Investigation of the Electrochemical Properties of Ni0.5Zn0.5Fe2O4 as Binder-Based and Binder-Free Electrodes of Supercapacitors
Abstract
1. Introduction
2. Synthesis Process
2.1. Materials
2.2. Treatment of the Nickel Foam
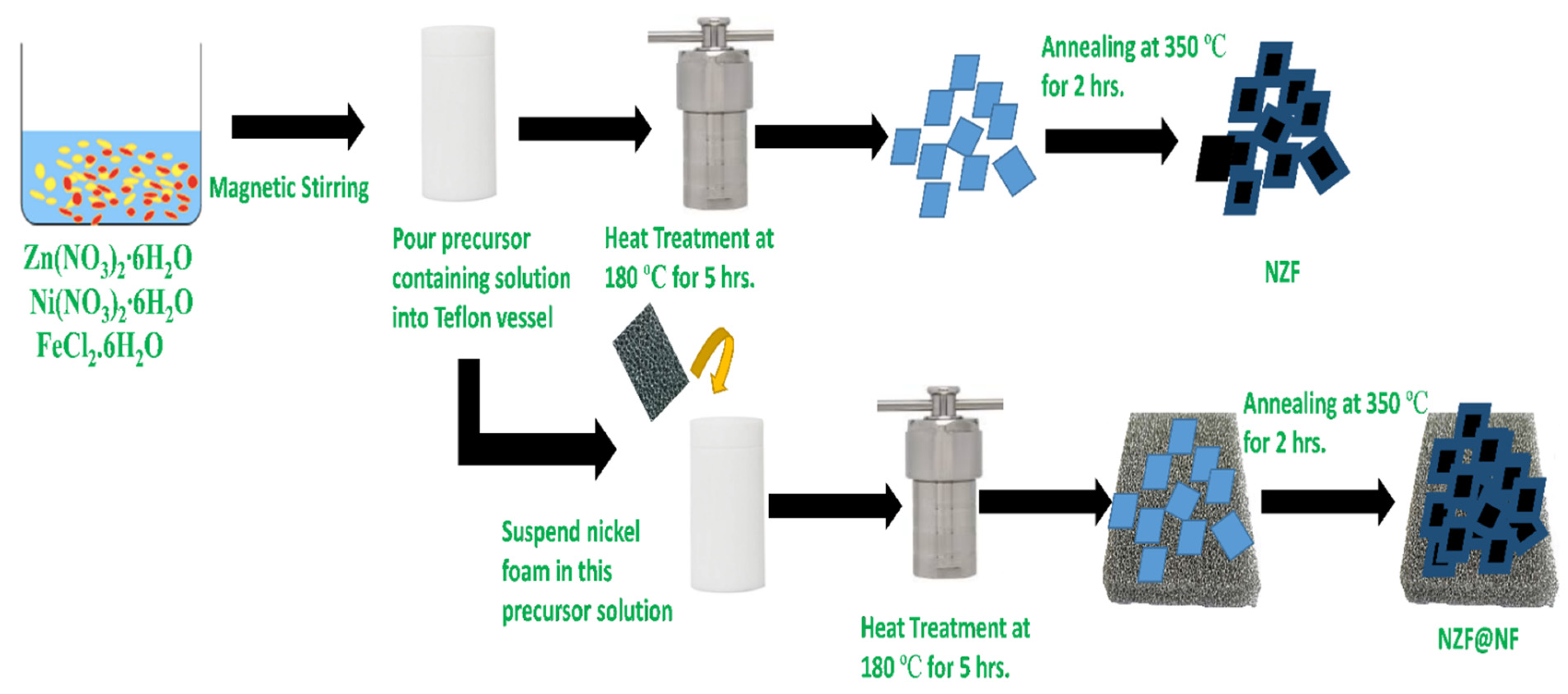
2.3. Synthesis of the Nickel Zinc Ferrite
2.4. Synthesis of Binder-Free Nickel Zinc Ferrite
2.5. Characterizations
2.6. Electrochemical Measurements
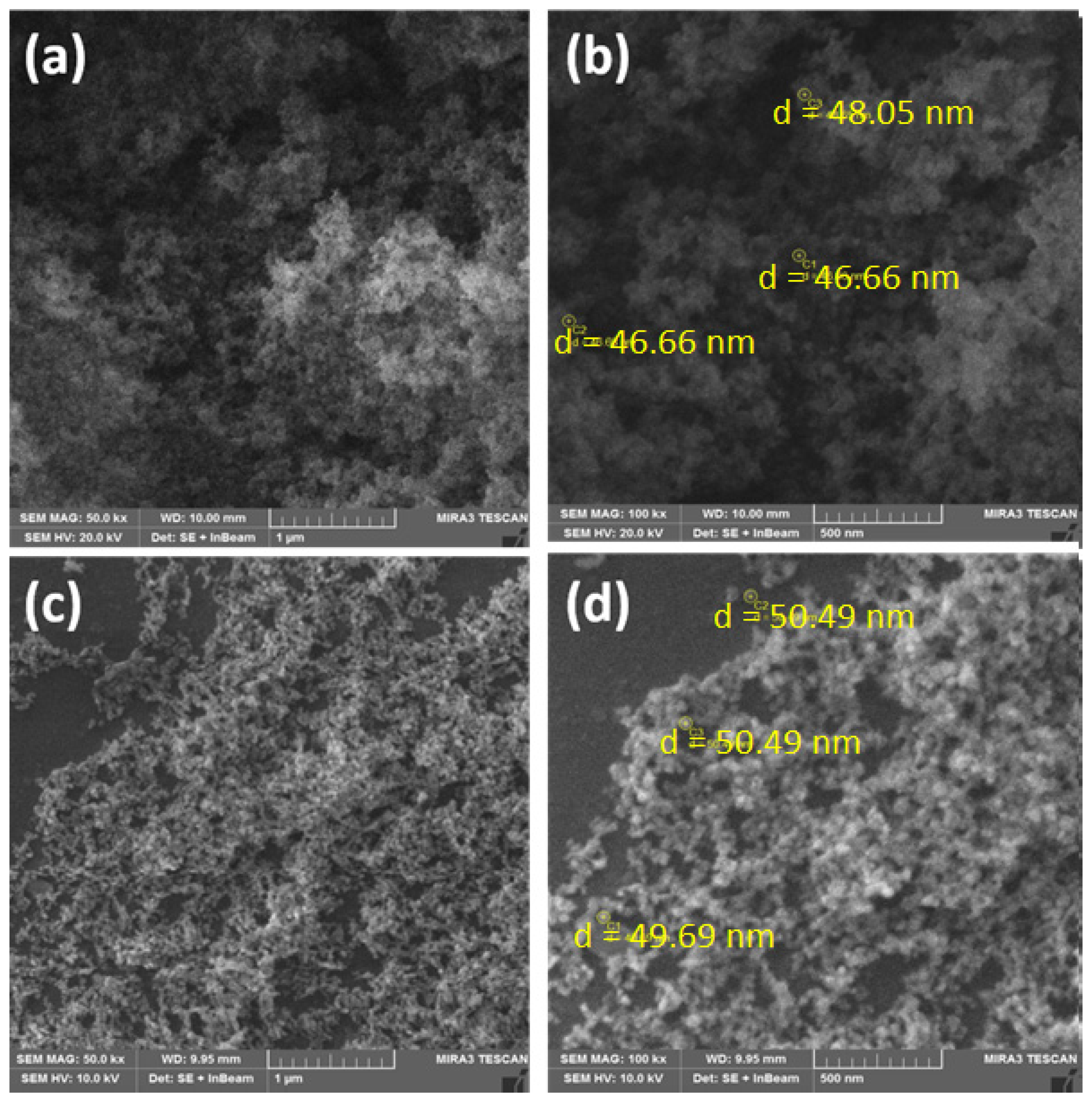
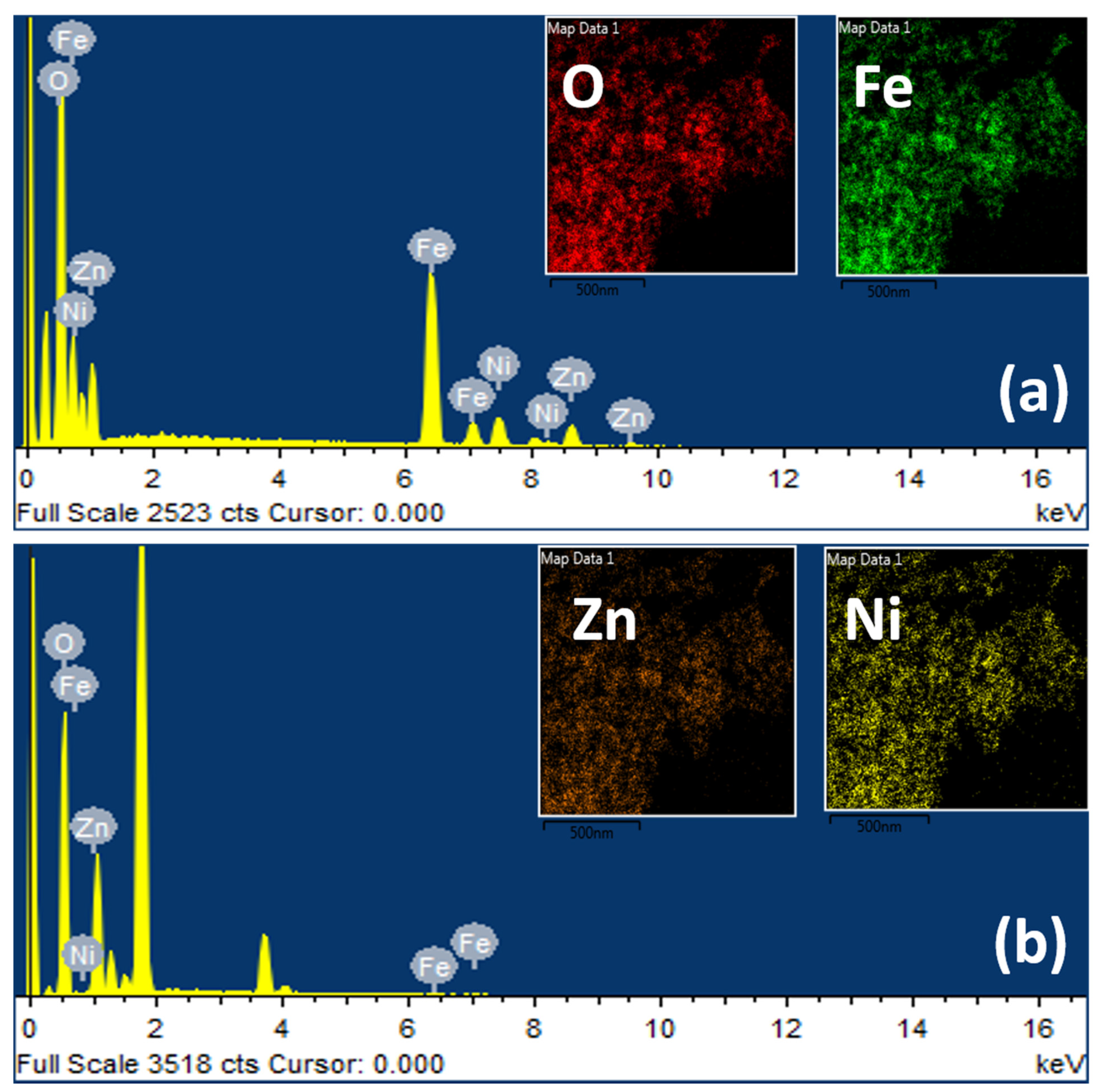
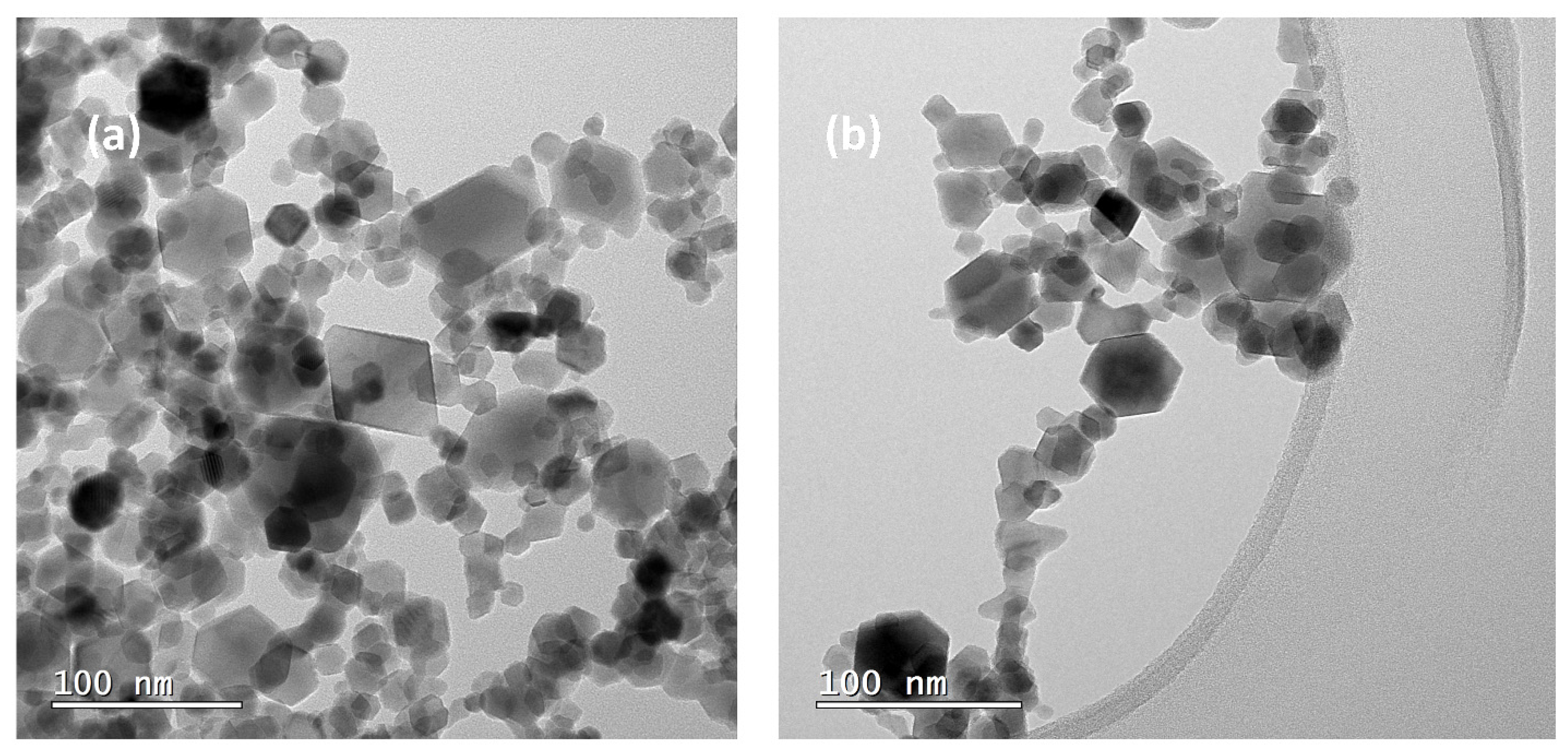
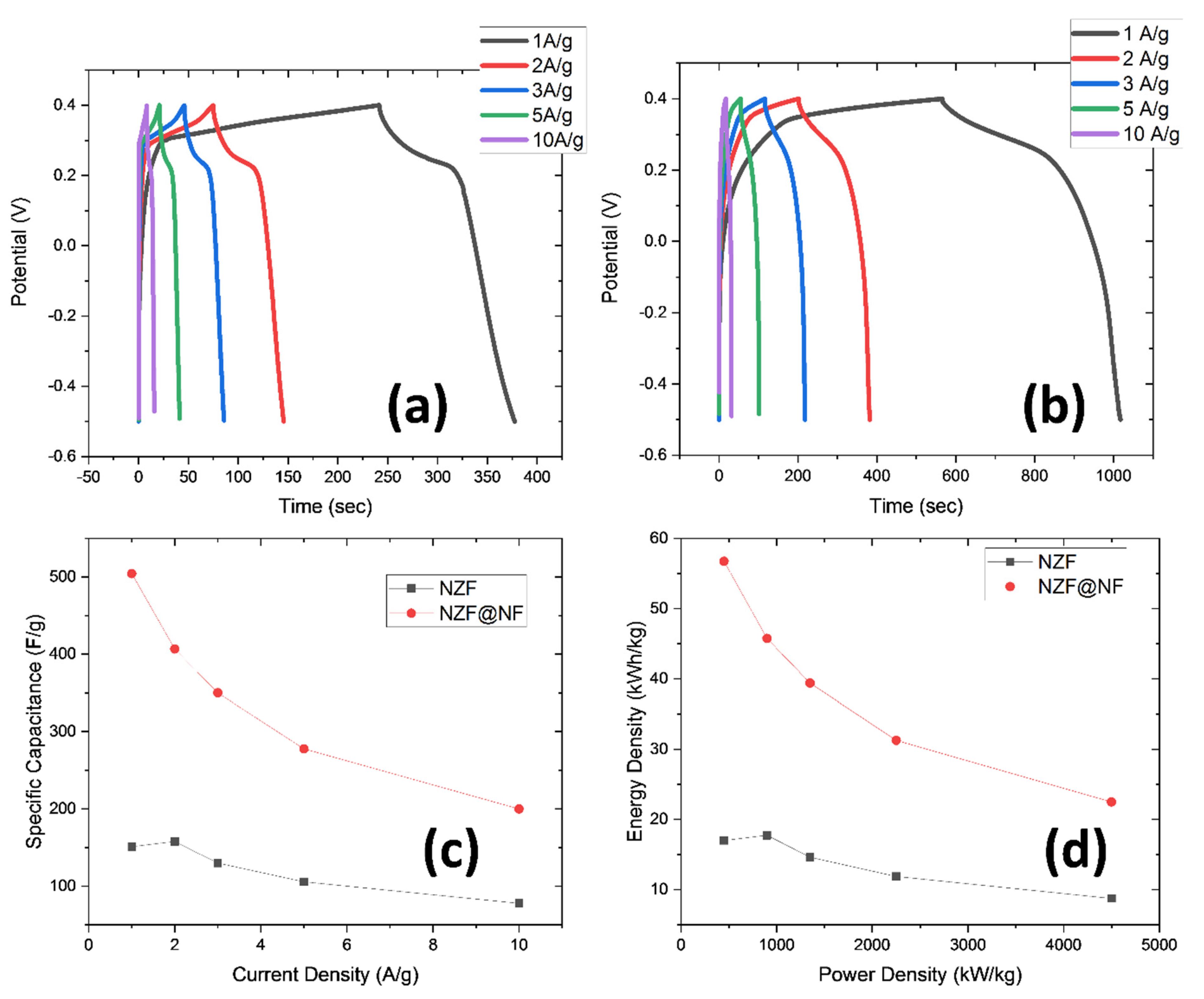
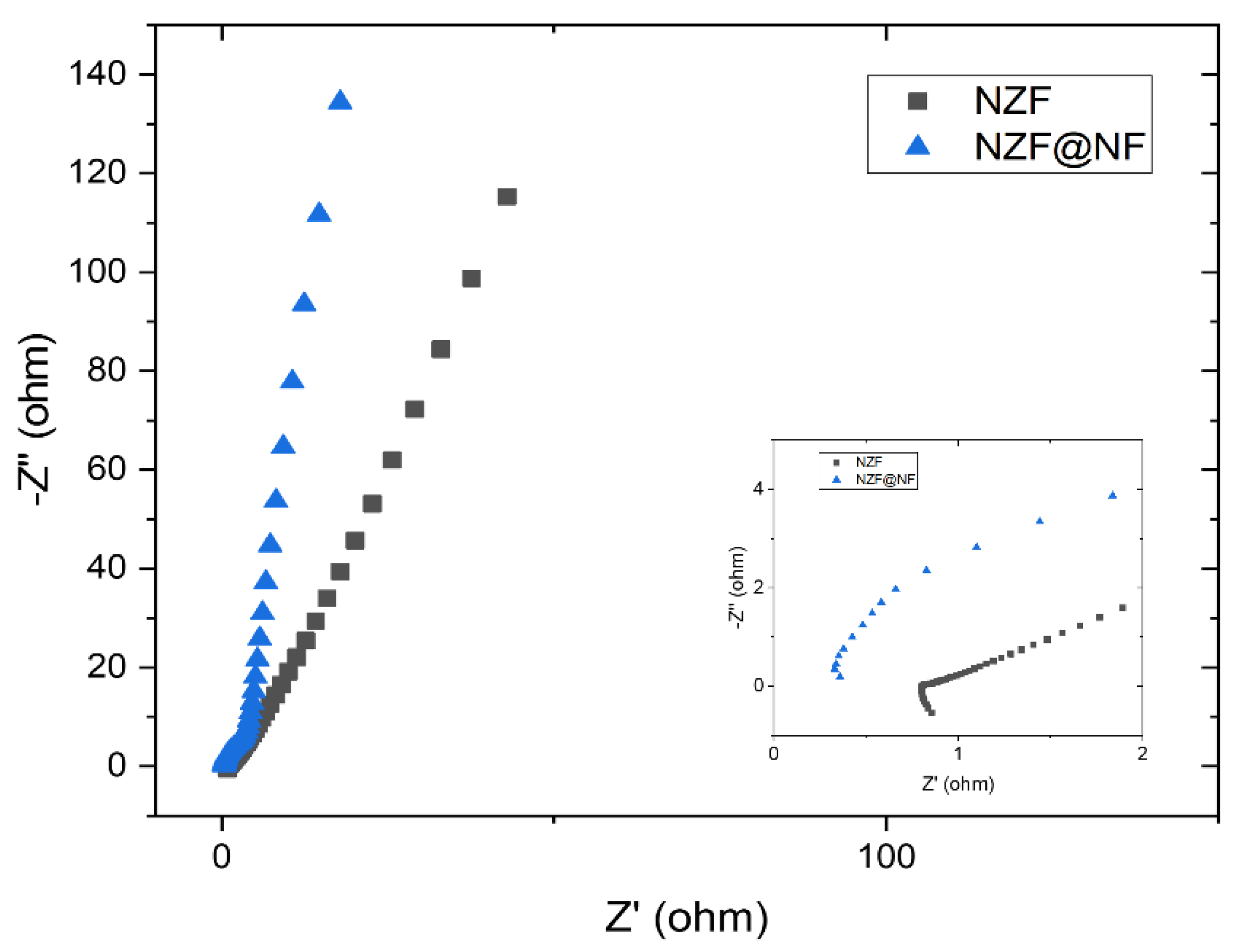
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iro, Z.S.; Subramani, C.; Dash, S. A brief review on electrode materials for supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Jin, T.; Han, Q.; Jiao, L. Binder-free electrodes for advanced sodium-ion batteries. Adv. Mater. 2020, 32, 1806304. [Google Scholar] [CrossRef]
- Vangari, M.; Pryor, T.; Jiang, L. Supercapacitors: Review of materials and fabrication methods. J. Energy Eng. 2013, 139, 72–79. [Google Scholar] [CrossRef]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef]
- Saha, S.; Samanta, P.; Murmu, N.C.; Kuila, T. A review on the heterostructure nanomaterials for supercapacitor application. J. Energy Storage 2018, 17, 181–202. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube-and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Manohar, A.; Chintagumpala, K.; Kim, K.H. Mixed Zn–Ni spinel ferrites: Structure, magnetic hyperthermia and photocatalytic properties. Ceram. Int. 2021, 47, 7052–7061. [Google Scholar] [CrossRef]
- Valenzuela, R. Novel applications of ferrites. Phys. Res. Int. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Harris, V.; Koon, N.; Williams, C.; Zhang, Q.; Abe, M.; Kirkland, J.; McKeown, D. Direct measurement of octahedral and tetrahedral site environments in NiZn-ferrites. IEEE Trans. Magn. 1995, 31, 3473–3475. [Google Scholar] [CrossRef]
- Mordina, B.; Kumar, R.; Neeraj, N.S.; Srivastava, A.K.; Setua, D.K.; Sharma, A. Binder free high performance hybrid supercapacitor device based on nickel ferrite nanoparticles. J. Energy Storage 2020, 31, 101677. [Google Scholar] [CrossRef]
- Bhujun, B.; Tan, M.T.; Shanmugam, A.S. Study of mixed ternary transition metal ferrites as potential electrodes for supercapacitor applications. Results Phys. 2017, 7, 345–353. [Google Scholar] [CrossRef]
- Cai, Y.-Z.; Cao, W.-Q.; Zhang, Y.-L.; He, P.; Shu, J.-C.; Cao, M.-S. Tailoring rGO-NiFe2O4 hybrids to tune transport of electrons and ions for supercapacitor electrodes. J. Alloy. Compd. 2019, 811, 152011. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Seifi, M.; Di Bartolomeo, A. ZnFe2O4 nanorods on reduced graphene oxide as advanced supercapacitor electrodes. J. Alloy. Compd. 2021, 860, 158497. [Google Scholar] [CrossRef]
- Bashir, B.; Shaheen, W.; Asghar, M.; Warsi, M.F.; Khan, M.A.; Haider, S.; Shakir, I.; Shahid, M. Copper doped manganese ferrites nanoparticles anchored on graphene nano-sheets for high performance energy storage applications. J. Alloy. Compd. 2017, 695, 881–887. [Google Scholar] [CrossRef]
- Hareesh, K.; Shateesh, B.; Joshi, R.P.; Dahiwale, S.; Bhoraskar, V.; Haram, S.; Dhole, S. PEDOT: PSS wrapped NiFe2O4/rGO tertiary nanocomposite for the super-capacitor applications. Electrochim. Acta 2016, 201, 106–116. [Google Scholar] [CrossRef]
- Fan, H.; Quan, L.; Yuan, M.; Zhu, S.; Wang, K.; Zhong, Y.; Chang, L.; Shao, H.; Wang, J.; Zhang, J. Thin Co3O4 nanosheet array on 3D porous graphene/nickel foam as a binder-free electrode for high-performance supercapacitors. Electrochim. Acta 2016, 188, 222–229. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, S.; Zhang, Z. Three-dimensional binder-free nanoarchitectures for advanced pseudocapacitors. Adv. Mater. 2017, 29, 1700515. [Google Scholar] [CrossRef]
- Obodo, R.M.; Shinde, N.M.; Chime, U.K.; Ezugwu, S.; Nwanya, A.C.; Ahmad, I.; Maaza, M.; Ejikeme, P.M.; Ezema, F.I. Recent advances in metal oxide/hydroxide on three-dimensional nickel foam substrate for high performance pseudocapacitive electrodes. Curr. Opin. Electrochem. 2020, 21, 242–249. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, X.; Zhao, S.; Wang, A.; Luo, J.; Wang, Z.L.; Kang, F.; Lin, Z.; Li, B. Advanced Matrixes for Binder-Free Nanostructured Electrodes in Lithium-Ion Batteries. Adv. Mater. 2020, 32, 1908445. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Feng, Y.; Wang, X.; Sun, Q.; Yu, D.; Tong, W.; Zhao, X.; Liu, X. Fabrication of porous ZnCo2O4 nanoribbon arrays on nickel foam for high-performance supercapacitors and lithium-ion batteries. Electrochim. Acta 2018, 260, 823–829. [Google Scholar] [CrossRef]
- Malaie, K.; Ganjali, M.; Alizadeh, T.; Norouzi, P. Hydrothermal growth of magnesium ferrite rose nanoflowers on Nickel foam; application in high-performance asymmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 650–657. [Google Scholar] [CrossRef]
- Chang, Z.; Li, T.; Li, G.; Wang, K. One-pot in-situ synthesis of Ni(OH)2–NiFe2O4 nanosheet arrays on nickel foam as binder-free electrodes for supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 600–608. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.; Xie, K.; Yu, F.; Huang, H. A One-Step and Binder-Free Method to Fabricate Hierarchical Nickel-Based Supercapacitor Electrodes with Excellent Performance. Adv. Funct. Mater. 2013, 23, 3675–3681. [Google Scholar] [CrossRef]
- Meng, F.; Yang, M.; Zhao, L.; Zhang, Y.; Shang, X.; Jin, P.; Zhang, W. A comparative study of the structural, magnetic and electrochemical properties of Al3+ and Cu2+ substituted NiZn ferrite/reduced graphene oxide nanocomposites. Ceram. Int. 2017, 43, 15959–15964. [Google Scholar] [CrossRef]
- Ivanova, R.; Tsyntsarski, B.; Issa, G.; Spassova, I.; Kovacheva, D.; Velinov, N.; Tsoncheva, T. M0.5Zn0.5Fe2O4 (M=Cu, Co, Mn, Ni) ferrites supported on activated carbon as catalysts for methanol decomposition. Bulg. Chem. Commun. 2021, 53, 93–100. [Google Scholar]
- Edison, T.N.J.I.; Atchudan, R.; Lee, Y.R. Binder-free electro-synthesis of highly ordered nickel oxide nanoparticles and its electrochemical performance. Electrochim. Acta 2018, 283, 1609–1617. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, X.; Huang, H.; Li, G.; Liang, X.; Zhou, W.; Guo, J.; Wei, W.; Tang, S. Hetero-structure arrays of NiCoO2 nanoflakes@ nanowires on 3D graphene/nickel foam for high-performance supercapacitors. Electrochim. Acta 2018, 289, 193–203. [Google Scholar] [CrossRef]
- Raut, S.S.; Sankapal, B.R.; Hossain, M.; Shahriar, A.; Pradhan, S.; Salunkhe, R.R.; Yamauchi, Y. Zinc ferrite anchored multiwalled carbon nanotubes for high-performance supercapacitor applications. Eur. J. Inorg. Chem. 2018, 2018, 137–142. [Google Scholar] [CrossRef]
- Cai, Y.-Z.; Cao, W.-Q.; He, P.; Zhang, Y.-L.; Cao, M.-S. NiFe2O4 nanoparticles on reduced graphene oxide for supercapacitor electrodes with improved capacitance. Mater. Res. Express 2019, 6, 105535. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, X.; Zhou, Y.; Zhuo, C.; Huang, J.; Li, S. Facile solvothermal synthesis of porous ZnFe₂O₄ microspheres for capacitive pseudocapacitors. RSC Adv. 2015, 5, 39270–39277. [Google Scholar] [CrossRef]
- Sen, P.; De, A. Electrochemical performances of poly (3, 4-ethylenedioxythiophene)–NiFe2O4 nanocomposite as electrode for supercapacitor. Electrochim. Acta 2010, 55, 4677–4684. [Google Scholar] [CrossRef]


| Electrode Material | Potential Window V | Current Density | Specific Capacitance | Reference |
|---|---|---|---|---|
| NiFe2O4 | 1 | 0.5 A g−1 | 50 F g−1 | [30] |
| NiFe2O4@NF | 0.4 | 1 A g−1 | 398 C g−1 | [11] |
| ZnFe2O4 microspheres | 0.45 | 0.1 A g−1 | 131 F g−1 | [31] |
| NiFe2O4 | 1 | 1 mA cm−2 | 127 F g−1 | [32] |
| Ni0.8Zn0.2Fe2O4 | 1 | 1 A g−1 | 12 F g−1 | [25] |
| Ni0.8Zn0.2Fe2O4/rGO | 1 | 1 A g−1 | 127.94 F g−1 | [25] |
| Ni0.5Zn0.5Fe2O4 | 0.9 | 1 A g−1 | 151.1 F g−1 | This work |
| Ni0.5Zn0.5Fe2O4 | 0.9 | 1 A g−1 | 504.4 F g−1 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, B.; Ali, G.; Ullah, M.O.; Rehman, S.; Abbas, F. Investigation of the Electrochemical Properties of Ni0.5Zn0.5Fe2O4 as Binder-Based and Binder-Free Electrodes of Supercapacitors. Energies 2021, 14, 3297. https://doi.org/10.3390/en14113297
Nawaz B, Ali G, Ullah MO, Rehman S, Abbas F. Investigation of the Electrochemical Properties of Ni0.5Zn0.5Fe2O4 as Binder-Based and Binder-Free Electrodes of Supercapacitors. Energies. 2021; 14(11):3297. https://doi.org/10.3390/en14113297
Chicago/Turabian StyleNawaz, Bushra, Ghulam Ali, Muhammad Obaid Ullah, Sarish Rehman, and Fazal Abbas. 2021. "Investigation of the Electrochemical Properties of Ni0.5Zn0.5Fe2O4 as Binder-Based and Binder-Free Electrodes of Supercapacitors" Energies 14, no. 11: 3297. https://doi.org/10.3390/en14113297
APA StyleNawaz, B., Ali, G., Ullah, M. O., Rehman, S., & Abbas, F. (2021). Investigation of the Electrochemical Properties of Ni0.5Zn0.5Fe2O4 as Binder-Based and Binder-Free Electrodes of Supercapacitors. Energies, 14(11), 3297. https://doi.org/10.3390/en14113297






