Abstract
Metallic oxides are considered promising candidates for supercapacitors owing to their inherent pseudocapacitive behavior and superior electrochemical properties. In this work, NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 composite electrodes are synthesized directly on a nickel foam substrate via the facile hydrothermal method. The phase of the prepared materials was analyzed using the X-ray diffraction method. The morphology of the prepared binder-free electrodes was observed by scanning electron microscopy. The electrochemical testing was done in a 2 M KOH solution against an Ag/AgCl reference electrode. The CuCo2O4@NiCo2O4 composite electrode demonstrated a value of specific capacitance as high as 422 F g−1 at a current density of 1 A g−1 and thus outperformed the NiCo2O4 and CuCo2O4 in terms of its electrochemical performance. The CuCo2O4@NiCo2O4 composite retained a specific capacitance of 278 F g−1 even with the increase of current density to 10 A g−1, which corresponds to a 34% loss of capacitance compared to 40% and 48% of individual NiCo2O4 and CuCo2O4 electrodes, respectively. Hence, the synergy in a composite material demonstrates it to be a potential candidate as an electrode in supercapacitors.
1. Introduction
Fossils fuels are becoming depleted at an accelerating rate, with increases in greenhouse gasses emissions resulting in severe environmental consequences. The world is in dire need of clean, green, and renewable energy systems. Supercapacitors are among such devices and have captured the attention of researchers around the globe due to their high power density, extraordinary cycling stability, and galvanostatic charge-discharge profile [1,2,3]. Despite all this, their low energy density has barred them from commercial application. Attractive materials as electrodes for supercapacitors are transition metal oxides as well as hydroxide and carbonaceous materials [4,5,6]. The transition materials have surpassed the others, owing to their high specific capacitance and high energy density with efficient redox reaction.
Many transition metals and their composites are being investigated as potential electrodes for supercapacitors [7]. Composites of metallic oxides have demonstrated better electrochemical performances than their single counterparts. Binder-free electrodes have demonstrated higher electrochemical performance because binder and additives decrease the sites for faradic reaction with reduced electrical conductivity [2,8,9]. Nickel foam has been researched comprehensively because of its 3D structure with numerous pores for faster ion diffusion with an improved surface area. Many metallic oxides and hydroxides composites anchored on a nickel foam substrate have displayed astonishing electrochemical properties, like Ni(OH)2 [10], Co3O4 [11], MnO2 [12], GO/MnO2 [13], ZnO@Co3O4 [14], (PPy/GO/ZnO) [15], bundle-like CuCo2O4 [16], and MgCo2O4@MnO2 [17].
Lili Ma et al., constructed a binder-free electrode from two binary transition metal oxides in a bid to take advantage of their large specific area and high theoretical capacitance [18]. The ZnCo2O4@NiWO4 nanowire/nanosheet arrays electrode outperformed a single material-based electrodes with the marvelous specific capacitance of 1782 F g−1. Moreover, the cycling performance of the composite electrode (95.4%) was much better than the single material (90.9%) because of its core-shell three-dimensional structure. In this structure, the ZnCo2O4 nanowires act as the core, with NiWO4 nanosheets as its shell; this core–shell structure has higher mechanical stability and enough space for ions diffusion.
Hai Chao Chen et al., synthesized amorphous and binder-free electrode NiCoMn–OH from the hydrothermal method [19]. The binder-free electrode demonstrated a higher specific capacity of 1124 C g−1 than the specific capacity of 749 C g−1 of its binder-based counterpart at the same current density of 1 A g−1. This surge in capacity is due to the porous structure of Ni foam, which provided faster channels for ions diffusion. The synergy between these transition metal hydroxides results in increased cycling stability, enhanced energy density, and specific capacitance closer to the theoretical value. While employed as an electrode with reduced graphene oxide anode, it has delivered an energy density of 42.8 Wh kg−1. The impact of different templates like Polyvinylpyrrolidone (PVP), cetyltrimethylammonium bromide (CTAB), and sodium dodecyl sulfate on the electrochemical properties of FeCo2O4 electrode was investigated [20]. Among all of these surfactants, the PVP template-based structure showed better performance than the others. In another work [21], the hybrid binder-free electrode consisted of MnCo2O4 nanosheets deposited on nanosheets of MnMoO4 to reap the benefits of both of these materials. This hybrid electrode gave the rate capability at 30 A g−1 and was shown to deliver a capacity of 629 C g−1, with 95% retention of capacity even after 5000 cycles.
Kuang et al. [22] synthesized MnO2 nanoflakes directly in the nanosheets of CuCo2O4 via a feasible hydrothermal method. A nickel foam served as the skeleton to anchor this core shell nanostructure. This binder-free electrode demonstrated a specific capacitance of 416 F g−1 at 1 A g−1, which is a testimonial to its remarkable electrochemical properties. The cycling stability of 92.1% was achieved after 4200 cycles in a wide voltage region. The specific energy density of 43.3 Wh Kg−1 was retained, thus it was demonstrated that this core shell architecture is of great use for energy storage devices. The ZnCo2O4 nanosheets arrays acted as the backbone for MnO2 nanosheets on nickel foam in order to achieve a larger specific surface area [23]. This synergistic combination resulted in a good value of capacitance (i.e., 929 F g−1). While at the higher current density of 40 mA cm−2, the specific capacitance of 751.1 F g−1 is still retained, owing to the enhanced specific surface of its core shell architecture.
Chen et al., utilized the chemical bath deposition technique to fabricate NiCoMn ternary oxide on nickel foam [24]. This binder-free electrode demonstrated, at a current density of 1 A g−1, the significant specific capacitance of 715 C g−1. The power density of 320 W kg−1 was attained at an energy density of 36.4 Wh kg−1 in a broad potential range of 1.6 V with activated carbon as an anode in a hybrid supercapacitor cell. Nickel foam was utilized as the backbone to reduce the agglomeration of CoMn layered double hydroxide [25]. This double layered hydroxide was synthesized via the hydrothermal method. In electrochemical testing, it has proven to be an incredible supercapacitor, with a high value of specific capacitance of 2422 F g−1 at a current density of 1 A g−1 with only negligible loss of 13.5% of capacitance after 3000 cycles. This astonishing prowess of its electrochemical activity can be attributed to a nickel mesh structure with plenty of electrochemical sites.
In this work, we have synthesized binder-free NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 electrodes through an efficient hydrothermal route. The phase formation is confirmed via X-ray diffraction and morphology by scanning electron microscopy. In order to assess their electrochemical prowess, techniques like cyclic voltammetry, galvanostatic charge–discharge curves, and electrochemical impedance spectroscopy are employed.
2. Materials and Synthesis
2.1. Materials
Ni(NO3)2·6H2O, Co(NO3)2·6H2O, NH4F, urea, KOH, and Cu(NO3)2·6H2O materials were procured from Sigma Aldrich with >98% purity and then consumed without any further modification. A nickel foam with 110 pores per inch (PPI) and 2 mm thickness was used in the experiment.
2.2. Synthesis
2.2.1. Treatment of Nickel Foam
First of all, nickel foam of size 1 × 2 cm2 was cut and then treated with 2 M HCl solution in a sonication dispenser for 30 min to remove the oxidation layer. Later, the nickel foam piece was cleaned with ethanol and distilled water in order to remove the impurities, followed by drying overnight at 80 °C.
2.2.2. Synthesis of NiCo2O4
In a bid to synthesize the NiCo2O4 nanoparticles, 0.048 M Ni(NO3)2·6H2O was put into 70 mL of water and then placed on a magnetic stirrer. Then, 0.096 M of Co(NO3)2·6H2O was added to 0.24 M of urea and 0.08 M of NH4F. The precursor contained solution was stirred for half an hour to make a clear pink homogenous solution. Afterwards, the solution was poured into a 100-mL Teflon-lined autoclave. The freshly etched nickel foam was suspended in this solution with Teflon tape. The autoclave was tightly closed and maintained at 120 °C for 6 h. When the reaction time was completed, the autoclave was kept at room temperature to cool down naturally. The nickel foam loaded with the precursors was cleansed by ethanol and deionized water repeatedly in a sonication bath. The foam was dried overnight at 80 °C with subsequent annealing at 350 °C for 3 h to synthesize NiCo2O4 on nickel foam.
2.2.3. Synthesis of CuCo2O4 and CuCo2O4@NiCo2O4 on Nickel Foam
In 70 mL of deionized water, 0.058 M of Co(NO3)2·6H2O and 0.028 M of Cu(NO3)2·6H2O was added. Next, 0.33 M of NH4F with 0.51 of M of urea was added to the above solution, then it was put on a magnetic stirrer for half an hour to obtain a clear solution. Later, the solution was transferred to a 100-mL autoclave and clean nickel was suspended with the help of Teflon tape. The autoclave was sealed and kept for 6 h at 120 °C. With the completion of the reaction time, the autoclave was kept at room temperature to cool down. The nickel foam was separated from the reaction mixture and washed with ethanol and deionized water multiple times in order to remove the loosened impurities. The nickel foam was dried overnight at 80 °C and later annealed at 350 °C for 3 h. For the synthesis of CuCo2O4@NiCo2O4 on nickel foam, the whole process remained the same, except instead of clean foam, NiCo2O4-loaded nickel foam was used in the reaction mixture. The whole synthesis process is illustrated in Figure 1.
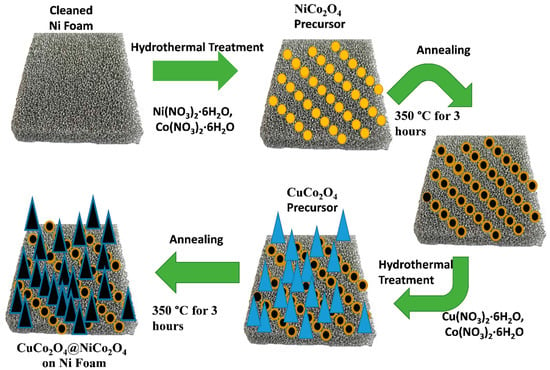
Figure 1.
Synthesis route of CuCo2O4@NiCo2O4 on nickel foam.
2.3. Characterizations
Bruker X-ray diffractometer (Cu Kα radiation) was used to examine the phase formation of the prepared samples. Two theta was varied starting from 10° and closing at 80° using a scanning size of 0.02° and count time of 0.5 s. The surface properties were examined by scanning electron microscopy (EVO50 ZEISS, USA).
2.4. Electrochemical Measurements
The 2 M KOH solution was employed as the electrolyte in a three electrodes electrochemical cell. In this cell, the Pt reference electrode was employed, the Ag/AgCl was designated as a counter electrode, while nickel foams with material loading were used as the working electrode. The area of the prepared nickel foam electrode was 1 × 2 cm2. The mass loading on NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 electrode was 1 mg, 1.2 mg, and 2 mg respectively. Electrochemical workstation (CHI 660e) was employed to take the cyclic voltammetry (CV) measurements and charge–discharge profile of the prepared samples. In a frequency range of 100 mHz to 100 kHz with an AC voltage and by utilizing 5 mV amplitude, the electrochemical impedance spectroscopy (EIS) testing was performed in open circuit potential.
3. Results and Discussion
The crystallographic phase and the structure of the prepared materials loaded on nickel foam are displayed in Figure 2. The diffraction peaks that appeared at 2θ of 31.09°, 36.94°, 59.80°, and 65.38° are assigned indexes of 220, 311, 511, and 440, respectively, which is in accordance with the reported literature on CuCo2O4 (JCPDS 01-1155) [26]. The diffraction peaks for CuCo2O4@NiCo2O4 appeared at 2θ of 31.09°, 36.94°, 59.80°, and 65.38°, which matched well with the NiCo2O4 (JCPDS 73-1702) and CuCo2O4 (JCPDS 01-1155) [27]. As anticipated, the NiCo2O4 and CuCo2O4 showed similar XRD patterns, with a slight shift of 2θ, owing to their spinel structure and similar ionic radii of Ni and Co. In composite, XRD pattern slight distortion of peaks were seen but no broadening of peaks was observed, thus indicating an intact crystalline structure.
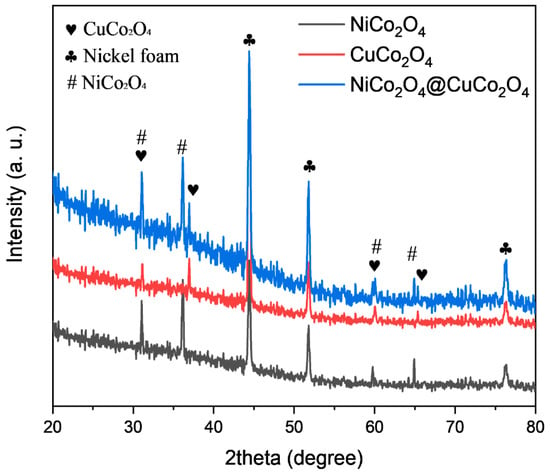
Figure 2.
X-ray diffraction of NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 electrode materials.
The SEM images of the NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 are shown in Figure 3a–c. In Figure 3a, the NiCo2O4 exhibits a uniform morphology with nanoparticles spherical in shape. In Figure 3b, the CuCo2O4 seems to possess a dandelion flower-like morphology with nanosheets that are vertically aligned. In Figure 3c, the SEM images of the CuCo2O4@NiCo2O4 is shown, in which it is evident that these dandelion nanoflowers of CuCo2O4 are directly deposited on the nanosphere of NiCo2O4. These observations clearly show that under hydrothermal treatment these metal oxides self-assemble and interconnect with each other. The uniform distribution and even sizes of these nanoparticles on the nickel foam surface in dandelion flower-like morphology can be attributed to the hydrothermal method. These vertically aligned nanoarrays of the CuCo2O4@NiCo2O4 structure are of great potential use for ions diffusion in electrochemical reactions, evident in the greater electrochemical activity of the composite electrode. The energy dispersive spectroscopy (EDS) of CuCo2O4@NiCo2O4 (Figure 3c) was measured to observe the elemental distribution in the prepared material. The EDS spectrum in Figure 3d illustrates the presence of Ni, Cu, Co, and oxygen atoms in the prepared material.
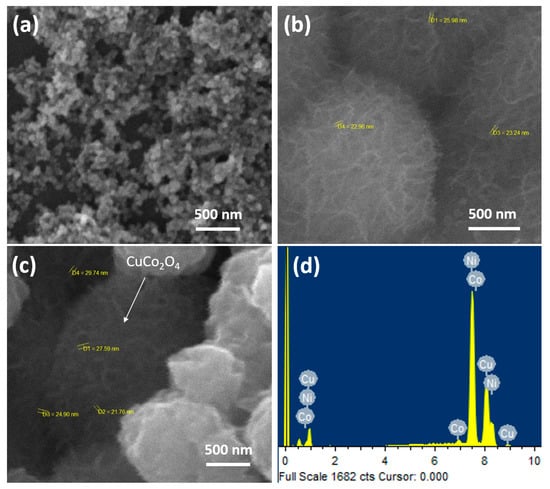
Figure 3.
SEM images of (a) NiCo2O4, (b) CuCo2O4, and (c) CuCo2O4@NiCo2O4 electrode materials loaded on nickel foam. (d) EDS spectra of CuCo2O4@NiCo2O4 electrode material.
In Figure 4, the CV plots of the materials are displayed within a potential window of −0.5 to 0.5 V at multiple scan rates. It is widely understood that the area enclosed in the CV curves is representative of the capacitive potential of the material [28,29]. It is evident that the area enclosed by NiCo2O4@CuCo2O4 is larger than that of the single NiCo2O4 or CuCo2O4. Figure 4a demonstrates the CV plots of NiCo2O4 at various scan rates (i.e., 5, 10, 15, 20, 50, and 100 mV s−1). When the rate of scanning potential increases, the reduction and oxidation peaks are shifted to a negative and positive potential, respectively, with the surge in the peak current. The increase in the peak current has slightly affected the polarization and intrinsic resistance of the electrode because with the increases of the scan rate, the insertion and extraction of the charge carriers will be increased in the electrolyte and on the surfaces of the electrode [30]. A pair of redox peaks are noted on the CV curves of the NiCo2O4, which is due to the Ni2+/Ni+ and Co3+/Co4+ redox couple [31]. Similar oxidation and reduction peaks are noted for the CuCo2O4 and composite electrode. These redox peak pairs emerged due to the direct growth of active material on the Ni foam and thus contributed to an increase in the electrochemical activity with enhanced surface area. It is apparent from all of the CV plots that the shape of the curves did not change with the surge in the scan rate; only slight polarization was observed. In Figure 4b,c, the CV curves of CuCo2O4 and the NiCo2O4@CuCo2O4 composite are given, which show identical behaviors like NiCo2O4. In Figure 4d, the comparison of CV curves of the CuCo2O4, NiCo2O4, and CuCo2O4@NiCo2O4 are presented, in which it can be observed that the CV curves of the composite are enlarged in comparison to those of the single cobaltite. The CV plots of the hybrid electrodes encompass more area as compared to the other two electrodes, demonstrating that the composite electrode has a larger capacitance. Thus, a fast redox reaction is possible due to the greater specific surface area of the prepared composite electrode. All CV curves of the materials in Figure 4a–c are well shaped, demonstrating their potent capacitive behavior.
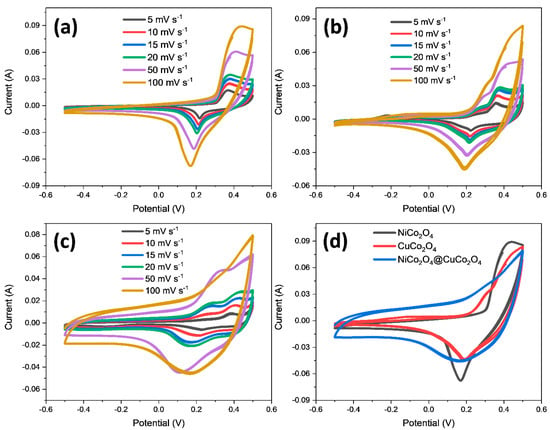
Figure 4.
CV curves at different scan rates in the potential window of -0.5 to 0.5 V of (a) NiCo2O4, (b) CuCo2O4, and (c) NiCo2O4@CuCo2O4. (d) Comparison of CV curves of NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 at 100 mV.
Further, in a bid to understand the electrochemical performance of bare and composite electrodes at various current densities, the galvanostatic charge–discharge test was performed. The charge–discharge curves are not straight and feature a slight plateau, which is a testimonial of the faradic behavior of the electrode, and in line with the above CV curves [32,33]. In Figure 5a, the charge–discharge curve of NiCo2O4 at 1, 2, 3, 5, and 10 A g−1 current densities is given with the specific capacitances of 334 F g−1, 288 F g−1, 276 F g−1, 244 F g−1, and 200 F g−1, respectively. In Figure 5b,c, the discharge curves of the CuCo2O4 electrode represents the specific capacitance of 320 F g−1, 316 F g−1, 273 F g−1, 233 F g−1, and 167 F g−1 at current densities of 1 A g−1, 2 A g−1, 3 A g−1, 5 A g−1, and 10 A g−1, respectively. The NiCo2O4@CuCo2O4 composite electrode shows a specific capacitance of 422 F g−1, 358 F g−1, 297 F g−1, 356 F g−1, and 278 F g−1 at current densities of 1 A g−1, 2 A g−1, 3 A g−1, 5 A g−1, and 10 A g−1, respectively.
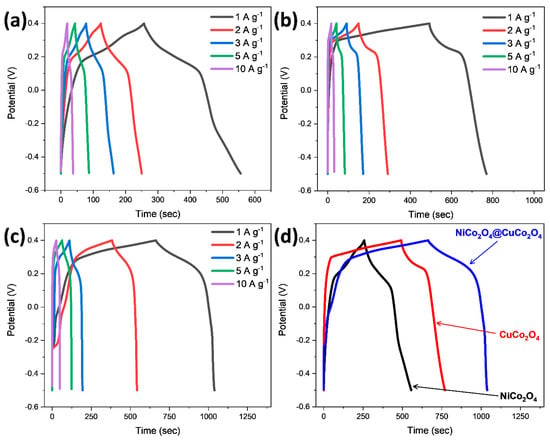
Figure 5.
Galvanostatic charge discharge (GCD) curves at different current densities in the potential window of −0.5 to 0.4 V of (a) NiCo2O4, (b) CuCo2O4, (c) NiCo2O4@CuCo2O4. (d) Comparison of GCD curves of NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 at 1 A g−1.
When the current density is increased, the capacitance of the electrode decreased because at higher current density, more OH− ions are required for diffusion across the electrolyte and the surface of the electrode. The whole process is dependent on ions diffusion, while the kinetics of ions are somewhat limited at a larger current density. So, the charge transfer will only happen at the outer surface, thus resulting in a decrease in the rate capability. In Figure 5a–c, it is apparent that the rate capability of the hybrid electrode is much greater than the standalone electrodes, owing to the synergistic effect between CuCo2O4 and NiCo2O4 particles. This enhancement in the electrochemical activity of the composite electrode is due to there being more sites for ion insertion and extraction. Thus, this composite electrode has overcome the limitation of diffusion kinetics with reduced diffusion resistance. The retention rate of the bare NiCo2O4 and CuCo2O4 electrodes was lower in comparison with the composite electrode, which is due to there being less loading of active mass on the nickel foam and less reaction sites. With the increase in the current density from the 1 A g−1 to 10 A g−1, the NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 electrodes demonstrate 60%, 52%, and 66% of their initial capacitance values, respectively. A comparison of the electrochemical data with earlier reported literature is given in Table 1.

Table 1.
Comparison of electrochemical data from earlier reported literature.
Figure 6a shows the specific capacitance of NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 electrodes at 1 A g−1, 2 A g−1, 3 A g−1, 5 A g−1, and 10 A g−1. Clearly, the CuCo2O4@NiCo2O4 electrode shows better capacitance compared to the individual NiCo2O4 and CuCo2O4 electrodes. The electrodes for supercapacitors should exhibit substantial energy and power densities for applicability. The values for the energy density are derived from E = 0.14x C x(ΔV)2 and power density is calculated by P = 3600 xE/Δt. It is common for the supercapacitor to show a decline in energy density with a rise in the power density. The CuCo2O4@NiCo2O4 electrode also shows high energy density at all current densities as displayed in the Ragone plot in Figure 6b. For instance, at 10 A g−1, the CuCo2O4@NiCo2O4 electrode displayed an energy density of 31.2 Wh kg−1 with a power density of 4500 W kg−1. Hence, the electrochemical performance of the CuCo2O4@NiCo2O4 electrode reflects excellent synergy among the NiCo2O4 and CuCo2O4 particles in the composite electrode.
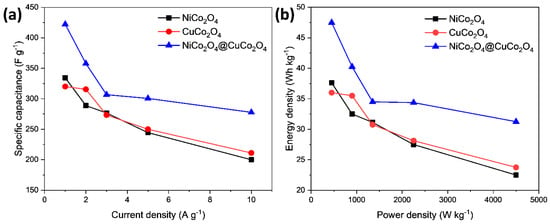
Figure 6.
(a) Specific capacitance vs. current density and (b) Ragone plot of NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 electrodes.
Electrochemical impedance spectroscopy (EIS) testing was done to assess the charge transfer resistance and ion diffusion of the prepared electrodes. The Nyquist plots of the NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 electrodes are displayed in Figure 7. Each Nyquist plot consists of a distorted semicircle and oblique line. The semicircle diameter represents the charge-transfer resistance because of the diffusion of the ions [39]. It is evident in Figure 7 that the slope line of all of the materials was less than 45°, while the diameter of the semicircle for CuCo2O4@NiCo2O4 is much smaller than the NiCo2O4 electrodes. The lower charge transfer resistance of CuCo2O4@NiCo2O4 resistance, as displayed in Figure 7, can be ascribed to the synthesis of the material on nickel foam with the enhanced reaction sites and diffusion rates. The inset of Figure 7 shows the Nyquist plot at a higher frequency region; the semicircle diameters of CuCo2O4@NiCo2O4 and CuCo2O4 are much smaller than that of NiCo2O4. The reduced resistance of the CuCo2O4@NiCo2O4 and CuCo2O4 is due to the better electrical conductivity of copper and increased surface area in the composite electrode.
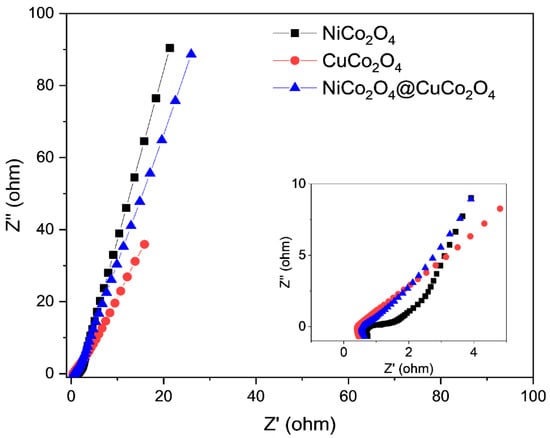
Figure 7.
Electrochemical impedance spectroscopy of NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 electrodes. The inset shows the magnified Nyquist plot.
4. Conclusions
In summary, binder-free electrodes were synthesized from NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 materials via a facile hydrothermal route using a nickel foam substrate. Their electrochemical properties were investigated via CV, GCD, and EIS. The phase formation and morphology were examined via XRD and SEM. The specific capacitance of NiCo2O4, CuCo2O4, and CuCo2O4@NiCo2O4 at 1 Ag−1 current density were 334 Fg−1, 320 F g−1, and 422 F g−1, respectively. The CuCo2O4@NiCo2O4 composite electrode outperformed the NiCo2O4 and CuCo2O4 in terms of its electrochemical performance with a high specific capacitance at various current densities, ranging from 1 A g−1 to 10 A g−1. The CuCo2O4@NiCo2O4 composite retained a specific capacitance of 278 F g−1 even when the current density was increased to 10 A g−1, with 34% loss of capacitance. The enhanced electrochemical properties of the composite electrode can be attributed to the increase in the specific surface area along with more electroactive sites and viable synergy between NiCo2O4 and CuCo2O4. These findings have proved the mettle of these electrode materials in terms of their possible utilization in energy storage devices.
Author Contributions
B.N. contributed to the conceptualization, methodology and formal analysis; G.A. and M.O.U. contributed to resources supervision and reviewed the manuscript; and all authors read, revised, and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This research was supported, funded, and conducted at UET Taxila, Rawalpindi and USPCAS-E, NUST, Islamabad, Pakistan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
Authors want to acknowledge USPCAS-E, NUST for providing electrochemical measurements.
Conflicts of Interest
The authors declare no conflicting interests.
References
- Conway, B. Transition from ‘supercapacitor’ to ‘battery’ behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539. [Google Scholar] [CrossRef]
- Ribeiro, P.; Johnson, B.; Crow, M.; Arsoy, A.; Liu, Y. Energy storage systems for advanced power applications. Proc. IEEE 2001, 89, 1744–1756. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Sci. Mag. 2008, 321, 651–652. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Iqbal, N.; Baig, M.M.; Noor, T.; Ali, G.; Gul, I.H. ZIF-67 derived nitrogen doped CNTs decorated with sulfur and Ni(OH)2 as potential electrode material for high-performance supercapacitors. Electrochim. Acta 2020, 364, 137147. [Google Scholar] [CrossRef]
- Kubra, K.T.; Sharif, R.; Patil, B.; Javaid, A.; Shahzadi, S.; Salman, A.; Siddique, S.; Ali, G. Hydrothermal synthesis of neodym-ium oxide nanoparticles and its nanocomposites with manganese oxide as electrode materials for supercapacitor application. J. Alloys Compd. 2020, 815, 152104. [Google Scholar] [CrossRef]
- Kubra, K.T.; Javaid, A.; Sharif, R.; Ali, G.; Iqbal, F.; Salman, A.; Shaheen, F.; Butt, A.; Iftikhar, F.J. Facile synthesis and electro-chemical study of a ternary hybrid PANI/GNP/MnO2 as supercapacitor electrode material. J. Mater. Sci. Mater. Electron. 2020, 31, 12455–12466. [Google Scholar] [CrossRef]
- Mordina, B.; Kumar, R.; Neeraj, N.S.; Srivastava, A.K.; Setua, D.K.; Sharma, A. Binder free high performance hybrid superca-pacitor device based on nickel ferrite nanoparticles. J. Energy Storage 2020, 31, 101677. [Google Scholar] [CrossRef]
- Obodo, R.M.; Shinde, N.; Chime, U.K.; Ezugwu, S.; Nwanya, A.C.; Ahmad, I.; Maaza, M.; Ejikeme, P.M.; Ezema, F.I. Recent advances in metal oxide/hydroxide on 3D nickel foam substrate for high performance pseudocapacitive electrodes. Curr. Opin. Electrochem. 2020, 21, 242–249. [Google Scholar] [CrossRef]
- Xiong, X.; Ding, D.; Chen, D.; Waller, G.; Bu, Y.; Wang, Z.; Liu, M. Three-dimensional ultrathin Ni(OH)2 nanosheets grown on nickel foam for high-performance supercapacitors. Nano Energy 2015, 11, 154–161. [Google Scholar] [CrossRef]
- Shim, J.-J. The 3D Co3O4/graphene/nickel foam electrode with enhanced electrochemical performance for supercapacitors. Mater. Lett. 2015, 139, 377–381. [Google Scholar]
- Yang, J.; Lian, L.; Ruan, H.; Xie, F.; Wei, M. Nanostructured porous MnO2 on Ni foam substrate with a high mass loading via a CV electrodeposition route for supercapacitor application. Electrochim. Acta 2014, 136, 189–194. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, T.; Liu, Z.; Zhong, Q.; Qin, Y. Facile fabrication of binder-free reduced graphene oxide/MnO2/Ni foam hybrid electrode for high-performance supercapacitors. J. Alloys Compd. 2020, 812, 152124. [Google Scholar] [CrossRef]
- Cai, D.; Huang, H.; Wang, D.; Liu, B.; Wang, L.; Liu, Y.; Li, Q.; Wang, T. High-performance supercapacitor electrode based on the unique ZnO@ Co3O4 core/shell heterostructures on nickel foam. ACS Appl. Mater. Interfaces 2014, 6, 15905–15912. [Google Scholar] [CrossRef]
- Chee, W.; Lim, H.; Harrison, I.; Chong, K.; Zainal, Z.; Ng, C.; Huang, N. Performance of flexible and binderless polypyr-role/graphene oxide/zinc oxide supercapacitor electrode in a symmetrical two-electrode configuration. Electrochim. Acta 2015, 157, 88–94. [Google Scholar] [CrossRef]
- Sun, J.; Du, X.; Wu, R.; Zhang, Y.; Xu, C.; Chen, H. Bundlelike CuCo2O4 Microstructures Assembled with Ultrathin Nanosheets As Battery-Type Electrode Materials for High-Performance Hybrid Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 8026–8037. [Google Scholar] [CrossRef]
- Teng, Y.; Yu, D.; Li, Y.; Meng, Y.; Wu, Y.; Feng, Y.; Hua, Y.; Wang, C.; Zhao, X.; Liu, X. Facile Synthesis of Hierarchical MgCo2O4@MnO2 Core-Shell Nanosheet Arrays on Nickel Foam as an Advanced Electrode for Asymmetric Supercapacitors. J. Electrochem. Soc. 2020, 167, 020510. [Google Scholar] [CrossRef]
- Ma, L.; Chang, Z.; Guo, L.; Li, T.; Li, G.; Wang, K. String-like core-shell ZnCo2O4@NiWO4 nanowire/nanosheet arrays on Ni foam for binder-free supercapacitor electrodes. Ionics 2020, 26, 2537–2547. [Google Scholar] [CrossRef]
- Chen, H.C.; Qin, Y.; Cao, H.; Song, X.; Huang, C.; Feng, H.; Zhao, X. Synthesis of amorphous nickel–cobalt–manganese hy-droxides for supercapacitor-battery hybrid energy storage system. Energy Storage Mater. 2019, 17, 194–203. [Google Scholar] [CrossRef]
- Du, C.; Han, E.; Gao, L.; Li, L.; Qiao, S.; Sun, L.; Liu, J. Soft-template and simple hydrothermal method to synthesize Fe-Co oxide on nickel foam and apply it to supercapacitors. Ionics 2020, 26, 4009–4018. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, A.; Che, H.; Mu, J.; Guo, Z.; Zhang, X.; Bai, Y.; Zhang, Z.; Wang, G.; Pei, Z. Three-dimensional interconnected MnCo2O4 nanosheets@ MnMoO4 nanosheets core-shell nanoarrays on Ni foam for high-performance supercapacitors. Chem. Eng. J. 2018, 336, 64–73. [Google Scholar] [CrossRef]
- Kuang, M.; Liu, X.Y.; Dong, F.; Zhang, Y.X. Tunable design of layered CuCo2O4 nanosheets@MnO2 nanoflakes core–shell arrays on Ni foam for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 21528–21536. [Google Scholar] [CrossRef]
- Lin, L.; Huang, M.; Ning, M.; Wu, K.; Li, H.; Hussain, S.; Zhao, S. Facile ordered ZnCo2O4@MnO2 nanosheet arrays for su-perior-performance supercapacitor electrode. Solid State Sci. 2018, 84, 51–56. [Google Scholar] [CrossRef]
- Chen, S.; Yang, G.; Zheng, H. Aligned Ni-Co-Mn oxide nanosheets grown on conductive substrates as binder-free electrodes for high capacity electrochemical energy storage devices. Electrochim. Acta 2016, 220, 296–303. [Google Scholar] [CrossRef]
- Su, D.; Tang, Z.; Xie, J.; Bian, Z.; Zhang, J.; Yang, D.; Zhang, D.; Wang, J.; Liu, Y.; Yuan, A.; et al. Co, Mn-LDH nanoneedle arrays grown on Ni foam for high performance supercapacitors. Appl. Surf. Sci. 2019, 469, 487–494. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Lian, J.; Pan, J.; Wei, T.; Sun, Y. Cedar leaf-like CuCo2O4 directly grow on nickel foam by a hydrother-mal/annealing process as an electrode for a high-performance symmetric supercapacitor. J. Alloys Compd. 2018, 735, 2046–2052. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Xia, X.; Shi, S.; Lu, Y.; Wang, X.; Gu, C.; Tu, J. Self-assembled porous NiCo2O4 hetero-structure array for electrochemical capacitor. J. Power Sources 2013, 239, 157–163. [Google Scholar] [CrossRef]
- Vishnukumar, P.; Saravanakumar, B.; Ravi, G.; Ganesh, V.; Guduru, R.K.; Yuvakkumar, R. Synthesis and characterization of NiO/Ni3V2O8 nanocomposite for supercapacitor applications. Mater. Lett. 2018, 219, 114–118. [Google Scholar] [CrossRef]
- Jung, N.; Kwon, S.; Lee, D.; Yoon, D.M.; Park, Y.M.; Benayad, A.; Choi, J.Y.; Park, J.S. Synthesis of chemically bonded gra-phene/carbon nanotube composites and their application in large volumetric capacitance supercapacitors. Adv. Mater. 2013, 25, 6854–6858. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, R.R.; Jang, K.; Lee, S.-w.; Ahn, H. Aligned nickel-cobalt hydroxide nanorod arrays for electrochemical pseudo-capacitor applications. RSC Adv. 2012, 2, 3190–3193. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.-J. Enhanced electrochemical performance of nanoplate nickel cobaltite (NiCo2O4) supercapacitor applications. RSC Adv. 2019, 9, 1115–1122. [Google Scholar] [CrossRef]
- Javed, M.S.; Dai, S.; Wang, M.; Xi, Y.; Lang, Q.; Guo, D.; Hu, C. Faradic redox active material of Cu7S4 nanowires with a high conductance for flexible solid state supercapacitors. Nanoscale 2015, 7, 13610–13618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.; Wang, Z.; Lin, T.; Zhu, X.; Luo, B.; Hu, H.; Xing, W.; Yan, Z.; Wang, L. Lithiation-Induced Vacancy Engi-neering of Co3O4 with Improved Faradic Reactivity for High-Performance Supercapacitor. Adv. Funct. Mater. 2020, 30, 2004172. [Google Scholar] [CrossRef]
- Kim, T.; Ramadoss, A.; Saravanakumar, B.; Veerasubramani, G.K.; Kim, S.J. Synthesis and characterization of NiCo2O4 nanoplates as efficient electrode materials for electrochemical supercapacitors. Appl. Surf. Sci. 2016, 370, 452–458. [Google Scholar] [CrossRef]
- Sethi, M.; Bhat, D.K. Facile solvothermal synthesis and high supercapacitor performance of NiCo2O4 nanorods. J. Alloys Compd. 2019, 781, 1013–1020. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, S.; Zheng, L.; Zhang, G.; Hao, Z.; Kang, L.; Liu, Z.-H. Preparation of NiMn2O4 with large specific surface area from an epoxide-driven sol-gel process and its capacitance. Electrochim. Acta 2013, 87, 546–553. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Priyadharshini, T.; Ravi, G.; Ganesh, V.; Sakunthala, A.; Yuvakkumar, R. Hydrothermal synthesis of spherical NiCO2O4 nanoparticles as a positive electrode for pseudocapacitor applications. J. Sol Gel Sci. Technol. 2017, 84, 297–305. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Reddy, M.; Harilal, M.; Vidyadharan, B.; Misnon, I.I.; Ab Rahim, M.H.; Ismail, J.; Jose, R. Characterization of MgCo2O4 as an electrode for high performance supercapacitors. Electrochim. Acta 2015, 161, 312–321. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Anitha, T.; Kim, H.-J. Fabrication of Hierarchical NiMoO4/NiMoO4 Nanoflowers on Highly Conductive Flexible Nickel Foam Substrate as a Capacitive Electrode Material for Supercapacitors with Enhanced Electrochemical Performance. Energies 2019, 12, 1143. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).