Valorization of Lignin Side-Streams into Polyols and Rigid Polyurethane Foams—A Contribution to the Pulp and Paper Industry Biorefinery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
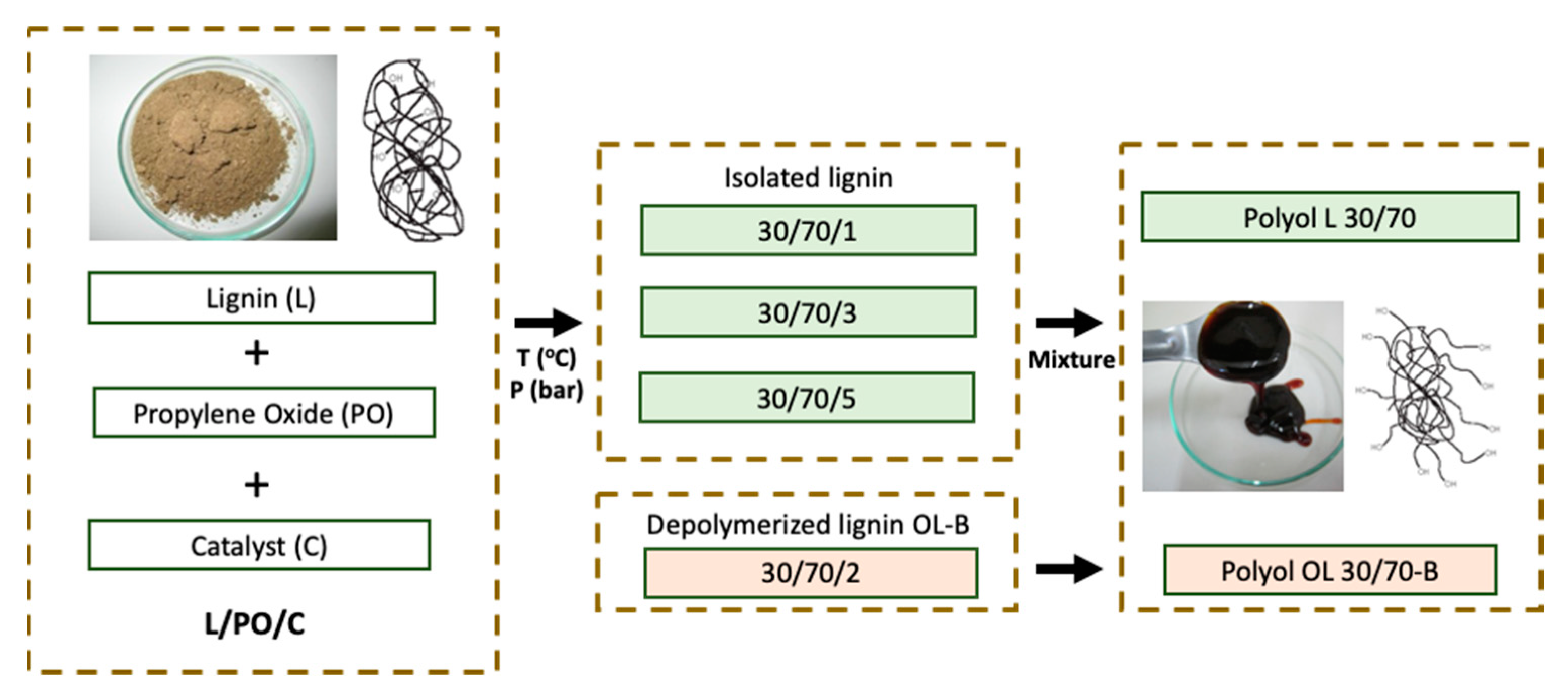
2.2. Oxypropylation Reaction Conditions and Procedure
2.3. Lignin-Based Polyol Characterization
2.4. RPU Foam Production
2.5. RPU Foams Characterization
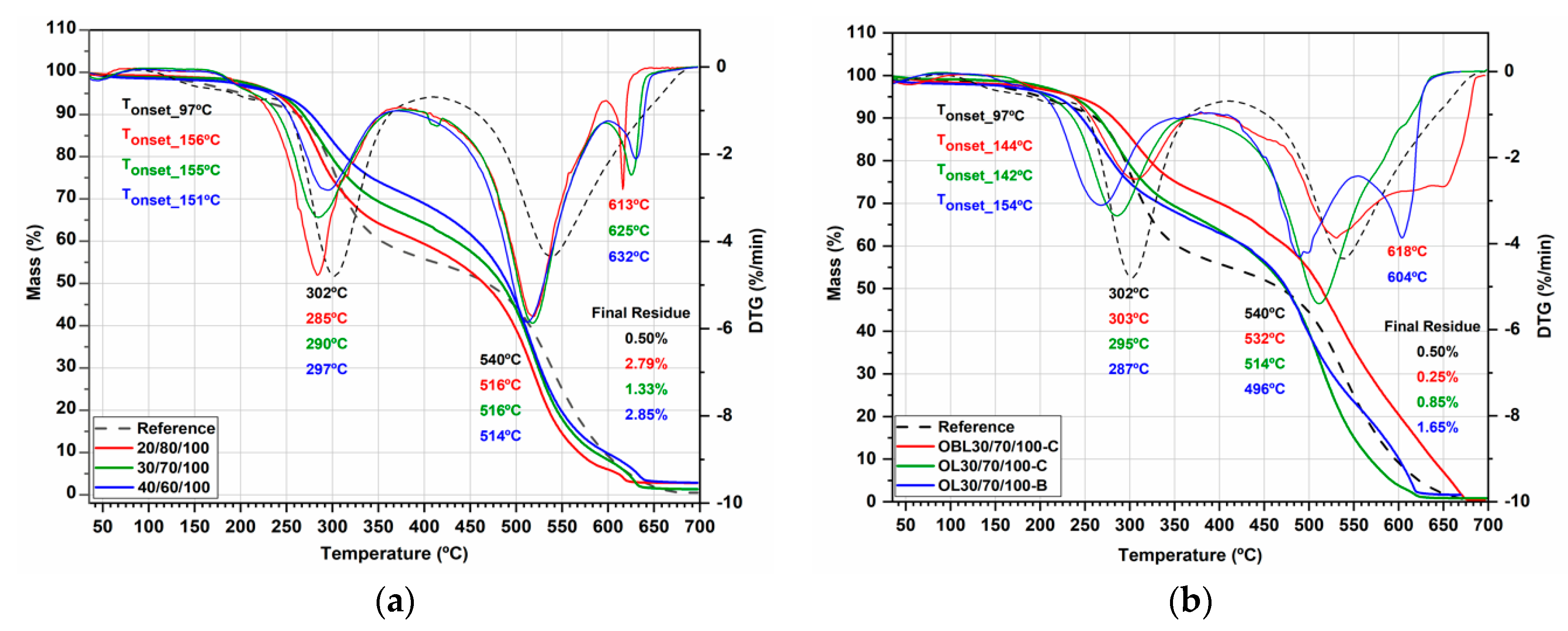
2.5.1. Thermogravimetric Analysis
2.5.2. SEM Analysis
2.5.3. Apparent Density Determination
2.5.4. Conductivity Measurements
2.5.5. Cone Calorimeter
3. Results and Discussion
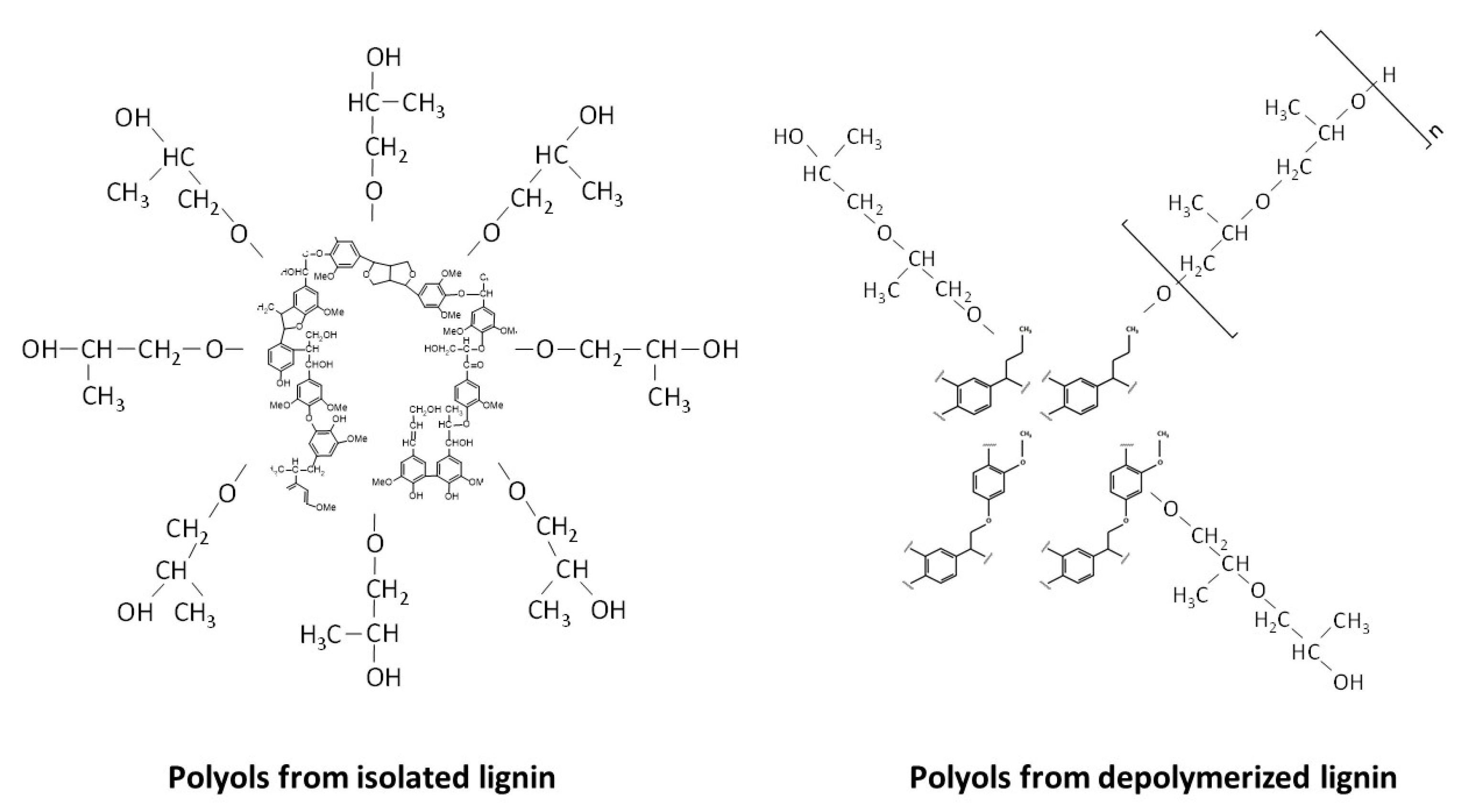
3.1. Lignin-Based Polyols
3.2. RPU Foam Synthesis and Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission Green Deal: Commission Adopts New Chemicals Strategy towards a Toxic-Free Environment. 2020. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 8 January 2021).
- Garlapati, V.K.; Chandel, A.K.; Kumar, S.J.; Sharma, S.; Sevda, S.; Ingle, A.P.; Pant, D. Circular economy aspects of lignin: Towards a lignocellulose biorefinery. Renew. Sustain. Energy Rev. 2020, 130, 109977. [Google Scholar] [CrossRef]
- Cadena, E.; Rocca, F.; Gutierrez, J.A.; Carvalho, A. Social life cycle assessment methodology for evaluating production process design: Biorefinery case study. J. Clean. Prod. 2019, 238, 117718. [Google Scholar] [CrossRef]
- Leibensperger, C.; Yang, P.; Zhao, Q.; Wei, S.; Cai, X. The synergy between stakeholders for cellulosic biofuel development: Perspectives, opportunities, and barriers. Renew. Sustain. Energy Rev. 2021, 137, 110613. [Google Scholar] [CrossRef]
- Falcone, P.M. Analysing stakeholders’ perspectives towards a socio-technical change: The energy transition journey in Gela Municipality. AIMS Energy 2018, 6, 645–657. [Google Scholar] [CrossRef]
- Glasser, W.G. About Making Lignin Great Again—Some Lessons from the Past. Front. Chem. 2019, 7, 565. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.E.; Pinto, P.C.D.O.R.; Barreiro, M.F.; Da Costa, C.A.E.; Da Mota, M.I.F.; Fernandes, I. An Integrated Approach for Added-Value Products from Lignocellulosic Biorefineries. In An Integrated Approach for Added-Value Products from Lignocellulosic Biorefineries; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- da Silva, E.B.; Zabkova, M.; Araújo, J.; Cateto, C.; Barreiro, M.; Belgacem, M.; Rodrigues, A. An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292. [Google Scholar] [CrossRef]
- Ma, C.; Mei, X.; Fan, Y.; Zhang, Z. Oxidative Depolymerizaton of Kraft Lignin and its Application in the Synthesis of Lignin-phenol- formaldehyde Resin. Bioresources 2017, 13, 1223–1234. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Tymchyshyn, M.; Xu, C.C. Hydrolytic liquefaction of hydrolysis lignin for the preparation of bio-based rigid polyurethane foam. Green Chem. 2016, 18, 2385–2398. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, Y.; Eberhardt, T.L.; Shmulsky, R. Lab-scale structural insulated panels with lignin-incorporated rigid polyurethane foams as core. Ind. Crop. Prod. 2019, 132, 292–300. [Google Scholar] [CrossRef]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef]
- Luo, S.; Gao, L.; Guo, W. Effect of incorporation of lignin as bio-polyol on the performance of rigid lightweight wood–polyurethane composite foams. J. Wood Sci. 2020, 66, 66. [Google Scholar] [CrossRef]
- Li, B.; Zhou, M.; Huo, W.; Cai, D.; Qin, P.; Cao, H.; Tan, T. Fractionation and oxypropylation of corn-stover lignin for the production of biobased rigid polyurethane foam. Ind. Crop. Prod. 2020, 143, 111887. [Google Scholar] [CrossRef]
- Zhang, X.; Jeremic, D.; Kim, Y.; Street, J.; Shmulsky, R. Effects of Surface Functionalization of Lignin on Synthesis and Properties of Rigid Bio-Based Polyurethanes Foams. Polymers 2018, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Optimization Study of Lignin Oxypropylation in View of the Preparation of Polyurethane Rigid Foams. Ind. Eng. Chem. Res. 2009, 48, 2583–2589. [Google Scholar] [CrossRef]
- Li, Y.; Ragauskas, A. Kraft Lignin-Based Rigid Polyurethane Foam. J. Wood Chem. Technol. 2012, 32, 210–224. [Google Scholar] [CrossRef]
- Kurańska, M.; Pinto, J.; Salach, K.; Barreiro, M.; Prociak, A. Synthesis of thermal insulating polyurethane foams from lignin and rapeseed based polyols: A comparative study. Ind. Crop. Prod. 2020, 143, 111882. [Google Scholar] [CrossRef]
- Abid, A.; Brosse, N.; Ziegler-Devin, I.; Gabsi, S. Production and characterization of rigid polyurethane foam by oxypropylation of organosolv lignin extracted from exhausted olive pomace. J. Polym. Res. 2020, 27, 1–7. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Preparation of bio-based rigid polyurethane foam using hydrolytically depolymerized Kraft lignin via direct replacement or oxypropylation. Eur. Polym. J. 2015, 68, 1–9. [Google Scholar] [CrossRef]
- Ihalainen, P.; Antunes, J.; Levée, T. Application of Enzymatically Treated Lignin Oligomers as Lignopolyols For A Full Replacement of Commercial Polyols in Polyurethane Foam Formulation. Biomed. J. Sci. Tech. Res. 2019, 24, 17898–17904. [Google Scholar] [CrossRef][Green Version]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Malucelli, G.; Guan, J.-P. An Overview on the Use of Lignin and Its Derivatives in Fire Retardant Polymer Systems. In Lignin-Trends and Applications; Poletto, M., Ed.; IntechOpen: London, UK, 2018; Volume 32, pp. 137–144. [Google Scholar]
- Lu, W.; Li, Q.; Zhang, Y.; Yu, H.; Hirose, S.; Hatakeyama, H.; Matsumoto, Y.; Jin, Z. Lignosulfonate/APP IFR and its flame retardancy in lignosulfonate-based rigid polyurethane foams. J. Wood Sci. 2018, 64, 287–293. [Google Scholar] [CrossRef]
- De Rezende, S.C.; Jo, A.P.; Fernandes, I.P.; Barreiro, F.V.L.A.M.-F. Oxypropylation of Brazilian Pine-Fruit Shell Evaluated by Principal Component Analysis. J. Renew. Mater. 2018, 6, 715–723. [Google Scholar] [CrossRef]
- Pavier, C.; Gandini, A. Oxypropylation of sugar beet pulp. 1. Optimisation of the reaction. Ind. Crop. Prod. 2000, 12, 1–8. [Google Scholar] [CrossRef]
- Pinto, J.A.; Prieto, M.A.; Ferreira, I.C.; Belgacem, M.N.; Rodrigues, A.E.; Barreiro, M.F. Analysis of the oxypropylation process of a lignocellulosic material, almond shell, using the response surface methodology (RSM). Ind. Crop. Prod. 2020, 153, 112542. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Szalaty, T.J.; Jesionowski, T. Depolymerization and Activation of Lignin: Current State of Knowledge and Perspectives. In Lignin-Trends and Applications; IntechOpen: London, UK, 2018; pp. 1–27. [Google Scholar]
- Fernández-Rodríguez, J.; Erdocia, X.; De Hoyos, P.L.; Alriols, M.G.; Labidi, J. Small phenolic compounds production from Kraft black liquor by lignin depolymerization with different catalytic agents. Chem. Eng. Trans. 2017, 57, 133–138. [Google Scholar]
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- He, J.-J.; Jiang, L.; Sun, J.-H.; Lo, S.M. Thermal degradation study of pure rigid polyurethane in oxidative and non-oxidative atmospheres. J. Anal. Appl. Pyrolysis 2016, 120, 269–283. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Hirose, S.; Kobashigawa, K.; Izuta, Y.; Hatakeyama, H. Thermal degradation of polyurethanes containing lignin studied by TG-FTIR. Polym. Int. 1998, 47, 247–256. [Google Scholar] [CrossRef]
- Shen, D.; Hu, J.; Xiao, R.; Zhang, H.; Li, S.; Gu, S. Online evolved gas analysis by Thermogravimetric-Mass Spectroscopy for thermal decomposition of biomass and its components under different atmospheres: Part I. Lignin. Bioresour. Technol. 2013, 130, 449–456. [Google Scholar] [CrossRef]
- Ahmad, Z.; Al Dajani, W.W.; Paleologou, M.; Xu, C.C. Sustainable Process for the Depolymerization/Oxidation of Softwood and Hardwood Kraft Lignins Using Hydrogen Peroxide under Ambient Conditions. Molecules 2020, 25, 2329. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]


| Formulation (L/PO/C, g/mL/%) | POO (%, w/w) | IOH (mg KOH/g) | Viscosity (20 °C, Pa·s) |
|---|---|---|---|
| L 20/80 | 42.7 | 302.4 | 4.8 |
| L 30/70 | 24.4 | 332.3 | 31.1 |
| L 40/60 | 6.3 | 387.7 | 221.5 |
| OL 30/70-B | 15.9 | 297.1 | 134.3 |
| OL 30/70-C | 16.8 | 349.8 | 169.5 |
| OBL 30/70-C | 10.9 | 397.5 | 456.5 |
| Formulation | Average Cell Diameter (µm) | Apparent Density (Kg/m3) | Thermal Conductivity (mW/mK) |
|---|---|---|---|
| 20/80/100 | 269 ± 49 | 44.7 | 37.2 |
| 30/70/100 | 274 ± 50 | 54.0 | 39.9 |
| 40/60/100 | 216 ± 75 | 71.7 | 40.1 |
| OL 30/70/100-B | 291 ± 107 | 81.3 | 44.6 |
| OL 30/70/100-C | 147 ± 28 | 46.8 | 38.5 |
| OBL 30/70/100-C | 252 ± 92 | 112.2 | 49.0 |
| Reference foam | 317 ± 98 | 70.9 | 41.1 |
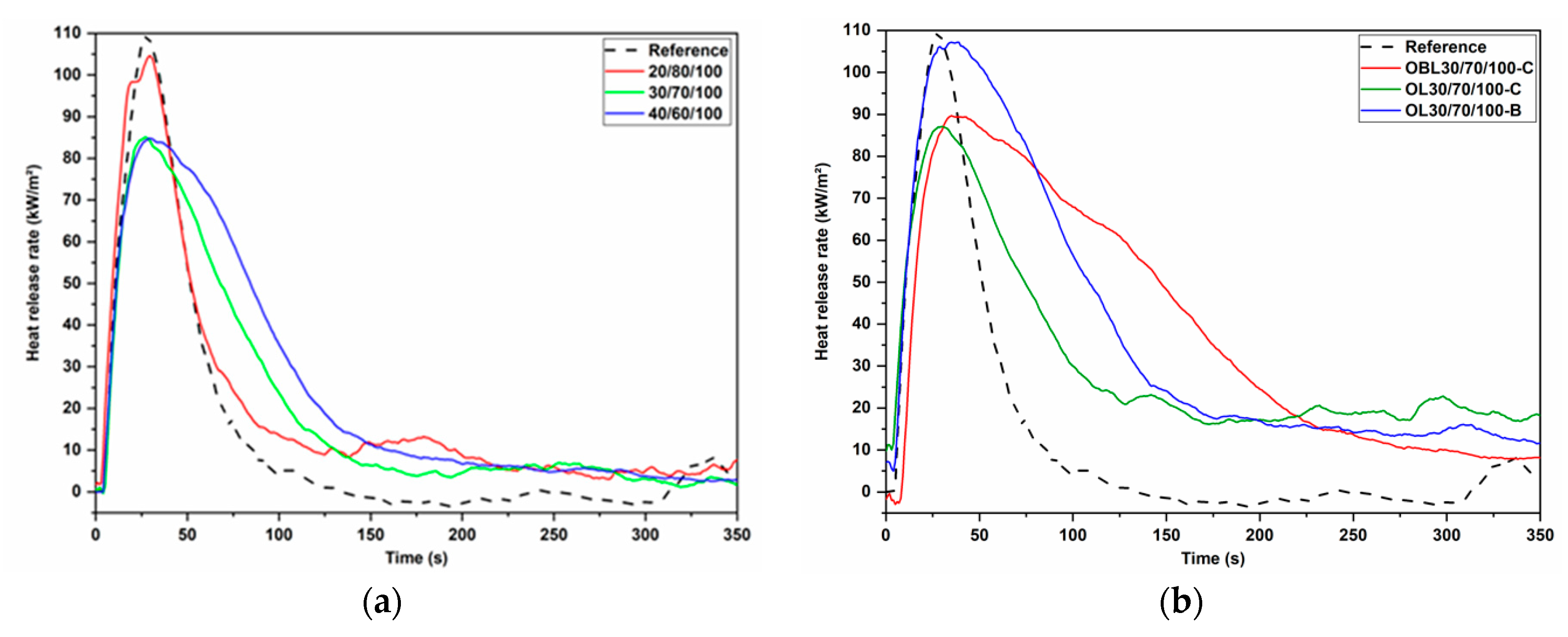
| Formulation | Time to Ignition (s) | Time to Flameout (s) | pHRR (kW/m²) |
|---|---|---|---|
| 20/80/100 | 2.0 | 47.0 | 104.5 |
| 30/70/100 | 4.0 | 106.0 | 85.1 |
| 40/60/100 | 2.0 | 122.0 | 84.8 |
| OL 30/70/100-B | 3.0 | 134.0 | 107.2 |
| OL 30/70/100-C | 2.0 | 105.0 | 87.1 |
| OLB 30/70/100-C | 7.0 | 244.0 | 89.7 |
| Reference foam | 3.0 | 60.0 | 109.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, J.A.; Fernandes, I.P.; Pinto, V.D.; Gomes, E.; Oliveira, C.F.; C. R. Pinto, P.; Mesquita, L.M.R.; Piloto, P.A.G.; Rodrigues, A.E.; Barreiro, M.-F. Valorization of Lignin Side-Streams into Polyols and Rigid Polyurethane Foams—A Contribution to the Pulp and Paper Industry Biorefinery. Energies 2021, 14, 3825. https://doi.org/10.3390/en14133825
Pinto JA, Fernandes IP, Pinto VD, Gomes E, Oliveira CF, C. R. Pinto P, Mesquita LMR, Piloto PAG, Rodrigues AE, Barreiro M-F. Valorization of Lignin Side-Streams into Polyols and Rigid Polyurethane Foams—A Contribution to the Pulp and Paper Industry Biorefinery. Energies. 2021; 14(13):3825. https://doi.org/10.3390/en14133825
Chicago/Turabian StylePinto, João A., Isabel P. Fernandes, Virginia D. Pinto, Elson Gomes, Cátia F. Oliveira, Paula C. R. Pinto, Luís M. R. Mesquita, Paulo A. G. Piloto, Alírio E. Rodrigues, and Maria-Filomena Barreiro. 2021. "Valorization of Lignin Side-Streams into Polyols and Rigid Polyurethane Foams—A Contribution to the Pulp and Paper Industry Biorefinery" Energies 14, no. 13: 3825. https://doi.org/10.3390/en14133825
APA StylePinto, J. A., Fernandes, I. P., Pinto, V. D., Gomes, E., Oliveira, C. F., C. R. Pinto, P., Mesquita, L. M. R., Piloto, P. A. G., Rodrigues, A. E., & Barreiro, M.-F. (2021). Valorization of Lignin Side-Streams into Polyols and Rigid Polyurethane Foams—A Contribution to the Pulp and Paper Industry Biorefinery. Energies, 14(13), 3825. https://doi.org/10.3390/en14133825










