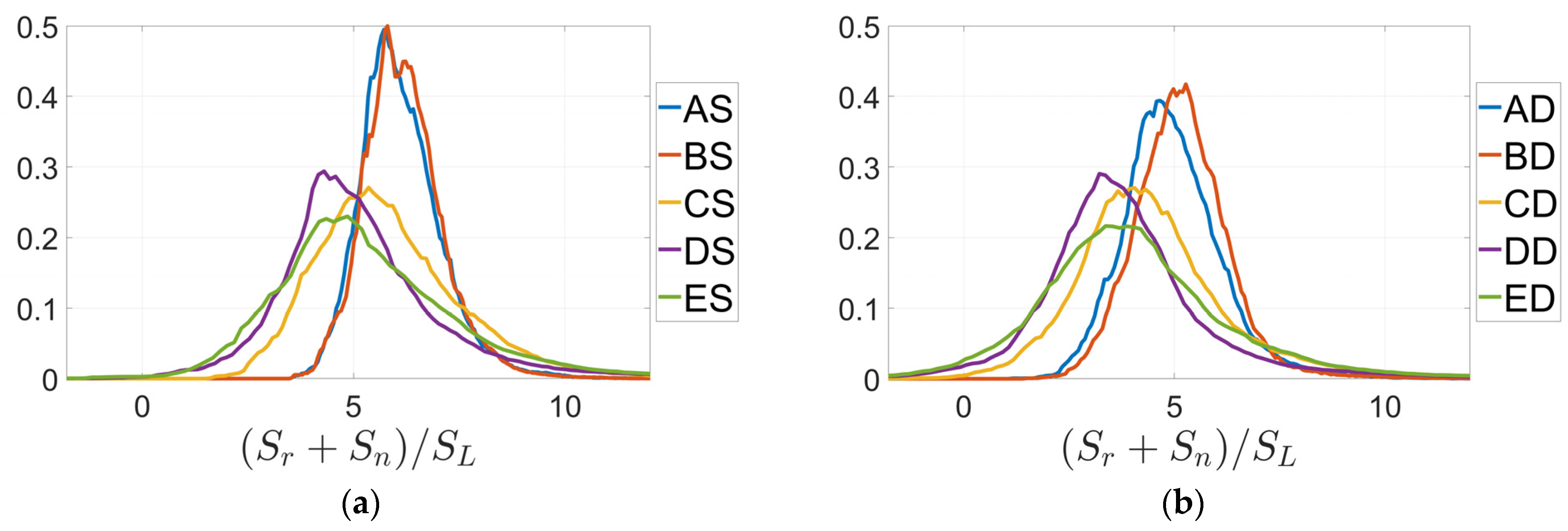
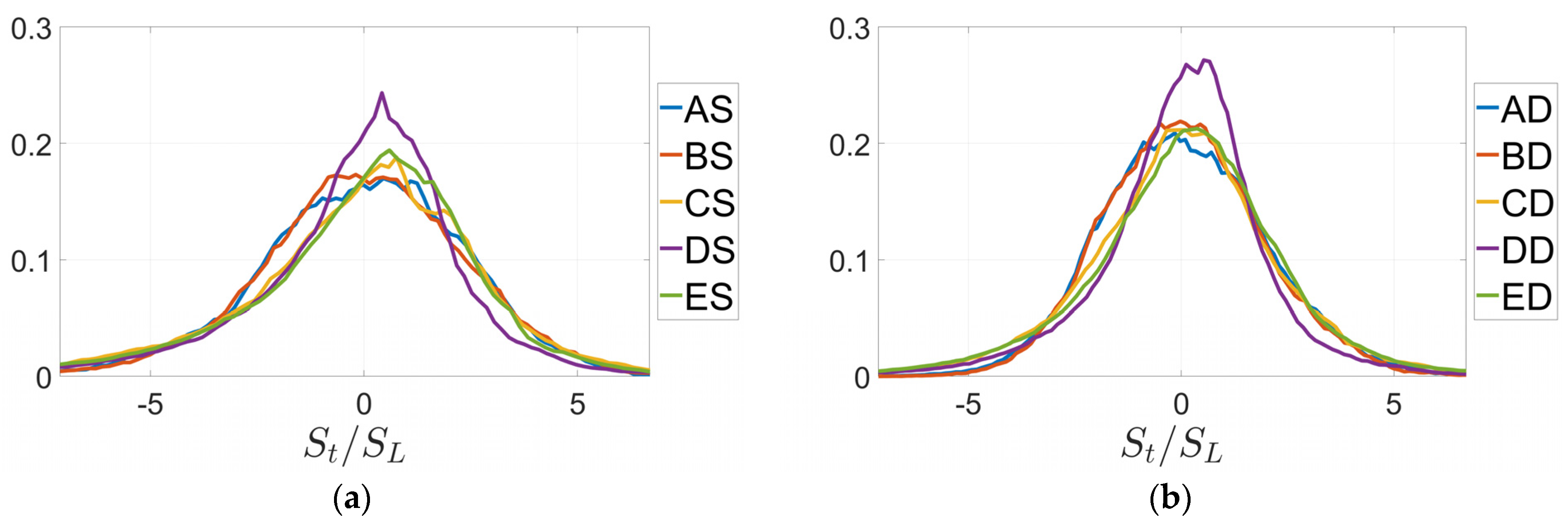
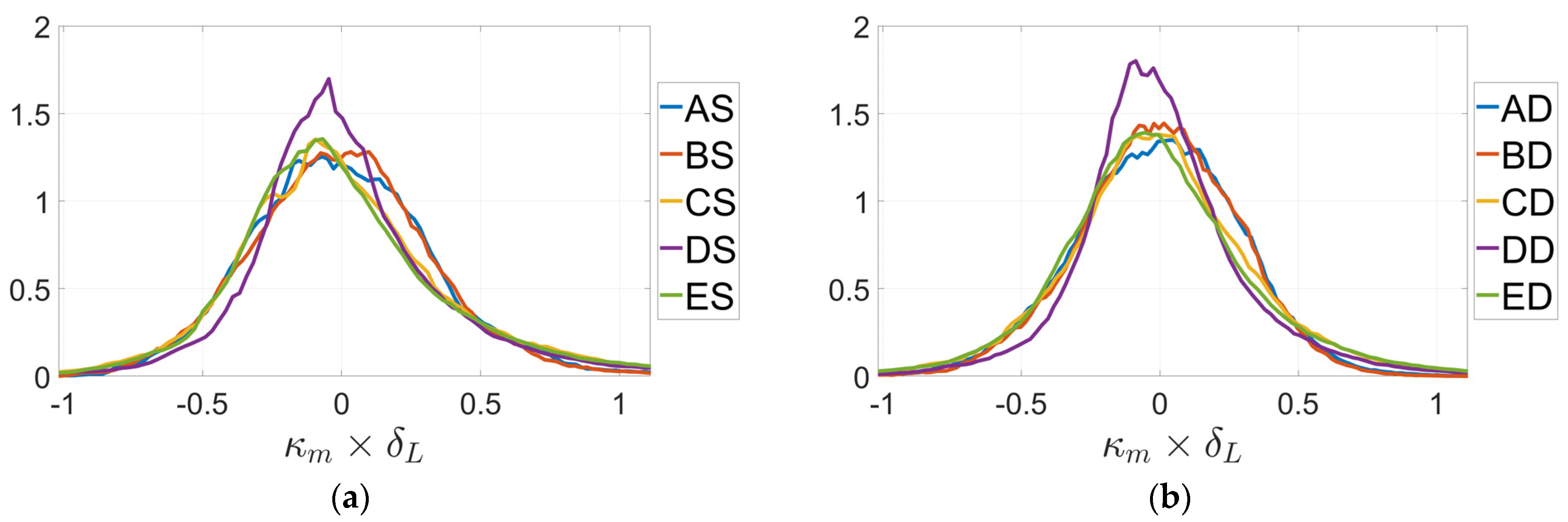
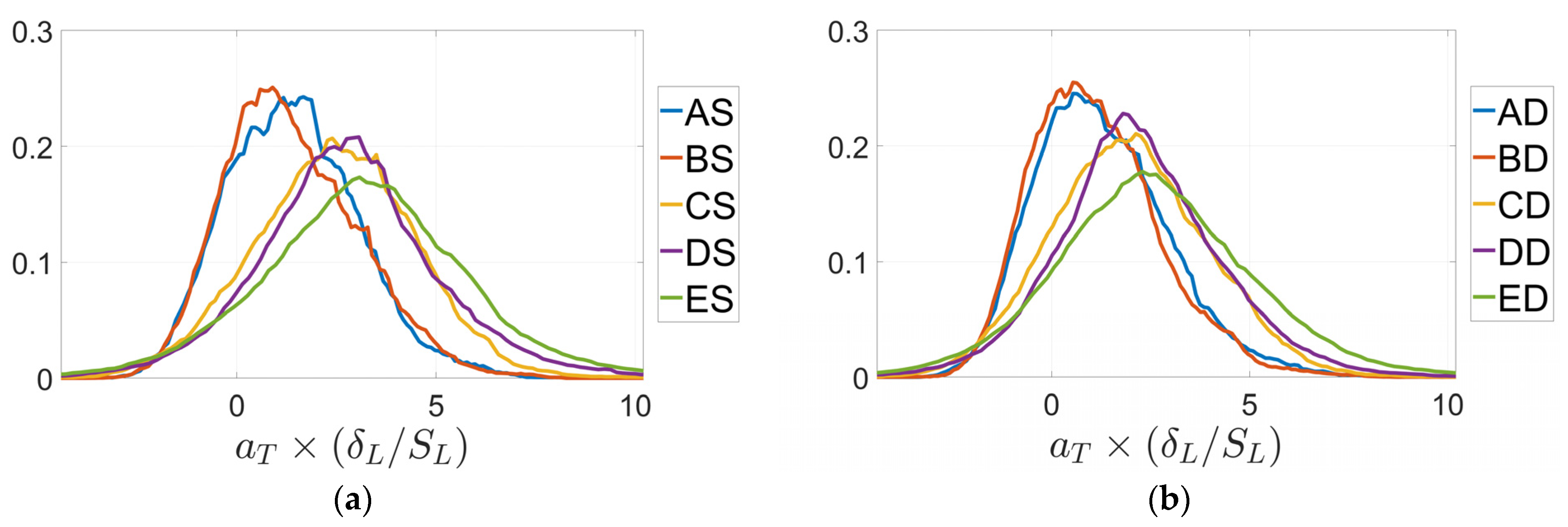
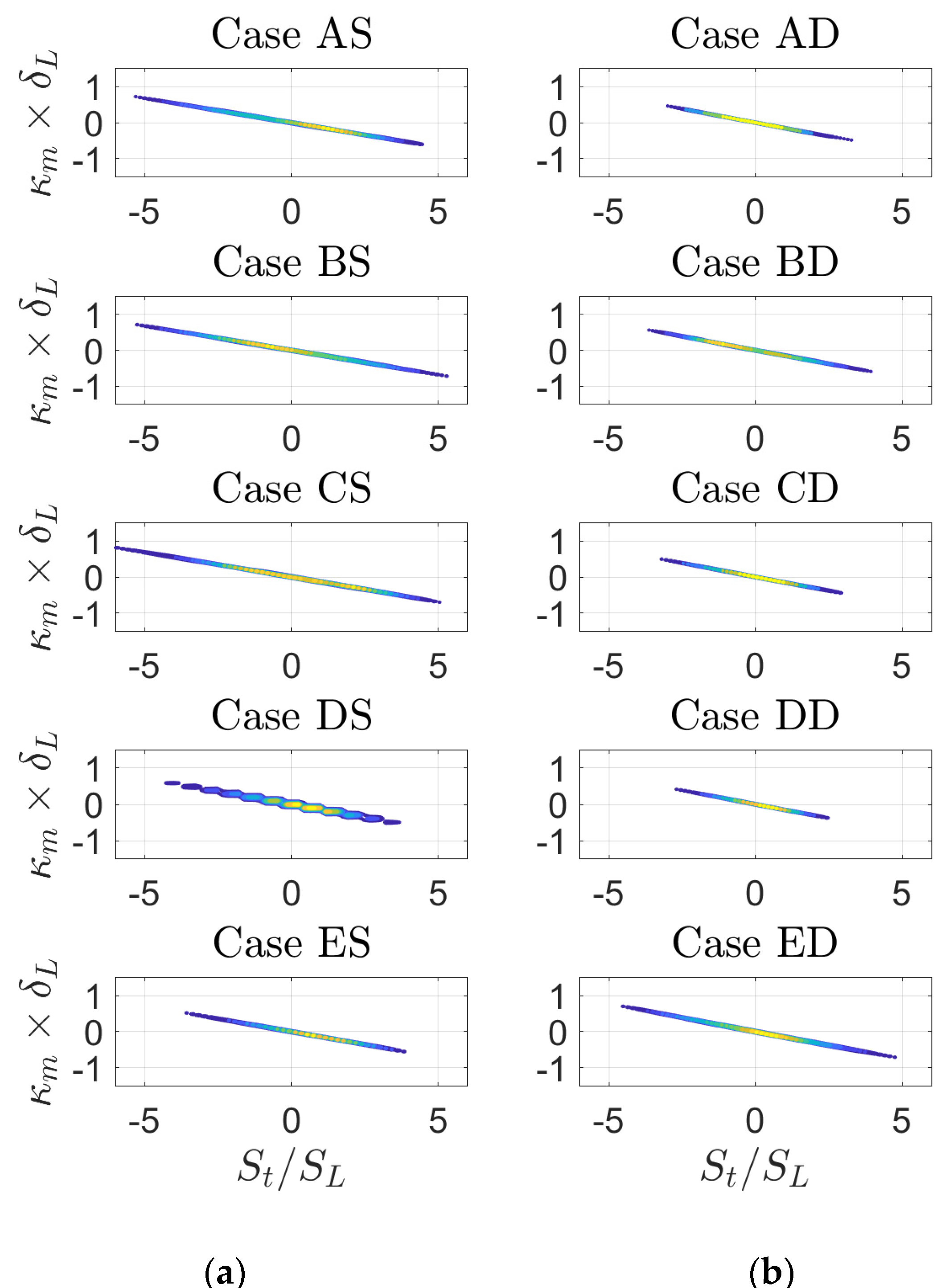
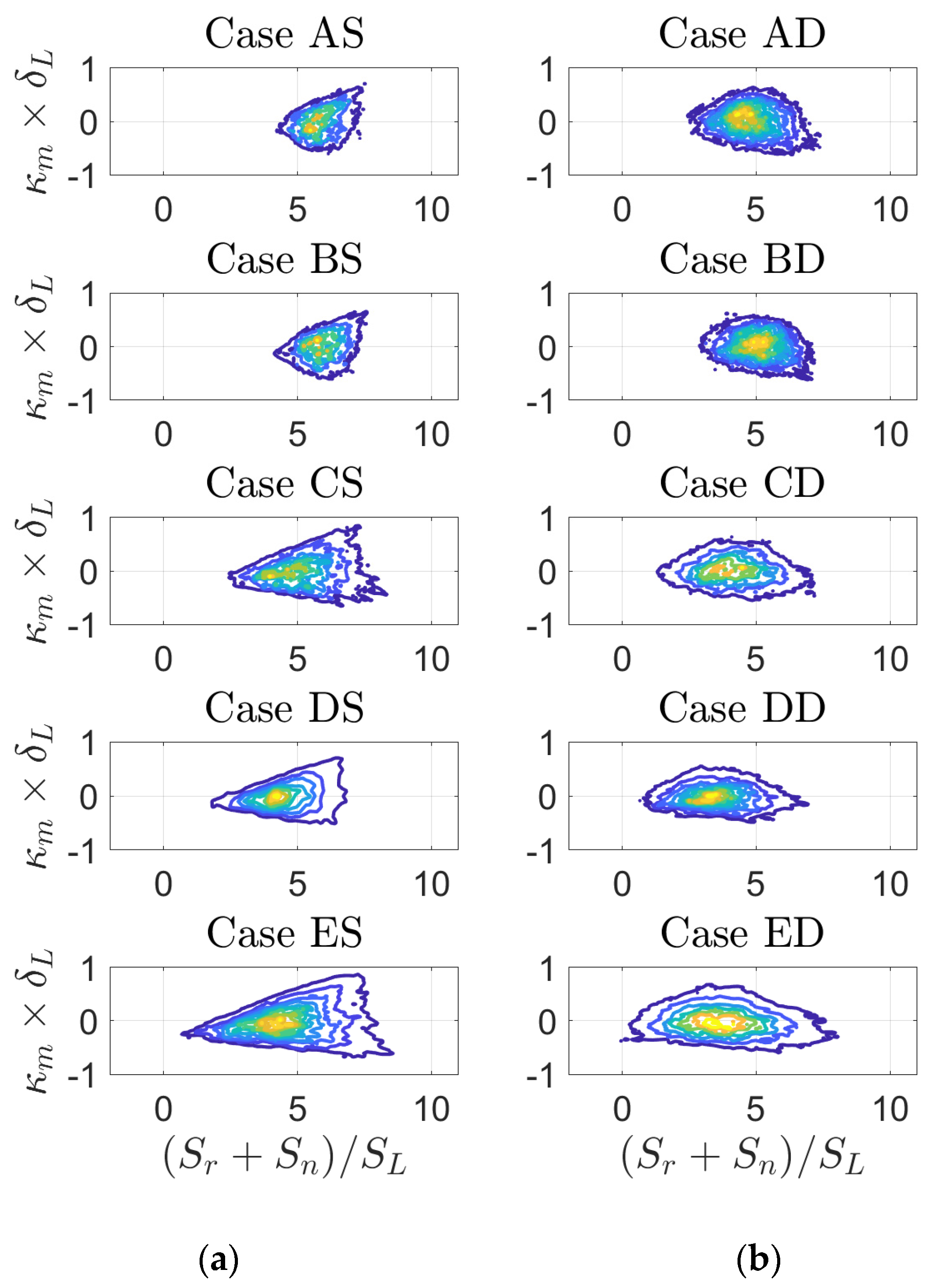
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trisjono, P.; Pitsch, H. Systematic Analysis Strategies for the Development of Combustion Models from DNS: A Review. Flow Turbul. Combust. 2015, 95, 231–259. [Google Scholar] [CrossRef]
- Poinsot, T.; Veynante, D. Theoretical and Numerical Combustion, R.T.; Edwards Inc.: Philadelphia, PA, USA, 2001. [Google Scholar]
- Chen, J.H. Petascale direct numerical simulation of turbulent combustion—Fundamental insights towards predictive models. Proc. Combust. Inst. 2011, 33, 99–123. [Google Scholar] [CrossRef]
- Burali, N.; Lapointe, S.; Bobbitt, B.; Blanquart, G.; Xuan, Y. Assessment of the constant non-unity Lewis number assumption in chemically-reacting flows. Combust. Theory Model. 2016, 20, 632–657. [Google Scholar] [CrossRef]
- Smooke, M.D.; Giovangigli, V. Premixed and Nonpremixed Test Flame Results, in Reduced Kinetic Mechanisms and Asymptotic Approximations for Methane-Air Flames; Springer: Berlin/Heidelberg, Germany, 1991; pp. 29–47. [Google Scholar]
- Hu, E.; Li, X.; Meng, X.; Chen, Y.; Cheng, Y.; Xie, Y.; Huang, Z. Laminar flame speeds and ignition delay times of methane–Air mixtures at elevated temperatures and pressures. Fuel 2015, 158, 1–10. [Google Scholar] [CrossRef]
- Pizzuti, L.; Martins, C.; dos Santos, L.R.; Guerra, D.R. Laminar Burning Velocity of Methane/Air Mixtures and Flame Propagation Speed Close to the Chamber Wall. Energy Procedia 2017, 120, 126–133. [Google Scholar] [CrossRef]
- Bhagatwala, A.; Sankaran, R.; Kokjohn, S.; Chen, J.H. Numerical investigation of spontaneous flame propagation under RCCI conditions. Combust. Flame 2015, 162, 3412–3426. [Google Scholar] [CrossRef] [Green Version]
- Stevens, A.R.H.; Bellstedt, S.; Elahi, P.J.; Murphy, M.T. The imperative to reduce carbon emissions in astronomy. Nat. Astron. 2020, 4, 843–851. [Google Scholar] [CrossRef]
- Zwart, S.P. The ecological impact of high-performance computing in astrophysics. Nat. Astron. 2020, 4, 819–822. [Google Scholar] [CrossRef]
- Icha, P. Entwicklung der Spezifischen Kohlendioxid Emissionen des Deutschen Strommix in den Jahren 1990–2018, Um-Weltbundesamt. Available online: https://www.umweltbundesamt.de/publikationen/entwicklung-der-spezifischen-kohlendioxid-4 (accessed on 2 May 2021).
- Chakraborty, N.; Cant, R.S. Influence of Lewis number on curvature effects in turbulent premixed flame propagation in the thin reaction zones regime. Phys. Fluids 2005, 17, 105105. [Google Scholar] [CrossRef]
- Han, I.; Huh, K.Y. Roles of displacement speed on evolution of flame surface density for different turbulent intensities and Lewis numbers in turbulent premixed combustion. Combust. Flame 2008, 152, 194–205. [Google Scholar] [CrossRef]
- Chakraborty, N. Comparison of displacement speed statistics of turbulent premixed flames in the regimes representing combustion in corrugated flamelets and thin reaction zones. Phys. Fluids 2007, 19, 105109. [Google Scholar] [CrossRef]
- Chakraborty, N.; Klein, M.; Cant, R. Stretch rate effects on displacement speed in turbulent premixed flame kernels in the thin reaction zones regime. Proc. Combust. Inst. 2007, 31, 1385–1392. [Google Scholar] [CrossRef]
- Peters, N.; Terhoeven, P.; Chen, J.H.; Echekki, T. Statistics of flame displacement speeds from computations of 2-D unsteady methane-air flames. Symp. Int. Combust. 1998, 27, 833–839. [Google Scholar] [CrossRef] [Green Version]
- Echekki, T.; Chen, J.H. Analysis of the contribution of curvature to premixed flame propagation. Combust. Flame 1999, 118, 308–311. [Google Scholar] [CrossRef]
- Chen, J.B.; Im, H.G. Stretch effects on the burning velocity of turbulent premixed hydrogen/air flames. Proc. Combust. Inst. 2000, 28, 211–218. [Google Scholar] [CrossRef]
- Chakraborty, N.; Cant, R.S. Effects of strain rate and curvature on surface density function transport in turbulent premixed flames in the thin reaction zones regime. Phys. Fluids 2005, 17, 065108. [Google Scholar] [CrossRef]
- Chakraborty, N.; Hawkes, E.R.; Chen, J.H.; Cant, R.S. Effects of strain rate and curvature on Surface Density Function transport in turbulent premixed CH4-air and H2-air flames: A comparative study. Combust. Flame 2008, 154, 259–280. [Google Scholar] [CrossRef]
- Lipatnikov, A.N.; Nishiki, S.; Hasegawa, T. A direct numerical study of vorticity transformation in weakly turbulent pre-mixed flames. Phys. Fluids 2014, 26, 105104. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Chakraborty, N.; Klein, M. Assessment of sub-grid scalar flux modelling in premixed flames for Large Eddy Simulations: A-priori Direct Numerical Simulation. Eur. J. Mech. Fluids-B 2015, 52, 97–108. [Google Scholar] [CrossRef]
- Papapostolou, V.; Wacks, D.H.; Klein, M.; Chakraborty, N.; Im, H.G. Enstrophy transport conditional on local flow topologies in different regimes of premixed turbulent combustion. Sci. Rep. 2017, 7, 11545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, M.; Kasten, C.; Chakraborty, N.; Mukhadiyev, N.; Im, H.G. Turbulent scalar fluxes in Hydrogen-Air premixed flames at low and high Karlovitz numbers. Combust. Theor. Model. 2018, 22, 1033–1048. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Chakraborty, N.; Swaminathan, N. Algebraic Closure of Scalar Dissipation Rate for Large Eddy Simulations of Turbulent Premixed Combustion. Combust. Sci. Technol. 2014, 186, 1309–1337. [Google Scholar] [CrossRef]
- Gao, Y.; Chakraborty, N.; Swaminathan, N. Scalar Dissipation Rate Transport in the Context of Large Eddy Simulations for Turbulent Premixed Flames with Non-Unity Lewis Number. Flow Turbul. Combust. 2014, 93, 461–486. [Google Scholar] [CrossRef]
- Gao, Y.; Minamoto, Y.; Tanahashi, M.; Chakraborty, N. A Priori Assessment of Scalar Dissipation Rate Closure for Large Eddy Simulations of Turbulent Premixed Combustion Using a Detailed Chemistry Direct Numerical Simulation Database. Combust. Sci. Technol. 2016, 188, 1398–1423. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, T.; Langella, I.; Doan, N.A.K.; Swaminathan, N.; Yu, R.; Bai, X.-S. A priori analysis of sub-grid variance of a reactive scalar using DNS data of high Ka flames. Combust. Theory Model. 2019, 23, 885–906. [Google Scholar] [CrossRef] [Green Version]
- Attili, A.; Lamioni, R.; Berger, L.; Kleinheinz, K.; Lapenna, P.E.; Pitsch, H.; Creta, F. The effect of pressure on the hydrodynamic stability limit of premixed flames. Proc. Combust. Inst. 2021, 38, 1973–1981. [Google Scholar] [CrossRef]
- Rasool, R.; Chakraborty, N.; Klein, M. Effect of non-ambient pressure conditions and Lewis number variation on direct numerical simulation of turbulent Bunsen flames at low turbulence intensity. Combust. Flame 2021, 231, 111500. [Google Scholar] [CrossRef]
- Klein, M.; Nachtigal, H.; Hansinger, M.; Pfitzner, M.; Chakraborty, N. Flame Curvature Distribution in High Pressure Turbulent Bunsen Premixed Flames. Flow Turbul. Combust. 2018, 101, 1173–1187. [Google Scholar] [CrossRef] [Green Version]
- Papapostolou, V.; Chakraborty, N.; Klein, M.; Im, H.G. Statistics of Scalar Flux Transport of Major Species in Different Premixed Turbulent Combustion Regimes for H2-air Flames. Flow Turb. Combust. 2019, 102, 931–955. [Google Scholar] [CrossRef] [Green Version]
- Papapostolou, V.; Chakraborty, N.; Klein, M.; Im, H.G. Effects of Reaction Progress Variable Definition on the Flame Surface Density Transport Statistics and Closure for Different Combustion Regimes. Combust. Sci. Technol. 2019, 191, 1276–1293. [Google Scholar] [CrossRef] [Green Version]
- Attili, A.; Luca, S.; Denker, D.; Bisetti, F.; Pitsch, H. Turbulent flame speed and reaction layer thickening in premixed jet flames at constant Karlovitz and increasing Reynolds numbers. Proc. Combust. Inst. 2021, 38, 2939–2947. [Google Scholar] [CrossRef]
- Bouvet, N.; Halter, F.; Chauveau, C.; Yoon, Y. On the effective Lewis number formulations for lean hydrogen/hydrocarbon/air mixtures. Int. J. Hydrog. Energy 2013, 38, 5949–5960. [Google Scholar] [CrossRef]
- Lipatnikov, A.; Chomiak, J. Molecular transport effects on turbulent flame propagation and structure. Prog. Energy Combust. Sci. 2005, 31, 1–73. [Google Scholar] [CrossRef]
- Chakraborty, N.; Herbert, A.; Ahmed, U.; Im, H.G.; Klein, M. Assessment of extrapolation relations of displacement speed for detailed chemistry Direct Numerical Simulation database of statistically planar turbulent premixed flames. Flow Turb. Combust. 2021. [Google Scholar] [CrossRef]
- Echekki, T.; Chen, J.H. Unsteady strain rate and curvature effects in turbulent premixed methane-air flames. Combust. Flame 1996, 106, 184–202. [Google Scholar] [CrossRef]
- Peters, N. Turbulent Combustion; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Pope, S. The evolution of surfaces in turbulence. Int. J. Eng. Sci. 1988, 26, 445–469. [Google Scholar] [CrossRef]
- Jenkins, K.W.; Cant, R.S. Direct Numerical Simulation of Turbulent Flame Kernels. In Recent Advances in DNS and LES. Fluid Mechanics and Its Applications; Knight, D., Sakell, L., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 54. [Google Scholar] [CrossRef]
- Wray, A.A. Minimal Storage Time Advancement Schemes for Spectral Methods; Tech. Rep. NASA Ames Research Center: Silicon Valley, CA, USA, 1990. [Google Scholar]
- Poisont, T.; Lele, S.K. Boundary conditions for direct simulation of compressible viscous flows. J. Comp. Phys. 1992, 101, 104–129. [Google Scholar]
- Cant, R.S. SENGA2 Manual, CUED-THERMO-2012/04, 2nd ed.; University of Cambridge: Cambridge, UK, 2013. [Google Scholar]
- Im, H.G.; Chen, J.H. Preferential diffusion effects on the burning rate of interacting turbulent premixed hydrogen-air flames. Combust. Flame 2002, 131, 246–258. [Google Scholar] [CrossRef]
- Kee, R.J.; Rupley, F.M.; Miller, J.A.; Coltrin, M.E.; Grcar, J.F.; Meeks, E.; Moffat, H.K.; Lutz, A.E.; Dixon-Lewis, G.; Smooke, M.D.; et al. CHEMKIN, Collection, Release 3.6; Reaction Design, Inc.: San Diego, CA, USA, 2000. [Google Scholar]
- Kennedy, C.A.; Carpenter, M.H.; Lewis, R. Low-storage, explicit Runge–Kutta schemes for the compressible Navier–Stokes equations. Appl. Numer. Math. 2000, 35, 177–219. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.; Chakraborty, N.; Ketterl, S. A Comparison of Strategies for Direct Numerical Simulation of Turbulence Chemistry Interaction in Generic Planar Turbulent Premixed Flames. Flow Turbul. Combust. 2017, 99, 955–971. [Google Scholar] [CrossRef]
- Rogallo, R.S. Numerical Experiments in Homogenous Turbulence, NASA Technical Memorandum 91416; NASA Ames Research Center: Silicon Valley, CA, USA, 1981. [Google Scholar]
- Cant, R.; Pope, S.; Bray, K. Modelling of flamelet surface-to-volume ratio in turbulent premixed combustion. Symp. Int. Combust. 1991, 23, 809–815. [Google Scholar] [CrossRef]
- Chakraborty, N.; Klein, M.; Cant, R.S. Effects of Turbulent Reynolds Number on the Displacement Speed Statistics in the Thin Reaction Zones Regime of Turbulent Premixed Combustion. J. Combust. 2011, 2011, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, K.; Klein, M.; Chakraborty, N.; Cant, R. Effects of strain rate and curvature on the propagation of a spherical flame kernel in the thin-reaction-zones regime. Combust. Flame 2006, 145, 415–434. [Google Scholar] [CrossRef]
- Butler, T.; O’Rourke, P. A numerical method for two dimensional unsteady reacting flows. Symp. Int. Combust. 1977, 16, 1503–1515. [Google Scholar] [CrossRef] [Green Version]
- Bray, K.; Libby, P.A.; Moss, J. Unified modeling approach for premixed turbulent combustion—Part I: General formulation. Combust. Flame 1985, 61, 87–102. [Google Scholar] [CrossRef]




| Method | Simple Chemistry and Transport | Detailed Chemistry and Transport |
|---|---|---|
| Carbon footprint, this database | ||
| CPU cost | CPU hours [30,31] | CPU hours [3] |
| Data volume | TB [30,31] | TB [3] |
| Parametric variations | Dozens possible [30,31] | Typically, very few (e.g., 1–4) [32,33,34]; more limited in terms of nondimensional numbers, pressure, or size of domain |
| Data analysis | Straightforward, unambiguous results. | Complex (due to re-evaluation of constitutive laws); results are not unambiguous due to different possibilities to define reaction progress variables [32,33] |
| Comparison with experiment | Possible in a qualitative manner, open questions remaining (e.g., effective Lewis number [35]). | Possible, more straightforward, but more limited in terms of nondimensional numbers or pressure. |
| Comparison with theory | Often easier because theory is based on similar assumptions (e.g., flame instabilities, flame stretch [36]). | Results are ambiguous due to different choices of reaction progress variables [32,33]. Often theory not available for DC (e.g., Markstein length [37]). |
| Turbulence–chemistry interaction | No limitation (this work and references herein) | No limitation |
| Flame propagation | No limitation (this work and references herein) | No limitation |
| Ignition (delay) | Not possible | No limitation |
| Emissions | Not possible | Possible, depending on complexity of mechanism |
| Differential diffusion | Differential diffusion of heat and mass of one deficient species possible [12,13,26,30]. | No limitation |
| Modeling of turbulent reacting flows | Possible, most models available in literature are originally based on SC assumptions [2]. | Possible, but more complex and potentially ambiguous. |
| Case | A | B | C | D | E |
|---|---|---|---|---|---|
| 5.0 | 6.25 | 7.5 | 9.0 | 11.25 | |
| 1.67 | 1.44 | 2.5 | 4.31 | 3.75 | |
| 0.33 | 0.23 | 0.33 | 0.48 | 0.33 | |
| 8.65 | 13.0 | 13.0 | 13.0 | 19.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keil, F.B.; Amzehnhoff, M.; Ahmed, U.; Chakraborty, N.; Klein, M. Comparison of Flame Propagation Statistics Extracted from Direct Numerical Simulation Based on Simple and Detailed Chemistry—Part 1: Fundamental Flame Turbulence Interaction. Energies 2021, 14, 5548. https://doi.org/10.3390/en14175548
Keil FB, Amzehnhoff M, Ahmed U, Chakraborty N, Klein M. Comparison of Flame Propagation Statistics Extracted from Direct Numerical Simulation Based on Simple and Detailed Chemistry—Part 1: Fundamental Flame Turbulence Interaction. Energies. 2021; 14(17):5548. https://doi.org/10.3390/en14175548
Chicago/Turabian StyleKeil, Felix Benjamin, Marvin Amzehnhoff, Umair Ahmed, Nilanjan Chakraborty, and Markus Klein. 2021. "Comparison of Flame Propagation Statistics Extracted from Direct Numerical Simulation Based on Simple and Detailed Chemistry—Part 1: Fundamental Flame Turbulence Interaction" Energies 14, no. 17: 5548. https://doi.org/10.3390/en14175548
APA StyleKeil, F. B., Amzehnhoff, M., Ahmed, U., Chakraborty, N., & Klein, M. (2021). Comparison of Flame Propagation Statistics Extracted from Direct Numerical Simulation Based on Simple and Detailed Chemistry—Part 1: Fundamental Flame Turbulence Interaction. Energies, 14(17), 5548. https://doi.org/10.3390/en14175548









