Energetic Value of Elymus elongatus L. and Zea mays L. Grown on Soil Polluted with Ni2+, Co2+, Cd2+, and Sensitivity of Rhizospheric Bacteria to Heavy Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Material Characteristics
2.2. Experimental Design
2.3. Resistance of Elymus elongatus L. and Zea mays L. to Heavy Metals and Analysis of Their Energetic Yield
2.4. Microbiological Analysis of Soil
- (1)
- The microbial colony development index (CD) acc. to Sarathchandra et al. [55]
- (2)
- The microbial ecophysiological diversity index (EP) acc. to De Leij et al. [56]
- (3)
- The resistance (RS) of soil microorganisms to pollution with Ni2+, Co2+, and Cd2+ acc. to the formula described by Orwin and Wardle [49]
- (4)
- The influence of heavy metals (IFHm) on counts of soil microorganisms acc. to the following formula
2.5. Metagenomic Soil Analysis
2.6. Statistical Analysis
3. Results
3.1. Sensitivity of Test Plants to Toxic Effects of Ni2+, Co2+, and Cd2+ and Their Energetic Value
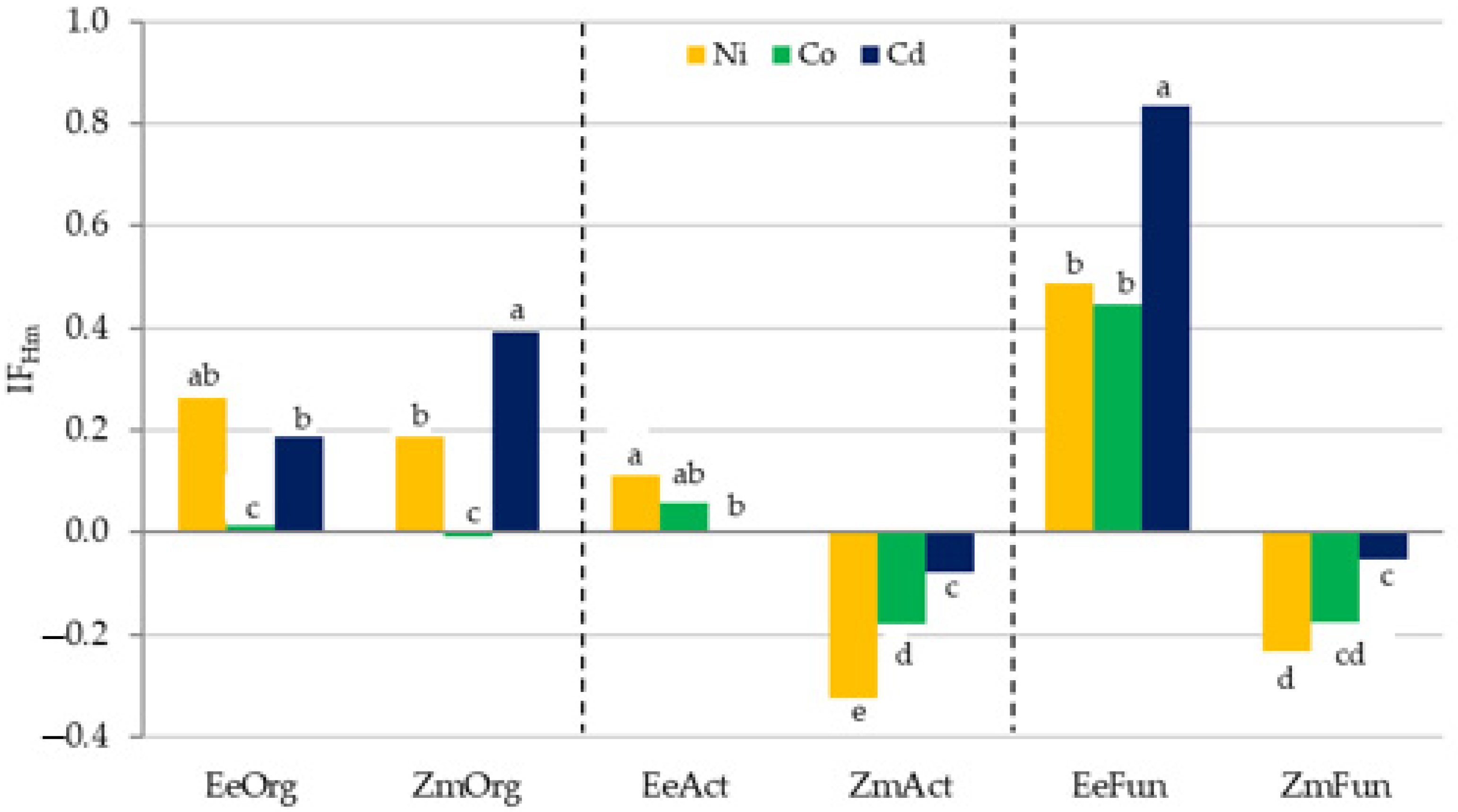
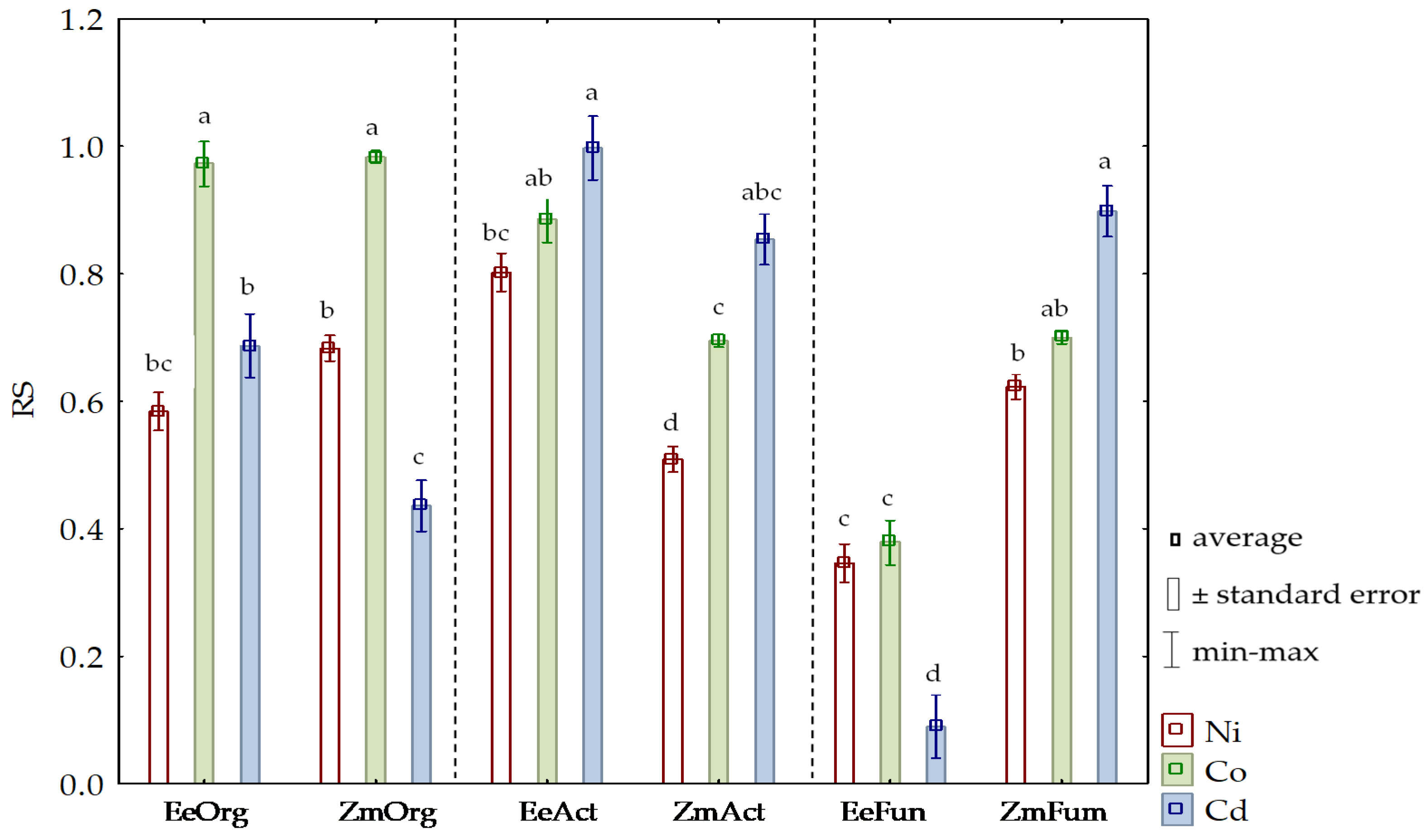
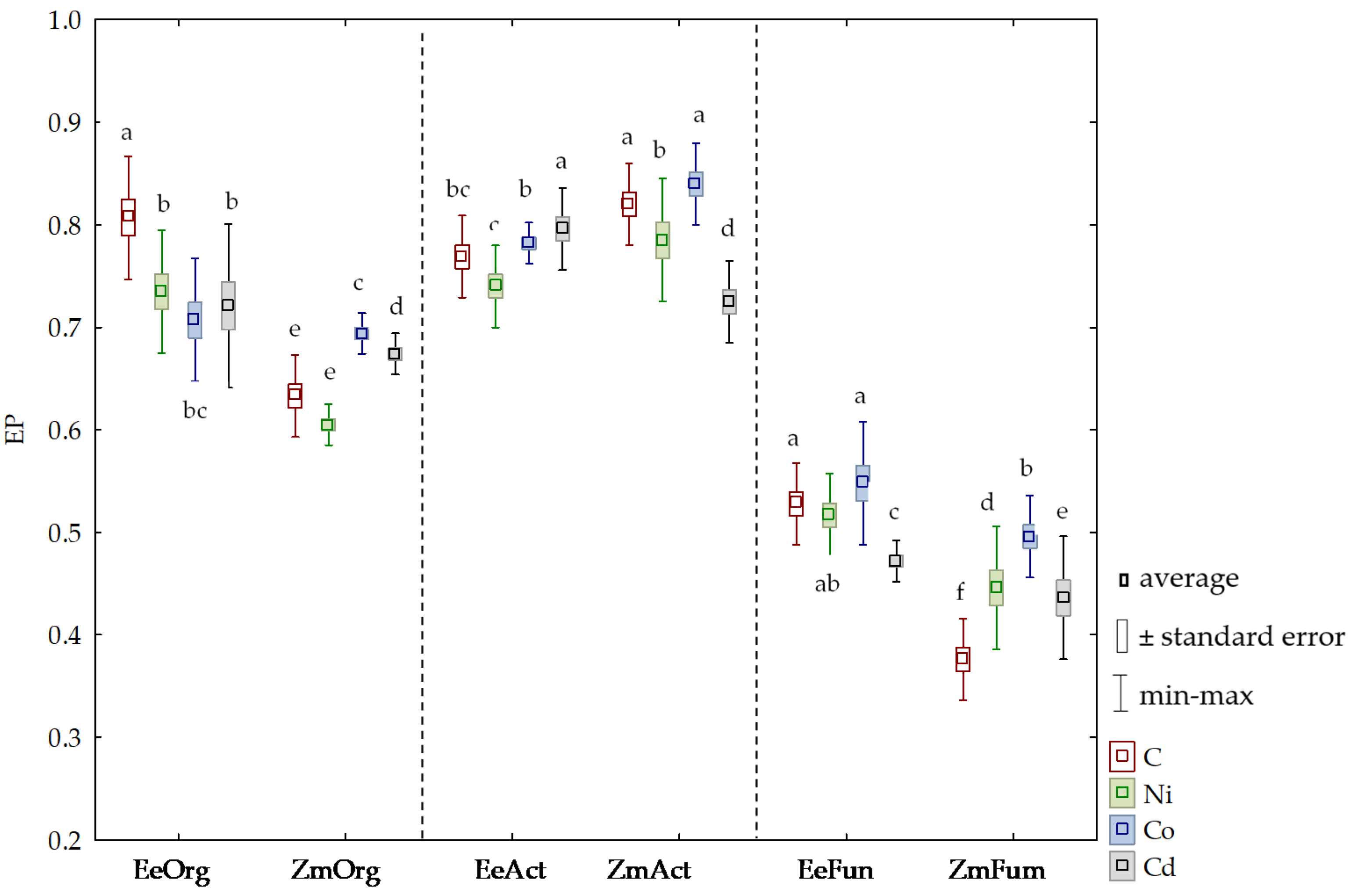
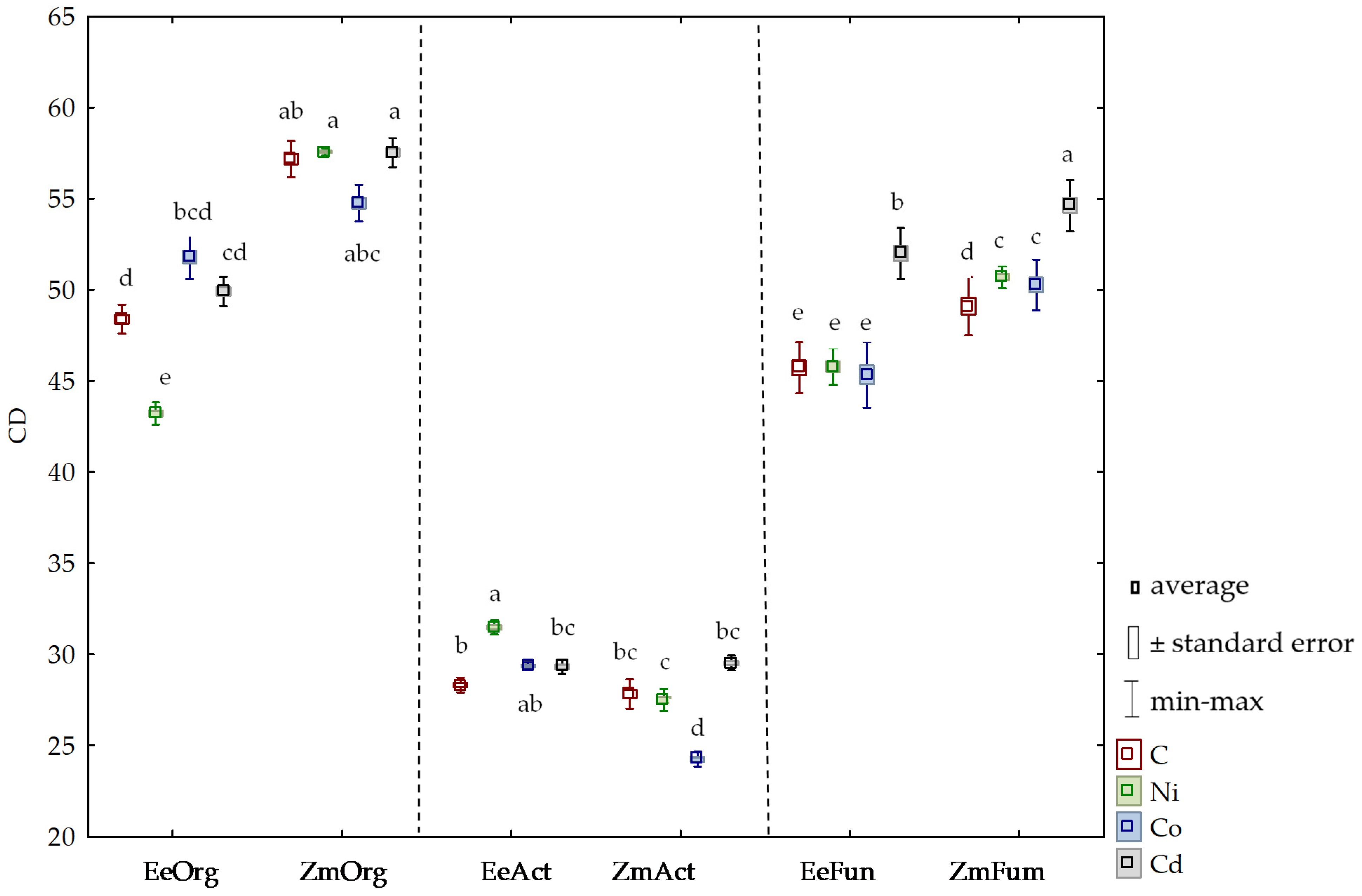
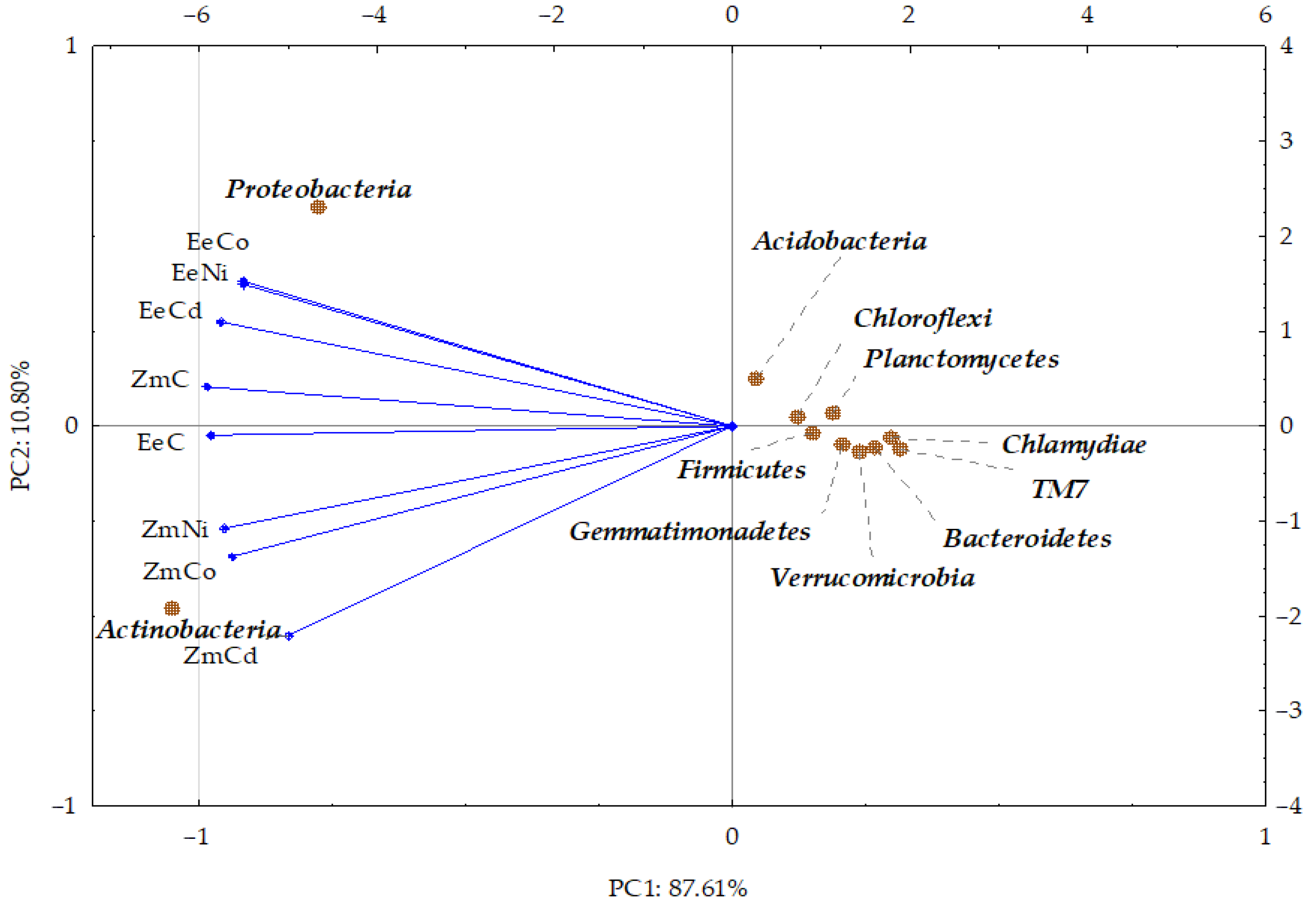
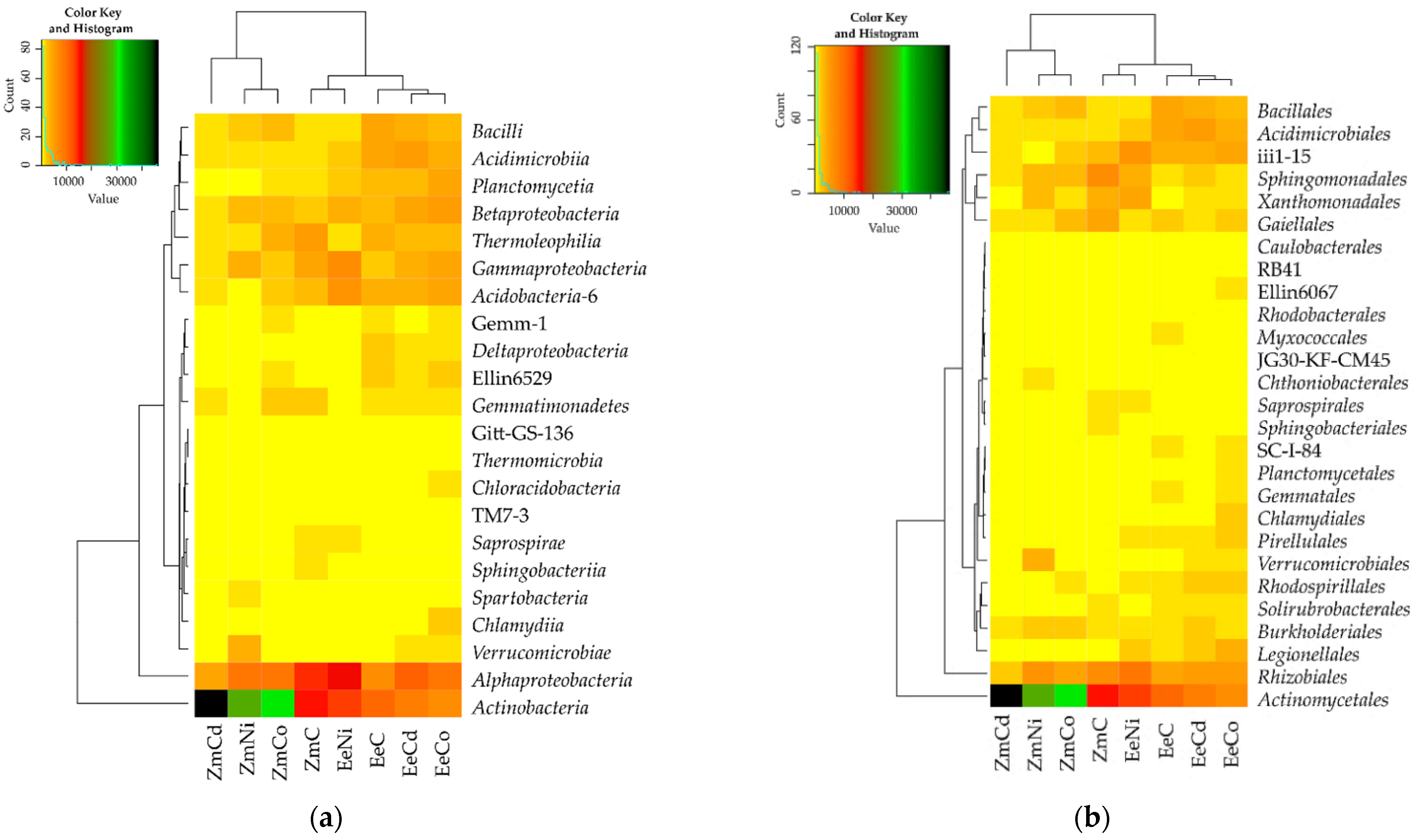
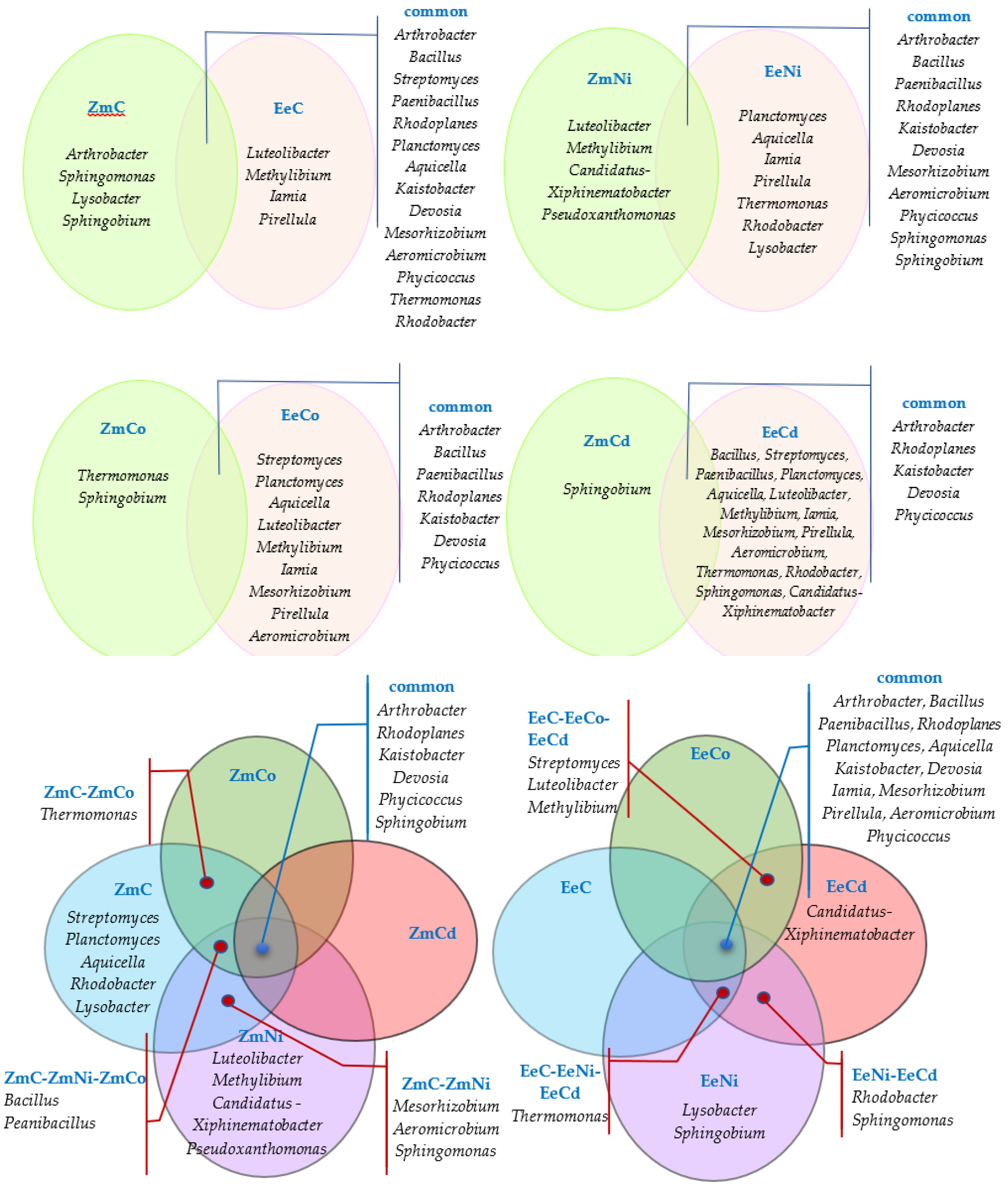
3.2. Sensitivity of Soil Microorganisms to Toxic Effects of Ni2+, Co2+, and Cd2+
4. Discussion
4.1. Sensitivity of Test Plants to the Effects of Ni2+, Co2+, and Cd2+ and Their Energetic Value
4.2. Sensitivity of Soil Microorganisms to the Effects of Ni2+, Co2+, and Cd2+
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Hewedy, O.A.; Lateif, K.S.A.; Seleiman, M.F.; Shami, A.; Albarakaty, F.M.; El-Meihy, R.M. Phylogenetic Diversity of Trichoderma Strains and Their Antagonistic Potential against Soil-Borne Pathogens under Stress Conditions. Biology 2020, 9, 189. [Google Scholar] [CrossRef]
- Ponomarova, O.; Patil, K.R. Metabolic interactions in microbial communities: Untangling the Gordian knot. Curr. Opin. Microbiol. 2015, 27, 37–44. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Borowik, A.; Kucharski, J. The Response of the Soil Microbiome to Contamination with Cadmium, Cobalt and Nickel in Soil Sown with Brassica napus. Minerals 2021, 11, 498. [Google Scholar] [CrossRef]
- Fiałkowski, K.; Kacprzak, M.; Grobelak, A.; Placek, A. The influence of selected soil parameters on the mobility of heavy metals in soils. Eng. Prot. Environ. 2012, 15, 81–92. [Google Scholar]
- Li, M.; Mohamed, I.; Raleve, D.; Chen, W.; Huang, Q. Retracted article: Field evaluation of intensive compost application on Cd fractionation and phytoavailability in a mining-contaminated soil. Environ. Geochem. Health 2015, 38, 1193–1201. [Google Scholar] [CrossRef]
- Rajkumar, M.; Sandhya, S.; Prasad, M.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef]
- Salem, H.M.; Abdel-Salam, A.; Abdel-Salam, M.A.; Seleiman, M.F. Phytoremediation of metal and metalloids from contami-nated soil. In Plants under Metal and Metalloid Stress; Springer: Singapore, 2018; pp. 249–262. [Google Scholar]
- De-Bashan, L.E.; Hernandez, J.; Nelson, K.N.; Bashan, Y.; Maier, R.M. Growth of Quailbush in Acidic, Metalliferous Desert Mine Tailings: Effect of Azospirillum brasilense Sp6 on Biomass Production and Rhizosphere Community Structure. Microb. Ecol. 2010, 60, 915–927. [Google Scholar] [CrossRef]
- Gómez-Sagasti, M.T.; Alkorta, I.; Becerril, J.; Epelde, L.; Anza, M.; Garbisu, C. Microbial Monitoring of the Recovery of Soil Quality During Heavy Metal Phytoremediation. Water Air Soil Pollut. 2012, 223, 3249–3262. [Google Scholar] [CrossRef]
- Pandey, V.C. Invasive species based efficient green technology for phytoremediation of fly ash deposits. J. Geochem. Explor. 2012, 123, 13–18. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Lu, B.R. Meiotic studies of Elymus nutans and E. jacquemontii (Poaceae, Triticeae) and their hybrids with Pseudoroegneria spicata and seventeen Elymus species. Plant Syst. Evol. 1993, 186, 193–211. [Google Scholar] [CrossRef]
- Ma, X.; Chen, S.; Zhang, X.; Bai, S.; Zhang, C. Assessment of Worldwide Genetic Diversity of Siberian Wild Rye (Elymus sibiricus L.) Germplasm Based on Gliadin Analysis. Molecules 2012, 17, 4424–4434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xie, W.; Zhang, J.; Zhao, X.; Zhao, Y.; Wang, Y. Phenotype- and SSR-Based Estimates of Genetic Variation between and within Two Important Elymus Species in Western and Northern China. Genes 2018, 9, 147. [Google Scholar] [CrossRef]
- Yan, X.; Guo, Y.; Zhou, H.; Wang, K. Isozyme variability among Elymus species indigenous to the Tibetan and Inner Mongolian Plateaus. Grassl. Sci. 2007, 53, 91–96. [Google Scholar] [CrossRef]
- Agostini, F.; Gregory, A.; Richter, G.M. Carbon Sequestration by Perennial Energy Crops: Is the Jury Still Out? BioEnergy Res. 2015, 8, 1057–1080. [Google Scholar] [CrossRef] [PubMed]
- Di Nasso, N.N.; Angelini, L.; Bonari, E. Influence of fertilisation and harvest time on fuel quality of giant reed (Arundo donax L.) in central Italy. Eur. J. Agron. 2010, 32, 219–227. [Google Scholar] [CrossRef]
- Prochnow, A.; Heiermann, M.; Plöchl, M.; Linke, B.; Idler, C.; Amon, T.; Hobbs, P. Bioenergy from permanent grassland—A review: 1. Biogas. Bioresour. Technol. 2009, 100, 4931–4944. [Google Scholar] [CrossRef] [PubMed]
- Ciria, C.S.; Barro, R.; Sanz, M.; Ciria, P. Long-Term Yield and Quality Performance of Perennial Energy Grasses (Agropyron spp.) on Marginal Land. Agronomy 2020, 10, 1051. [Google Scholar] [CrossRef]
- Gillespie, G.D.; Farrelly, D.J.; Everard, C.D.; McDonnell, K. The use of near infrared hyperspectral imaging for the prediction of processing parameters associated with the pelleting of biomass feedstocks. Fuel Process. Technol. 2016, 152, 343–349. [Google Scholar] [CrossRef]
- Sipos, G.; Solti, A.; Czech, V.; Vashegyi, I.; Tóth, B.; Cseh, E.; Fodor, F. Heavy metal accumulation and tolerance of energy grass (Elymus elongatus subsp. ponticus cv. Szarvasi-1) grown in hydroponic culture. Plant Physiol. Biochem. 2013, 68, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Santanen, A.; Jaakkola, S.; Ekholm, P.; Hartikainen, H.; Stoddard, F.; Mäkelä, P.S. Biomass yield and quality of bioenergy crops grown with synthetic and organic fertilizers. Biomass Bioenergy 2013, 59, 477–485. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A.; Thimmanagari, M.; Defersha, F. Techno-economic assessment of corn stover for hybrid bioenergy production: A sustainable approach. Case Stud. Therm. Eng. 2019, 13, 100408. [Google Scholar] [CrossRef]
- Etumuluru, J.S. Comparison of chemical composition and energy property of torrefied switchgrass and corn stover. Front. Energy Res. 2015, 3. [Google Scholar] [CrossRef]
- Ambrosio, R.; Pauletti, V.; Barth, G.; Povh, F.P.; Da Silva, D.A.; Blum, H. Energy potential of residual maize biomass at different spacings and nitrogen doses. Agric. Sci. 2017, 41, 626–633. [Google Scholar] [CrossRef][Green Version]
- Jutakridsada, P.; Sriprasoed, R.; Patikarnmonthon, N.; Kamwilaisak, K. Comparison study of sugarcane leaves and corn stover as a potential energy source in pyrolysis process. Energy Procedia 2016, 100, 26–29. [Google Scholar] [CrossRef]
- Lizotte, P.-L.; Savoie, P.; De Champlain, A. Ash Content and calorific energy of corn stover components in eastern canada. Energies 2015, 8, 4827–4838. [Google Scholar] [CrossRef]
- Bothe, H.; Słomka, A. Divergent biology of facultative heavy metal plants. J. Plant Physiol. 2017, 219, 45–61. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Liang, H.-M.; Lin, T.-H.; Chiou, J.-M.; Yeh, K.-C. Model evaluation of the phytoextraction potential of heavy metal hyperaccumulators and non-hyperaccumulators. Environ. Pollut. 2009, 157, 1945–1952. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, R.P. Cadmium tolerance and its phytoremediation by two oil yielding plants Ricinus Communis (L.) and Brassica Juncea (L.) from the contaminated soil. Int. J. Phytoremediat. 2012, 14, 772–785. [Google Scholar] [CrossRef]
- Laporte, M.-A.; Sterckeman, T.; Dauguet, S.; Denaix, L.; Nguyen, C. Variability in cadmium and zinc shoot concentration in 14 cultivars of sunflower (Helianthus annuus L.) as related to metal uptake and partitioning. Environ. Exp. Bot. 2015, 109, 45–53. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Wang, J. Cadmium phytoextraction from loam soil in tropical southern China by Sorghum bicolor. Int. J. Phytoremediat. 2017, 19, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, Y.-C.; Shen, C.; He, C.-T.; Yuan, J.-G.; Yang, Z.-Y. Comparative analysis between low- and high-cadmium-accumulating cultivars of Brassica parachinensis to identify difference of cadmium-induced microRNA and their targets. Plant Soil 2017, 420, 223–237. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Phytoremediation of soil contaminated with nickel, cadmium and cobalt. Int. J. Phytoremediat. 2020, 23, 252–262. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Q.; An, J.; Sun, Y.; Liu, R. Variations in cadmium accumulation among Chinese cabbage cultivars and screening for Cd-safe cultivars. J. Hazard. Mater. 2010, 173, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Nehnevajova, E.; Herzig, R.; Federer, G.; Erismann, K.-H.; Schwitzguébel, J.-P. Screening of sunflower cultivars for metal phytoextraction in a contaminated field prior to mutagenesis. Int. J. Phytoremediat. 2005, 7, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Santanen, A.; Kleemola, J.; Stoddard, F.; Mäkelä, P.S. Improved sustainability of feedstock production with sludge and interacting mycorrhiza. Chemosphere 2013, 91, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Coleman-Derr, D.; Tringe, S.G.; Coleman-Derr, D.; Tringe, S.G. Building the crops of tomorrow: Advantages of symbiont-based approaches to improving abiotic stress tolerance. Front. Microbiol. 2014, 5, 283. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources 2006; World Soil Research Report No.103; FAO: Rome, Italy, 2014. [Google Scholar]
- Regulation of the Minister of the Environment of 1 September 2016 Applicable in Poland (Journal of Laws 2016 Item 1395). ISAP—Internet System of Legal Acts. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001395 (accessed on 11 March 2020).
- Kabata-Pendias, A.; Mukherjee, A. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Nadgórska-Socha, A.; Ptasiński, B.; Kita, A. Heavy metal bioaccumulation and antioxidative responses in Cardaminopsis arenosa and Plantago lanceolata leaves from metalliferous and non-metalliferous sites: A field study. Ecotoxicology 2013, 22, 1422–1434. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Kandziora-Ciupa, M.; Ciepał, R. Element accumulation, distribution, and phytoremediation potential in selected metallophytes growing in a contaminated area. Environ. Monit. Assess. 2015, 187, 1–15. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Kandziora-Ciupa, M.; Ciepał, R.; Barczyk, G. Robinia pseudoacacia and Melandrium album in trace elements biomonitoring and air pollution tolerance index study. Int. J. Environ. Sci. Technol. 2016, 13, 1741–1752. [Google Scholar] [CrossRef]
- Barbieri, M. The importance of enrichment factor (EF) and geoaccumulation index (Igeo) to evaluate the soil contamination. J. Geol. Geophys. 2016, 5, 237. [Google Scholar] [CrossRef]
- Marchand, L.; Pelosi, C.; González-Centeno, M.R.; Maillard, A.; Ourry, A.; Galland, W.; Teissedre, P.-L.; Bessoule, J.-J.; Mongrand, S.; Morvan-Bertrand, A.; et al. Trace element bioavailability, yield and seed quality of rapeseed (Brassica napus L.) modulated by biochar incorporation into a contaminated technosol. Chemosphere 2016, 156, 150–162. [Google Scholar] [CrossRef]
- Orwin, K.; Wardle, D. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biol. Biochem. 2004, 36, 1907–1912. [Google Scholar] [CrossRef]
- PN-EN. ISO 18125:2017-07: Solid Biofuels—Determination of Calorific Value; European Committee for Standardization: Brussels, Belgium, 2010; Available online: https://pkn.pl/pn-en-iso-18125-2017-07 (accessed on 10 May 2021).
- Kopetz, H.; Jossart, J.; Ragossnig, H.; Metschina, C. European Biomass Statistics 2007; European Biomass Association: Brussels, Belgium, 2007. [Google Scholar]
- Alexander, M. Microorganisms and Chemical Pollution. BioScience 1973, 23, 509–515. [Google Scholar] [CrossRef]
- Parkinson, D.; Gray, F.R.G.; Williams, S.T. Methods of studying ecology of soil microorganism. In IBP Handbook; Blackweel Scientific Publication: Oxford, UK; Endinburgh, UK, 1971; p. 19. [Google Scholar]
- Martin, J.P. Use of acid, rose bengal, and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Sarathchandra, S.; Burch, G.; Cox, N. Growth patterns of bacterial communities in the rhizoplane and rhizosphere of white clover (Trifolium repens L.) and perennial ryegrass (Lolium perenne L.) in long-term pasture. Appl. Soil Ecol. 1997, 6, 293–299. [Google Scholar] [CrossRef]
- De Leij, F.A.A.M.; Whipps, J.M.; Lynch, J.M. The use of colony development for the characterization of bacterial communities in soil and on roots. Microb. Ecol. 1994, 27, 81–97. [Google Scholar] [CrossRef]
- Dell Inc. Dell Statistica (Data Analysis Software System); Version 13.1; Dell Inc.: Tulsa, OK, USA, 2016. [Google Scholar]
- Parks, D.H.; Tyson, G.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Team, R. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com (accessed on 10 May 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 10 May 2021).
- Sofy, M.R.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.M.; Mohamed, H.I. Minimizing adverse effects of Pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Hasan, K.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [PubMed]
- Ovečka, M.; Takáč, T. Managing heavy metal toxicity stress in plants: Biological and biotechnological tools. Biotechnol. Adv. 2014, 32, 73–86. [Google Scholar] [CrossRef]
- Jasiewicz, C.; Antonkiewicz, J. Heavy metal contents in maize cultivated in soils contaminated with these metals. Ecol. Chem. Eng. S 2002, 9, 373–377. [Google Scholar]
- Barbosa, D.B.P.; Nabel, M.; Jablonowski, N.D. Biogas-digestate as nutrient source for biomass production of Sida Hermaphrodita, Zea Mays L. and Medicago sativa L. Energy Procedia 2014, 59, 120–126. [Google Scholar] [CrossRef]
- Kopecky, J.M., Jr.; Bernas, J.; Suchy, K. The environmental aspects of energy crops growing in the condition of the Czech Republic. Agric. Sci. 2017. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Kucharski, J. Maintenance of soil homeostasis under exposure to cadmium. Commun. Soil Sci. Plant Anal. 2015, 46, 2051–2069. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Boros-Lajszner, E.; Borowik, A.; Baćmaga, M.; Kucharski, J.; Tomkiel, M. Implication of zinc excess on soil health. J. Environ. Sci. Health Part B 2016, 51, 261–270. [Google Scholar] [CrossRef]
- Raj, S.K.; Syriac, E.K. Herbicidal effect on the bio-indicators of soil health—A review. J. Appl. Nat. Sci. 2017, 9, 2438–2448. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; McGrath, S. Heavy metals and soil microbes. Soil Biol. Biochem. 2009, 41, 2031–2037. [Google Scholar] [CrossRef]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Biological activity of soil contaminated with cobalt, tin, and molybdenum. Environ. Monit. Assess. 2016, 188, 1–10. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Use of a zeolite and molecular sieve to restore homeostasis of soil contaminated with cobalt. Minerals 2020, 10, 53. [Google Scholar] [CrossRef]
- Xu, F.F.; Imlay, J.A. Silver(I), mercury(ii), cadmium(ii), and zinc(ii) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 3614–3621. [Google Scholar] [CrossRef]
- Bruins, M.R.; Kapil, S.; Oehme, F.W. Microbial Resistance to Metals in the Environment. Ecotoxicol. Environ. Saf. 2000, 45, 198–207. [Google Scholar] [CrossRef]
- Hobman, J.; Crossman, L.C. Bacterial antimicrobial metal ion resistance. J. Med. Microbiol. 2015, 64, 471–497. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.L.; Liu, W.; Tang, C.; Franks, A.E. Microorganisms in heavy metal bioremediation: Strategies for applying microbial-community engineering to remediate soils. AIMS Environ. Sci. 2016, 3, 211–229. [Google Scholar] [CrossRef]
- Gołębiewski, M.; Deja-Sikora, E.; Cichosz, M.; Tretyn, A.; Wróbel, B. 16S rDNA Pyrosequencing Analysis of Bacterial Community in Heavy Metals Polluted Soils. Microb. Ecol. 2014, 67, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Franke-Whittle, I.H.; Manici, L.M.; Insam, H.; Stres, B. Rhizosphere bacteria and fungi associated with plant growth in soils of three replanted apple orchards. Plant Soil 2015, 395, 317–333. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, F.; He, L.; Karthik, L.; Li, Z. Actinomycetes from the South China Sea sponges: Isolation, diversity, and potential for aromatic polyketides discovery. Front. Microbiol. 2015, 6, 1048. [Google Scholar] [CrossRef] [PubMed]
- Greening, C.; Carere, C.; Rushton-Green, R.; Harold, L.; Hards, K.; Taylor, M.; Morales, S.; Stott, M.B.; Cook, G.M. Persistence of the dominant soil phylum Acidobacteria by trace gas scavenging. Proc. Natl. Acad. Sci. USA 2015, 112, 10497–10502. [Google Scholar] [CrossRef]
- De, J.; Ramaiah, N.; Vardanyan, L. Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar. Biotechnol. 2008, 10, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Lingua, G.; Berta, G.; Glick, B.R. Beneficial role of plant growth promoting bacteria and arbuscular mycorrhizal fungi on plant responses to heavy metal stress. Can. J. Microbiol. 2009, 55, 501–514. [Google Scholar] [CrossRef]
- Kidd, P.; Barceló, J.; Bernal, M.P.; Navari-Izzo, F.; Poschenrieder, C.; Shilev, S.; Clemente, R.; Monterroso, C. Trace element behaviour at the root–soil interface: Implications in phytoremediation. Environ. Exp. Bot. 2009, 67, 243–259. [Google Scholar] [CrossRef]
- Zhang, G.; Cao, T.; Ying, J.; Yang, Y.; Ma, L. Diversity and novelty of actinobacteria in Arctic Marine sediments. Antonie Van Leeuwenhoek 2014, 105, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Lee, J.E.; Kim, Y.M.; Park, S.W.; Park, S.T. Pseudonocardia carboxydivorans sp. nov., a carbon monoxide-oxidizing actinomycete, and an emended description of the genus Pseudonocardia. Int. J. Syst. Evol. Microbiol. 2008, 58, 2475–2478. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Tang, Y.-L.; Lin, S.-M. Efficient bioconversion of compactin to pravastatin by the quinoline-degrading microorganism Pseudonocardia carboxydivorans isolated from petroleum-contaminated soil. Bioresour. Technol. 2011, 102, 10187–10193. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Oszust, K. Functional diversity of fungal communities in soil contaminated with diesel oil. Front. Microbiol. 2017, 8, 1862. [Google Scholar] [CrossRef]
- Deng, F.; Dou, R.; Sun, J.; Li, J.; Dang, Z. Phenanthrene degradation in soil using biochar hybrid modified bio-microcapsules: Determining the mechanism of action via comparative metagenomic analysis. Sci. Total Environ. 2021, 775, 145798. [Google Scholar] [CrossRef]
- Lodha, T.D.; Srinivas, A.; Sasikala, C.; Ramana, C.V. Hopanoid inventory of Rhodoplanes spp. Arch. Microbiol. 2015, 197, 861–867. [Google Scholar] [CrossRef]
- Belin, B.J.; Busset, N.; Giraud, E.; Molinaro, A.; Silipo, A.; Newman, D.K. Hopanoid lipids: From membranes to plant-bacteria interactions. Nat. Rev. Genet. 2018, 16, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gao, G.; Wang, Y. Effects of soil properties, heavy metals, and PBDEs on microbial community of e-waste contaminated soil. Ecotoxicol. Environ. Saf. 2019, 180, 705–714. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Response of soil microorganisms and enzymes to the foliar application of Helicur 250 EW fungicide on Horderum vulgare L. Chemosphere 2019, 242, 125163. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, J. Microbiological study in petrol-spiked soil. Molecules 2021, 26, 2664. [Google Scholar] [CrossRef]
- Talwar, C.; Nagar, S.; Kumar, R.; Scaria, J.; Lal, R.; Negi, R.K. Defining the environmental adaptations of genus Devosia: Insights into its expansive short peptide transport system and positively selected genes. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93, 1–9. [Google Scholar] [CrossRef]
- Jansson, J.; Hofmockel, K.S. The soil microbiome—From metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.-L.; Bassler, B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef]
- Huang, J.; Shi, Y.; Zeng, G.; Gu, Y.; Chen, G.; Shi, L.; Hu, Y.; Tang, B.; Zhou, J. Acyl-homoserine lactone-based quorum sensing and quorum quenching hold promise to determine the performance of biological wastewater treatments: An overview. Chemosphere 2016, 157, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Genet. 2016, 14, 576–588. [Google Scholar] [CrossRef] [PubMed]


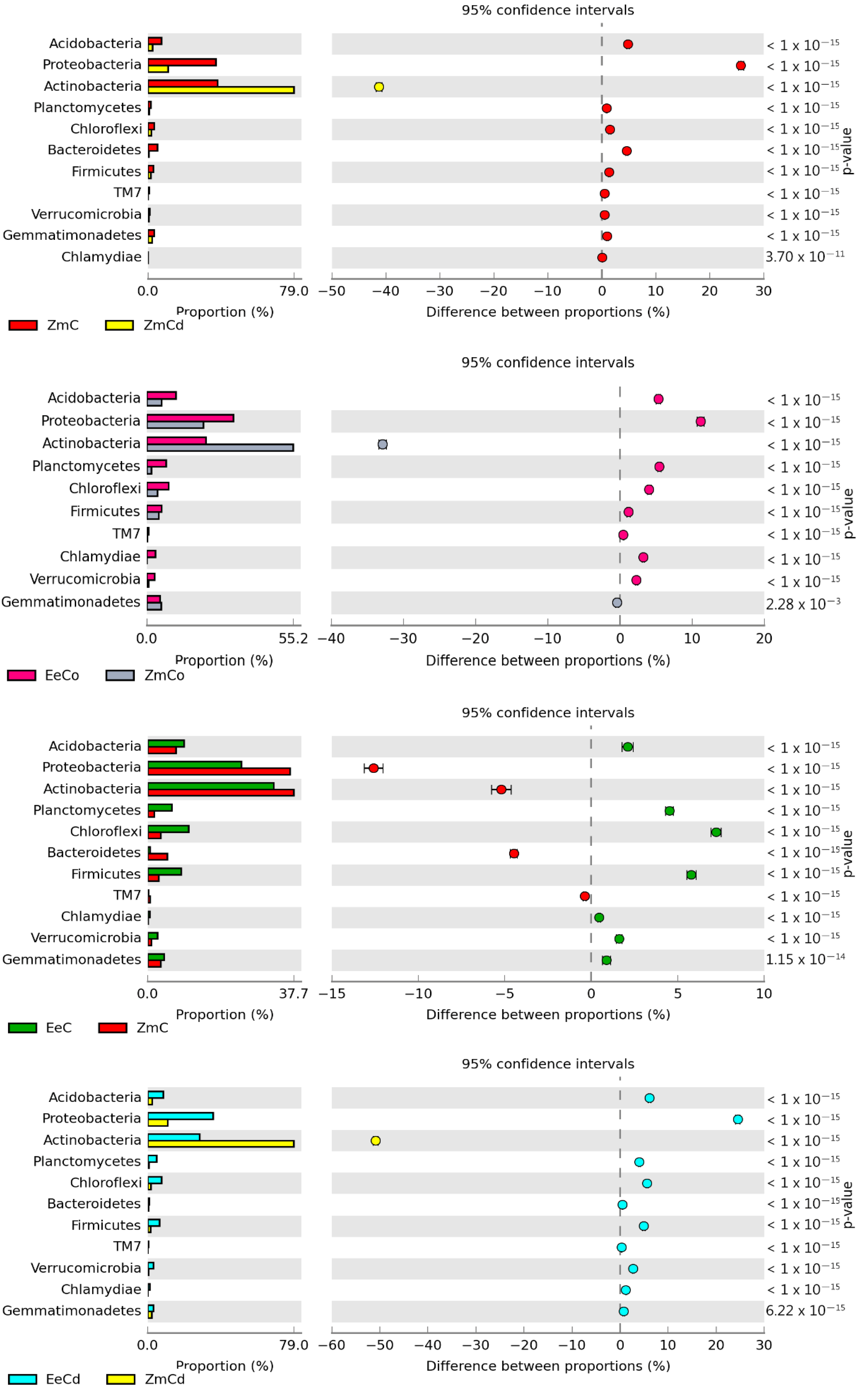
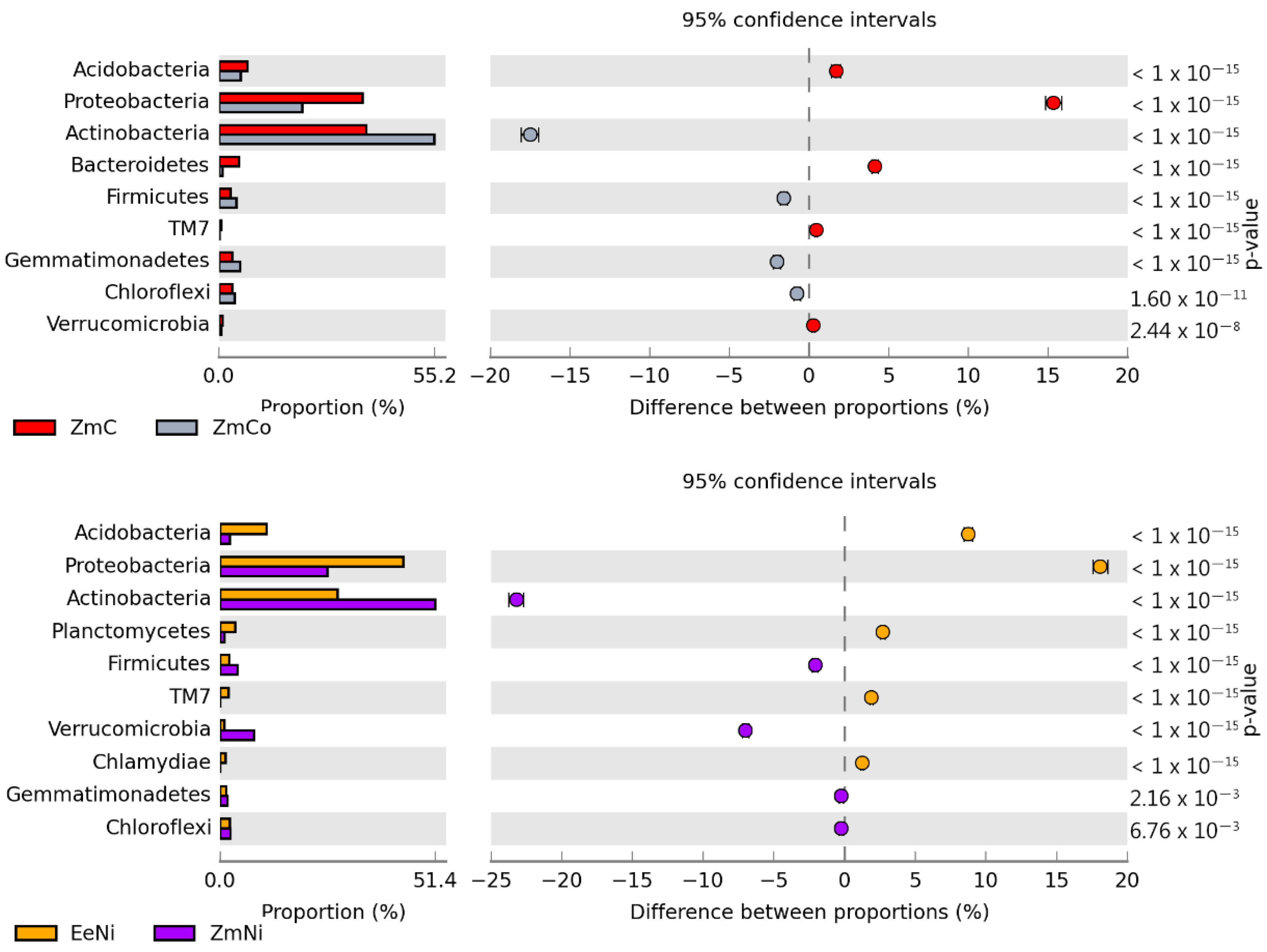
| Heavy Metals | Energy Production (Q) | Heating Value (Hv) | Plant Energy Yield (YEP) MJ kg−1 |
|---|---|---|---|
| MJ kg−1 Air-Dry Matter Plants | |||
| Elymus elongatus L. | |||
| C | 19.087 a ± 0.201 | 15.052 a ± 0.201 | 0.101 cd ± 0.020 |
| Ni2+ | 18.836 ab ± 0.200 | 14.604 a ± 0.200 | 0.059 d ± 0.010 |
| Co2+ | 18.889 ab ± 0.201 | 14.891 a ± 0.200 | 0.068 d ± 0.010 |
| Cd2+ | 18.789 ab ± 0.201 | 14.810 a ± 0.200 | 0.091 cd ± 0.020 |
| Zea mays L. | |||
| C | 18.351 b ± 0.201 | 14.791 a ± 0.200 | 0.265 a ± 0.030 |
| Ni2+ | 18.546 ab ± 0.201 | 14.953 a ± 0.200 | 0.110 cd ± 0.020 |
| Co2+ | 18.497 b ± 0.201 | 14.913 a ± 0.200 | 0.127 c ± 0.020 |
| Cd2+ | 18.562 ab ± 0.201 | 14.967 a ± 0.200 | 0.194 b ± 0.030 |
| Taxon | Object | |||||||
|---|---|---|---|---|---|---|---|---|
| EeC | EeNi | EeCo | EeCd | ZmC | ZmNi | ZnCo | ZnCd | |
| Phylum | 2.02 b | 1.78 c | 2.16 a | 2.00 b | 1.67 cd | 1.54 d | 1.54 d | 0.90 e |
| Class | 3.08 b | 2.60 c | 3.18 a | 3.04 b | 2.51 c | 2.19 d | 2.24 d | 1.28 e |
| Order | 3.19 c | 3.00 d | 3.43 a | 3.35 b | 2.86 e | 2.39 f | 2.45 f | 1.38 g |
| Family | 3.29 a | 3.25 a | 3.11 b | 3.31 a | 3.26 a | 2.82 cb | 2.72 c | 1.84 d |
| Genus | 2.01 bc | 2.13 b | 1.86 c | 2.06 bc | 2.19 b | 2.37 a | 1.93 c | 1.55 d |
| Variables | Shannon–Wiener Index (H’) | ||||
|---|---|---|---|---|---|
| Phylum | Class | Order | Family | Genus | |
| Control soil | |||||
| Org | –0.529 | –0.752 * | –0.444 | –0.073 | 0.375 |
| Act | –0.588 | –0.738 * | –0.525 | –0.229 | 0.167 |
| CDOrg | 0.563 | 0.791 * | 0.474 | 0.089 | –0.379 |
| CDAct | –0.647 | –0.860 * | –0.562 | –0.180 | 0.306 |
| EPOrg | 0.592 | 0.814 * | 0.505 | 0.123 | –0.351 |
| EPAct | 0.315 | 0.494 | 0.248 | –0.028 | –0.344 |
| Soil contaminated with Ni2+ | |||||
| Org | –0.504 | –0.458 | –0.300 | 0.494 | 0.494 |
| Act | 0.237 | 0.201 | 0.077 | –0.482 | –0.482 |
| CDOrg | –0.112 | –0.149 | –0.266 | –0.670 | –0.670 |
| CDAct | –0.225 | –0.233 | –0.255 | –0.248 | –0.248 |
| EPOrg | –0.767 * | –0.767 * | –0.751 * | –0.395 | –0.395 |
| EPAct | –0.449 | –0.434 | –0.376 | 0.018 | 0.018 |
| Soil contaminated with Co2+ | |||||
| Org | –0.406 | –0.655 | –0.737 * | –0.725 * | 0.325 |
| Act | 0.414 | 0.735 * | 0.845 * | 0.829 * | –0.466 |
| CDOrg | –0.787 * | –0.644 | –0.540 | –0.559 | –0.640 |
| CDAct | 0.399 | 0.365 | 0.328 | 0.335 | 0.247 |
| EPOrg | –0.257 | 0.115 | 0.284 | 0.257 | –0.867 * |
| EPAct | –0.096 | 0.133 | 0.234 | 0.218 | –0.507 |
| Soil contaminated with Cd2+ | |||||
| Org | –0.912 * | –0.952 * | –0.959 * | –0.946 * | –0.806 * |
| Act | –0.828 * | –0.880 * | –0.889 * | –0.871 * | –0.706 |
| CDOrg | –0.190 | –0.256 | –0.270 | –0.244 | –0.073 |
| CDAct | –0.757 * | –0.836 * | –0.851 * | –0.822 * | –0.593 |
| EPOrg | 0.524 | 0.546 | 0.549 | 0.542 | 0.465 |
| EPAct | 0.899 * | 0.950 * | 0.959 * | 0.942 * | 0.773 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boros-Lajszner, E.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Energetic Value of Elymus elongatus L. and Zea mays L. Grown on Soil Polluted with Ni2+, Co2+, Cd2+, and Sensitivity of Rhizospheric Bacteria to Heavy Metals. Energies 2021, 14, 4903. https://doi.org/10.3390/en14164903
Boros-Lajszner E, Wyszkowska J, Borowik A, Kucharski J. Energetic Value of Elymus elongatus L. and Zea mays L. Grown on Soil Polluted with Ni2+, Co2+, Cd2+, and Sensitivity of Rhizospheric Bacteria to Heavy Metals. Energies. 2021; 14(16):4903. https://doi.org/10.3390/en14164903
Chicago/Turabian StyleBoros-Lajszner, Edyta, Jadwiga Wyszkowska, Agata Borowik, and Jan Kucharski. 2021. "Energetic Value of Elymus elongatus L. and Zea mays L. Grown on Soil Polluted with Ni2+, Co2+, Cd2+, and Sensitivity of Rhizospheric Bacteria to Heavy Metals" Energies 14, no. 16: 4903. https://doi.org/10.3390/en14164903
APA StyleBoros-Lajszner, E., Wyszkowska, J., Borowik, A., & Kucharski, J. (2021). Energetic Value of Elymus elongatus L. and Zea mays L. Grown on Soil Polluted with Ni2+, Co2+, Cd2+, and Sensitivity of Rhizospheric Bacteria to Heavy Metals. Energies, 14(16), 4903. https://doi.org/10.3390/en14164903








