Biocellulose for Treatment of Wastewaters Generated by Energy Consuming Industries: A Review
Abstract
:1. Introduction
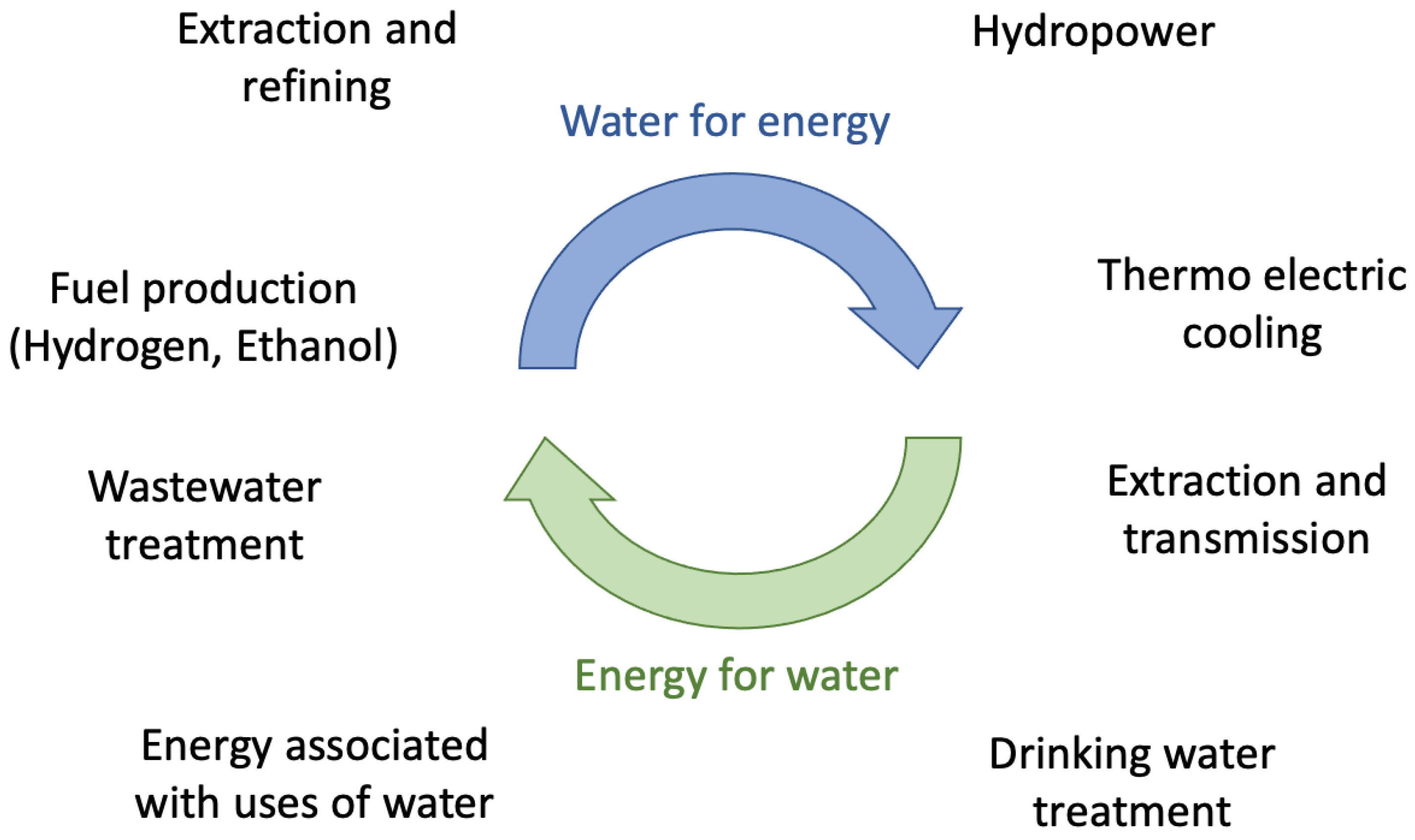
2. Water Resources and Energy Management
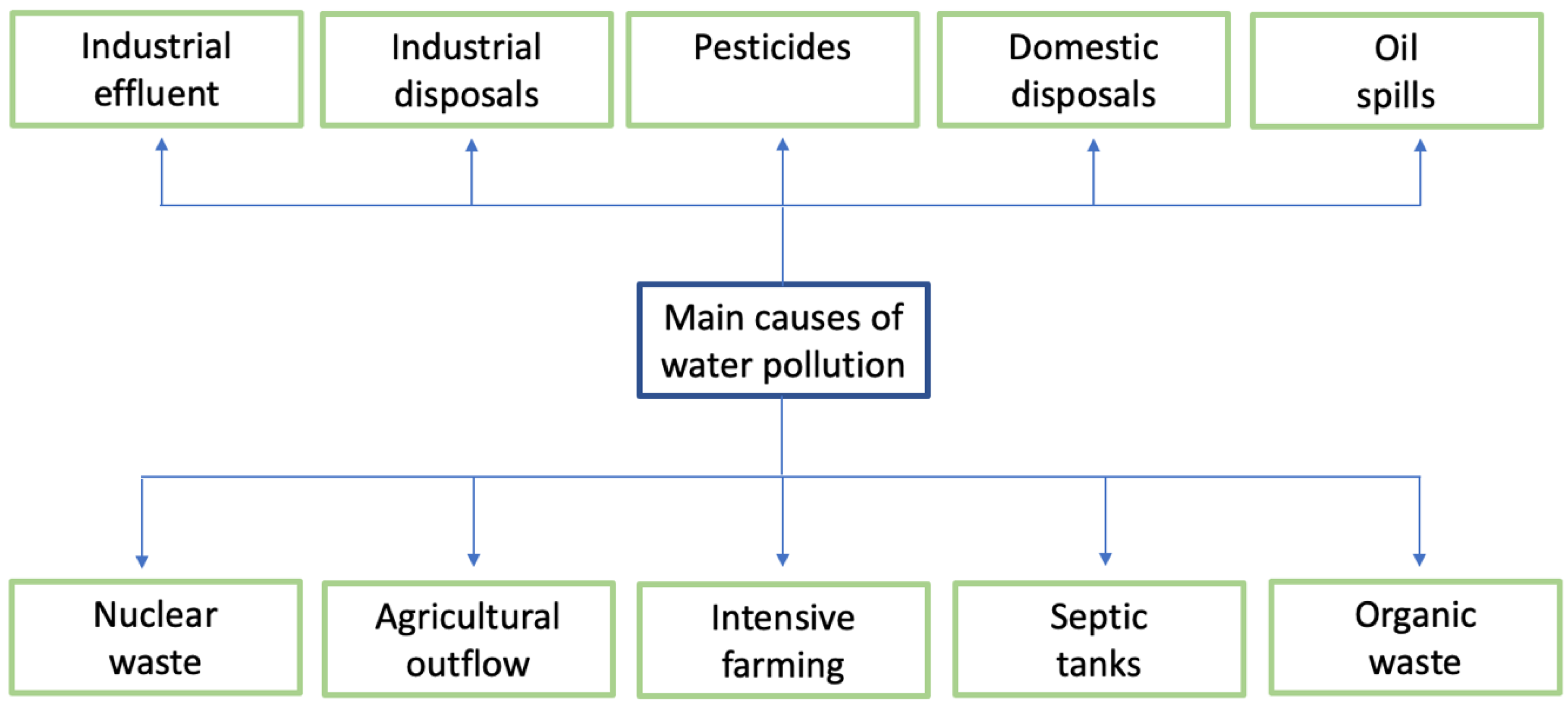
3. Water Contamination
4. Filtration Membranes
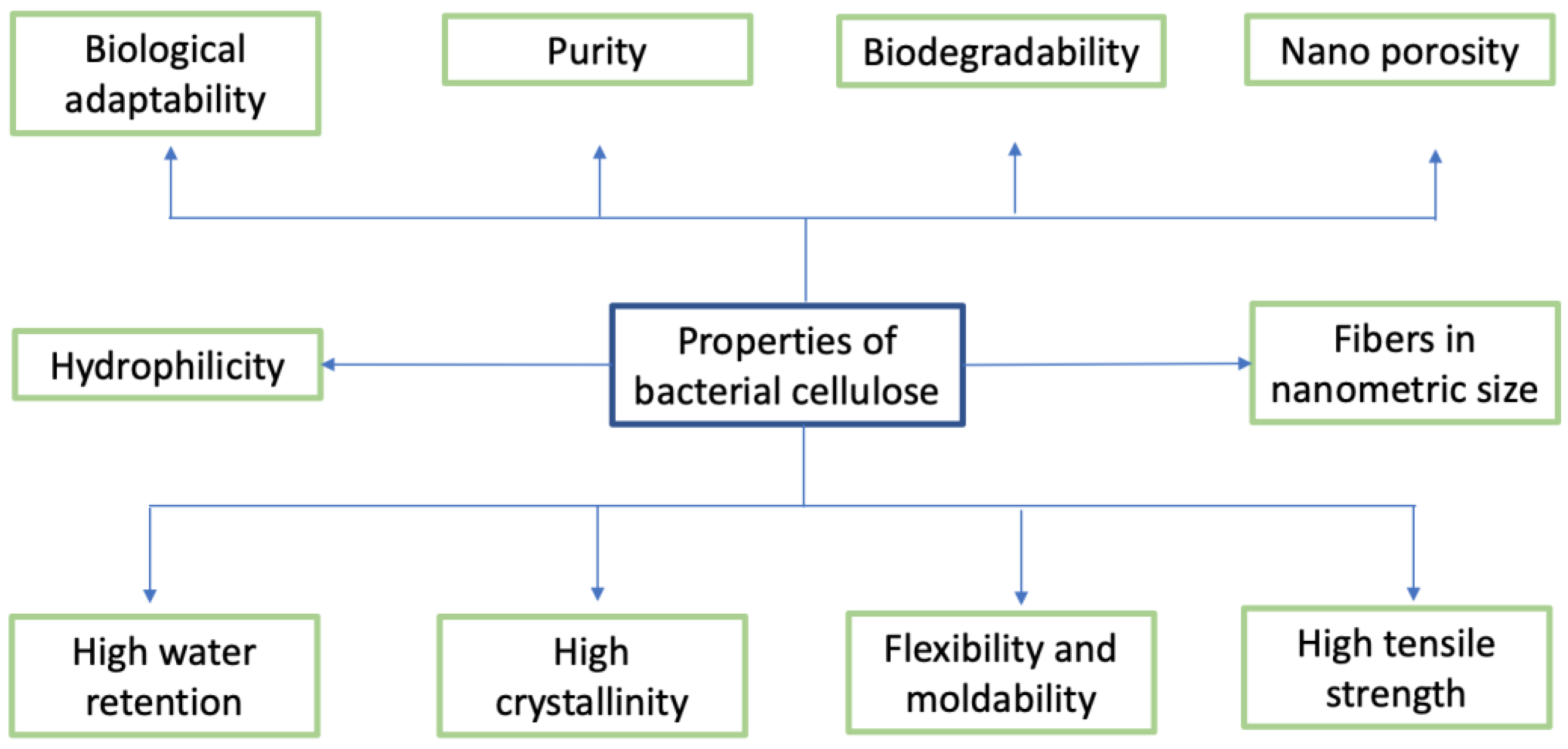
5. Bacterial Cellulose Membranes
6. Influence of Culture Conditions on Bacterial Cellulose Production
7. Bacterial Cellulose in Wastewater Treatment
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Riordon, J.; Sovilj, D.; Sanner, S.; Sinton, D.; Young, E.W.K. Deep learning with microfluidics for biotechnology. Trends Biotechnol. 2019, 37, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Fröhling, M.; Hiete, M. Sustainability and life cycle assessment in industrial biotechnology: A review of current approaches and future needs. In Sustainability and Life Cycle Assessment in Industrial Biotechnology. Advances in Biochemical Engineering/Biotechnology; Fröhling, M., Hiete, M., Eds.; Springer: Cham, Switzerland, 2020; Volume 173, pp. 143–203. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Silva, J.F.; Barbosa, N.P.S.R.; França, M.T.N.; Houllou, L.M.; Malafaia, C.B. Reuse of polluting agroindustrial waste for ethanol production by Kluyveromyces marxianus. J. Environ. Anal. Prog. 2019, 4, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Del Borghi, A.; Moreschi, L.; Gallo, M. Circular economy approach to reduce water–energy–food nexus. Curr. Opin. Environ. Sci. Health 2020, 13, 23–28. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G.; Adeniran, J.A.; Ogunniyi, S. A systematic literature analysis of the nature and regional distribution of water pollution sources in Nigeria. J. Clean. Prod. 2021, 283, 124566–124602. [Google Scholar] [CrossRef]
- Mikulčić, H.; Baleta, J.; Klemel, J.J. Sustainability through combined development of energy, water and environment systems. J. Clean. Prod. 2020, 251, 119727–119762. [Google Scholar] [CrossRef]
- Kumar, P.; Saroj, D.P. Water–energy–pollution nexus for growing cities. Urban Clim. 2014, 10, 846–853. [Google Scholar] [CrossRef]
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Dissolved air flotation combined to biosurfactants: A clean and efficient alternative to treat industrial oily water. Rev. Environ. Sci. Biotechnol. 2018, 17, 591–602. [Google Scholar] [CrossRef]
- Galdino, C.J.S.; Maia, A.D.M.; Meira, H.M.; Souza, T.C.; Amorim, J.D.P.; Almeida, F.C.G.; Costa, A.F.S.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2020, 91, 288–296. [Google Scholar] [CrossRef]
- Chaudhry, F.N.; Malik, M.F. Factors affecting water pollution: A review. J. Ecosyst. Ecography 2017, 7, 1000225–1000228. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water–A comprehensive review. Resour. Eff. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef] [Green Version]
- El-Gawad, H.S.A. Oil and grease removal from industrial wastewater using new utility approach. Adv. Environ. Chem. 2014, 2014, 916878. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lautz, L.S.; Nolte, T.M.; Posthuma, L.; Koopman, K.R.; Leuven, R.S.W.; Hendriks, A.J. Towards a systematic method for assessing the impact of chemical pollution on ecosystem services of water systems. J. Environ. Manag. 2021, 281, 111873–111882. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.; Anderson, S.M. Biogenesis of bacterial cellulose. Crit. Rev. Microbiol. 1991, 17, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Rachtanapun, P.; Jantrawut, P.; Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S. Carboxymethyl bacterial cellulose from nata de coco: Effects of NaOH. Polymers 2021, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.D.P.; Souza, K.C.; Duarte, C.R.; Duarte, I.S.; Ribeiro, F.A.S.; Silva, G.S.; Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Wanichapichart, P.; Kaewnopparat, S.; Buaking, K.; Puthai, W. Characterization of cellulose membranes produced by Acetobacter xyllinum. Songklanakarin J. Sci. Technol. 2002, 24, 855–862. [Google Scholar]
- Mautner, A.; Lee, K.Y.; Lahtinen, P.; Hakalahti, M.; Tammelin, T.; Li, K.; Bismarck, A. Nanopapers for organic solvent nanofiltration. Chem. Commun. 2014, 50, 5778–5781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, A.W.; Lannoy, S.-F.; Wiesner, M.R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef] [PubMed]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7738. [Google Scholar] [CrossRef] [PubMed]
- Gordić, D.; Babić, M.; Jovičić, N.; Šušteršič, V.; Končalović, D.; Jelić, D. Development of energy management system–Case study of Serbian car manufacturer. Energy Convers. Manag. 2010, 51, 2783–2790. [Google Scholar] [CrossRef]
- Hussey, K.; Pittock, J. The energy–water nexus: Managing the links between energy and water for a sustainable future. Ecol. Soc. 2012, 17, 31–40. [Google Scholar] [CrossRef]
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable energy and geopolitics: A review. Renew. Sustain. Energy Rev. 2020, 122, 109547–109559. [Google Scholar] [CrossRef]
- Khan, I.; Hou, F.; Zakari, A.; Tawiah, V.K. The dynamic links among energy transitions, energy consumption, and sustainable economic growth: A novel framework for IEA countries. Energy 2021, 222, 119935. [Google Scholar] [CrossRef]
- Calvin, K.; Patel, P.; Clarke, L.; Asrar, G.; Bond-Lamberty, B.; Cui, R.Y.; Di Vittorio, A.; Dorheim, K.; Edmonds, J.; Hartin, C.; et al. GCAM v5.1: Representing the linkages between energy, water, land, climate, and economic systems. Geosci. Model. Dev. 2019, 12, 677–698. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, R.A.; Molden, D.J.; Shrestha, A.B.; Wagle, N.; Tortajada, C. The role of hydropower in South Asia’s energy future. Int. J. Water Resour. Dev. 2021, 37, 367–391. [Google Scholar] [CrossRef]
- Xiong, W.; Li, Y.; Pfister, S.; Zhang, W.; Wang, C.; Wang, P. Improving water ecosystem sustainability of urban water system by management strategies optimization. J. Environ. Manag. 2020, 254, 109766–109774. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Li, M.; Peng, H.; Wang, D.; Fu, S. Cost-effective resource utilization for waste biomass: A simple preparation method of photo-thermal biochar cakes (BCs) toward dye wastewater treatment with solar energy. Environ. Res. 2021, 194, 11–720. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Rajaei, V.; Glass, J.B.; Hud, N.V.; Williams, L.D. Water and life: The medium is the message. J. Mol. Evol. 2021, 89, 2–11. [Google Scholar] [CrossRef]
- Azimi, S.; Rocher, V. Energy consumption reduction in a wastewater treatment plant. Water Pract. Technol. 2017, 12, 104–116. [Google Scholar] [CrossRef]
- Markham, A.C. A Brief History of Pollution, 1st ed.; Routledge: Abingdon, UK, 1994; pp. 1–178. [Google Scholar] [CrossRef]
- Spash, C.L. The History of Pollution ‘Externalities’ in Economic Thought, 1st ed.; Social-Ecological Research In Economics, Vienna University of Economics and Business: Vienna, Austria, 2021; pp. 1–44. [Google Scholar]
- Pandey, A.; Brauer, M.; Cropper, M.L.; Balakrishnan, K.; Mathur, P.; Dey, S.; Turkgulu, B.; Kumar, G.A.; Khare, M.; Beig, G. Health and economic impact of air pollution in the states of India: The global burden of disease study 2019. Lancet Planet. Health 2021, 5, 25–38. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Aravinthasamy, P.; Deepali, M.; Subramani, M.; Shankar, K. Groundwater pollution and human health risks in an industrialized region of southern India: Impacts of the Covid-19 lockdown and the monsoon seasonal cycles. Arch. Environ. Contam. Toxicol. 2021, 80, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh-Hesary, F.; Taghizadeh-Hesary, F. The impacts of air pollution on health and economy in Southeast Asia. Energies 2020, 13, 1812. [Google Scholar] [CrossRef] [Green Version]
- Malik, D.S.; Sharma, A.K.; Thakur, R.; Sharma, M. A review on impact of water pollution on freshwater fish species and their aquatic environment. In Advances in Environmental Pollution Management: Wastewater Impacts and Treatment Technologies, 1st ed.; Kumar, V., Kamboj, N., Payum, T., Eds.; Agro Environ Media—Agriculture and Ennvironmental Science Academy: Haridwar, India, 2020; pp. 10–28. [Google Scholar]
- Huang, S.; Wei, J.; Ning, S.; Fang, H.; Li, S.; Ye, S.; Zhou, X. Comprehensive pollution analysis of contaminated sediment in an urban river, China. IOP Conf. Ser. Earth Environ. Sci. 2021, 69, 012003–012011. [Google Scholar] [CrossRef]
- Fabrício, S.A.; Ferreira, D.D.M.; Borba, J.A. A panorama of Mariana and Brumadinho disasters: What do we know so far? Rev. Eletrôn. Adm. 2021, 27, 128–152. [Google Scholar] [CrossRef]
- Guimarães, A.T.B.; Charlie-Silva, I.; Malafaia, G. Toxic effects of naturally-aged microplastics on zebrafish juveniles: A more realistic approach to plastic pollution in freshwater ecosystems. J. Hazard. Mater. 2021, 407, 124833. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.I.; Avranas, A. Treatment of oil-in-water emulsions by coagulation and dissolved air flotation. Colloids Surf. A Physicochem. Eng. Asp. 2000, 172, 153–161. [Google Scholar] [CrossRef]
- Demore, J.P. Aspectos Sedimentares do Estuário da Lagoa dos Patos e Sua Interação Com a Poluição por Petróleo: Subsídios Para um Plano de Contingência. Bachelor’s Thesis, Fundação Universidade Federal do Rio Grande, Rio Grande, RS, Brazil, 2001. [Google Scholar]
- Luo, F.; He, L.; He, N. Simulation and experimental study of working characteristics of an improved bioreactor for degrading oily sludge. Process Saf. Environ. Prot. 2021, 147, 1201–1208. [Google Scholar] [CrossRef]
- Silva, W.E.; Belian, M.F.; Lima, L.S.G.L.; Galembeck, A.; Alves, A.A. BR 10 2018 009736 9 A2–Filtros à Base de Membrana de Celulose Bacteriana Modificada; INPI: Rio de Janeiro, Brazil, 2018. [Google Scholar]
- Lehtonen, J.; Chen, X.; Beaumont, M.; Hassinen, J.; Orelma, H.; Dumée, L.F.; Tardy, B.L.; Rojas, O.J. Impact of incubation conditions and post-treatment on the properties of bacterial cellulose membranes for pressure-driven filtration. Carbohydr. Polym. 2021, 251, 117073–117082. [Google Scholar] [CrossRef]
- Mo, X.; Ni, Y.; Liu, F. Preparation of different scale firous membranes and their filtration properties. Therm. Sci. 2021, 25, 1453–1459. [Google Scholar] [CrossRef]
- Gao, H.; He, W.; Zhao, Y.-B.; Opris, D.M.; Xu, G.; Wang, J. Electret mechanisms and kinetics of electrospun nanofiber membranes and lifetime in filtration applications in comparison with corona-charged membranes. J. Membr. Sci. 2020, 600, 117879. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Microfiltration membrane processes: A review of research trends over the past decade. J. Water Process Eng. 2019, 32, 100941. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro-food by-products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef] [Green Version]
- Hassan, E.; Hassan, M.; Abou-Zeid, R.; Berglund, L.; Oksman, K. Use of bacterial cellulose and crosslinked cellulose nanofibers membranes for removal of oil from oil-in-water emulsions. Polymers 2017, 9, 388. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Amorim, J.D.P.; Gomes, E.A.S.; Araujo, L.M.; Sarubbo, L. Residue from the production of sugar cane: An alternative nutrient used in biocellulose production by Gluconacetobacter hansenii. Chem. Eng. Trans. 2019, 64, 7–12. [Google Scholar] [CrossRef]
- Albuquerque, R.M.B.; Meira, H.M.; Silva, I.D.; Silva, C.J.G.; Almeida, F.C.G.; Amorim, J.D.P.; Vinhas, G.M.; Costa, A.F.S.; Sarubbo, L.A. Production of a bacterial cellulose/poly(3-hydroxybutyrate) blend activated with clove essential oil for food packaging. Polym. Polym. Compos. 2020, 29, 259–270. [Google Scholar] [CrossRef]
- Donini, Í.A.N.; Salvi, D.T.B.; Fukumoto, F.K.; Lustri, W.R.; Barud, H.S.; Marchetto, R.; Messaddeq, Y.; Ribeiro, S.J.L. Biossíntese e recentes avanços na produção de celulose bacteriana. Eclet. Quim. J. 2010, 35, 165–178. [Google Scholar] [CrossRef]
- Jardine, A.; Sayed, S. Challenges in the valorization of chitinous biomass within the refinery concept. Sustain. Chem. 2016, 2, 34–39. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown Jr, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Batra, H.V. Occurrence of cellulose-producing Gluconacetobacter spp. in fruit samples and kombucha tea, and production of the biopolymer. Appl. Biochem. Biotechnol. 2015, 176, 1162–1173. [Google Scholar] [CrossRef]
- Lin, D.; Lopez-Sanchez, P.; Li, R.; Li, Z. Production of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917 using only waste beer yeast as nutrient source. Bioresour. Technol. 2014, 151, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mohite, B.V.; Patil, S.V. Physical, structural, mechanical and thermal characterization of bacterial cellulose by G. hansenii NCIM 2529. Carbohydr. Polym. 2014, 106, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Adrio, J.L.; Demain, A.L. Microbial enzymes: Tools for biotechnological processes. Biomolecules 2014, 1, 117–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from indústrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Galdino, C.J.S. Avaliação do Potencial da Celulose Bacteriana no Tratamento de Águas Oleosas. Master’s Thesis, Universidade Católica de Pernambuco, Recife, Brazil, 2020. [Google Scholar]
- Stasiak-Różańska, L.; Płoska, J. Study on the use of microbial cellulose as a biocarrier for 1,3-dihydroxy-2-propanone and its potential application in industry. Polymers 2018, 10, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecoraro, E.; Manzani, D.; Messaddeq, Y.; Ribeiro, S.J.L. Bacterial cellulose from Glucanacetobacter xylinus: Preparation, properties and applications. In Monomers, Polymers and Composites from Renewable Resources, 1st ed.; Belgacem, M., Gandini, A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2008; pp. 369–383. [Google Scholar]
- Costa, A.F.S.; Rocha, M.A.V.; Sarubbo, L.A. Bacterial cellulose: An ecofriendly biotextile. Int. J. Text. Fash. Technol. 2017, 7, 11–26. [Google Scholar]
- Amorim, J.D.P. Obtenção de Celulose Bacteriana Aditivada com Extrato de Própolis Para Aplicação em Cosméticos. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2020. [Google Scholar]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2015, 23, 57–91. [Google Scholar] [CrossRef]
- Fernandes, I.A.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial cellulose: From production optimization to new applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611. [Google Scholar] [CrossRef] [PubMed]
- Hungund, B.S.; Gupta, S.G. Improved production of bacterial cellulose from Gluconacetobacter persimmonis GH-2. J. Microb. Biochem. Technol. 2010, 2, 127–133. [Google Scholar] [CrossRef]
- Amorim, J.D.P.; Galdino, C.J.S.J.; Costa, A.F.S.; Sarubbo, L.A.; Melo, J.F.H.M. Avaliação do potencial da celulose bacteriana para aplicação em cosméticos. Blucher Chem. Eng. Proc. 2018, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; JiI, H.; Yang, Y.; Guo, B.; Luo, L.; Meng, Z.; Fan, L.; Xu, J. Tempo-oxidized bacterial cellulose nanofiber membranes as high-performance separators for lithium-ion batteries. Carbohydr. Polym. 2020, 230, 115570. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, X.; Alfred, M.; Li, D.; Huang, F.; Wei, Q. Bacterial cellulose hydrogel: A promising electrolyte for flexible zinc-air batteries. J. Power Sources 2021, 482, 228963. [Google Scholar] [CrossRef]
- Nizam, P.A.; Gopakumar, D.A.; Pottathara, Y.B.; Pasquini, D.; Nzihou, A.; Thomas, S. Nanocellulose-based composites. In Nanocellulose Based Composites for Electronics, 1st ed.; Thomas, S., Pottathara, Y.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 15–29. [Google Scholar] [CrossRef]
- Haghighi, H.; Gullo, M.; Lachina, S.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. characterization of bio-nanocomposite films based on gelatin/polyvinyl alcohol blend reinforced with bacterial cellulose nanowhiskers for food packaging applications. Food Hydrocoll. 2021, 113, 106454. [Google Scholar] [CrossRef]
- Hongyan, W.; Nan, Z.X.; Yingchen, Z. CN103301815B–Preparation Method for Bacterial Cellulose Water Ultrafiltration Adsorption Material; China, Zhongyuan University of Technology: Hainan, China, 2014; Available online: https://worldwide.espacenet.com/patent/search/family/049127790/publication/CN103301815B?q=pn%3DCN103301815B (accessed on 20 January 2021).
- Wang, F.-P.; Zhao, X.-J.; Wahid, F.; Zhao, X.-Q.; Qin, X.-T.; Bai, H.; Xie, Y.-Y.; Zhong, C.; Jia, S.-R. Sustainable, superhydrophobic membranes based on bacterial cellulose for gravity-driven oil/water separation. Carbohydr. Polym. 2021, 253, 117220. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.Y.; Park, J.K.; Chang, H.N. Bacterial cellulose production by Gluconacetobacter hansenii in an agitated culture without living non-cellulose producing cells. Enzyme Microb. Technol. 2005, 37, 347–354. [Google Scholar] [CrossRef]
- Zywicka, A.; Peitler, D.; Rakoczy, F.; Konopacki, M.; Kordas, M.; Fijałkowski, K. The effect of different agitation modes on bacterial cellulose synthesis by Gluconacetobacter xylinus strains. Acta Sci. Pol. Zootech. 2015, 14, 137–150. [Google Scholar]
- Sani, A.; Dahman, Y. Improvements in the production of bacterial synthesized biocellulose nanofibers using different culture methods. J. Chem. Technol. Biotechnol. 2010, 85, 151–164. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Yousefi, M. Biotechnological production of cellulose by Gluconacetobacter xylinus from agricultural waste. Iran. J. Biotechnol. 2011, 9, 94–101. [Google Scholar]
- Klemm, D.; Schumann, D.; Kramer, F.; Heßler, N.; Hornung, M.; Schmauder, H.P.; Marsch, S. Nanocelluloses as innovative polymers in research and application. Adv. Polym. Sci. 2006, 205, 49–96. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Sheykhnazaria, S.; Tabarsaa, T.; Ashorib, A.; Shakeric, A.; Golalipourd, M. Bacterial synthesized cellulose nanofibers; effects of growth times and culture mediums on the structural characteristics. Carbohydr. Polym. 2011, 86, 1187–1191. [Google Scholar] [CrossRef]
- Borzani, W.; Souza, S.J. Mechanism of the film thickness increasing during the bacterial production of cellulose on non-agitated liquid media. Biotechnol. Lett. 1995, 17, 1271–1272. [Google Scholar] [CrossRef]
- Amorim, J.D.P.; Costa, A.F.S.; Galdino, C.J.S.J.; Vinhas, G.M.; Santos, E.M.S.; Sarubbo, L.A. Bacterial cellulose production using industrial fruit residues as subtract to industrial application. Chem. Eng. Trans. 2019, 74, 1165–1170. [Google Scholar] [CrossRef]
- Tanskul, S.; Amornthatree, K.; Jaturonlak, N. A new cellulose-producing bacterium, Rhodococcus sp. MI 2: Screening and optimization of culture conditions. Carbohydr. Polym. 2013, 92, 421–428. [Google Scholar] [CrossRef]
- Dudman, W.F. Cellulose production by Acetobacter strains in submerged culture. J. Gen. Microbiol. 1960, 22, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Hirai, A.; Tsuji, M.; Horii, F. Culture conditions producing structure entities composed of cellulose I and II in bacterial cellulose. Cellulose 1997, 4, 239–245. [Google Scholar] [CrossRef]
- Kroon-Batenburg, L.M.J.; Kroon, J. The crystal and molecular structures of cellulose I and II. Glycoconj. J. 1997, 14, 677–690. [Google Scholar] [CrossRef]
- Shirai, A.; Takahashi, M.; Kaneko, H.; Nishimura, S.-I.; Ogawa, M.; Nishi, N.; Tokura, S. Biosynthesis of a novel polysaccharide by Acetobacter xylinum. Int. J. Biol. Macromol. 1994, 16, 297–300. [Google Scholar] [CrossRef]
- Huang, C.; Guo, H.J.; Xiong, L.; Wang, B.; Shi, S.L.; Chen, X.F.; Chen, X.D. Using wastewater after lipid fermentation as substrate for bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr. Polym. 2016, 136, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, L.; Tang, J.; Jönsson, L.J.; Hong, F.F. Production of bacterial nanocellulose and enzyme from [AMIM]Cl-pretreated waste cotton fabrics: Effects of dyes on enzymatic saccharification and nanocellulose production. J. Chem. Technol. Biotechnol. 2015, 91, 1413–1421. [Google Scholar] [CrossRef]
- Molina-Ramírez, C.; Castro, C.; Zuluaga, R.; Gañán, P. Physical characterization of bacterial cellulose produced by Komagataeibacter medellinensis using food supply chain waste and agricultural by-products as alternative low-cost feedstocks. J. Polym. Environ. 2017, 26, 830–837. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; Saied, H.E. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, D.K.; Bansal, V.; Mehta, D.; Sangwan, R.S.; Yadav, S.K. Efficient and economic process for the production of bacterial cellulose from isolated strain of Acetobacter pasteurianus of RSV-4 bacterium. Bioresour. Technol. 2019, 275, 430–433. [Google Scholar] [CrossRef]
- Barshan, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization and characterization of bacterial cellulose produced by Komagataeibacter xylinus PTCC 1734 using vinasse as a cheap cultivation medium. Int. J. Biol. Macromol. 2019, 136, 1188–1195. [Google Scholar] [CrossRef]
- Souza, E.F.; Furtado, M.R.; Carvalho, C.W.P.; Freitas-Silva, O.; Gottschalk, L.M.F. Production and characterization of Gluconacetobacter xylinus bacterial cellulose using cashew apple juice and soybean molasses. Int. J. Biol. Macromol. 2020, 146, 285–289. [Google Scholar] [CrossRef]
- Güzel, M.; Akpđnar, Ö. Preparation and characterization of bacterial cellulose produced from fruit and vegetable peels by Komagataeibacter hansenii GA2016. Int. J. Biol. Macromol. 2020, 162, 1597–1604. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, D.K.; Sandhu, P.P.; Jadaun, J.; Sangwan, R.S.; Yadav, S.K. Sustainable process for the production of cellulose by an Acetobacter pasteurianus RSV-4 (MTCC 25117) on whey medium. Cellulose 2020, 28, 103–116. [Google Scholar] [CrossRef]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-bacterial cellulose bioadsorbent for effective removal of copper and lead ions from aqueous solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Song, H.; Cui, Y.; Zhu, W.; Du, K.; Yao, S. Porous spherical cellulose carrier modified with polyethyleneimine and its adsorption for Cr(III) and Fe(III) from aqueous solutions. Chin. J. Chem. Eng. 2014, 22, 984–990. [Google Scholar] [CrossRef]
- Wang, Q.; Asoh, T.-A.; Uyama, H. Facile fabrication of flexible bacterial cellulose/silica composite aerogel for oil/water separation. Bull. Chem. Soc. Jpn. 2018, 91, 1138–1140. [Google Scholar] [CrossRef]
- Zhijiang, C.; Ping, X.; Cong, Z.; Tingting, Z.; Jie, G.; Kongyin, Z. Preparation and characterization of a bi-layered nano-filtration membrane from a chitosan hydrogel and bacterial cellulose nanofiber for dye removal. Cellulose 2018, 25, 5123–5137. [Google Scholar] [CrossRef]
- Urbina, L.; Guaresti, O.; Requies, J.; Gabilondo, N.; Eceiza, A.; Corcuera, M.A.; Retegi, A. Design of reusable novel membranes based on bacterial cellulose and chitosan for the filtration of copper in wastewaters. Carbohydr. Polym. 2018, 193, 362–372. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, J. Removal of U(VI) from aqueous solution using phosphate functionalized bacterial cellulose as efficient adsorbent. Radiochim. Acta 2019, 107, 459–467. [Google Scholar] [CrossRef]
- Alves, A.A.; Silva, W.E.; Belian, M.F.; Lins, L.S.G.; Galembeck, A. Bacterial cellulose membranes for environmental water remediation and industrial wastewater treatment. Int. J. Environ. Sci. Technol. 2020, 17, 3997–4008. [Google Scholar] [CrossRef]
- Liu, F.; Chen, C.; Qian, J. Film-like bacterial cellulose/cyclodextrin oligomer composites with controllable structure for the removal of various persistent organic pollutants from water. J. Hazard. Mater. 2021, 405, 124122. [Google Scholar] [CrossRef]
- Jahan, K.; Tyeb, S.; Kumar, N.; Verma, V. Bacterial cellulose-polyaniline porous mat for removal of methyl orange and bacterial pathogens from potable water. J. Polym. Environ. 2020, 29, 1257–1270. [Google Scholar] [CrossRef]
- Hou, Y.; Duan, C.; Zhu, G.; Luo, H.; Liang, S.; Jin, Y.; Zhao, N.; Xu, J. Functional bacterial cellulose membranes with 3D porous architectures: Conventional drying, tunable wettability and water/oil separation. J. Membr. Sci. 2019, 591, 117312. [Google Scholar] [CrossRef]
- Sai, H.; Jin, Z.; Wang, Y.; Fu, R.; Wang, Y.; Ma, L. Facile and green route to fabricate bacterial cellulose membrane with superwettability for oil–water separation. Adv. Sustain. Syst. 2020, 4, 2000042. [Google Scholar] [CrossRef]
- Song, L.; Song, S. CN110354693A–Bacterial Cellulose Filtering Membrane, Preparation Method and Applications Thereof; Xiamen University: Fujian, China, 2019; Available online: https://patents.google.com/patent/CN1063425A/fr (accessed on 20 January 2021).



| Classification | Application | Pore Size (nm) | Reference |
|---|---|---|---|
| Microfiltration (MF) | Removal of suspended solids, protozoa, and bacteria | 100–5000 | [50] |
| Ultrafiltration (UF) | Removal of viruses and colloids | 2–100 | [51] |
| Nanofiltration (NF) | Removal of water hardness, heavy metals, and dissolved organic matter | 0.5–2 | [51] |
| Reverse osmosis | Desalination, water reuse and ultra-pure water production | 0.2–1 | [52] |
| Culture Medium | Microorganism | Time (Days) | Temperature (°C) | pH | Dry Weight Yield (g/L) | Reference |
|---|---|---|---|---|---|---|
| Lipid fermentation wastewater | Gluconacetobacter xylinus CH001 | 5 | 28 | 6.0 | 0.66 | [95] |
| Hydrolysate of dyed waste cotton fabrics | Gluconacetobacter xylinus ATCC 23,770 | 10 | 30 | 5.0 | 12.80 | [96] |
| Corn Steep Liquor | Gluconacetobacter hansenii UCP1619 | 10 | 30 | 6.0 | 9.63 | [54] |
| Cheese whey | Komagataeibacter medellinensis NBRC 3288 | 10 | 30 | 3.5 | 2.37 | [97] |
| Rotten banana juice | Komagataeibacter medellinensis NBRC 3288 | 10 | 30 | 3.5 | 4.81 | [97] |
| Rotten mango juice | Komagataeibacter medellinensis NBRC 3288 | 10 | 30 | 3.5 | 1.95 | [97] |
| Potato peel wastes | Gluconacetobacter xylinum ATCC 10,245 | 6 | 35 | 9.0 | 4.70 | [98] |
| Tomato juice | Acetobacterpasteurianus MTCC 25,117 | 7 | 30 | 4.5 | 7.80 | [99] |
| Tropical fruit residues | Gluconacetobacter hansenii UCP 1619 | 10 | 30 | 6.0 | 7.60 | [89] |
| Vinasse | Komagatacibacter xylinus | 10 | 30 | 6.0 | 1.80 | [100] |
| Cashew apple juice and soybean molasses | Gluconacetobacter xylinus | 7 | 30 | 5.5 | 4.54 | [101] |
| Fruit and vegetable peels (cucumber, melon, kiwifruit, tomato, apple, quince and pomegranate) | Komagataeibacter hansenii | 21 | 30 | 4.5 | 1.40 | [102] |
| Whey | Acetobacter pasteurianus | 8 | 30 | 4.0 | 5.60 | [103] |
| Title | Description | Reference |
|---|---|---|
| Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation | Nanofibers of BC aerogels were modified on their surfaces by trimethylsilylation derivatization followed by freeze-drying. The resulting hydrophobic and oleophilic aerogels were shown to remove a wide range of organic solvents and oils, with potential use in cleaning up oil spills in the marine environment. | [22] |
| Polyethyleneimine-bacterial cellulose bioadsorbent for effective removal of copper and lead ions from aqueous solution | Reductive amination with polyethyleneimine allowed to transform the BC membrane into a bioadsorbent for the removal of heavy metal ions [Cu (II) and Pb (II)] from wastewater. | [104] |
| Facile fabrication of flexible bacterial cellulose/silica composite aerogel for oil/water separation | A silica aerogel composite was prepared by BC modification with methylene diphenyl diisocyanate to increase its hydrophobicity and flexibility, thus making it a promising oil sorbent. | [106] |
| Preparation and characterization of a bi-layered nanofiltration membrane from a chitosan hydrogel and bacterial cellulose nanofiber for dye removal | A membrane was developed by grafting multi-walled carbon nanotubes into BC molecular chains. The BC powder was dissolved in a solution of LiCl and N,N-dimethylacetamide, and stannous octoate was used as a reaction catalyst. The membrane exhibited greater tensile strength, Young’s modulus and pressure resistance, which practically tripled its flow rate and allowed for a yield of dye removal above 90%. | [107] |
| Design of reusable novel membranes based on bacterial cellulose and chitosan for the filtration of copper in wastewaters | Chitosan-modified BC membranes were developed by ex situ (BC immersed in solutions with different chitosan concentrations) or in situ (addition of chitosan solutions to BC production medium) techniques for Cu (II) ions adsorption. The membrane produced by the ex situ technique showed greater efficiency in removing ions. | [108] |
| Removal of U(VI) from aqueous solution using phosphate functionalized bacterial cellulose as efficient adsorbent | BC membranes were modified by grafting phosphate functional groups soaking them in dimethylacetamide and urea. Membrane characterization confirmed the successful incorporation of phosphate groups. Due to the presence of polar hydroxyl groups and electrostatic attraction, the membranes at pH between 4 and 8 were able to adsorb 9 mg/g of U (IV) ions. | [109] |
| Bacterial cellulose membranes for environmental water remediation and industrial wastewater treatment | BC was produced and cleaned with NaOH to be used as a filter membrane for the treatment of microbiologically contaminated effluents (Escherichia coli) and dyes from the textile industry. BC membranes showed better results than the commercial ones, removing 100% of cells present in the effluent and being able to be reused for 10 cycles without loss of efficiency. | [110] |
| Impact of incubation conditions and post-treatment on the properties of bacterial cellulose membranes for pressure-driven filtration | Studies on the permeation properties of BC derivatized with poly-oxyethylene were carried out to determine the filtration efficiency of both dry and wet membranes at different pressures and water flow rates. | [46] |
| Film-like bacterial cellulose/cyclodextrin oligomer composites with controllable structure for the removal of various persistent organic pollutants from water | A film-like water purifier, prepared by loading cyclodextrin oligomer onto ultrafine BC, was described. The system showed high and stable adsorption capacity toward various target pollutants such as phenol, bisphenol A, glyphosate and 2,4-dichlorophenol. | [111] |
| Bacterial cellulose-polyaniline porous mat for removal of methyl orange and bacterial pathogens from potable water | BC membranes were modified with polyaniline by in situ oxidative polymerization and posterior lyophilization. BC was applied to remove methyl orange dye and bacterial cells present in drinking water. Membranes showed an absorption capacity of approximately 300 mg/g and antimicrobial activity, reducing the microbial load present in the effluent by up to four times. | [112] |
| Title | Description | Reference |
|---|---|---|
| Use of bacterial cellulose and crosslinked cellulose nanofibers membranes for removal of oil from oil-in-water emulsions | Wet BC and crosslinked cellulose nanofibers were used for the removal of oil from stabilized and non-stabilized oil-in-water emulsions with droplet size of less than 1 µm. The efficiency of oil removal from stabilized and non-stabilized emulsions was higher than 92%. | [53] |
| Functional bacterial cellulose membranes with 3D porous architectures: Conventional drying, tunable wettability and water/oil separation | A BC membrane was functionalized by the hydrolysis of alkoxysilanes. This procedure was able to preserve the 3D nanofibrillar architecture of the membrane even after the drying process and increased its surface wettability and ability to separate oily emulsions. | [113] |
| Use of a bacterial cellulose filter for the removal of oil from wastewater | BC membranes produced in an alternative medium based on corn steep liquor were cleaned with NaOH without further treatment. When used as filters, they made it possible to retain almost 100% of the oil present in the emulsion. | [10] |
| Facile and green route to fabricate bacterial cellulose membrane with superwettability for oil–water separation | A simple method was described to weave BC fibers and BC nanofiber clusters in aqueous dispersion on a stainless-steel mesh, which resulted in an increase in the roughness and consequently in the wettability of the biopolymer. The oil–water separation process showed 99% efficiency. | [114] |
| Sustainable, superhydrophobic membranes based on bacterial cellulose for gravity-driven oil/water separation | Needle-leaf bleached kraft pulp was added to BC to increase the biopolymer pore size, thus forming a superhydrophobic/super-oleophilic membrane. The membrane showed not only an oil–water separation yield by gravity >95%, but also an excellent recyclability, as it was washed and reused without significant structural changes after 10 separations. | [79] |
| Patent | Description | Reference |
|---|---|---|
| CN103301815B | A method for preparing a BC filter to purify water was described. The membrane nanofibrils incorporated a silica solution to assist in the refinement of the filtration process. BC membranes also acted as a material for drainage and removal of bacterial cells present in water. | [78] |
| BR 1020180097369A2 | BC membranes functionalized with silica solution, using sodium tetraborate to bind the silica to cellulose nanofibrils, were used in chromatographic analyses. BC did not clog (saturated) even after 10 filtrations, and no biological matter was detected in the filtered material. | [45] |
| CN110354693A | BC membrane was modified by incorporating gelatin microspheres to improve the filtration of reactive and acid dyes used in textile processes. | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Medeiros, A.D.M.; da Silva Junior, C.J.G.; de Amorim, J.D.P.; do Nascimento, H.A.; Converti, A.; Costa, A.F.d.S.; Sarubbo, L.A. Biocellulose for Treatment of Wastewaters Generated by Energy Consuming Industries: A Review. Energies 2021, 14, 5066. https://doi.org/10.3390/en14165066
de Medeiros ADM, da Silva Junior CJG, de Amorim JDP, do Nascimento HA, Converti A, Costa AFdS, Sarubbo LA. Biocellulose for Treatment of Wastewaters Generated by Energy Consuming Industries: A Review. Energies. 2021; 14(16):5066. https://doi.org/10.3390/en14165066
Chicago/Turabian Stylede Medeiros, Alexandre D’Lamare Maia, Cláudio José Galdino da Silva Junior, Julia Didier Pedrosa de Amorim, Helenise Almeida do Nascimento, Attilio Converti, Andréa Fernanda de Santana Costa, and Leonie Asfora Sarubbo. 2021. "Biocellulose for Treatment of Wastewaters Generated by Energy Consuming Industries: A Review" Energies 14, no. 16: 5066. https://doi.org/10.3390/en14165066
APA Stylede Medeiros, A. D. M., da Silva Junior, C. J. G., de Amorim, J. D. P., do Nascimento, H. A., Converti, A., Costa, A. F. d. S., & Sarubbo, L. A. (2021). Biocellulose for Treatment of Wastewaters Generated by Energy Consuming Industries: A Review. Energies, 14(16), 5066. https://doi.org/10.3390/en14165066









