Experimental Evaluation of Aging Indicators for Lithium–Iron–Phosphate Cells
Abstract
:1. Introduction
- Compensating the power fluctuation of the renewable source;
- Compensating the peak powers occurring at the first intervals of vehicle charging operations.
2. The Test Procedure and Experimental Set-Up
2.1. The Reference Discharge Chosen
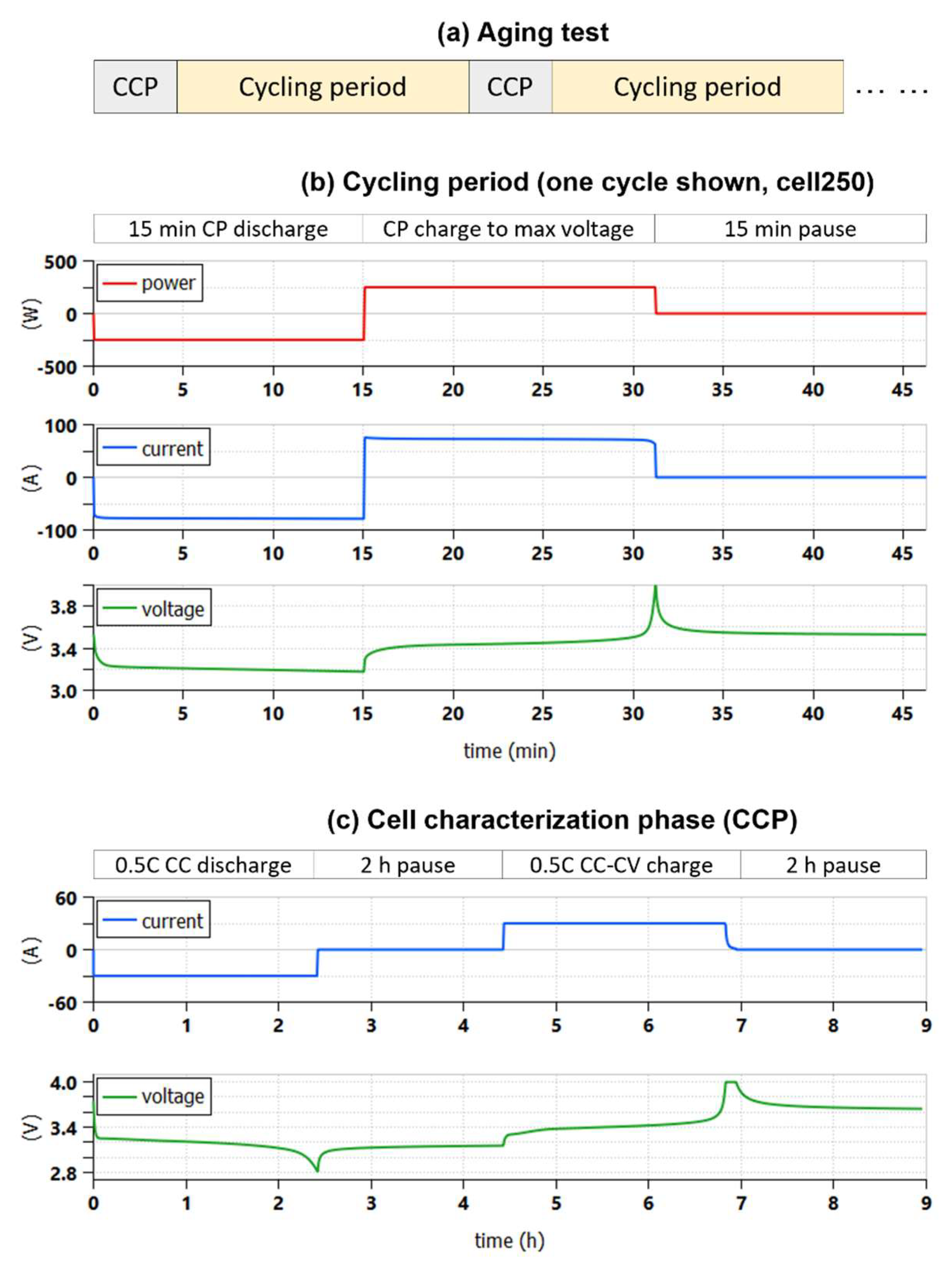
2.2. The Cycle-life Test Pattern
- The cell available capacity Ca,n; measured during the nth CCP;
- The cell internal resistances evaluated during the cycling periods, at the beginning and at the end of each discharge;
- The cell case temperature, always measured;
- The end-of-discharge voltage reached VEOD,n measured during the cycling periods at the end of every discharge.
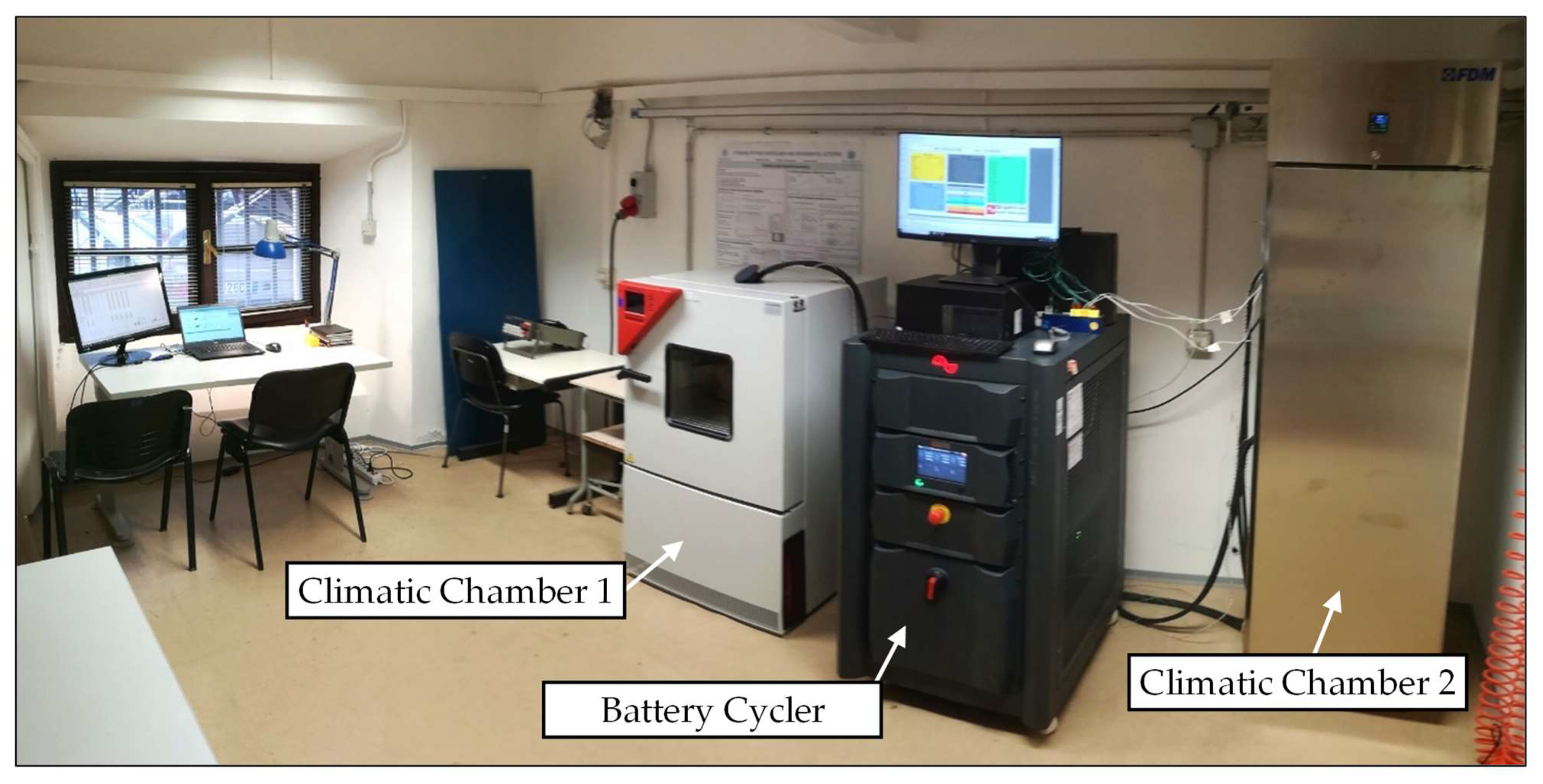
2.3. Experimental Setup
2.4. Device under Test
3. Aging Indicators
3.1. Available Capacity
3.2. Heat Generation
3.3. End-of-Discharge Voltage
3.4. Internal Resistance
4. Results
4.1. Aging Indicators
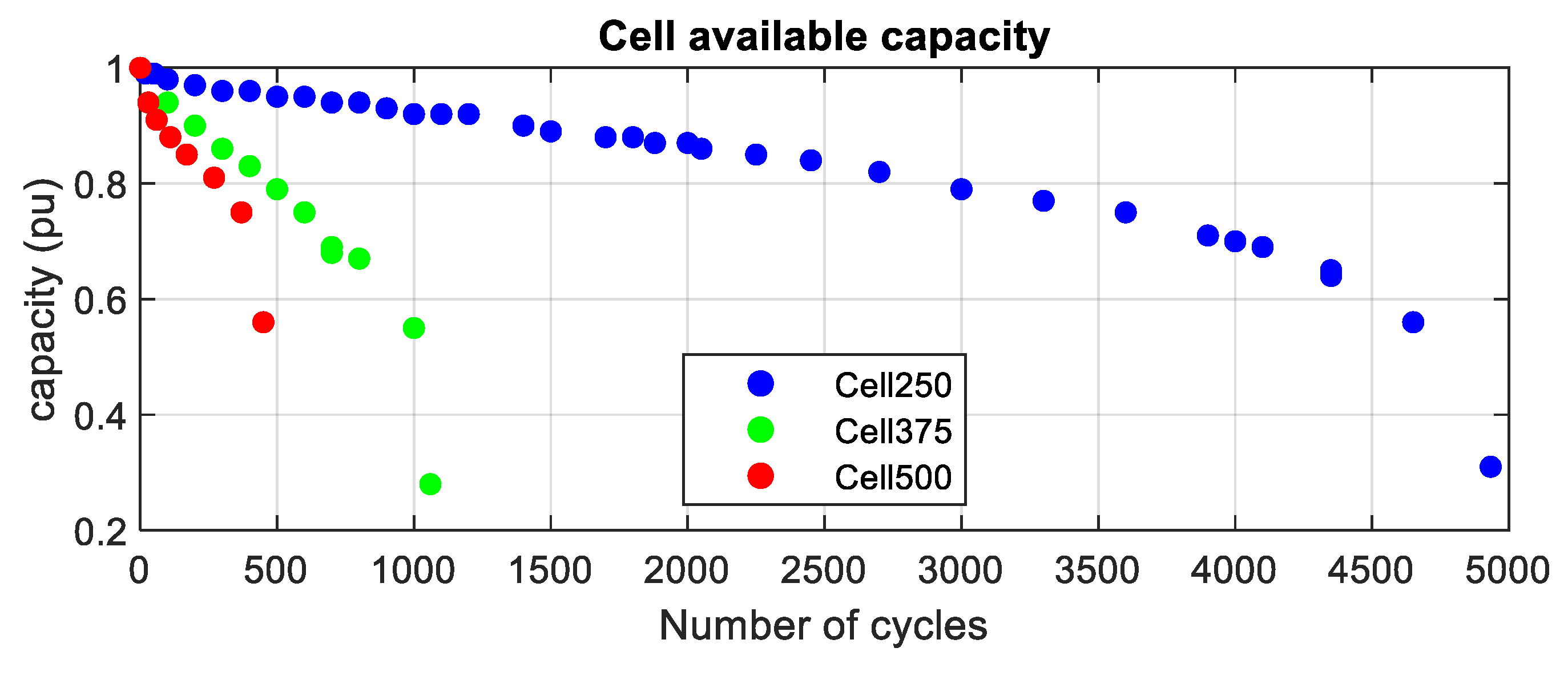
4.1.1. Available Capacity
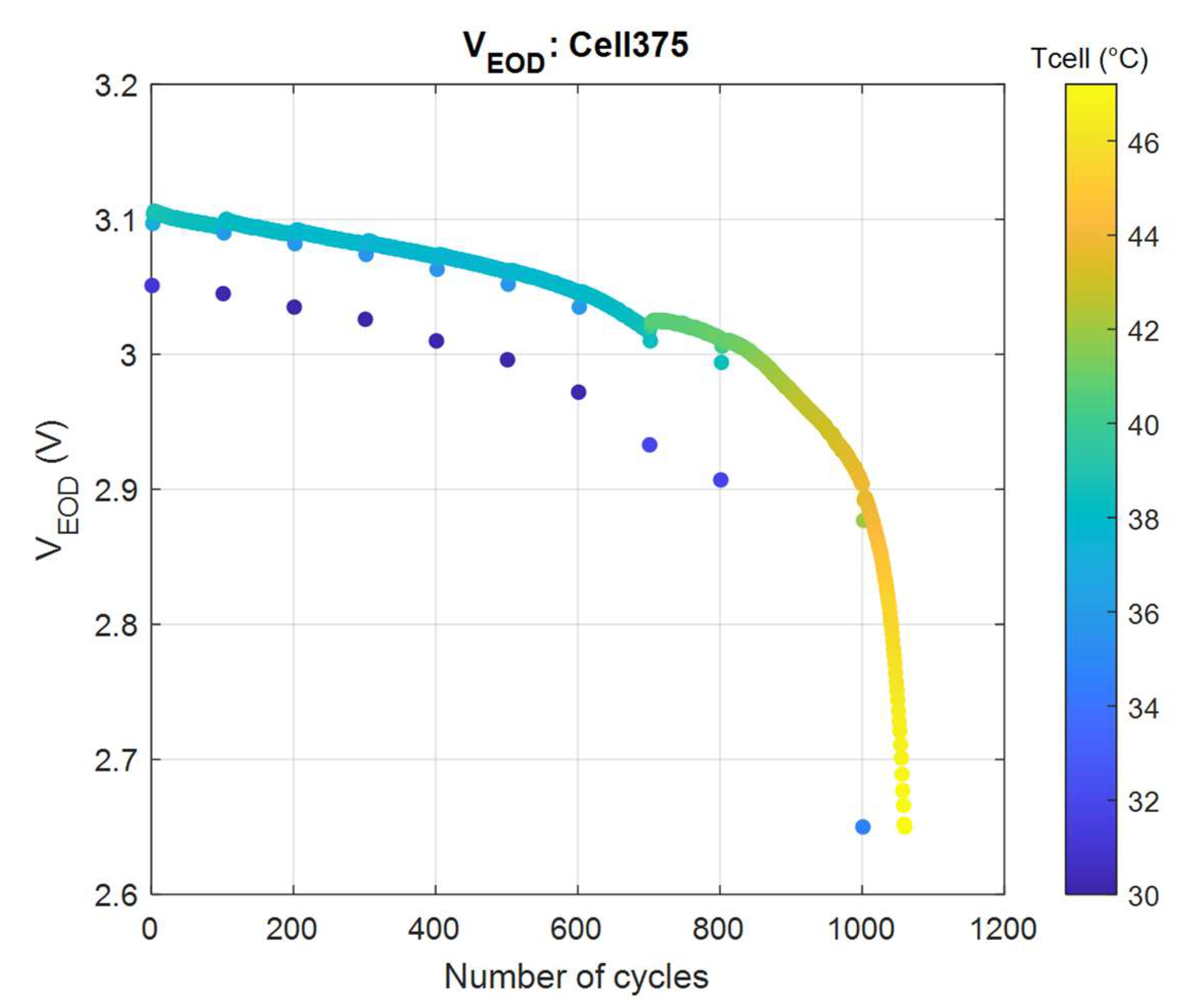
4.1.2. End-of-Discharge (EOD) Voltage and Heat Generation
- Throughout the entire cell cycle life, the end-of-discharge voltage is a good indicator of the cell’s SOL, and for a given temperature, a linear interpolation may be used;
- At low SOLs (large cycle numbers), a significant temperature rise is a good indicator, in conjunction with a steep fall of VEOD of the imminent cell end-of-life.
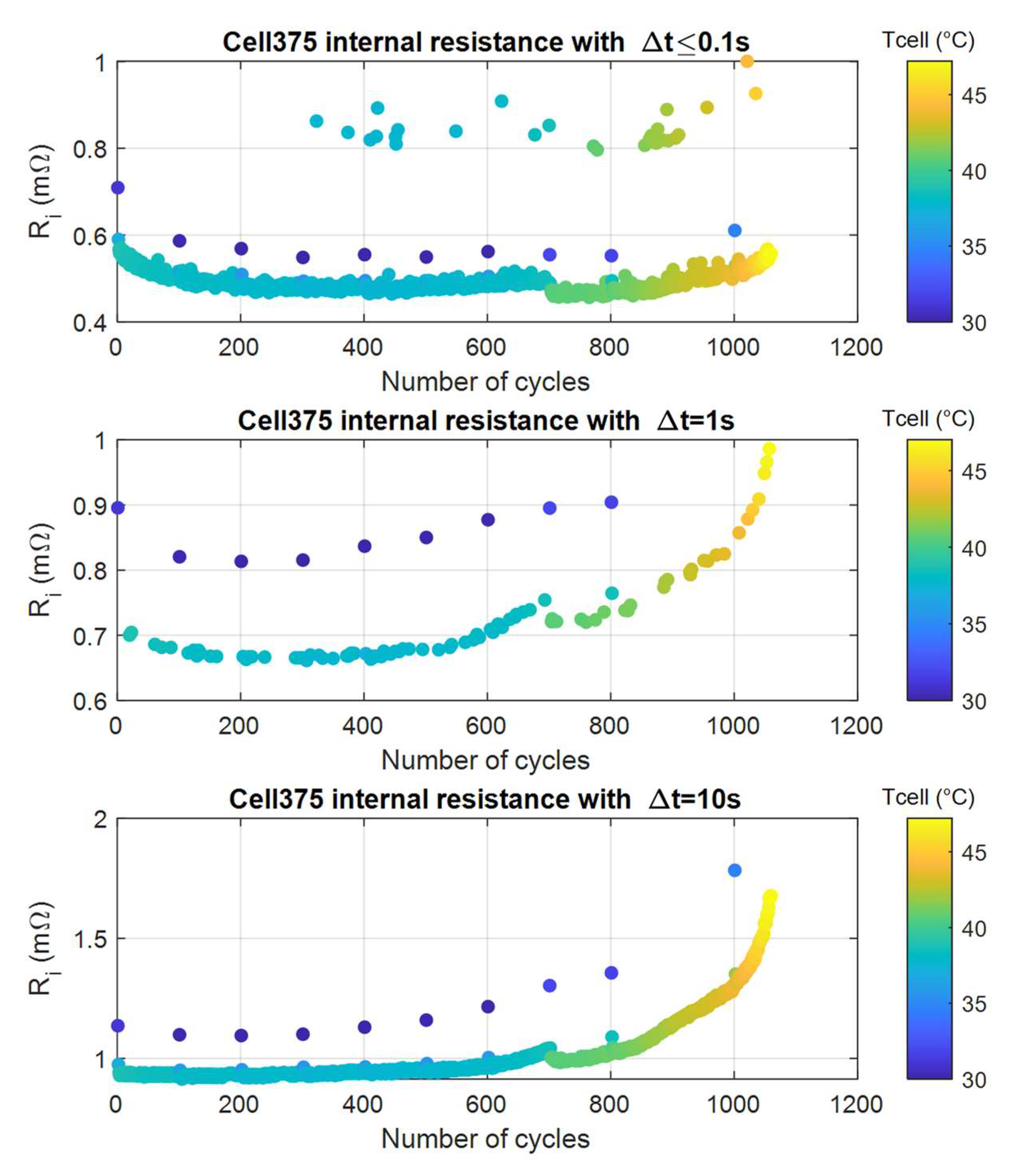
4.1.3. Internal Resistance and Heat Generation
- The resistance evaluation must be paired with the cell case temperature. Because these values are taken immediately after a CCP, where, as already mentioned, the cell’s temperature is lower, the corresponding resistances are significantly larger.
- There are some resistance changes after the cell has been rested without cycling. Indeed, during our tests, cell375 was left inactive for a few months after cycle number 700 and a few days after the 1000th cycle. Correspondingly, we saw a deviation from the previous trends, apparently due to internal battery state changes, not captured by temperature alone. It is reasonable to assume that the inactive periods between cycling phases impact the cell behavior by increasing the availability to deliver power.
- If we do not consider the extra points mentioned in the first bullet, the measured resistance over time had a rather constant behavior. Its trend over the entire cycle life displayed meaningful variation only if Δt = 10 s was used. However, the usage of this value was tricky, since some battery rests offsets the larger life-related Ri change, as discussed in the previous bullet point.
- The case with Δt = 0.1 s is rather useless. We had a cloud with resistances above 0.8 mΩ, which was probably due to some weakness of our data storing mechanism; moreover, we had some cool samples (blue dots) that were out of the bulk of the points. Even though we found an algorithm to take all these values out, the general trend of most of the points, the low curve, showed that the variation in resistance was not very large; moreover, rests in the battery operation (as we said the battery was at rest a few months at cycle number 700) caused significant changes in Δt = 0.1 s resistances.
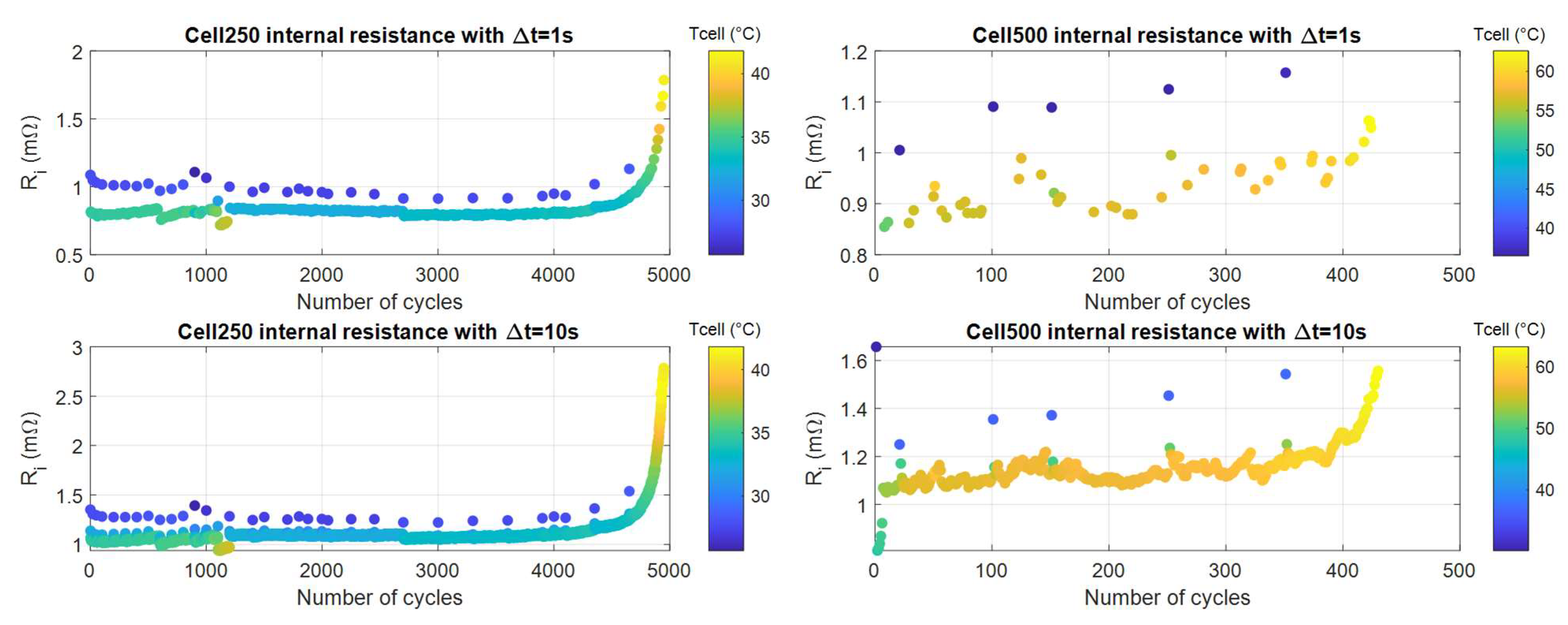
- The cases with Δt = 1 s and Δt = 10 s instead showed useful results. As with all the previous cases, we must combine the Ri measure with temperature, since the trend with resistance values measured immediately after CCP, where the cell was cooler, showed much larger resistances values (blue dots) than the others. Once we were able to select data that were thermally homogeneous (blue points discarded), we saw a significant variation of the internal resistance in function of the cell number of cycles, which was by far larger than the variation occurring due to cell resting, (e.g., after the cell rest at cycle number 700. Between the first and the last internal resistance values, the increase was 43% for the case Δt = 1 s and 79% for the Δt = 10 s. Therefore, the end-of-discharge resistance was a good indicator of the battery aging, especially when SOL was at least 60%.
- The cell250 value showed a significant change only starting from 88% SOL.
- The cell500 had less regular behavior and showed significant increase, for the best case with Δt = 10 s, starting from around 80% SOL.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Battery Cycler | |
|---|---|
| Manufacturer | Digatron |
| Voltage range (V) | 0 ÷ 6 |
| Max current (A) | 250 |
| Voltage accuracy (% of full scale) | 0.05 |
| Current accuracy (% of full scale) | 0.1 |
| Min acquisition timestep (ms) | 10 |
| Thermocouples | |
| Type | “K” |
| Expanded uncertainty (°C) | 0.3 (95%) |
| Climatic Chamber 2 | |
| Manufacturer | Binder |
| Temperature range (°C) | −40 ÷ +180 |
| Internal volume (L) | 53 |
| Max set-point temperature oscillation (°C) | ± 0.3 |
| Climatic Chamber 1 | |
| Manufacturer | FDM |
| Temperature range (°C) | 0 ÷ +70 |
| Internal volume (L) | 370 |
| Max set-point temperature oscillation (°C) | ±0.5 |
References
- Batteries Europe Strategic Research Agenda for Batteries 2020 Report. Available online: https://ec.europa.eu/energy/topics/technology-and-innovation/batteries-europe/news-articles-and-publications/sra_en (accessed on 6 August 2021).
- Warner, J. The Handbook of Lithium-Ion Battery Pack Design; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Wikner, E.; Thiringer, T. Extending Battery Lifetime by Avoiding High SOC. Appl. Sci. 2018, 8, 1825. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Oudalov, A.; Ulbig, A.; Andersson, G.; Kirschen, D.S. Modeling of Lithium-Ion Battery degradation for Cell Life Assessment. IEEE Trans. Smart Grid 2018, 9, 1131–1140. [Google Scholar] [CrossRef]
- Khalik, Z.; Bergveld, H.J.; Donkers, M.C.F. Ageing-Aware Charging of Lithium-Ion Batteries Using an Electrochemistry-Based Model with Capacity-Loss Side Reactions. In Proceedings of the American Control Conference (ACC), Denver, CO, USA, 1–3 July 2020; pp. 2213–2218. [Google Scholar]
- Motapon, S.N.; Lachance, E.; Dessaint, L.A.; Al-Haddad, K. A generic cycle life model for lithium batteries based on fatigue theory and equivalent cycle counting. IEEE Open J. Ind. Electron. Soc. 2020, 1, 207–217. [Google Scholar] [CrossRef]
- SUMA Project. Available online: https://www.destec.unipi.it/ricerca/progetti-nazionali-ed-internazionali/far-fas-2014/568-progetto-suma (accessed on 4 May 2021).
- Ceraolo, M.; Lutzemberger, G.; Poli, D.; Scarpelli, C. Experimental analysis of LFP lithium cells aging. In Proceedings of the IEEE International Conference on Environment and Electrical Engineering and 2020 IEEE Industrial and Commercial Power Systems Europe (EEEIC/I&CPS Europe), Madrid, Spain, 9–12 June 2020; pp. 1–6. [Google Scholar]
- Digatron Official Site. Available online: https://www.digatron.com/en-us/Solutions/Battery-Laboratory (accessed on 4 May 2021).
- Binder Official Site. Available online: https://www.binder-world.com/it (accessed on 4 May 2021).
- Winston Batteries Official Site. Available online: http://en.winston-battery.com/ (accessed on 4 May 2021).
- Jaguemont, J.; Boulon, L.; Venet, P.; Dubé, Y.; Sari, A. Low temperature aging tests for lithium-ion batteries. In Proceedings of the IEEE 24th International Symposium on Industrial Electronics (ISIE), Rio de Janeiro, Brazil, 3–5 June 2015; pp. 1284–1289. [Google Scholar]
- Lee, H.; Park, J.; Kim, J. Comparative analysis of the SOH estimation based on various resistance parameters for LiCoO2 cells. In Proceedings of the IEEE Transportation Electrification Conference and Expo, Asia-Pacific (ITEC Asia-Pacific), Busan, Korea, 1–4 June 2016; pp. 788–792. [Google Scholar]
- Jarraya, I.; Loukil, J.; Masmoudi, F.; Chabchoub, M.H.; Trabelsi, H. Modeling and Parameters Estimation for Lithium-Ion Cells in Electric Drive Vehicle. In Proceedings of the 15th International Multi-Conference on Systems, Signals & Devices (SSD), Hammamet, Tunisia, 19–22 March 2018; pp. 1128–1132. [Google Scholar]
- Gurjer, L.; Chaudhary, P.; Verma, H.K. Detailed Modelling Procedure for Lithium-ion Battery Using Thevenin Equivalent. In Proceedings of the IEEE International Conference on Electrical, Computer and Communication Technologies (ICECCT), Coimbatore, India, 20–22 February 2019; pp. 1–6. [Google Scholar]
- Tan, X.; Tan, Y.; Zhan, D.; Yu, Z.; Fan, Y.; Qiu, J.; Li, J. Real-Time State-of-Health Estimation of Lithium-Ion Batteries Based on the Equivalent Internal Resistance. IEEE Access 2020, 8, 56811–56822. [Google Scholar] [CrossRef]
- Mathew, M.; Janhunen, S.; Rashid, M.; Long, F.; Fowler, M. Comparative Analysis of Lithium-Ion Battery Resistance Estimation Techniques for Battery Management Systems. Energies 2018, 11, 1490. [Google Scholar] [CrossRef] [Green Version]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A review on lithium-ion battery ageing mechanisms and estimations for automotive applications. J. Power Sources 2013, 2013, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, V. Standard for the Performance and Durability Assessment of Electric Vehicles Batteries; JRC Technical Reports; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Barai, A.; Uddin, K.; Widanage, W.D.; McGordon, A.; Jennings, P. A study of the influence of measurement timescale on internal resistance characterisation methodologies for lithium-ion cells. Sci. Rep. 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.J.; Lee, J.M.; Cho, B.H. A New Direct Current Internal Resistance and State of Charge Relationship for the Li-Ion Battery Pulse Power Estimation. In Proceedings of the IEEE International Conference on Power Electronics, Daegu, Korea, 22–26 October 2007; p. 1173. [Google Scholar]




| Manufacturer | Winston Battery |
| Model name | WB-LYP060AHA |
| Chemistry | LFP |
| Nominal capacity (Ah) | 60 |
| Nominal voltage (V) | 3.2 |
| Operating voltage (V) | max 4.0–min 2.8 |
| Deep discharge voltage (V) | 2.5 (damage if below) |
| Optimal discharge current (A) | 30 |
| Max discharge current (A) | 180 |
| Max peak discharge current (A) | 600 (5 s max) |
| Optimal charge current (A) | 30 |
| Max charge current (A) | 180 |
| Max operating temperature (°C) | 80 |
| Dimension (mm) | 114 × 203 × 61 |
| Weight (kg) | 2.3 |
| Cell under Test | Test Power (W) | DOD (%) | Average Discharge Current (A) | |
|---|---|---|---|---|
| Cell250 | 250 | 33 | Cycle #10 | Cycle #4950 |
| 78.0 | 84.9 | |||
| Cell375 | 375 | 50 | Cycle #10 | Cycle #1060 |
| 118.1 | 124.9 | |||
| Cell500 | 500 | 70 | Cycle #10 | Cycle #430 |
| 161.0 | 167.0 | |||
| Test Power (W) | Number of Cycles Performed | Lifetime Energy (kWh) |
|---|---|---|
| 500 | 430 | 53.8 |
| 375 | 1060 | 100.3 |
| 250 | 4950 | 309.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceraolo, M.; Lutzemberger, G.; Poli, D.; Scarpelli, C. Experimental Evaluation of Aging Indicators for Lithium–Iron–Phosphate Cells. Energies 2021, 14, 4813. https://doi.org/10.3390/en14164813
Ceraolo M, Lutzemberger G, Poli D, Scarpelli C. Experimental Evaluation of Aging Indicators for Lithium–Iron–Phosphate Cells. Energies. 2021; 14(16):4813. https://doi.org/10.3390/en14164813
Chicago/Turabian StyleCeraolo, Massimo, Giovanni Lutzemberger, Davide Poli, and Claudio Scarpelli. 2021. "Experimental Evaluation of Aging Indicators for Lithium–Iron–Phosphate Cells" Energies 14, no. 16: 4813. https://doi.org/10.3390/en14164813
APA StyleCeraolo, M., Lutzemberger, G., Poli, D., & Scarpelli, C. (2021). Experimental Evaluation of Aging Indicators for Lithium–Iron–Phosphate Cells. Energies, 14(16), 4813. https://doi.org/10.3390/en14164813








