Multi-Objective Optimization and Fluid Selection of Different Cogeneration of Heat and Power Systems Based on Organic Rankine Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. System Description
2.1.1. Basic Organic Rankine Cycle
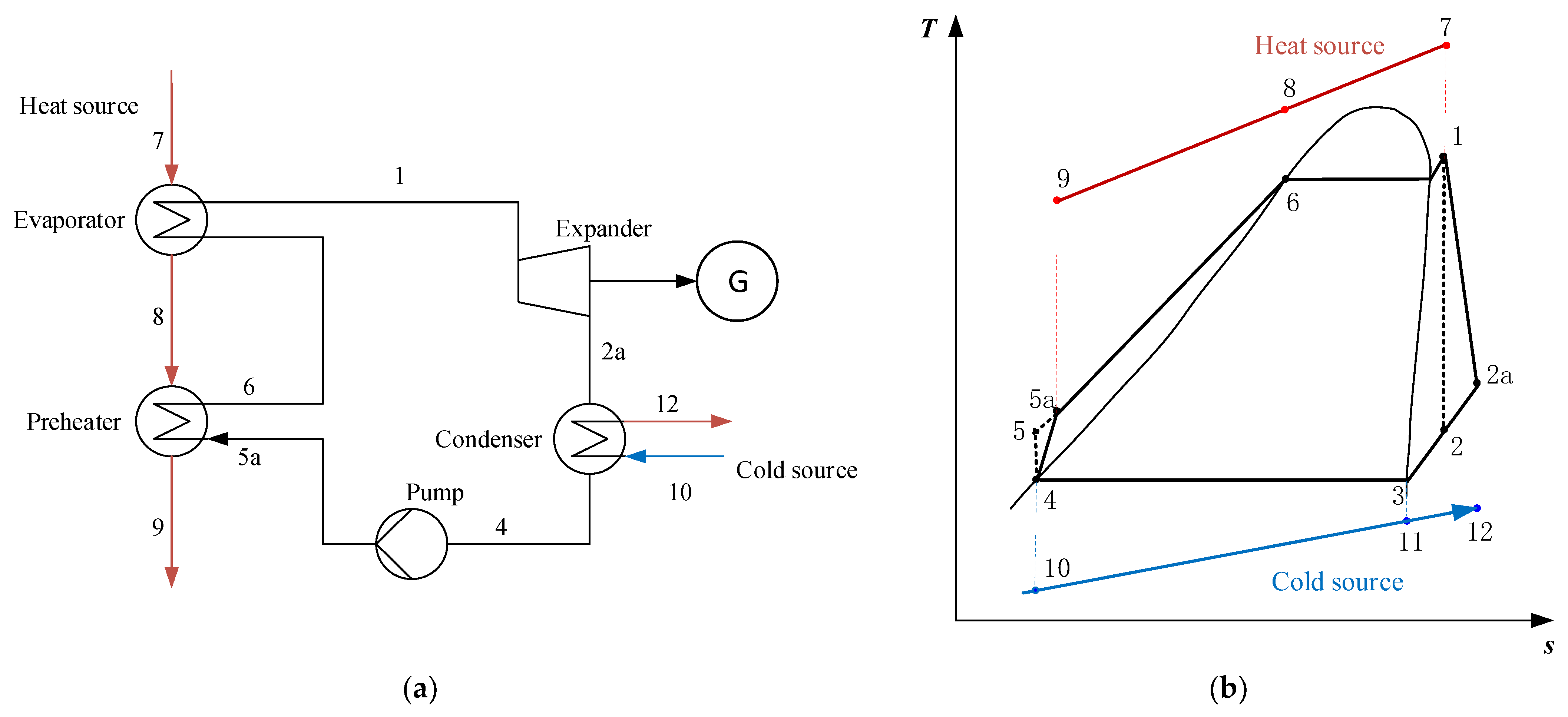
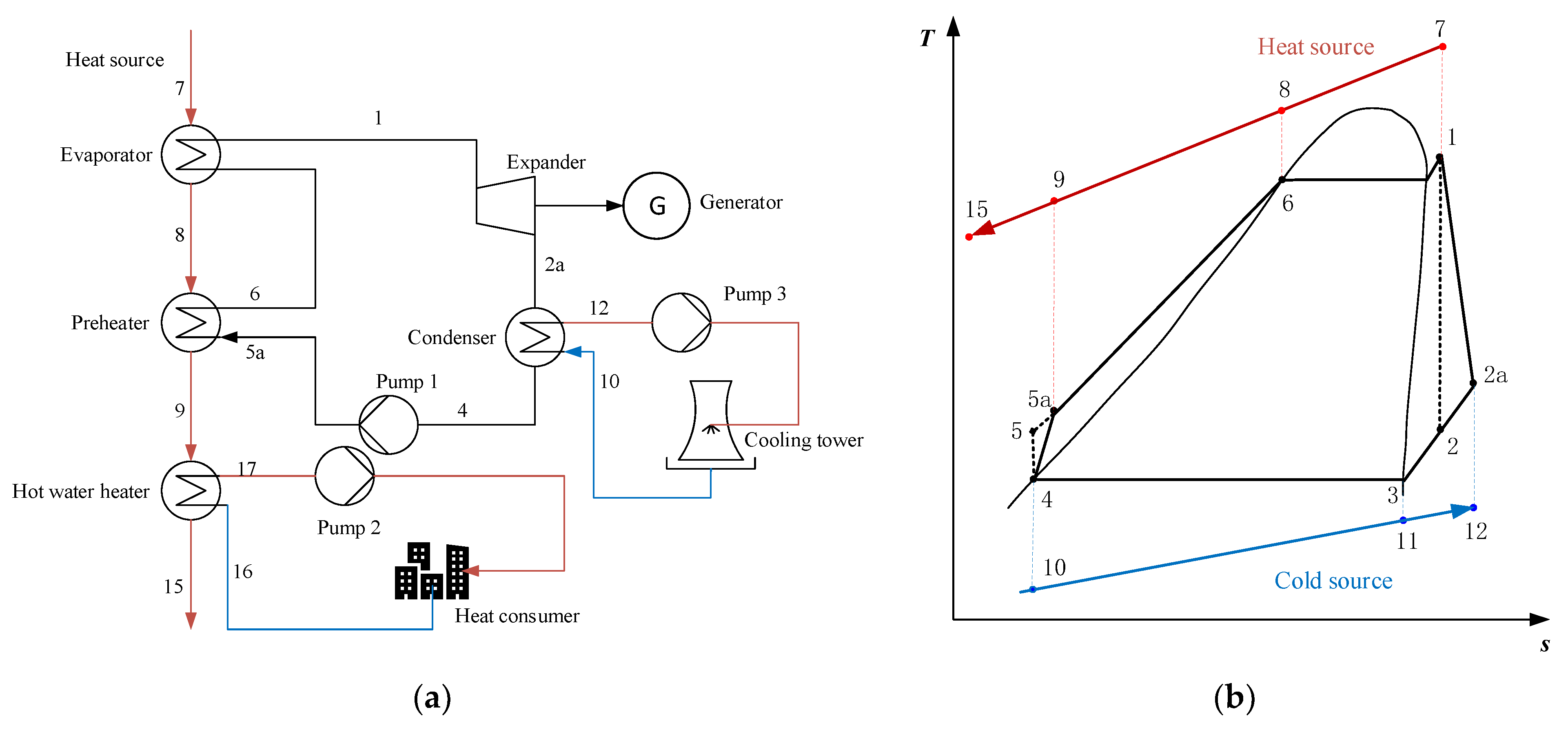
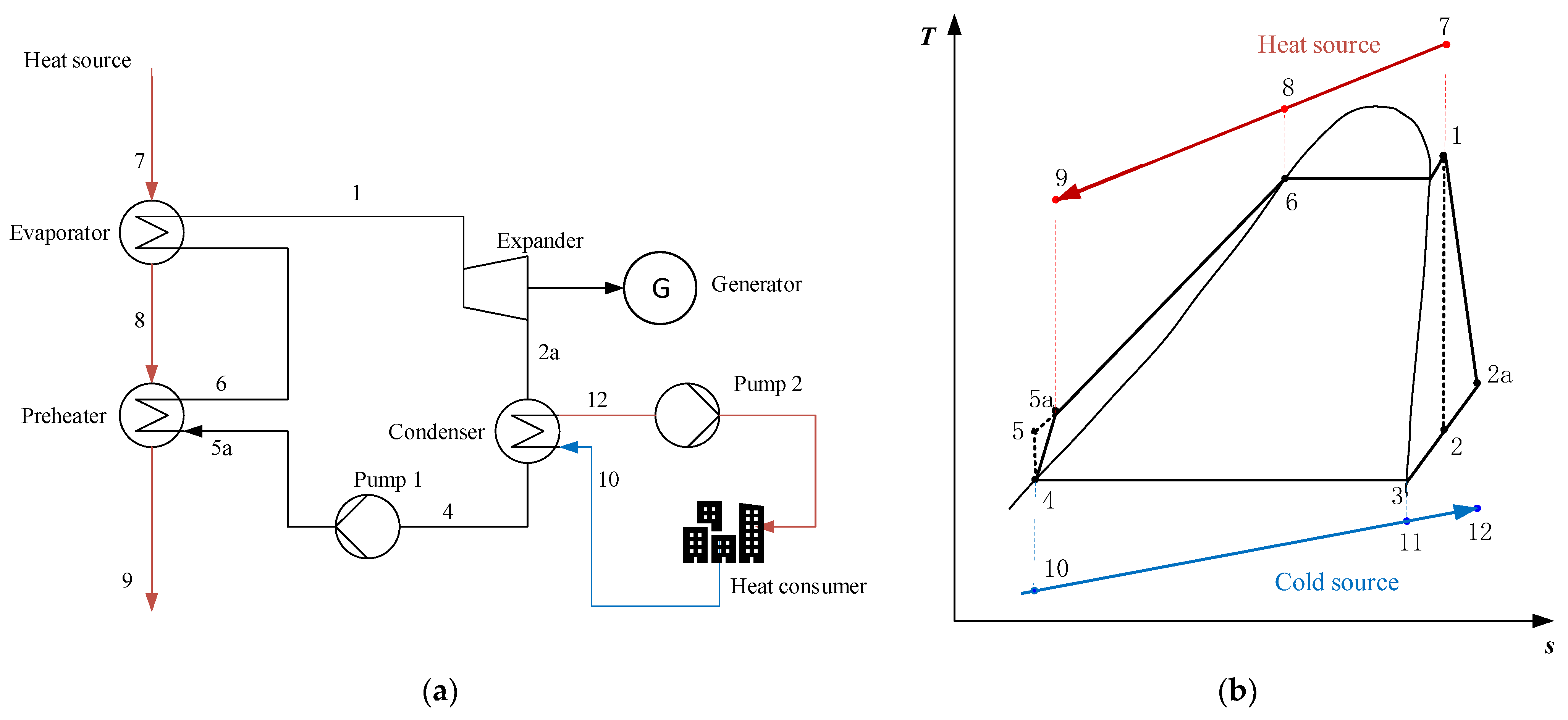
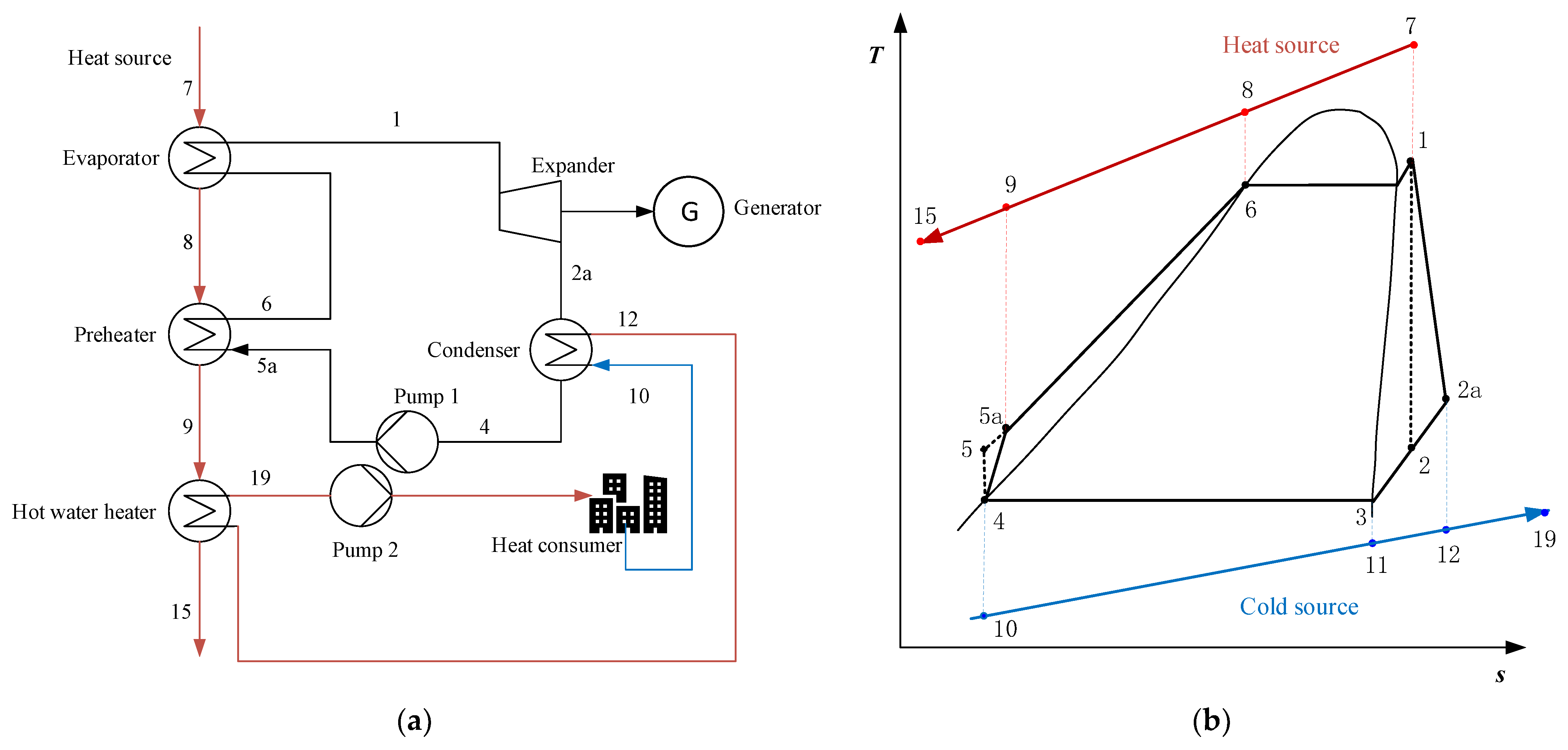
2.1.2. Serial System (SS)
2.1.3. Condensation System (CS)
2.1.4. Serial/Condensation System (SS/CS)
2.2. Mathematical Methods
2.2.1. Selection of Working Fluid
2.2.2. Exergy Analysis
2.2.3. Economic Analysis
2.3. Optimization Method
2.3.1. Multi-Objectives Optimization
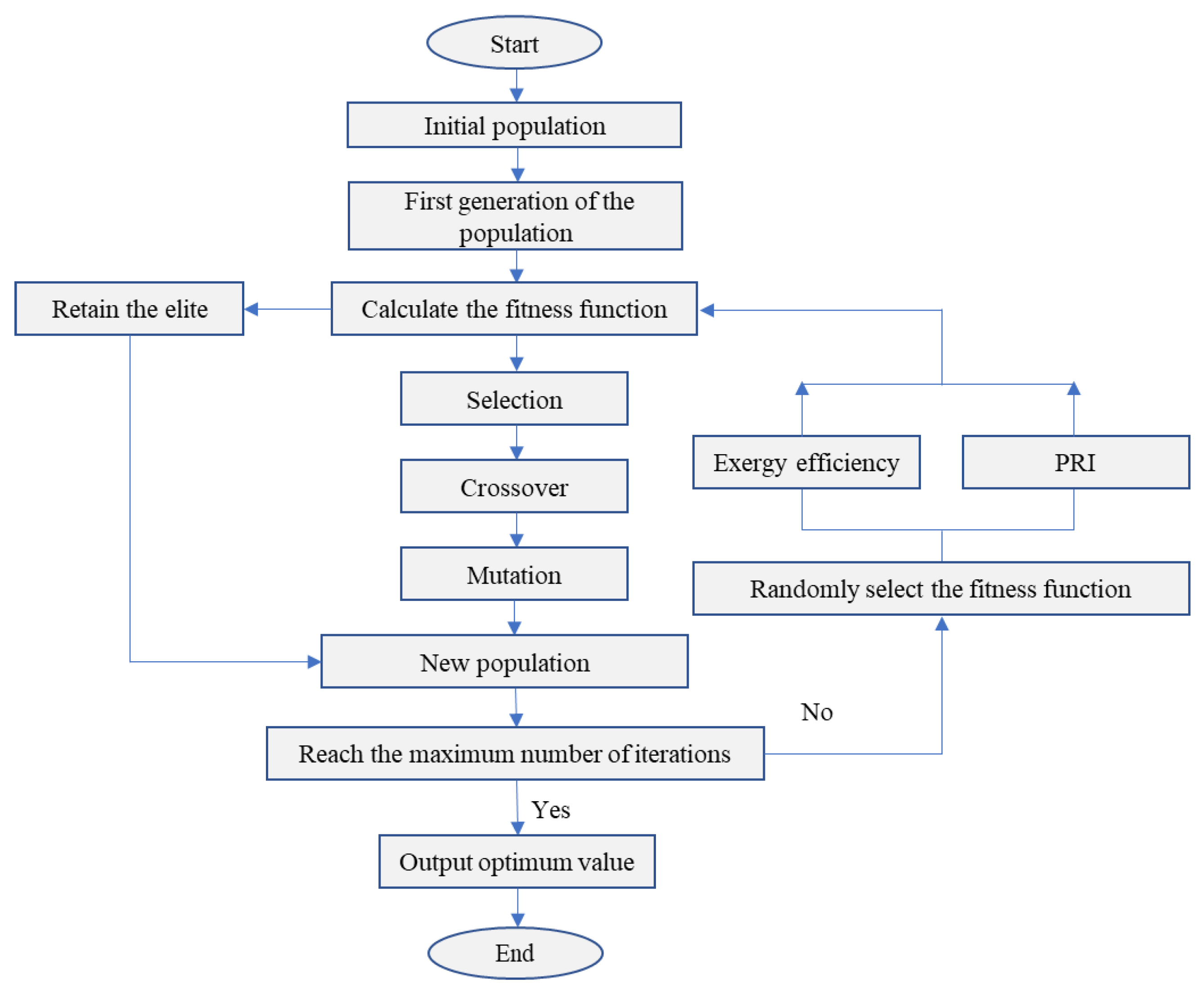
2.3.2. Multi-Objective Genetic Algorithm
3. Results and Discussion
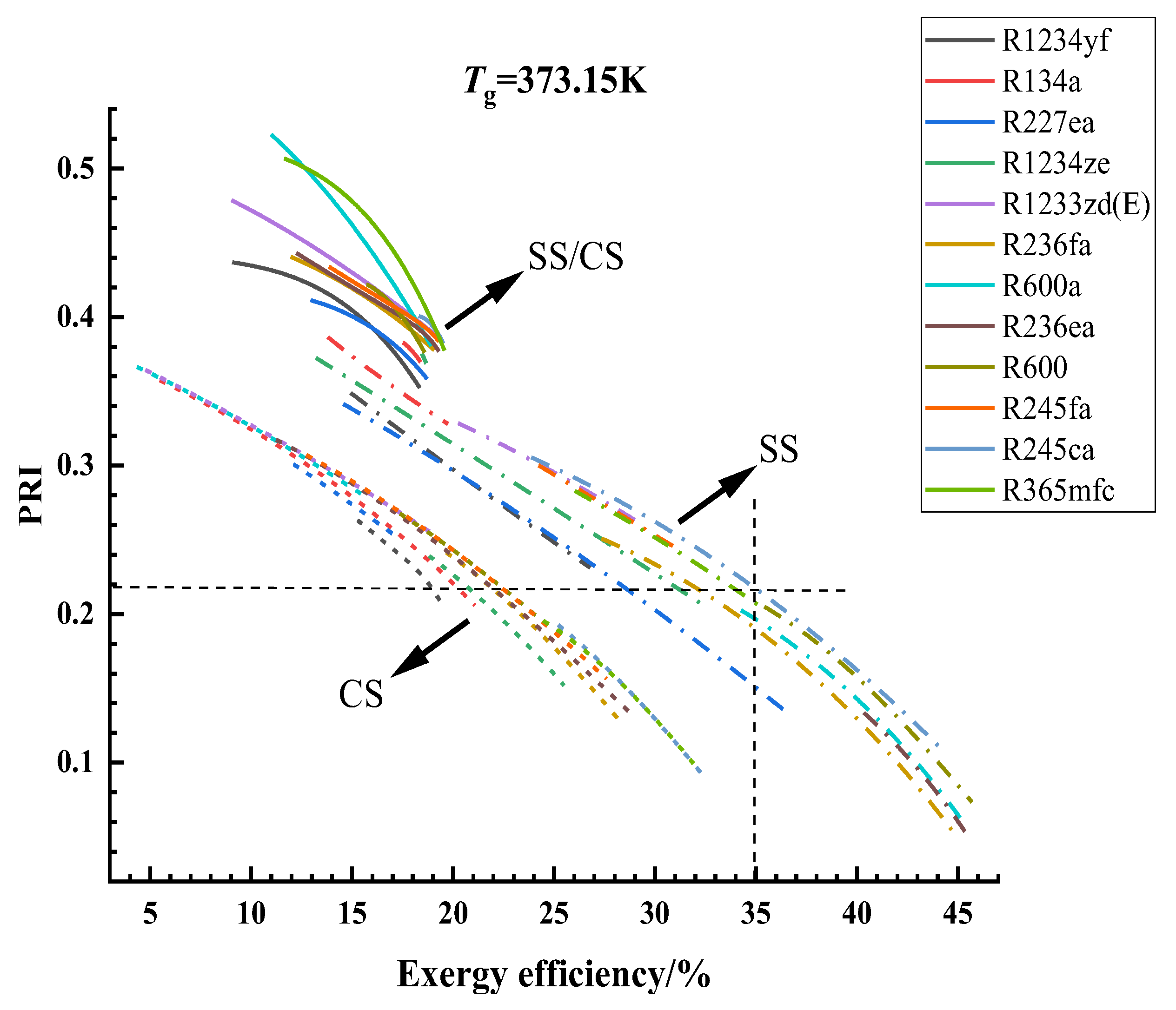
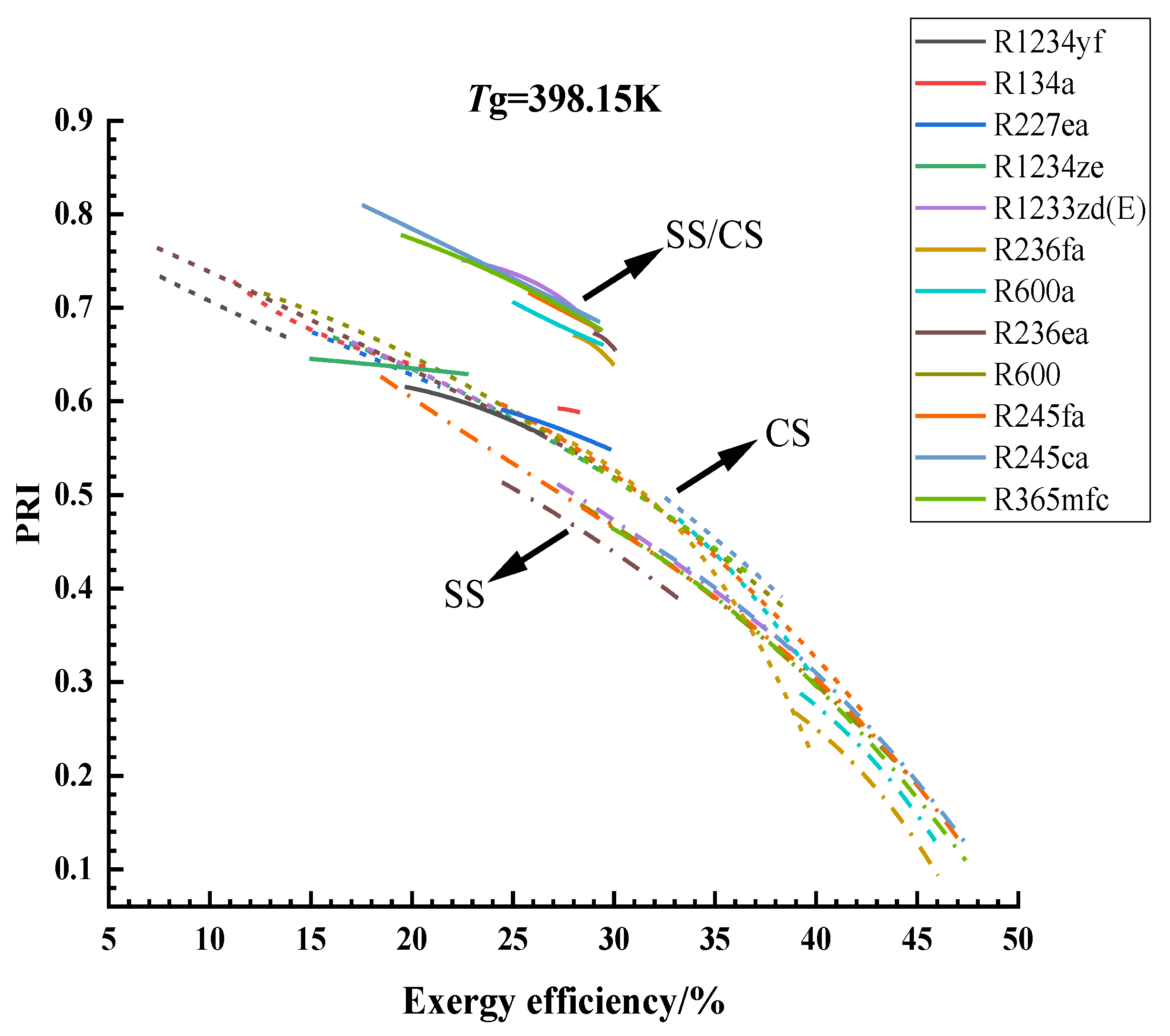
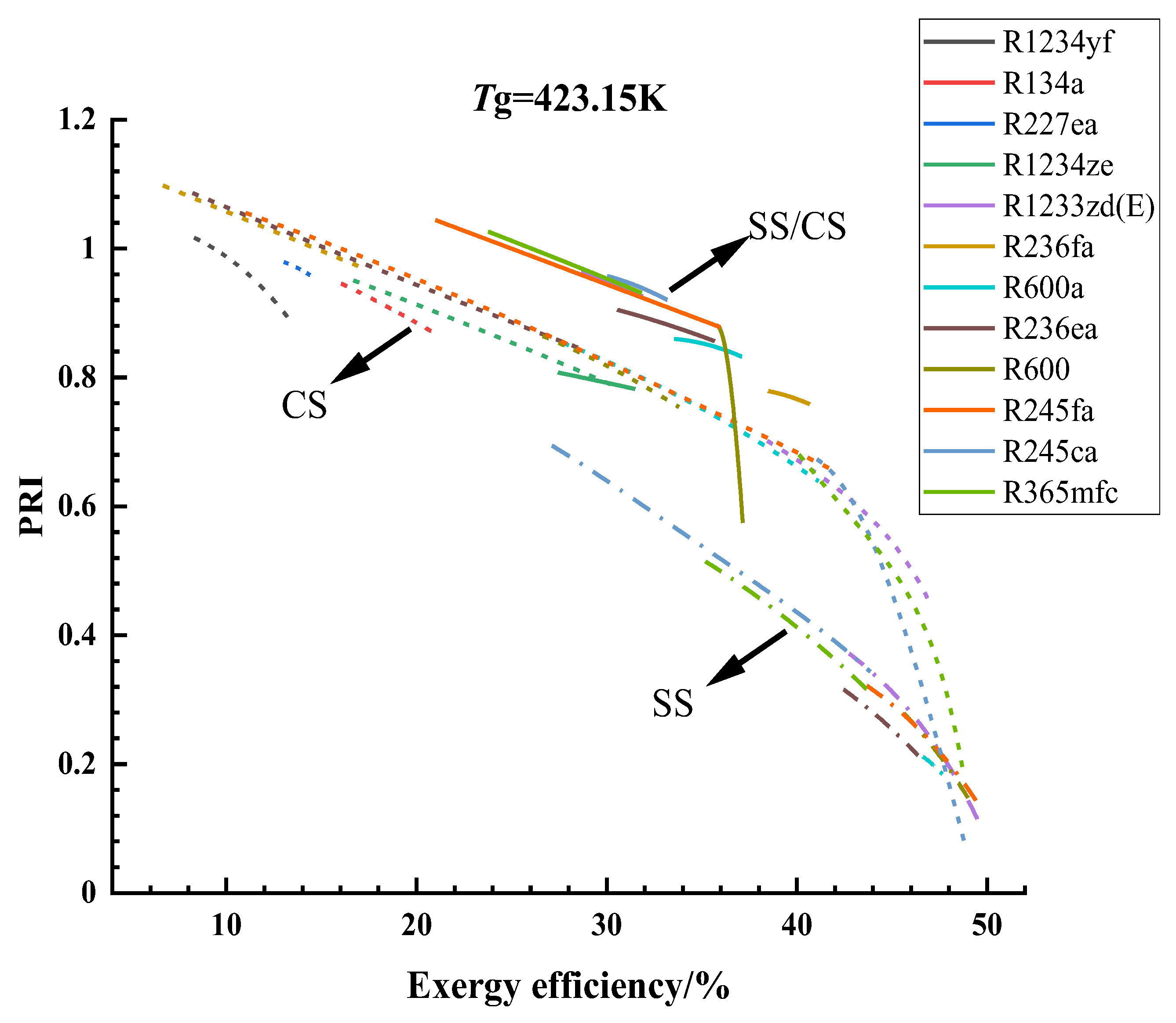
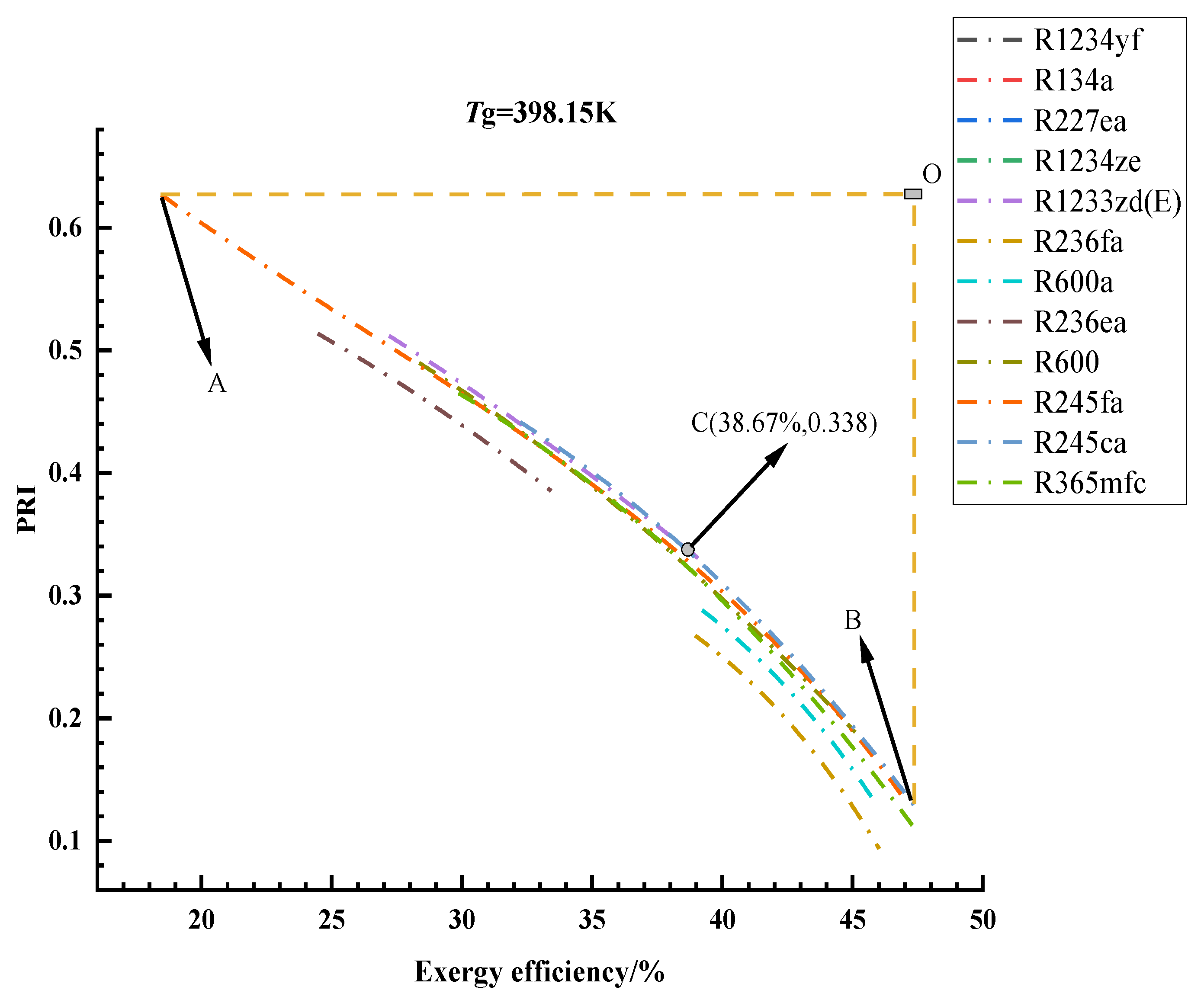
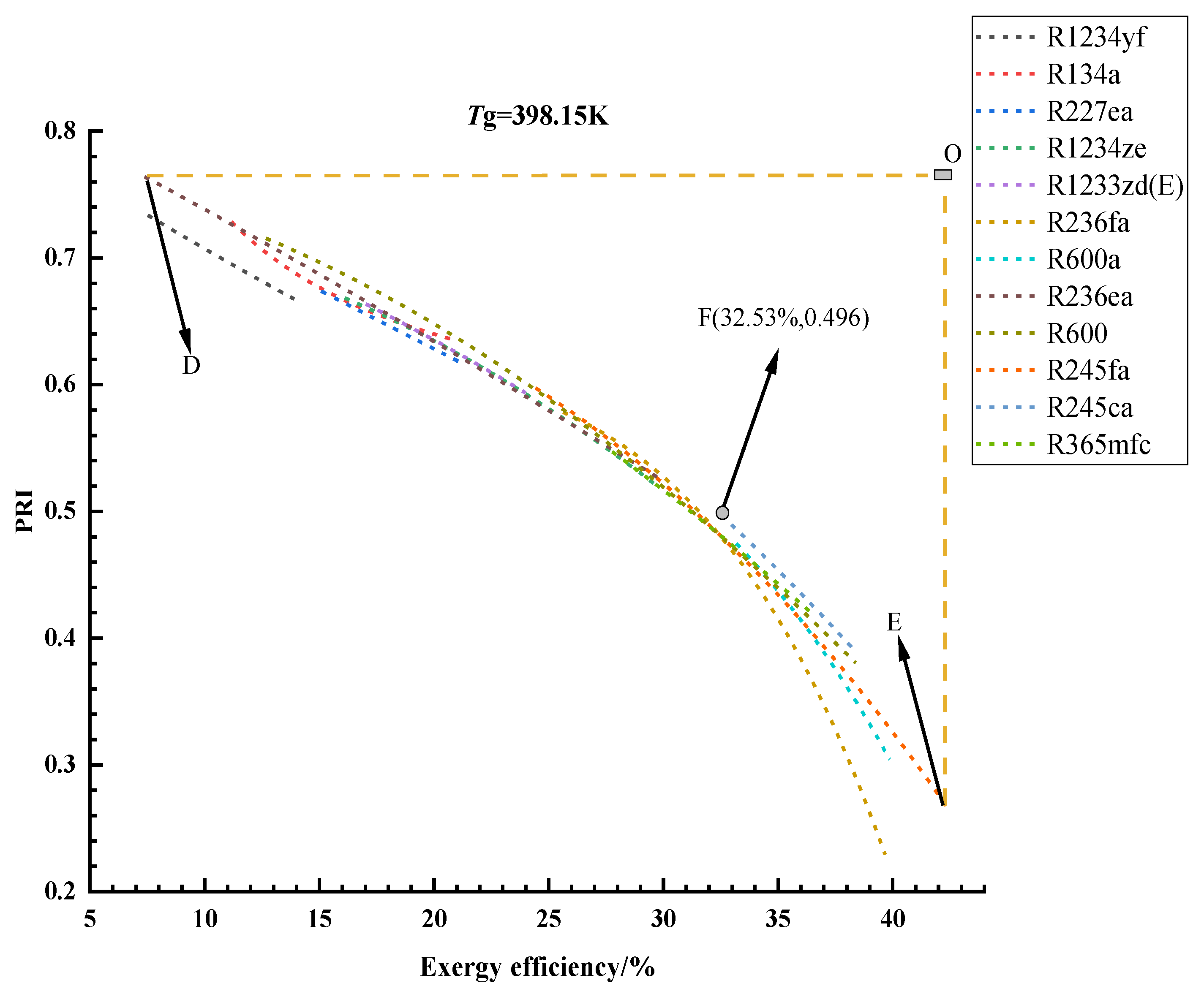
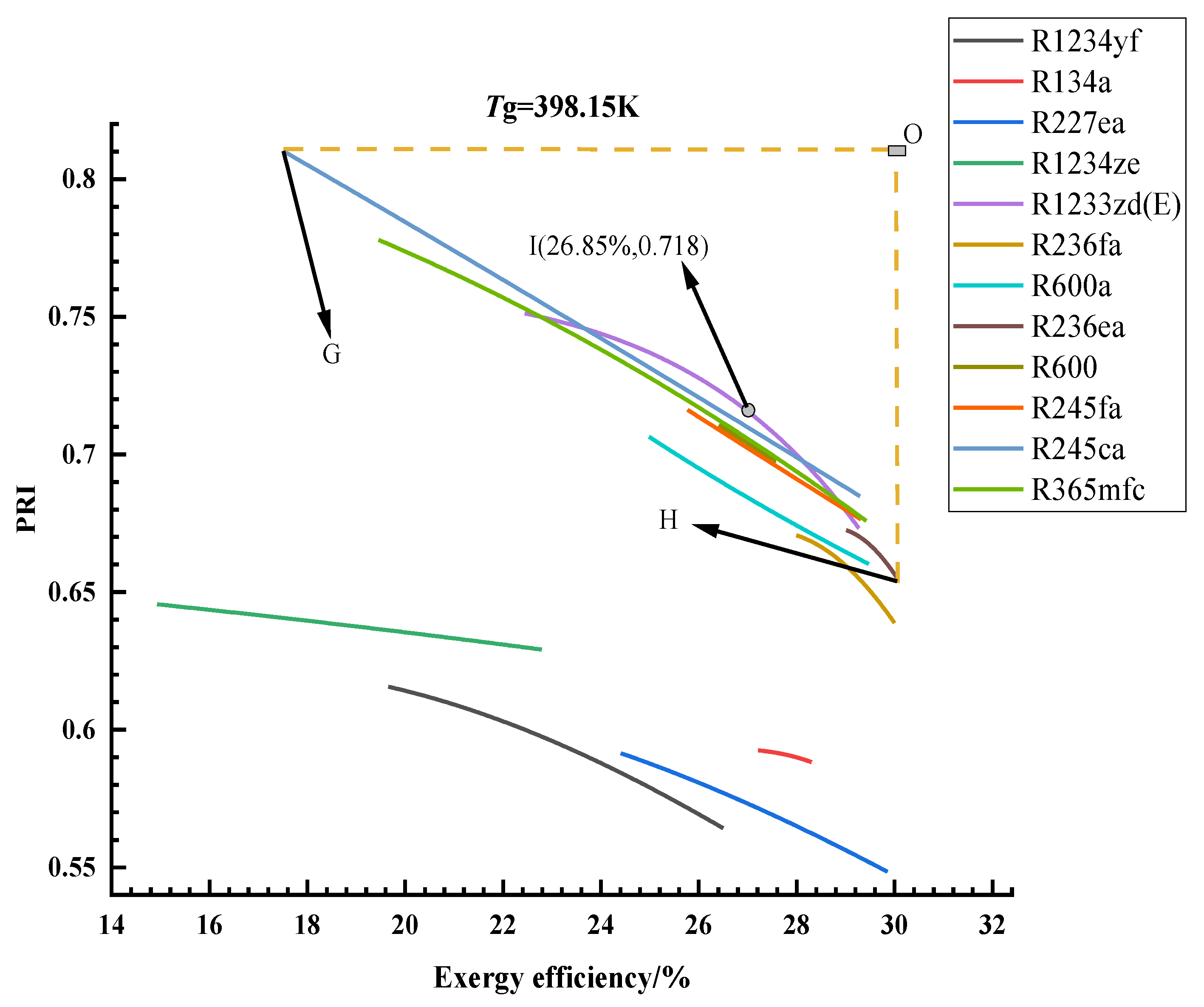
3.1. Optimization Results and Analysis
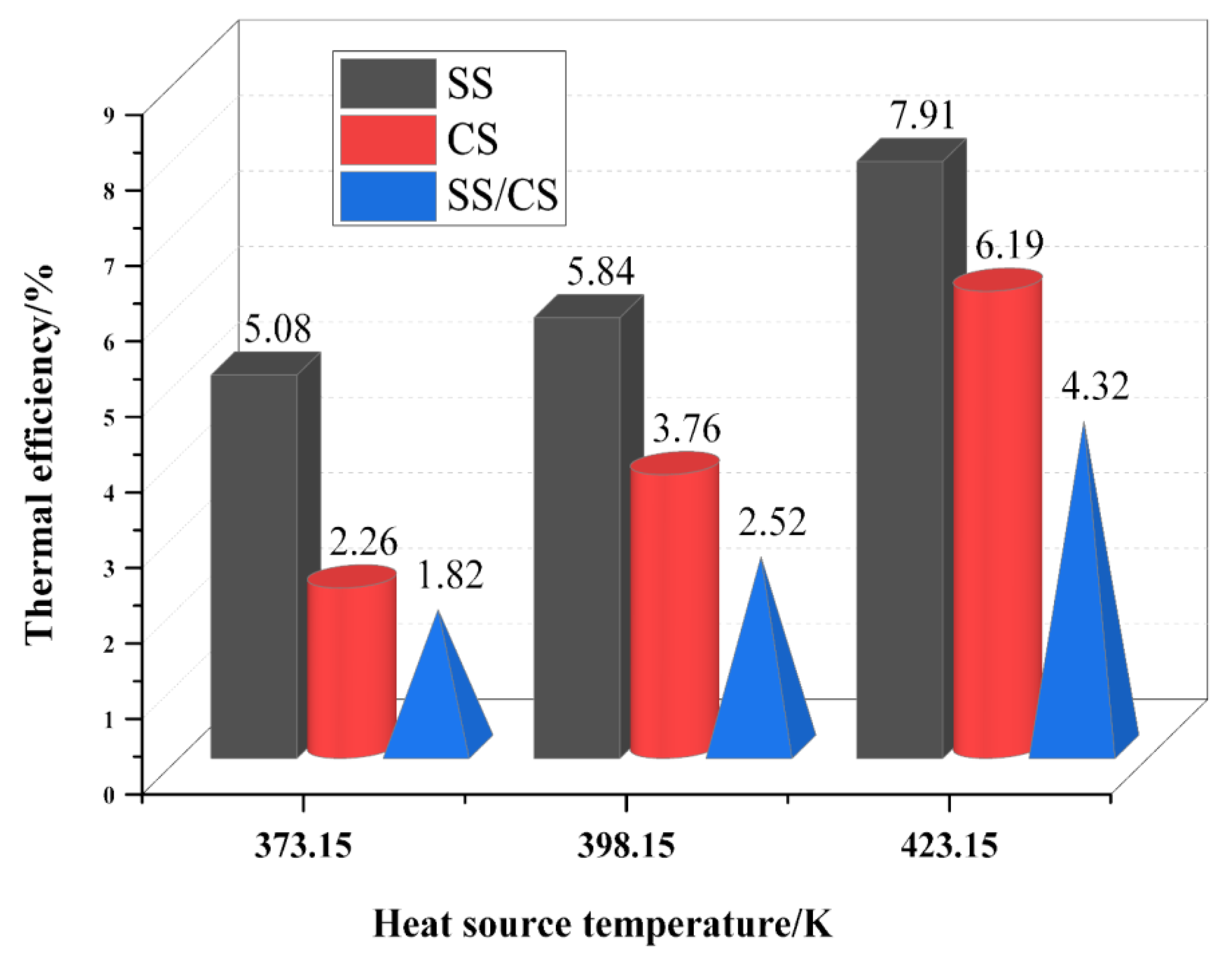
3.2. Thermodynamic Cycle Efficiencies of the Optimal Solutions
3.3. Exergy Analysis of the Optimal Solutions
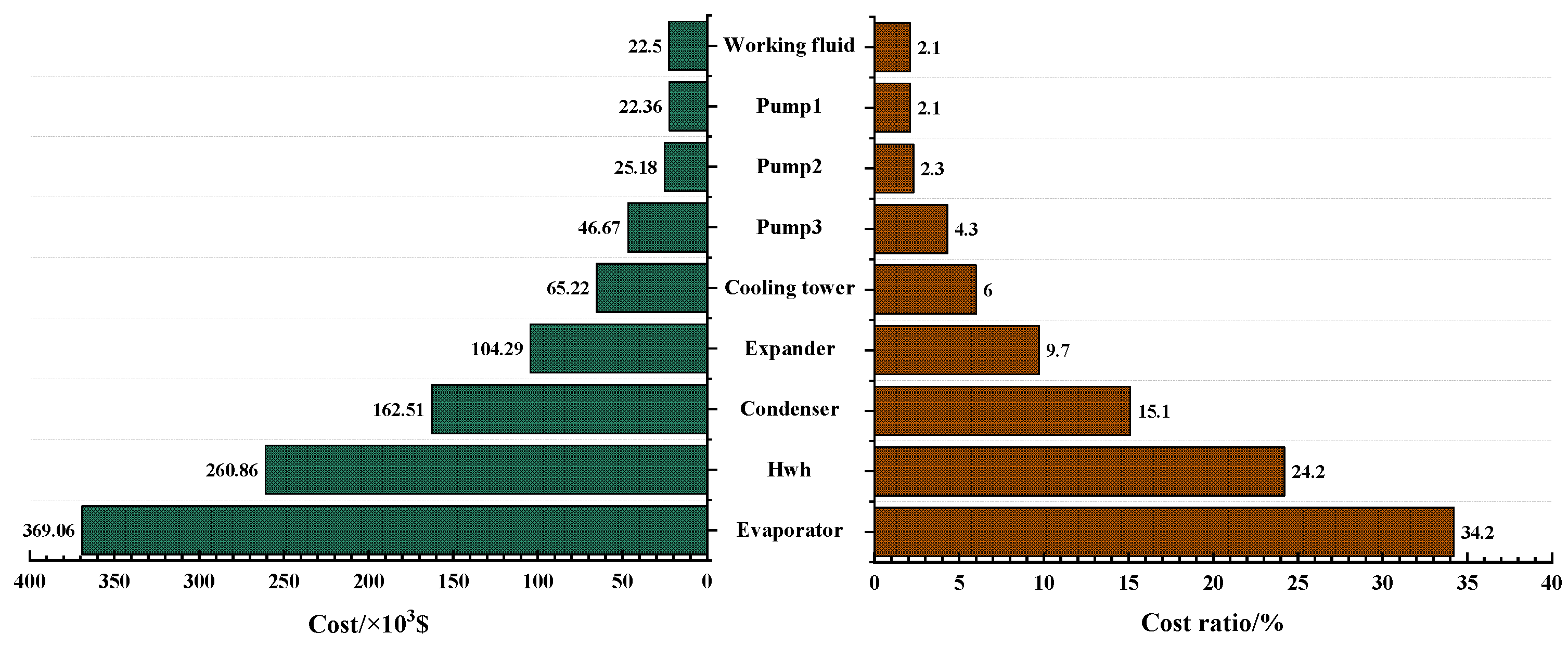
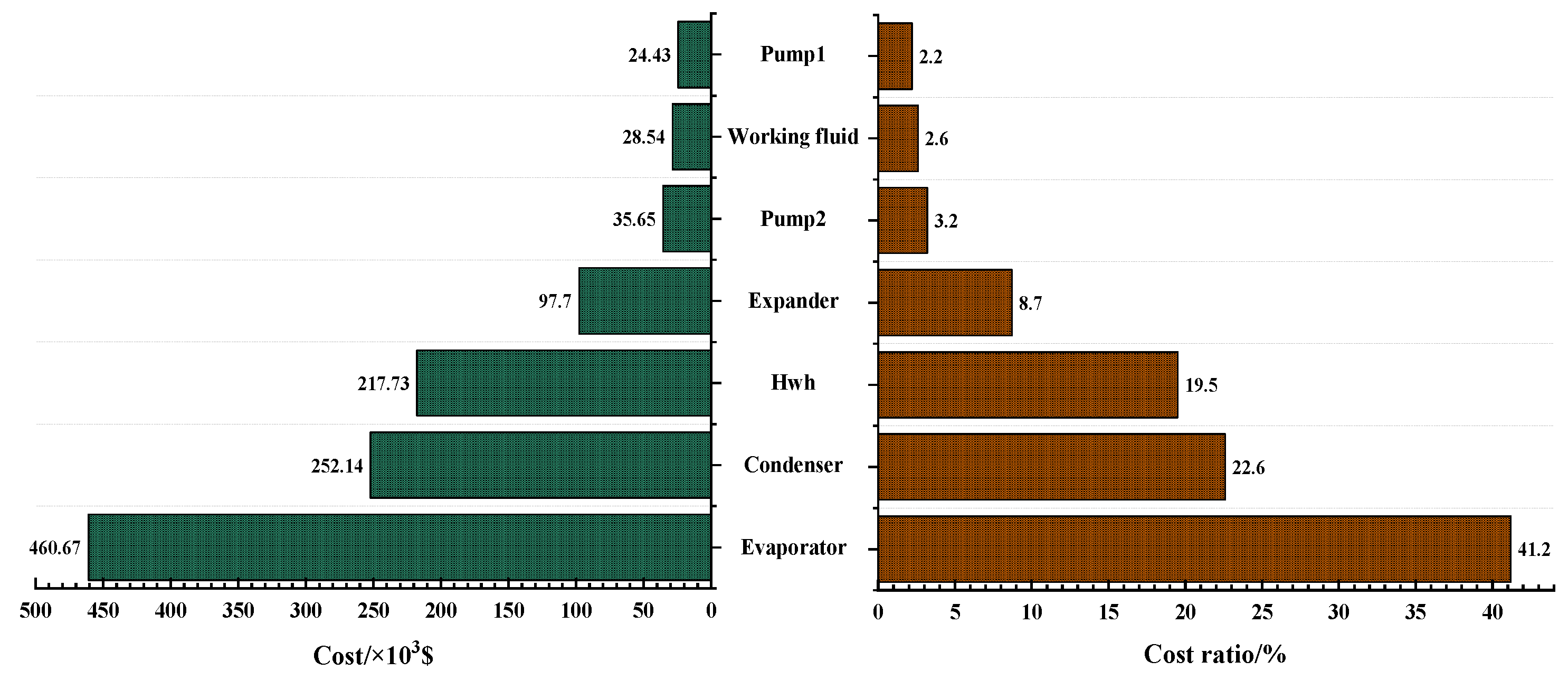
3.4. Economic Analysis of the Optimal Solutions
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, A.; Prasher, R.; Majumdar, A. Five thermal energy grand challenges for decarbonization. Nat. Energy 2020, 5, 635–637. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Y.; Wang, X.; He, Z.; Hung, T.; Wang, Q.; Xi, H. Performance prediction and optimization of an organic Rankine cycle (ORC) for waste heat recovery using back propagation neural network. Energy Convers. Manag. 2020, 226, 113552. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhou, D.; Sun, K. Key issues and novel optimization approaches of industrial waste heat recovery in district heating systems. Energy 2019, 188, 116005. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, G.; Li, L.; Yang, Q.; Tang, B.; Liu, Y. Expansion devices for organic Rankine cycle (ORC) using in low temperature heat recovery: A review. Energy Convers. Manag. 2019, 199, 111944. [Google Scholar] [CrossRef]
- Usman, M.; Imran, M.; Yang, Y.; Lee, D.H.; Park, B. Thermo-economic comparison of air-cooled and cooling tower based Organic Rankine Cycle (ORC) with R245fa and R1233zde as candidate working fluids for different geographical climate conditions. Energy 2017, 123, 353–366. [Google Scholar] [CrossRef]
- Li, T.; Meng, N.; Liu, J.; Zhu, J.; Kong, X. Thermodynamic and economic evaluation of the organic Rankine cycle (ORC) and two-stage series organic Rankine cycle (TSORC) for flue gas heat recovery. Energy Convers. Manag. 2019, 183, 816–829. [Google Scholar] [CrossRef]
- Bin Wan Ramli, W.R.; Pesyridis, A.; Gohil, D.; Alshammari, F. Organic Rankine Cycle Waste Heat Recovery for Passenger Hybrid Electric Vehicles. Energies 2020, 13, 4532. [Google Scholar] [CrossRef]
- Saleh, B.; Koglbauer, G.; Wendland, M.; Fischer, J. Working fluids for low-temperature organic Rankine cycles. Energy 2007, 32, 1210–1221. [Google Scholar] [CrossRef]
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste heat recovery technologies and applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
- Xia, X.X.; Wang, Z.Q.; Zhou, N.J.; Hu, Y.H.; Zhang, J.P.; Chen, Y. Working fluid selection of dual-loop organic Rankine cycle using multi-objective optimization and improved grey relational analysis. Appl. Therm. Eng. 2020, 171, 115028. [Google Scholar] [CrossRef]
- Xi, H.; Li, M.; He, Y.; Zhang, Y. Economical evaluation and optimization of organic Rankine cycle with mixture working fluids using R245fa as flame retardant. Appl. Therm. Eng. 2017, 113, 1056–1070. [Google Scholar] [CrossRef]
- Xi, H.; Li, M.; He, Y.; Tao, W. A graphical criterion for working fluid selection and thermodynamic system comparison in waste heat recovery. Appl. Therm. Eng. 2015, 89, 772–782. [Google Scholar] [CrossRef]
- Xi, H.; Zhang, H.; He, Y.; Huang, Z. Sensitivity analysis of operation parameters on the system performance of organic rankine cycle system using orthogonal experiment. Energy 2019, 172, 435–442. [Google Scholar] [CrossRef]
- Xi, H.; He, Y.; Wang, J.; Huang, Z. Transient response of waste heat recovery system for hydrogen production and other renewable energy utilization. Int. J. Hydrog. Energy 2019, 44, 15985–15996. [Google Scholar] [CrossRef]
- Xi, H.; Li, M.; Zhang, H.; He, Y. Experimental studies of organic Rankine cycle systems using scroll expanders with different suction volumes. J. Clean Prod. 2019, 218, 241–249. [Google Scholar] [CrossRef]
- Oyewunmi, O.A.; Kirmse, C.J.W.; Pantaleo, A.M.; Markides, C.N. Performance of working-fluid mixtures in ORC-CHP systems for different heat-demand segments and heat-recovery temperature levels. Energy Convers. Manag. 2017, 148, 1508–1524. [Google Scholar] [CrossRef]
- Eyerer, S.; Dawo, F.; Wieland, C.; Spliethoff, H. Advanced ORC architecture for geothermal combined heat and power generation. Energy 2020, 205, 117967. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Li, J.; Li, H.; Wang, Y.; Li, S. Thermodynamic analysis and economic assessment of biomass-fired organic Rankine cycle combined heat and power system integrated with CO2 capture. Energy Convers. Manag. 2020, 204, 112310. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, W.; He, Z. Thermodynamic analysis and optimization of a novel organic Rankine cycle-based micro-scale cogeneration system using biomass fuel. Energy Convers. Manag. 2019, 198, 111803. [Google Scholar] [CrossRef]
- Xi, H.; Li, M.; Huang, Z.; Wang, M. Energy, exergy and economic analyses and performance assessment of a trigeneration system for power, freshwater and heat based on supercritical water oxidation and organic Rankine cycle. Energy Convers. Manag. 2021, 243, 114395. [Google Scholar] [CrossRef]
- Van Erdeweghe, S.; Van Bael, J.; Laenen, B.; D’Haeseleer, W. Optimal configuration for a low-temperature geothermal CHP plant based on thermoeconomic optimization. Energy 2019, 179, 323–335. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, J.; Gao, L. Parametric optimization and comparative study of organic Rankine cycle (ORC) for low grade waste heat recovery. Energy Convers. Manag. 2009, 50, 576–582. [Google Scholar] [CrossRef]
- Lecompte, S.; Oyewunmi, O.A.; Markides, C.N.; Lazova, M.; Kaya, A.; Van den Broek, M.; De Paepe, M. Case Study of an Organic Rankine Cycle (ORC) for Waste Heat Recovery from an Electric Arc Furnace (EAF). Energies 2017, 10, 649. [Google Scholar] [CrossRef]
- Zhao, D.; Deng, S.; Zhao, L.; Xu, W.; Wang, W.; Nie, X.; Chen, M. Overview on artificial intelligence in design of Organic Rankine Cycle. Energy AI 2020, 1, 100011. [Google Scholar] [CrossRef]
- Muhammad, U.; Imran, M.; Lee, D.H.; Park, B.S. Design and experimental investigation of a 1kW organic Rankine cycle system using R245fa as working fluid for low-grade waste heat recovery from steam. Energy Convers. Manag. 2015, 103, 1089–1100. [Google Scholar] [CrossRef]
- Khennich, M.; Galanis, N.; Sorin, M. Comparison of combined heat and power systems using an organic Rankine cycle and a low-temperature heat source. Int. J. Low-Carbon Tec. 2013, 8 (Suppl. S1), i42–i46. [Google Scholar] [CrossRef] [Green Version]
- Couvreur, K.; Beyne, W.; De Paepe, M.; Lecompte, S. Hot water storage for increased electricity production with organic Rankine cycle from intermittent residual heat sources in the steel industry. Energy 2020, 200, 117501. [Google Scholar] [CrossRef]
- Garg, P.; Kumar, P.; Srinivasan, K.; Dutta, P. Evaluation of isopentane, R-245fa and their mixtures as working fluids for organic Rankine cycles. Appl. Therm. Eng. 2013, 51, 292–300. [Google Scholar] [CrossRef]
- Xi, H.; Li, M.; Xu, C.; He, Y. Parametric optimization of regenerative organic Rankine cycle (ORC) for low grade waste heat recovery using genetic algorithm. Energy 2013, 58, 473–482. [Google Scholar] [CrossRef]
- Darvish, K.; Ehyaei, M.A.; Atabi, F.; Rosen, M.A. Selection of Optimum Working Fluid for Organic Rankine Cycles by Exergy and Exergy-Economic Analyses. Sustainability 2015, 7, 15362–15383. [Google Scholar] [CrossRef] [Green Version]
- Kolasiński, P. The Method of the Working Fluid Selection for Organic Rankine Cycle (ORC) Systems Employing Volumetric Expanders. Energies 2020, 13, 573. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Torres, A.M.; García-Rodríguez, L. Preliminary assessment of solar organic Rankine cycles for driving a desalination system. Desalination 2007, 216, 252–275. [Google Scholar] [CrossRef]
- Velders, G.J.M.; Andersen, S.O.; Daniel, J.S.; Fahey, D.W.; McFarland, M. The importance of the Montreal Protocol in protecting climate. Proc. Natl. Acad. Sci. USA 2007, 104, 4814–4819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dincer, I.; Cengel, Y.A. Energy, Entropy and Exergy Concepts and Their Roles in Thermal Engineering. Entropy 2001, 3, 116–149. [Google Scholar] [CrossRef]
- Mignard, D. Correlating the chemical engineering plant cost index with macro-economic indicators. Chem. Eng. Res. Des. 2014, 92, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Toffolo, A.; Lazzaretto, A.; Manente, G.; Paci, M. A multi-criteria approach for the optimal selection of working fluid and design parameters in Organic Rankine Cycle systems. Appl. Energy 2014, 121, 219–232. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 9.1; National Institute of Standard Technology: Boulder, CO, USA, 2017.
- He, Z.; Yen, G.G. Many-objective evolutionary algorithm: Objective space reduction and diversity improvement. IEEE Trans. Evolut. Comput. 2015, 20, 145–160. [Google Scholar] [CrossRef]
- Zolpakar, N.A.; Lodhi, S.S.; Pathak, S.; Sharma, M.A. Application of Multi-objective Genetic Algorithm (MOGA) Optimization in Machining Processes. In Optimization of Manufacturing Processes; Gupta, K., Gupta, M.K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 185–199. [Google Scholar]
- Rayegan, R.; Tao, Y.X. A procedure to select working fluids for Solar Organic Rankine Cycles (ORCs). Renew. Energy 2011, 36, 659–670. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Wang, R.Z.; Yang, C. Perspectives for low-temperature waste heat recovery. Energy 2019, 176, 1037–1043. [Google Scholar] [CrossRef]
- Yari, M.; Mehr, A.S.; Zare, V.; Mahmoudi, S.M.S.; Rosen, M.A. Exergoeconomic comparison of TLC (trilateral Rankine cycle), ORC (organic Rankine cycle) and Kalina cycle using a low grade heat source. Energy 2015, 83, 712–722. [Google Scholar] [CrossRef]
- Santos, M.; André, J.; Costa, E.; Mendes, R.; Ribeiro, J. Design strategy for component and working fluid selection in a domestic micro-CHP ORC boiler. Appl. Therm. Eng. 2020, 169, 114945. [Google Scholar] [CrossRef]
- Yadav, K.; Sircar, A. Selection of working fluid for low enthalpy heat source Organic Rankine Cycle in Dholera, Gujarat, India. Case Stud. Therm. Eng. 2019, 16, 100553. [Google Scholar] [CrossRef]
- Tempesti, D.; Fiaschi, D. Thermo-economic assessment of a micro CHP system fuelled by geothermal and solar energy. Energy 2013, 58, 45–51. [Google Scholar] [CrossRef]
- Kai, Z.; Mi, Z.; Yabo, W.; Zhili, S.; Shengchun, L.; Jinghong, N. Parametric Optimization of Low Temperature ORC System. Energy Procedia 2015, 75, 1596–1602. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.H.; Zhang, H.G.; Fan, B.Y.; Ouyang, M.G.; Zhao, Y.; Mu, Q.H. Study of working fluid selection of organic Rankine cycle (ORC) for engine waste heat recovery. Energy 2011, 36, 3406–3418. [Google Scholar] [CrossRef]
- Mateu-Royo, C.; Mota-Babiloni, A.; Navarro-Esbrí, J.; Peris, B.; Molés, F.; Amat-Albuixech, M. Multi-objective optimization of a novel reversible High-Temperature Heat Pump-Organic Rankine Cycle (HTHP-ORC) for industrial low-grade waste heat recovery. Energy Convers. Manag. 2019, 197, 111908. [Google Scholar] [CrossRef]
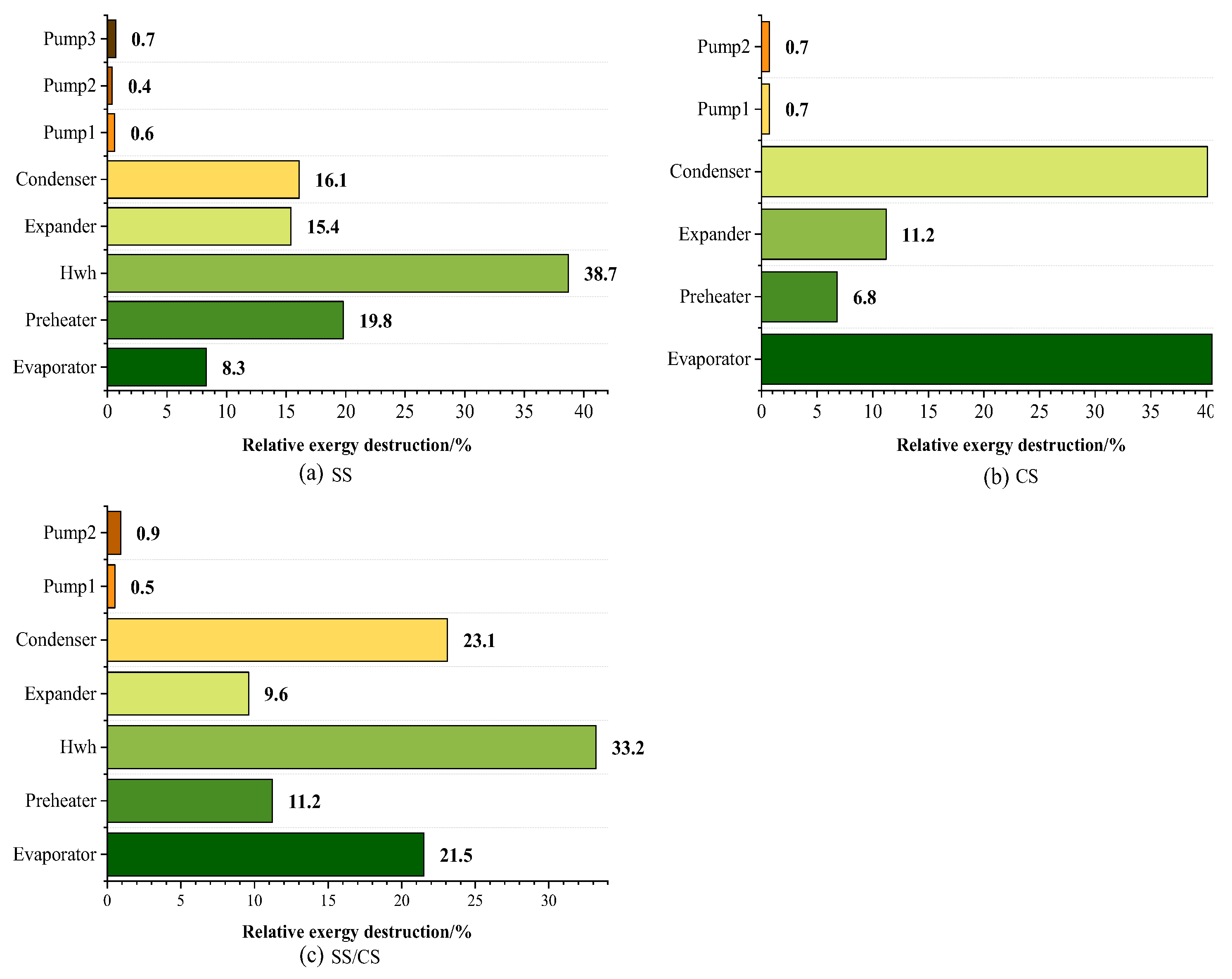
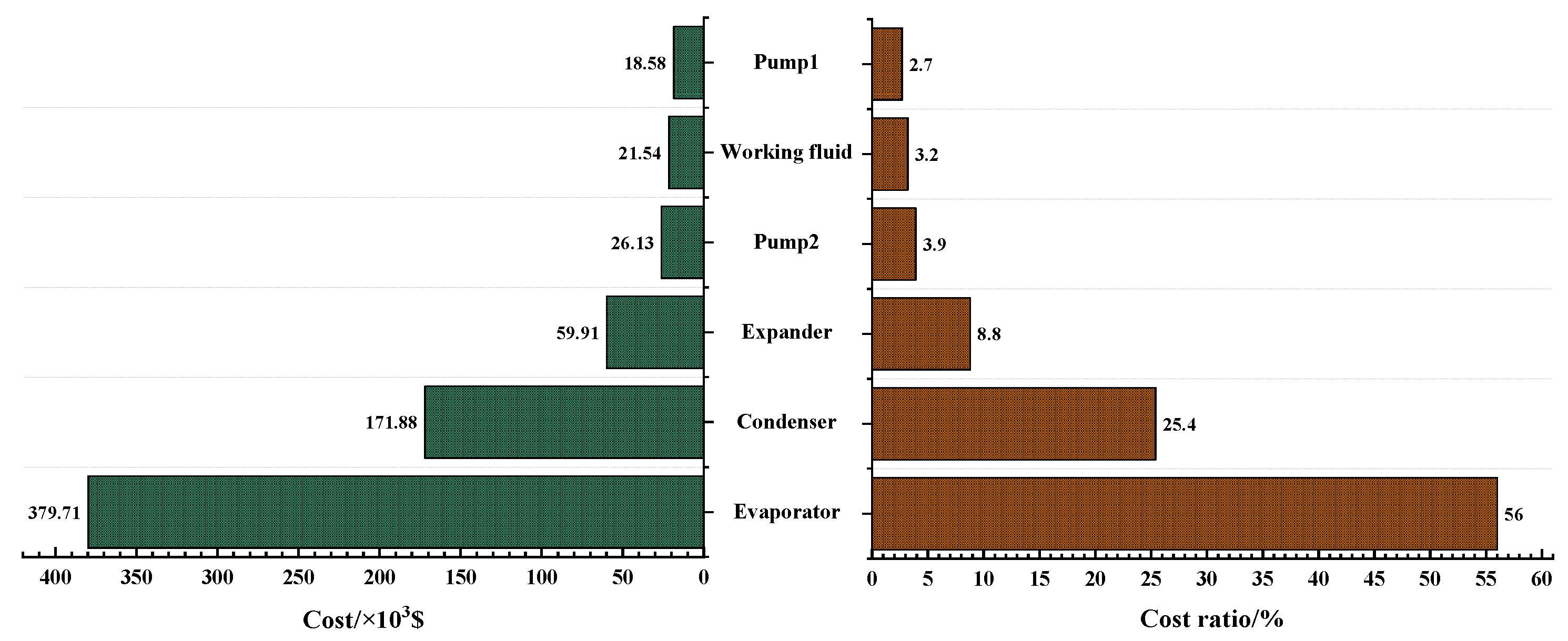

| Parameter (Unit) | Value | Reference |
|---|---|---|
| Heat source temperature (K) | 373.15/398.15/423.15 | [28] |
| Mass flow rate of the heat source (kg/s) | 14.0 | [29] |
| Specific heat at constant pressure for the hot gas (kJ·kg−1·K−1) | 1.1 | [29] |
| Supply temperature in heating network (K) | 343.15 | [26] |
| Return temperature in heating network (K) | 328.15 | [26] |
| Environment temperature (K) | 298.15 | - |
| Environment pressure (kPa) | 101.3 | - |
| Condensing temperature (SS) (K) | 303.15 | - |
| Condensing temperature (CS) (K) | 348.15 | - |
| Condensing temperature (SS/CS) (K) | 343.15 | - |
| Expander isentropic efficiency | 0.8 | [29] |
| Pump isentropic efficiency | 0.7 | [29] |
| Cooling water temperature (K) | 293.15 | - |
| Minimum temperature difference of heat exchangers (K) | 8 | [29] |
| No. | Working Fluid | M (kg/kmol) | |||
|---|---|---|---|---|---|
| 1 | R1234yf | 114.04 | 367.85 | 3.3822 | 475.55 |
| 2 | R134a | 102.03 | 374.21 | 4.0593 | 511.9 |
| 3 | R227ea | 170.03 | 374.9 | 2.925 | 594.25 |
| 4 | R1234ze | 114.04 | 382.52 | 3.6363 | 489.24 |
| 5 | R236fa | 152.04 | 398.07 | 3.2 | 551.29 |
| 6 | R600a | 58.122 | 407.81 | 3.6290 | 225.5 |
| 7 | R236ea | 152.04 | 412.44 | 3.502 | 563.0 |
| 8 | R600 | 58.122 | 425.13 | 3.796 | 228.0 |
| 9 | R245fa | 134.05 | 427.16 | 3.651 | 516.08 |
| 10 | R1233zd(E) | 130.5 | 438.75 | 3.5709 | 476.31 |
| 11 | R245ca | 134.05 | 447.57 | 3.925 | 523.6 |
| 12 | R365mfc | 148.07 | 460.0 | 3.266 | 473.84 |
| System | Total Exergy Loss |
|---|---|
| SS | |
| CS | |
| SS/CS |
| Parameter | Value |
|---|---|
| Population size | 200 |
| Crossover probability | 0.4 |
| Mutation probability | 0.2 |
| Number of elites | 20 |
| Stop generation | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, S.; Feng, Y.-Q.; Hung, T.-C.; Xi, H. Multi-Objective Optimization and Fluid Selection of Different Cogeneration of Heat and Power Systems Based on Organic Rankine Cycle. Energies 2021, 14, 4967. https://doi.org/10.3390/en14164967
Teng S, Feng Y-Q, Hung T-C, Xi H. Multi-Objective Optimization and Fluid Selection of Different Cogeneration of Heat and Power Systems Based on Organic Rankine Cycle. Energies. 2021; 14(16):4967. https://doi.org/10.3390/en14164967
Chicago/Turabian StyleTeng, Shiyang, Yong-Qiang Feng, Tzu-Chen Hung, and Huan Xi. 2021. "Multi-Objective Optimization and Fluid Selection of Different Cogeneration of Heat and Power Systems Based on Organic Rankine Cycle" Energies 14, no. 16: 4967. https://doi.org/10.3390/en14164967
APA StyleTeng, S., Feng, Y.-Q., Hung, T.-C., & Xi, H. (2021). Multi-Objective Optimization and Fluid Selection of Different Cogeneration of Heat and Power Systems Based on Organic Rankine Cycle. Energies, 14(16), 4967. https://doi.org/10.3390/en14164967









