Hydrothermal Pretreatment of Water-Extracted and Aqueous Ethanol-Extracted Quinoa Stalks for Enzymatic Saccharification of Cellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Saponin Removal
2.3. Hydrothermal Pretreatment
2.4. Determination of the Composition of the Raw Material and Processed Solids
2.5. Enzymatic Hydrolysis
2.6. Analysis of Liquid Samples
3. Results
3.1. Saponin Removal
3.2. Pretreatment of Pre-Extracted Quinoa Stalks
3.3. Yield and Composition of the Pretreated Solids
3.4. Composition of the Pretreatment Liquids
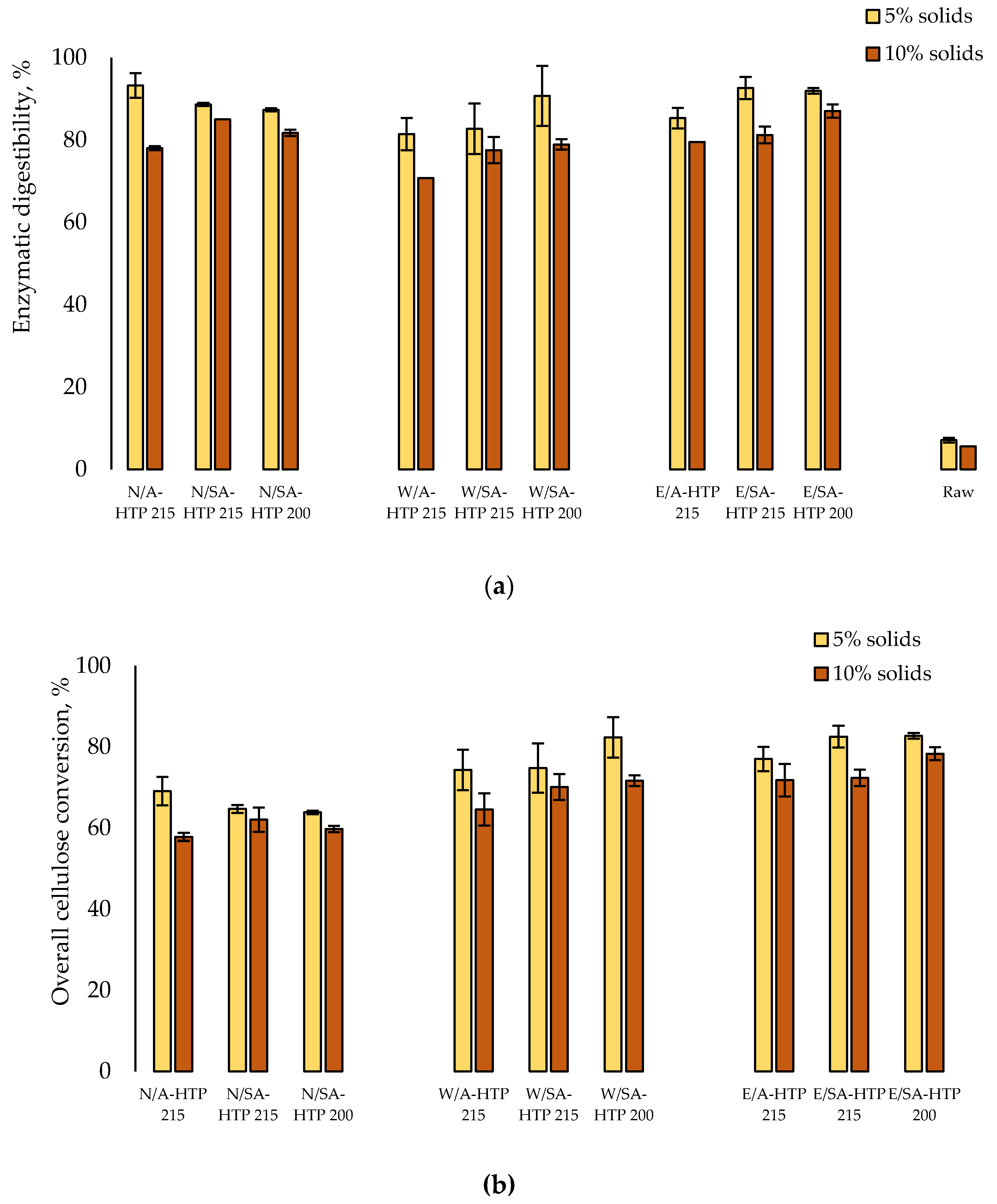
3.5. Enzymatic Hydrolysis of Cellulose
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Boboescu, I.Z.; Chemarin, F.; Beigbeder, J.-B.; de Vasconcelos, B.R.; Munirathinam, R.; Ghislain, T.; Lavoie, J.-M. Making next-generation biofuels and biocommodities a feasible reality. Curr. Opin. Green Sustain. Chem. 2019, 20, 25–32. [Google Scholar] [CrossRef]
- Gandla, M.L.; Martín, C.; Jönsson, L.J. Analytical enzymatic saccharification of lignocellulosic biomass for conversion to biofuels and bio-based chemicals. Energies 2018, 11, 2936. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, I.; Flury, K.; Jungbluth, N.; Rigarlsford, G.; Canals, L.M.i.; King, H. Life cycle assessment of bio-based ethanol produced from different agricultural feedstocks. Int. J. Life Cycle Assess. 2014, 19, 109–119. [Google Scholar] [CrossRef]
- Martín, C.; Wei, M.; Xiong, S.; Jönsson, L.J. Enhancing saccharification of cassava stems by starch hydrolysis prior to pretreatment. Ind. Crops Prod. 2017, 97, 21–31. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Huang, H.; Dien, B.S.; Singh, V. The costs of sugar production from different feedstocks and processing technologies. Biofuels Bioprod. Biorefin. 2019, 13, 723–739. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic biorefineries in Europe: Current state and prospects. Trends Biotechnol. 2019, 37, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci. Total Environ. 2020, 743, 140630. [Google Scholar] [CrossRef] [PubMed]
- Martín, C. Pretreatment of crop residues for bioconversion. Agronomy 2021, 11, 924. [Google Scholar] [CrossRef]
- FAOSTAT Food and Agricultural Organization of the United Nations Statistical Database. Rome. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 25 January 2021).
- Carrasco, C.; Cuno, D.; Carlqvist, K.; Galbe, M.; Lidén, G. SO2-catalysed steam pretreatment of quinoa stalks. J. Chem. Technol. Biotechnol. 2015, 90, 64–71. [Google Scholar] [CrossRef]
- Chambi, D.; Romero-Soto, L.; Villca, R.; Orozco-Gutiérrez, F.; Vega-Baudrit, J.; Quillaguamán, J.; Hatti-Kaul, R.; Martín, C.; Carrasco, C. Exopolysaccharides production by cultivating a bacterial isolate from the hypersaline environment of Salar de Uyuni (Bolivia) in pretreatment liquids of steam-exploded quinoa stalks and enzymatic hydrolysates of Curupaú sawdust. Fermentation 2021, 7, 33. [Google Scholar] [CrossRef]
- Giummarella, N.; Pu, Y.; Ragauskas, A.J.; Lawoko, M. A critical review on the analysis of lignin carbohydrate bonds. Green Chem. 2019, 21, 1573–1595. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.; Ragauskas, A. Pretreatment and lignocellulosic chemistry. Bioenergy Res. 2012, 5, 1043–1066. [Google Scholar] [CrossRef]
- Martin, C.; Alriksson, B.; Sjöde, A.; Nilvebrant, N.-O.; Jönsson, L.J. Dilute sulfuric acid pretreatment of agricultural and agro-industrial residues for ethanol production. Appl. Biochem. Biotechnol. 2007, 137–140, 339–352. [Google Scholar] [CrossRef]
- López, Y.; Gullón, B.; Puls, J.; Parajó, J.C.; Martín, C. Dilute acid pretreatment of starch-containing rice hulls for ethanol production: 11th EWLP, Hamburg, Germany, August 16–19, 2010. Holzforschung 2011, 65, 467–473. [Google Scholar] [CrossRef]
- Silveira, M.H.L.; Morais, A.R.C.; da Costa Lopes, A.M.; Olekszyszen, D.N.; Bogel-Łukasik, R.; Andreaus, J.; Pereira Ramos, L. Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. ChemSusChem 2015, 8, 3366–3390. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.S.; Jahangir, M.; ul Hussan, S.S.; Choudhary, M.I. Inhibition of α-glucosidase by oleanolic acid and its synthetic derivatives. Phytochemistry 2002, 60, 295–299. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Miranda, M.; Lemus-Mondaca, R.; Lozano, M.; An-Hen, K. A kinetic approach to saponin extraction during washing of quinoa (Chenopodium quinoa Willd.) Seeds. J. Food Process Eng. 2013, 36, 202–210. [Google Scholar] [CrossRef]
- Lim, J.G.; Park, H.-M.; Yoon, K.S. Analysis of saponin composition and comparison of the antioxidant activity of various parts of the quinoa plant (Chenopodium quinoa Willd.). Food Sci. Nutr. 2020, 8, 694–702. [Google Scholar] [CrossRef] [Green Version]
- Gil-Ramirez, A.; Salas-Veizaga, D.M.; Grey, C.; Karlsson, E.N.; Rodriguez-Meizoso, I.; Linares-Pastén, J.A. Integrated process for sequential extraction of saponins, xylan and cellulose from quinoa stalks (Chenopodium quinoa Willd.). Ind. Crops Prod. 2018, 121, 54–65. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Herrera, T.; García-Risco, M.R.; Fornari, T.; Reglero, G.; Martin, D. Ultrasound-assisted extraction and bioaccessibility of saponins from edible seeds: Quinoa, lentil, fenugreek, soybean and lupin. Food Res. Int. 2018, 109, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Lozano, M.; Tícona, E.; Carrasco, C.; Flores, Y.; Almanza, G.R. Cuantificación de saponinas en residuos de quinua real Chenopodium quinoa Willd. Rev. Boliv. Quím. 2012, 29, 131–138. [Google Scholar]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A.; Hartley, B.S.; Broda, P.M.A.; Senior, P.J. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Sluiter, A. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); Technical Report for National Renewable Energy Laboratory: Golden, CO, USA, 2008; p. 18. [Google Scholar]
- Koziol, M.J. Afrosimetric estimation of threshold saponin concentration for bitterness in quinoa (Chenopodium quinoa Willd). J. Sci. Food Agric. 1991, 54, 211–219. [Google Scholar] [CrossRef]
- Monje, C.Y.; Raffaillac, J.P. Determinación de saponina total en quinua (Chenopodium quinoa Willd.) método espectrofotométrico. In Proceedings of the Memorias del IV Congreso Nacional de la Asociación Boliviana de Protección Vegetal, Oruro, Bolivia, 5–7 April 2006; p. 217. [Google Scholar]
- Martín, C.; Peinemann, J.C.; Wei, M.; Stagge, S.; Xiong, S.; Jönsson, L.J. Dilute-sulfuric acid pretreatment of de-starched cassava stems for enhancing the enzymatic convertibility and total glucan recovery. Ind. Crops Prod. 2019, 132, 301–310. [Google Scholar] [CrossRef]
- Salas-Veizaga, D.M.; Villagomez, R.; Linares-Pastén, J.A.; Carrasco, C.; Álvarez, M.T.; Adlercreutz, P.; Nordberg Karlsson, E. Extraction of glucuronoarabinoxylan from quinoa stalks (Chenopodium quinoa Willd.) and Evaluation of xylooligosaccharides produced by GH10 and GH11 xylanases. J. Agric. Food Chem. 2017, 65, 8663–8673. [Google Scholar] [CrossRef] [PubMed]
- Chum, H.L.; Johnson, D.K.; Black, S.K.; Overend, R.P. Pretreatment-catalyst effects and the combined severity parameter. Appl. Biochem. Biotechnol. 1990, 24, 1–14. [Google Scholar] [CrossRef]
- Shinde, S.D.; Meng, X.; Kumar, R.; Ragauskas, A.J. Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem. 2018, 20, 2192–2205. [Google Scholar] [CrossRef] [Green Version]
- Ilanidis, D.; Stagge, S.; Jönsson, L.J.; Martín, C. Effects of operational conditions on auto-catalyzed and sulfuric-acid-catalyzed hydrothermal pretreatment of sugarcane bagasse at different severity factor. Ind. Crops Prod. 2021, 159, 113077. [Google Scholar] [CrossRef]
- Ilanidis, D.; Stagge, S.; Jönsson, L.J.; Martín, C. Hydrothermal pretreatment of wheat straw: Effects of temperature and acidity on byproduct formation and inhibition of enzymatic hydrolysis and ethanolic fermentation. Agronomy 2021, 11, 487. [Google Scholar] [CrossRef]
- Du, J.; Cao, Y.; Liu, G.; Zhao, J.; Li, X.; Qu, Y. Identifying and overcoming the effect of mass transfer limitation on decreased yield in enzymatic hydrolysis of lignocellulose at high solid concentrations. Bioresour. Technol. 2017, 229, 88–95. [Google Scholar] [CrossRef]
- Weiss, N.D.; Felby, C.; Thygesen, L.G. Enzymatic hydrolysis is limited by biomass–water interactions at high-solids: Improved performance through substrate modifications. Biotechnol. Biofuels 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Väljamäe, P.; Pettersson, G.; Johansson, G. Mechanism of substrate inhibition in cellulose synergistic degradation. Eur. J. Biochem. 2001, 268, 4520–4526. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lei, F.; Cristhian, C.; Liu, Z.; Yu, H.; Jiang, J. Enhancement of fermentable sugar yield by competitive adsorption of non-enzymatic substances from yeast and cellulase on lignin. BMC Biotechnol. 2014, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Oliva-Taravilla, A.; Carrasco, C.; Jönsson, L.J.; Martín, C. Effects of biosurfactants on enzymatic saccharification and fermentation of pretreated softwood. Molecules 2020, 25, 3559. [Google Scholar] [CrossRef] [PubMed]


| Codification 1 | Solvent | Temperature, °C | Time, min | H2SO4 Load, % (w/w) | Log R0(IT) 2 |
|---|---|---|---|---|---|
| N/A-HTP 215 | None | 215 | 1.8 | - | 3.6 |
| N/SA-HTP 215 | None | 215 | 1.8 | 0.2 | 3.6 |
| N/SA-HTP 200 | None | 200 | 5.0 | 0.2 | 3.6 |
| W/A-HTP 215 | H2O | 215 | 1.8 | - | 3.6 |
| W/SA-HTP 215 | H2O | 215 | 1.8 | 0.2 | 3.6 |
| W/SA-HTP 200 | H2O | 200 | 5.0 | 0.2 | 3.6 |
| E/A-HTP 215 | Aq. ethanol | 215 | 1.8 | - | 3.6 |
| E/SA-HTP 215 | Aq. ethanol | 215 | 1.8 | 0.2 | 3.6 |
| E/SA-HTP 200 | Aq. ethanol | 200 | 5.0 | 0.2 | 3.6 |
| Extracting Solvent | Yield of Solids | Saponins | Glucan | Xylan | Arabinan | Galactan | Mannan | Lignin |
|---|---|---|---|---|---|---|---|---|
| None | - | 5.2 (0.2) | 34.6 (1.3) | 22.7 (<0.1) | 2.2 (0.1) | 2.0 (<0.1) | 1.5 (<0.1) | 19.5 (0.1) |
| Water | 86.2 | 2.5 (<0.1) | 39.0 (0.5) | 25.7 (5.0) | 1.9 (0.3) | 2.0 (0.3) | 1.8 (0.2) | 22.0 (0.7) |
| Ethanol | 92.3 | 1.3 (0.1) | 36.8 (0.6) | 23.2 (0.5) | 2.1 (0.2) | 2.0 (<0.1) | 1.7 (0.1) | 20.6 (0.8) |
| Pretreatment Codification 2 | Log R0(O) 3 | pH | CS 4 | Yield of Pretreated Solids, % (w/w) | Volume of the Pretreatment Liquid, mL |
|---|---|---|---|---|---|
| N/A-HTP 215 | 4.1 | 3.5 | 0.6 | 59.6 | 98 |
| N/SA-HTP 215 | 4.0 | 2.7 | 1.3 | 57.0 | 196 |
| N/SA-HTP 200 | 3.7 | 2.6 | 1.1 | 56.8 | 187 |
| W/A-HTP 215 | 4.0 | 3.5 | 0.5 | 60.0 | 123 |
| W/SA-HTP 215 | 4.0 | 2.2 | 1.8 | 58.4 | 162 |
| W/SA-HTP 200 | 3.7 | 2.2 | 1.5 | 59.9 | 140 |
| E/A-HTP 215 | 3.9 | 3.0 | 0.9 | 61.7 | 75 |
| E/SA-HTP 215 | 3.9 | 2.2 | 1.7 | 59.5 | 151 |
| E/SA-HTP 200 | 3.7 | 2.2 | 1.5 | 60.9 | 129 |
| Pretreatment Codification 1 | Glucan | Xylan | Arabinan | Galactan | Mannan | Lignin 2 |
|---|---|---|---|---|---|---|
| N/A-HTP 215 | 49.5 (0.3) | 2.9 (0.6) | 0.7 (<0.1) | 0.8 (0.1) | 1.3 (0.1) | 44.0 (1.8) |
| N/SA-HTP 215 | 52.1 (0.4) | 2.1 (0.2) | ND | 0.6 (<0.1) | 0.9 (0.1) | 44.7 (0.3) |
| N/SA-HTP 200 | 52.3 (0.2) | 2.7 (0.5) | ND | 0.6 (<0.1)) | 1.0 (0.3) | 43.0 (<0.1) |
| W/A-HTP 215 | 56.0 (1.2) | 3.1 (0.2) | ND | 0.7 (0.1) | 1.2 (0.1) | 40.1 (0.9) |
| W/SA-HTP 215 | 57.0 (0.9) | 3.2 (0.3) | ND | 0.6 (<0.1) | 1.1 (0.1) | 41.1 (1.9) |
| W/SA-HTP 200 | 56.2 (1.0) | 2.2 (0.1) | ND | ND | 0.8 (<0.1) | 41.1 (2.6) |
| E/A-HTP 215 | 48.3 (0.3) | 3.2 (0.1) | 0.7 (<0.1) | 0.8 (<0.1) | 1.6 (<0.1) | 43.6 (0.9) |
| E/SA-HTP 215 | 51.8 (5.7) | 2.6 (0.1) | ND | 0.7 (<0.1) | 1.2 (<0.1) | 42.6 (<0.1) |
| E/SA-HTP 200 | 50.3 (2.7) | 2.4 (<0.1) | ND | 0.7 (<0.1) | 1.0 (<0.1) | 44.0 (1.8) |
| Pretreatment Codification 1 | Glucose | Xylose | Arabinose | Galactose | Mannose |
|---|---|---|---|---|---|
| N/A-HTP 215 | 0.2 | 1.1 | 0.1 | 0.1 | 0.1 |
| N/SA-HTP 215 | 0.8 | 3.0 | 0.3 | 0.5 | 0.2 |
| N/SA-HTP 200 | 1.0 | 4.5 | 0.5 | 0.4 | 0.6 |
| W/A-HTP 215 | 0.1 | 1.4 | 0.1 | 0.1 | 0.2 |
| W/SA-HTP 215 | 0.7 | 5.9 | 0.4 | 0.4 | 0.8 |
| W/SA-HTP 200 | 0.5 | 8.2 | 0.6 | 0.7 | 1.0 |
| E/A-HTP 215 | 0.1 | 1.2 | 0.1 | 0.1 | 0.2 |
| E/SA-HTP 215 | 0.6 | 4.8 | 0.4 | 0.4 | 0.7 |
| E/SA-HTP 200 | 0.5 | 8.4 | 0.6 | 0.6 | 0.9 |
| Pretreatment Codification 1 | Furfural | HMF | Formic Acid | Levulinic Acid | Acetic Acid |
|---|---|---|---|---|---|
| N/A-HTP 215 | 0.9 | 0.2 | 0.9 | 0.5 | 1.6 |
| N/SA-HTP 215 | 3.3 | 0.8 | 2.5 | 2.2 | 3.9 |
| N/SA-HTP 200 | 1.6 | 0.4 | 2.4 | 1.3 | 3.7 |
| W/A-HTP 215 | 1.0 | 0.1 | 0.5 | 0.4 | 1.8 |
| W/SA-HTP 215 | 2.6 | 0.3 | 0.6 | 0.5 | 2.4 |
| W/SA-HTP 200 | 1.9 | 0.2 | 0.6 | 0.5 | 2.2 |
| E/A-HTP 215 | 0.6 | 0.1 | 0.2 | 0.1 | 0.6 |
| E/SA-HTP 215 | 3.1 | 0.4 | 0.4 | 0.2 | 1.2 |
| E/SA-HTP 200 | 1.6 | 0.2 | 0.4 | 0.2 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco, C.; Jönsson, L.J.; Martín, C. Hydrothermal Pretreatment of Water-Extracted and Aqueous Ethanol-Extracted Quinoa Stalks for Enzymatic Saccharification of Cellulose. Energies 2021, 14, 4102. https://doi.org/10.3390/en14144102
Carrasco C, Jönsson LJ, Martín C. Hydrothermal Pretreatment of Water-Extracted and Aqueous Ethanol-Extracted Quinoa Stalks for Enzymatic Saccharification of Cellulose. Energies. 2021; 14(14):4102. https://doi.org/10.3390/en14144102
Chicago/Turabian StyleCarrasco, Cristhian, Leif J. Jönsson, and Carlos Martín. 2021. "Hydrothermal Pretreatment of Water-Extracted and Aqueous Ethanol-Extracted Quinoa Stalks for Enzymatic Saccharification of Cellulose" Energies 14, no. 14: 4102. https://doi.org/10.3390/en14144102
APA StyleCarrasco, C., Jönsson, L. J., & Martín, C. (2021). Hydrothermal Pretreatment of Water-Extracted and Aqueous Ethanol-Extracted Quinoa Stalks for Enzymatic Saccharification of Cellulose. Energies, 14(14), 4102. https://doi.org/10.3390/en14144102








