Storage and Upgrading of Biogas by Physicochemical Purification in a Sudano-Sahelian Context
Abstract
:1. Introduction
2. Materials and Methods
2.1. Background of Biogas Production and Purification Preparation
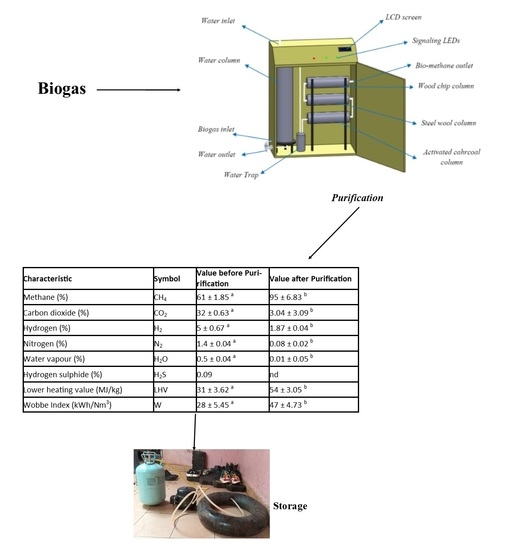
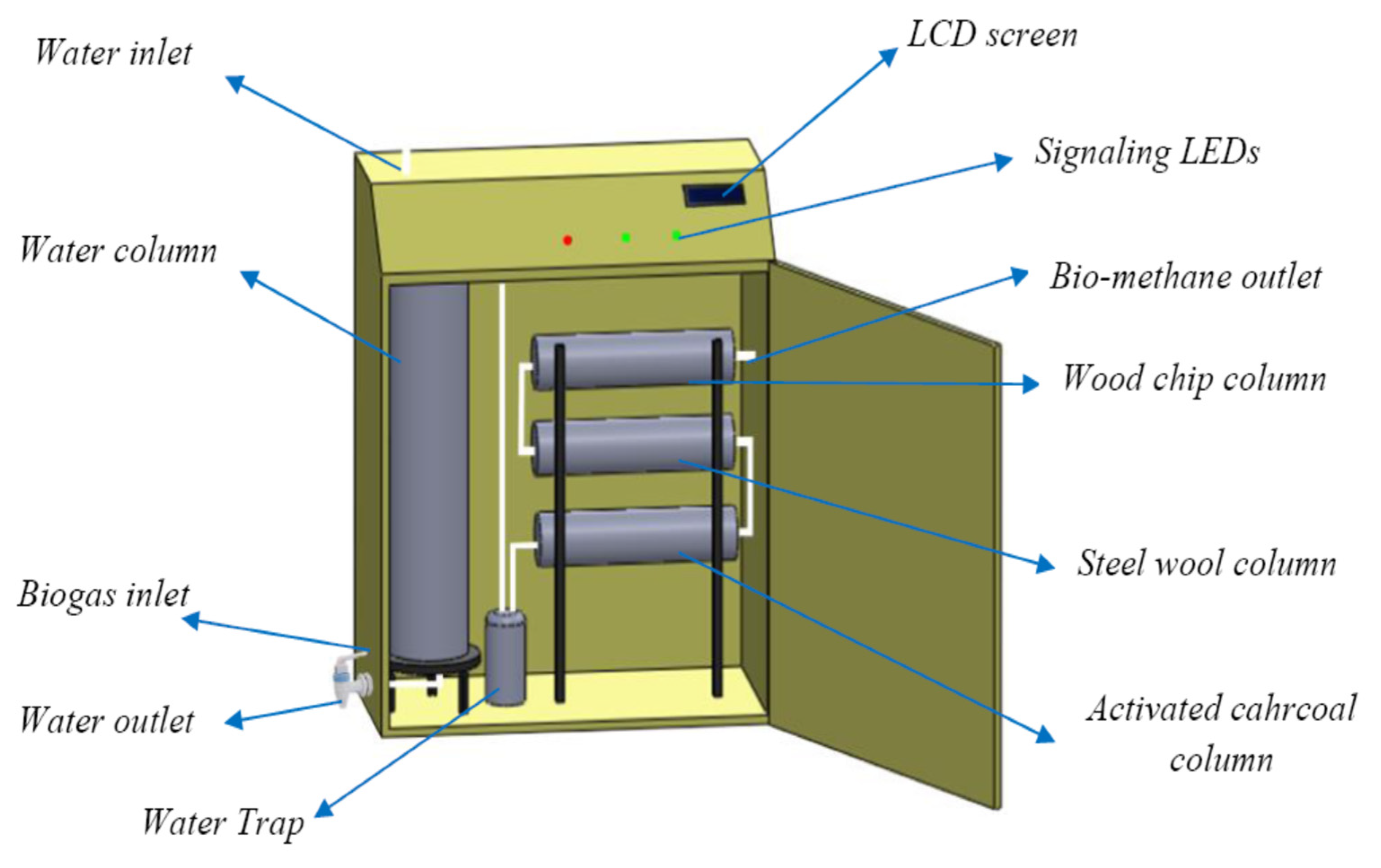
2.2. Biogas Purification
2.3. Chemical Analysis
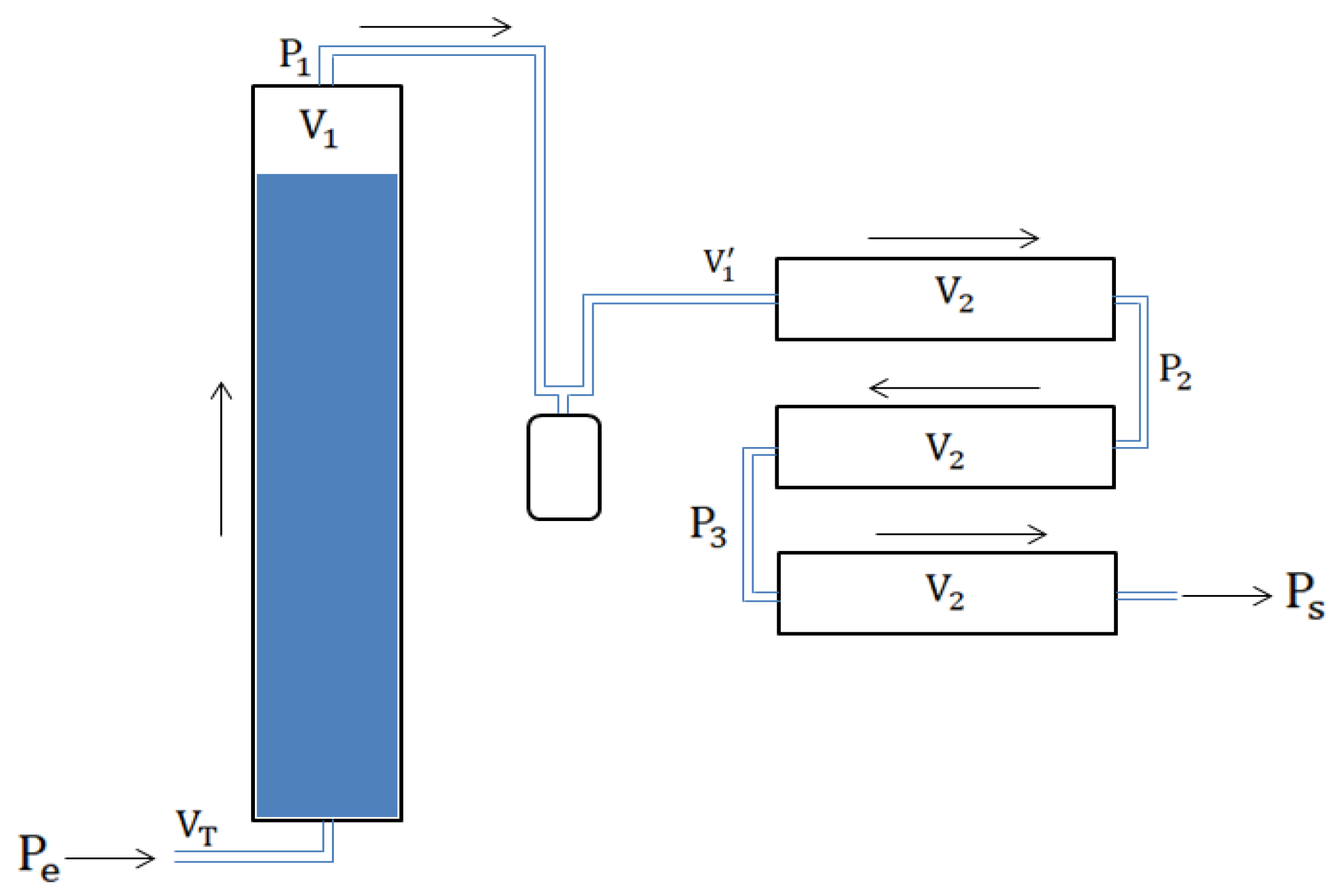
2.3.1. Estimation of the Pressure of the Biomethane at the Outlet of the System
2.3.2. Biogas Storage in an Inflatable Balloon
2.3.3. Storage by Compression in a Bottle
3. Results and Discussion
3.1. Characteristics of Lignocellulosic Biomasses in Sudano-Sahelian Context
3.2. Composition of Biogas before and after Purification in Sudano-Sahelian Context
3.3. Biomethane Storage in Sudano-Sahelian Context
3.3.1. Storage in the Balloon
3.3.2. Storage by Compression in a Bottle
Compressor Characteristics
Bottle Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BP Statistical Review of World Energy. Centre for Energy Economics Research and Policy, Heriot-Watt University. 2019, p. 64. Available online: Ceerp.hw.ac.uk (accessed on 5 September 2021).
- Ghasemian, S.; Faridzad, A.; Abbaszadeh, P.; Taklif, A.; Ghasemi, A.; Hafezi, R. An overview of global energy scenarios by 2040: Identifying the driving forces using cross-impact analysis method. Int. J. Environ. Sci. Technol. 2020, 1–24. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.; Fahl, F. Biogas: Development and perspectives in Europe. Renew. Energy 2020, 129, 457–472. [Google Scholar] [CrossRef]
- Herbes, C.; Halbherr, V.; Braun, L. Factors influencing prices for heat from biogas plants. Appl. Energy 2018, 221, 308–318. [Google Scholar] [CrossRef]
- Martikainen, E. Mustankorkea Oy—Biological Waste Treatment and Making Products (and Profit) out of It; Mustankorkea: Laauka, Finland, 2018. [Google Scholar]
- Kapoor, R.; Ghosh, P.; Kumar, M.; Vijay, V.K. Evaluation of biogas upgrading technologies and future perspectives: A review. Environ. Sci. Pollut. Res. 2019, 26, 11631–11661. [Google Scholar] [CrossRef]
- Mustankorkea Oy, J.; Merkle, W.; Baer, K.; Haag, N.L.; Zielonka, S.; Ortloff, F.; Graf, F.; Lemmer, A. High-pressure anaerobic digestion up to 100 bar: Influence of initial pressure on production kinetics and specific methane yields. Environ. Technol. 2017, 38, 337–344. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- The Biogaz Handbook. In Sciences, Production and Applications; Woodheat Publishing Series in Energy; Eds Arthur Wellinger and Co., Woodhead: Cambridge, UK, 2013.
- Mrosso, R.; Machunda, R.; Pogrebnaya, T. Removal of Hydrogen Sulphide from Biogas Using a Red Rock. Hindawi J. Energy 2020, 2020, 2309378. [Google Scholar]
- Okoro, O.; Sun, Z. Desulphurisation of Biogas: A Systematic Qualitative and Economic-Based Quantitative Review of Alternative Strategies. Chem. Eng. 2019, 3, 76. [Google Scholar] [CrossRef] [Green Version]
- Naik, L.; Gebreegziabher, Z.; Tumwesige, V.; Balana, B.B.; Mwirigi, J.; Austin, G. Factors determining the stability and productivity of small scale anaerobic digesters. Biomass Bioenergy 2014, 70, 51–57. [Google Scholar] [CrossRef]
- Al-Imarah, K.A.; Lafta, T.M.; Jabr, A.K.; Mohammad, A.N. Desulphurization for Biogas Gen-erated by Lab Anaerobic Digestion Unit. IOSR J. Agric. Vet. Sci. 2017, 10, 66–73. [Google Scholar]
- Arya, A.; Divekar, S.; Rawat, R.; Gupta, P.; Garg, M.O.; Dasgupta, S.; Nanoti, A.; Singh, R.; Xiao, P.; Webley, P.A. Upgrading Biogas at Low Pressure by Vacuum Swing Adsorption. Ind. Eng. Chem. Res. 2015, 54, 404–413. [Google Scholar] [CrossRef]
- Sahota, S.; Shah, G.; Ghosh, P.; Kapoor, R.; Sengupta, S.; Sing, P.; Vijay, V.; Sahay, A.; Vijay, V.K.; Thakur, I.S. Bioresource Technology Reports; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 79–88. [Google Scholar]
- Santos-Clotas, E.; Cabrera-Codony, A.; Castillo, A.; Martín, M.J.; Poch, M.; Monclús, H. En-vironmental decision support system for biogas upgrading to feasible fuel. Energies 2019, 12, 1546. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. Overview of Recent Progress Towards In-situ Biogas Upgrading Techniques. Fuel 2018, 226, 686–697. [Google Scholar] [CrossRef]
- Wilken, D.; Strippel, F.; Hofmann, F.; Maciejczyk, M.; Klinkmüller, L.; Wagner, L.; Bontempo, G.; Münch, J.; Scheidl, S.; Conton, M.; et al. Bio-Gas to Biomethane; Fachverband Biogas e. V: Bonn, Germany, 2017. [Google Scholar]
- Ohannessian, A. Composés Organiques Volatils du Silicium: Un Frein à la Valorisation Énergétique des Biogaz: “Genèse et Mécanismes de Formation”. Ph.D. Thesis, INSA de Lyon, Villeurbanne, France, 4 December 2018; p. 244. [Google Scholar]
- IEA. Outlook for Biogas and Biomethane: Prospects for Organic Growth. 2020. Available online: https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth/sustainable-supply-potential-and-costs (accessed on 23 July 2020).
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing pipe line quality biomethane via anaerobic digestion of sludge amended with corn Stover biochar with in-situ CO2 removal. Appl. Energy 2015, 158, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Brauman, A.; Fonty, G.; Roger, P. La méthanisation dans les écosystèmes Naturels et cultivés. In Moletta, R. La Méthanisation; Editions Tec & Doc. Paris: Lavoisier, France, 2008; pp. 9–59. [Google Scholar]
- Wedraogo Tarsida, N. Valorisation du Biogaz par Purification et par Reformage. Ph.D. Thesis, Génie des Procédés et des Produits. NNT, Lorraine, France, 2018; p. 201. [Google Scholar]
- Abanades, S.; Abbaspour, H.; Ahmadi, A.; Das, B.; Ehyaei, M.A.; Esmaeilion, F.; Assad, M.E.; Hajilounezhad, T.; Jamali, D.H.; Hmida, A.; et al. A critical review of biogas production and usage with legislations framework across the globe. Int. J. Environ. Sci. Technol. 2021, 1–24. [Google Scholar] [CrossRef]
- Wang, T.; Shao, L.; Li, T.; Lü, F.; He, P. Digestion and dewatering characteristics of waste activated sludge treated by an anaerobic biofilm system. Bioresour. Technol. 2014, 153, 131–136. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [Green Version]

| Elements | Units | Characteristic |
|---|---|---|
| Type | - | Borosilicate glass |
| Nature of the substrate | - | Mixed waste |
| Gas production | m3/day | 0.05 |
| Digester volume | m3 | 0.01 |
| Pre-treatment tank volume | m3 | 0.25 |
| Volume of discharge tank | m3 | 0.02 |
| Characteristics (%) | Bil-Bil Residue | Arki Residue | Straw Rice | Rice Husks | Cattle Dung |
|---|---|---|---|---|---|
| Total solid | 87.0 ± 3.7 | 86.4 ± 2.5 | 85.7 ± 4.2 | 86.8 ± 3.7 | 87.0 ± 3.7 |
| Volatile compounds | 84.0 ± 4.0 | 83.0 ± 5.2 | 66.2 ± 2.8 | 78.0 ± 4.1 | 84.0 ± 4.0 |
| Carbon | 56.8 ± 2.9 | 58.0 ± 0.8 | 34.5 ± 5.1 | 34.8 ± 8.2 | 56.8 ± 2.9 |
| Notrigen | 0.732 ± 0.023 | 0.662 ± 0.054 | 1.023 ± 0.093 | 0.504 ± 0.027 | 0.732 ± 0.023 |
| * C:N before mixing | 77.6 ± 7.2 | 87.7 ± 6.0 | 33.7 ± 4.7 | 69.1 ± 0.03 | 77.6 ± 7.2 |
| ** C:N after mixing | 27.8 ± 4.7 | 28.5 ± 3.0 | 23.5 ± 5.7 | 27.2 ± 0.8 | 27.8 ± 4.7 |
| Elements | Units | Value |
|---|---|---|
| Length | m | 105 × 10−2 |
| Width | m | 75 × 10−2 |
| Depth | m | 35 × 10−2 |
| Screen tilt | m | 14 × 10−2 |
| Water tank column volume | m3 | 10−2 |
| Activated charcoal column volume | m3 | 10−3 |
| Steel wool storage volume | m3 | 10−3 |
| Specific surface area charcoal (BET) | m2/g | 231.02 |
| Specific surface area steel wool (BET) | m2/g | 343.528 |
| Characteristic | Symbol | Value before Purirification | Value after Purification |
|---|---|---|---|
| Methane (%) | CH4 | 61 ± 1.85 a | 95 ± 6.83 b |
| Carbon dioxide (%) | CO2 | 32 ± 0.63 a | 3.04 ± 3.09 b |
| Hydrogen (%) | H2 | 5 ± 0.67 a | 1.87 ± 0.04 b |
| Nitrogen (%) | N2 | 1.4 ± 0.04 a | 0.08 ± 0.02 b |
| Water vapour (%) | H2O | 0.5 ± 0.04 a | 0.01 ± 0.05 b |
| Hydrogen sulphide (%) | H2S | 0.09 | nd |
| Lower heating value (MJ/kg) | LHV | 31 ± 3.62 a | 54 ± 3.05 b |
| Wobbe Index (kWh/Nm3) | W | 28 ± 5.45 a | 47 ± 4.73 b |
| Elements | Units | Value |
|---|---|---|
| Total volume of the balloon | m3 | 3 |
| Balloon length | m | 1.5 |
| Balloon width | m | 1.5 |
| Balloon height | m | 1 |
| Material | - | Rubber |
| Elements | Units | Value |
|---|---|---|
| Generated power | HP | 1/8 |
| Power supply | W | 97 |
| Sucked gas flow | m/s | 0.17 × 10−3 |
| maximum pressure allowed | Bar | 25 |
| Elements | Units | Value |
|---|---|---|
| Volume | L | 13.4 × 10−3 |
| Working pressure | Bar | 28 |
| Filling capacity | kg | 13.6 |
| Critical pressure | Bar | 40.7 |
| Critical temperature | °C | 101.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djomdi; Mintsop Nguela, L.J.; Bakari, H.; Fadimatou, H.; Christophe, G.; Michaud, P. Storage and Upgrading of Biogas by Physicochemical Purification in a Sudano-Sahelian Context. Energies 2021, 14, 5855. https://doi.org/10.3390/en14185855
Djomdi, Mintsop Nguela LJ, Bakari H, Fadimatou H, Christophe G, Michaud P. Storage and Upgrading of Biogas by Physicochemical Purification in a Sudano-Sahelian Context. Energies. 2021; 14(18):5855. https://doi.org/10.3390/en14185855
Chicago/Turabian StyleDjomdi, Leonel Junior Mintsop Nguela, Hamadou Bakari, Hamadou Fadimatou, Gwendoline Christophe, and Philippe Michaud. 2021. "Storage and Upgrading of Biogas by Physicochemical Purification in a Sudano-Sahelian Context" Energies 14, no. 18: 5855. https://doi.org/10.3390/en14185855
APA StyleDjomdi, Mintsop Nguela, L. J., Bakari, H., Fadimatou, H., Christophe, G., & Michaud, P. (2021). Storage and Upgrading of Biogas by Physicochemical Purification in a Sudano-Sahelian Context. Energies, 14(18), 5855. https://doi.org/10.3390/en14185855