Technical and Commercial Challenges of Proton-Exchange Membrane (PEM) Fuel Cells
Abstract
1. Introduction
2. Applications of Fuel Cells
2.1. Advantages and Disadvantages of Fuel Cells
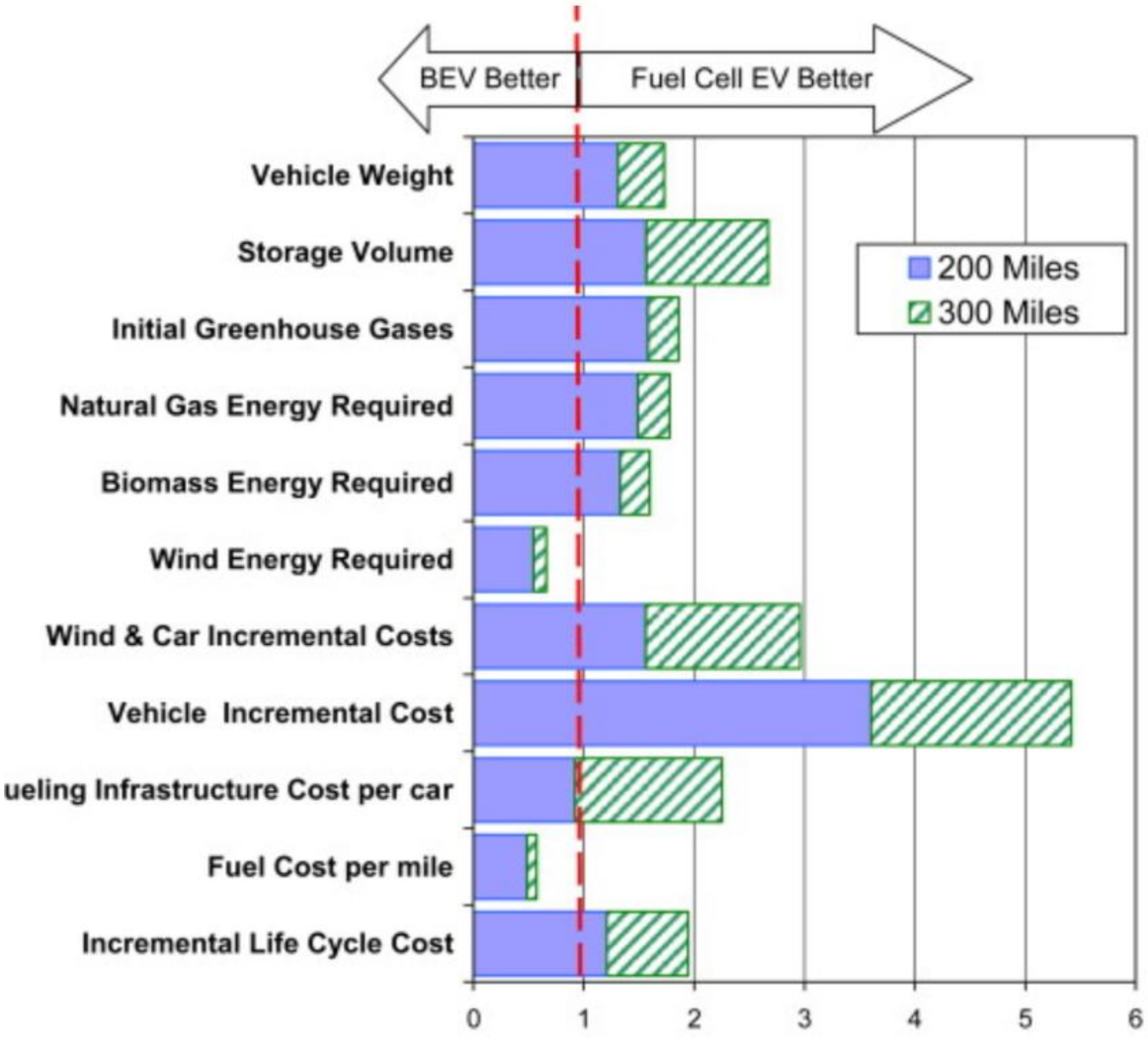
2.2. Comparison between Fuel Cells and Batteries
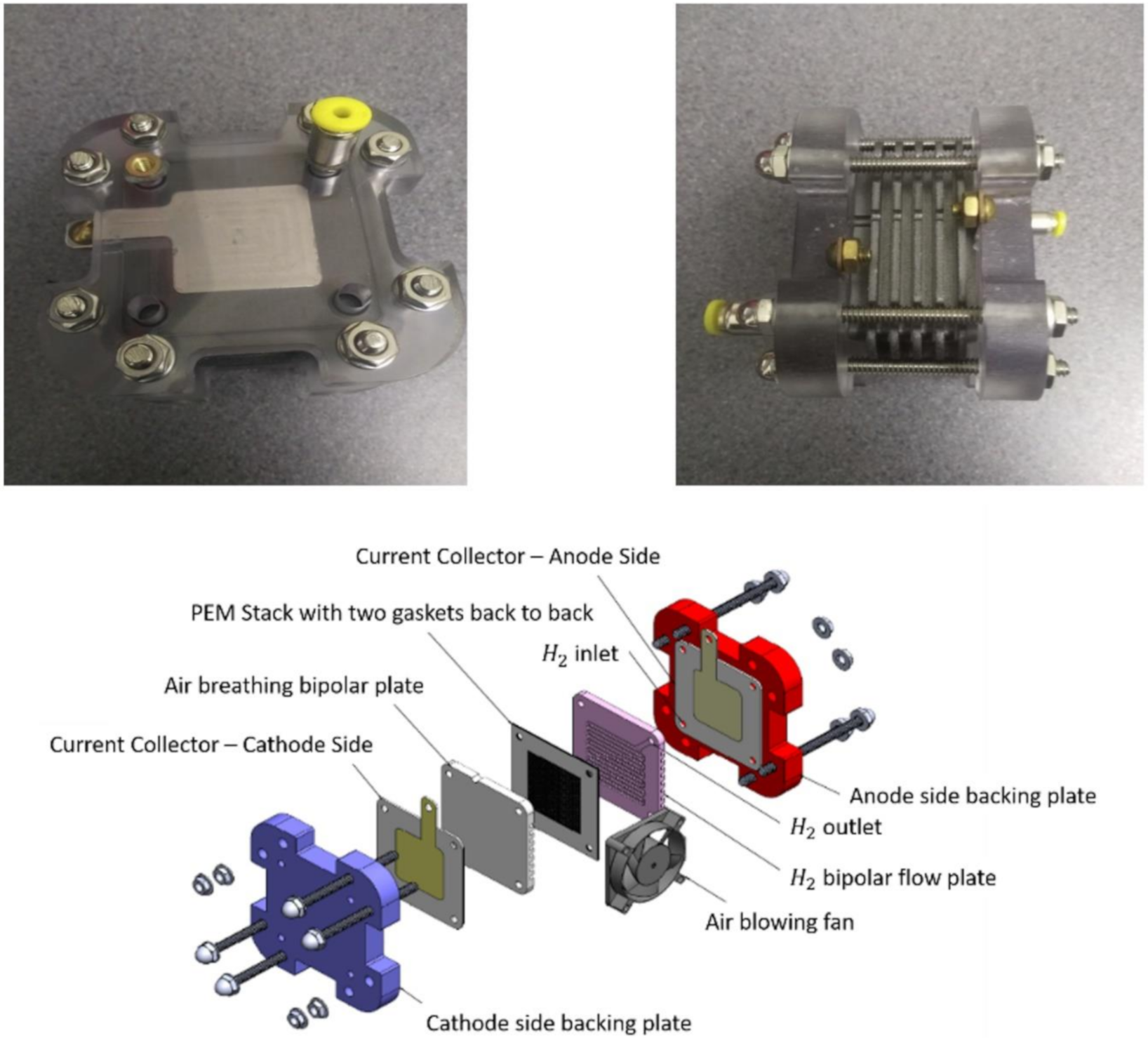
3. Proton-Exchange Membrane Fuel Cells (PEMFCs)
Composition of Proton-Exchange Membrane Fuel Cells
4. Prospects of Fuel Cell in the Automotive Industry
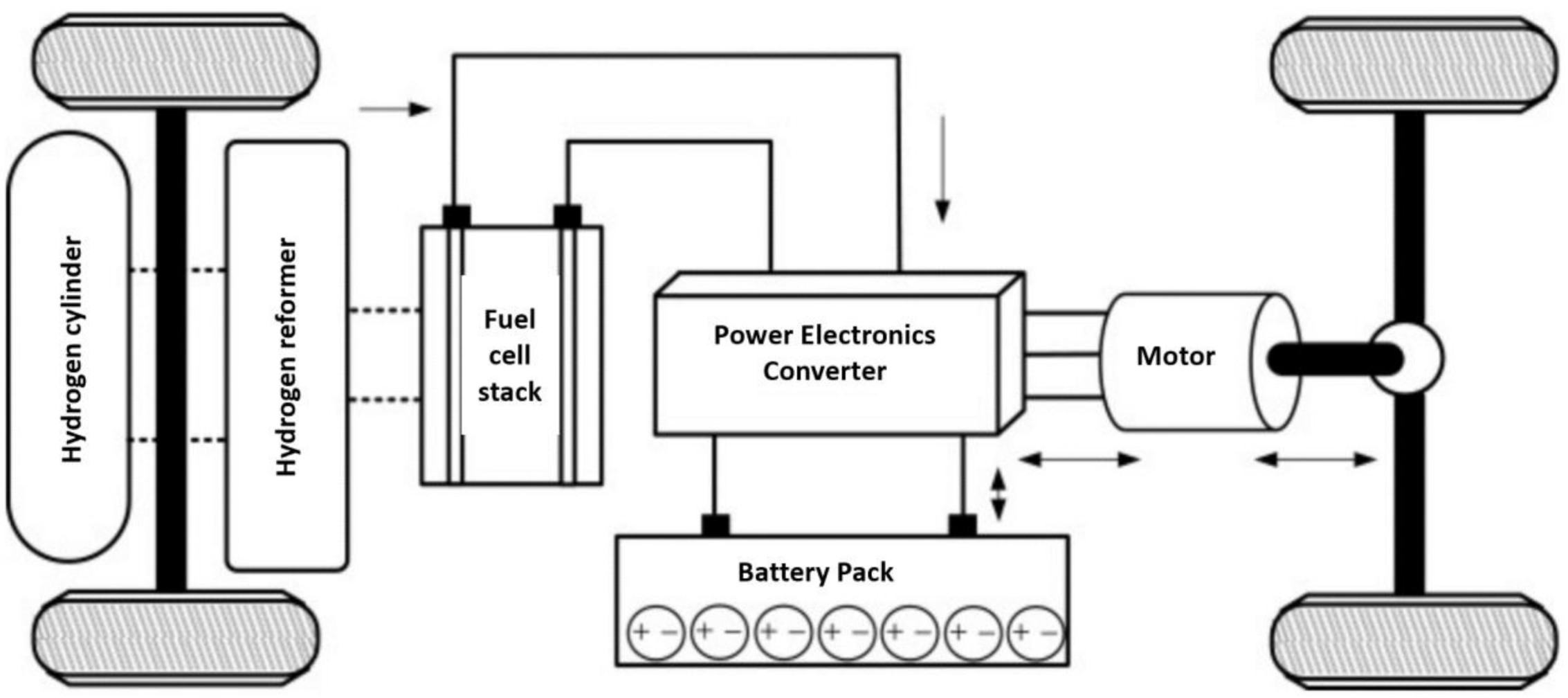
4.1. Some Applications of Fuel Cells in the Automotive Industry
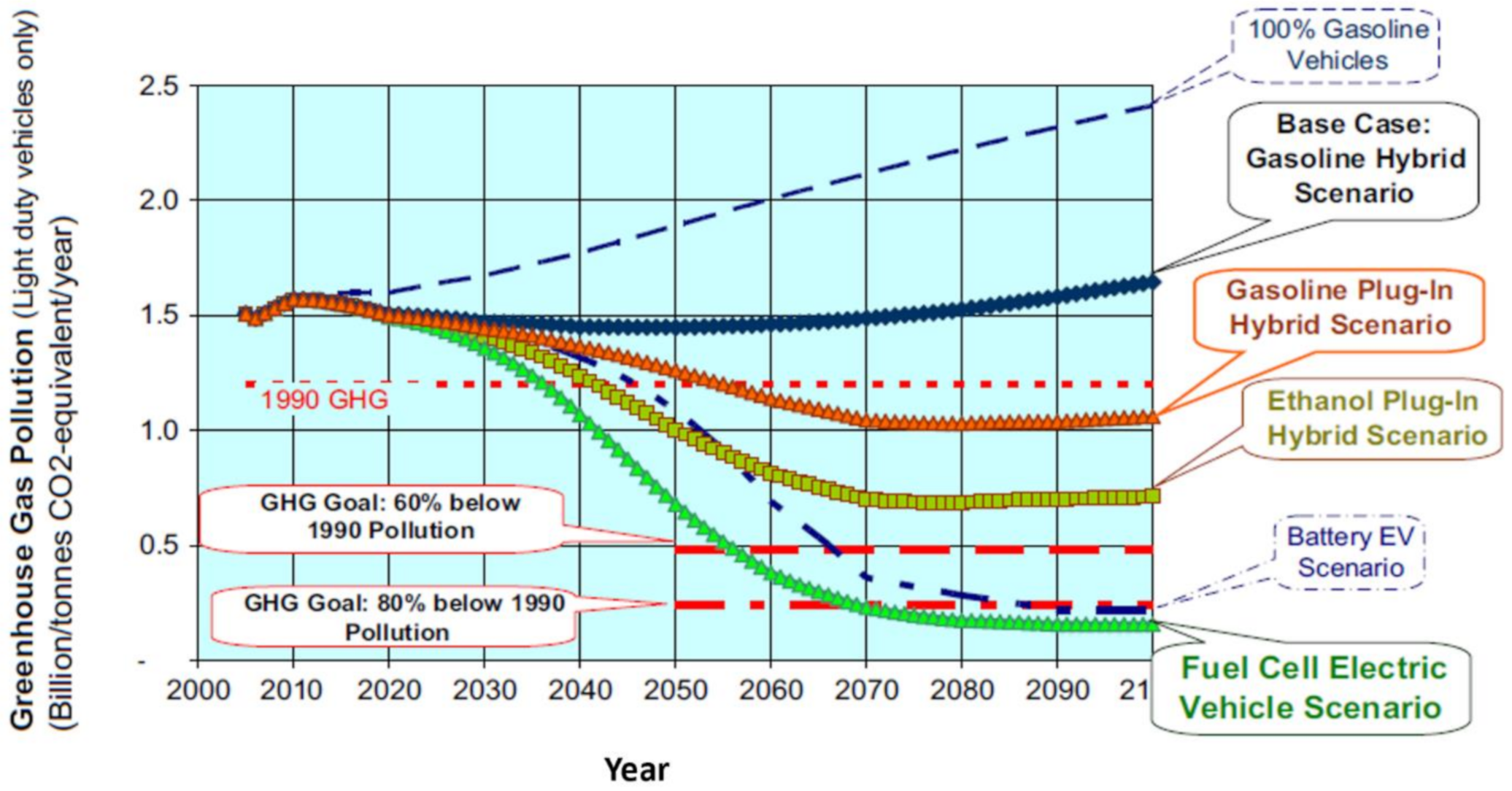
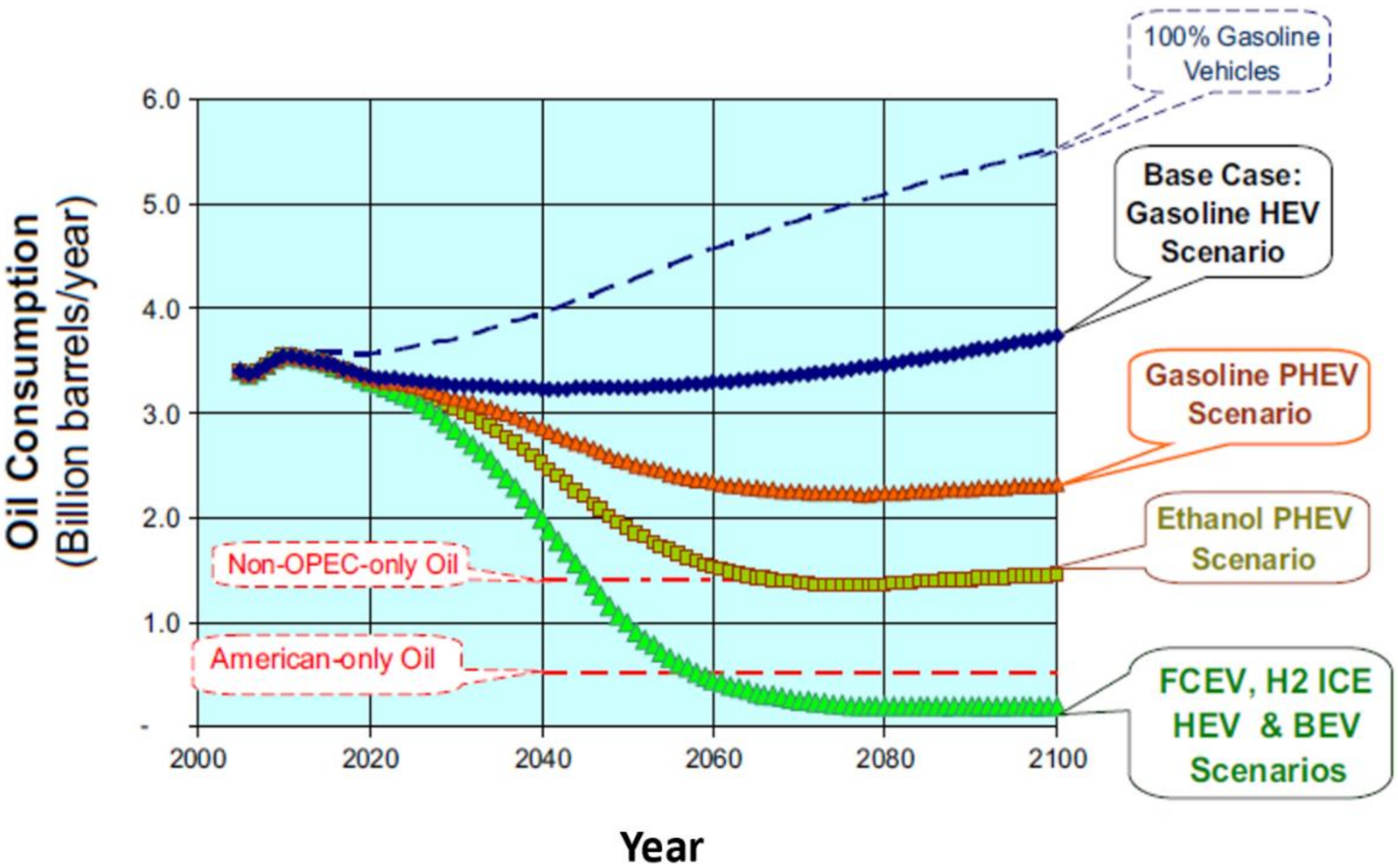
4.2. Light Duty Fuel Cell Electric Vehicles
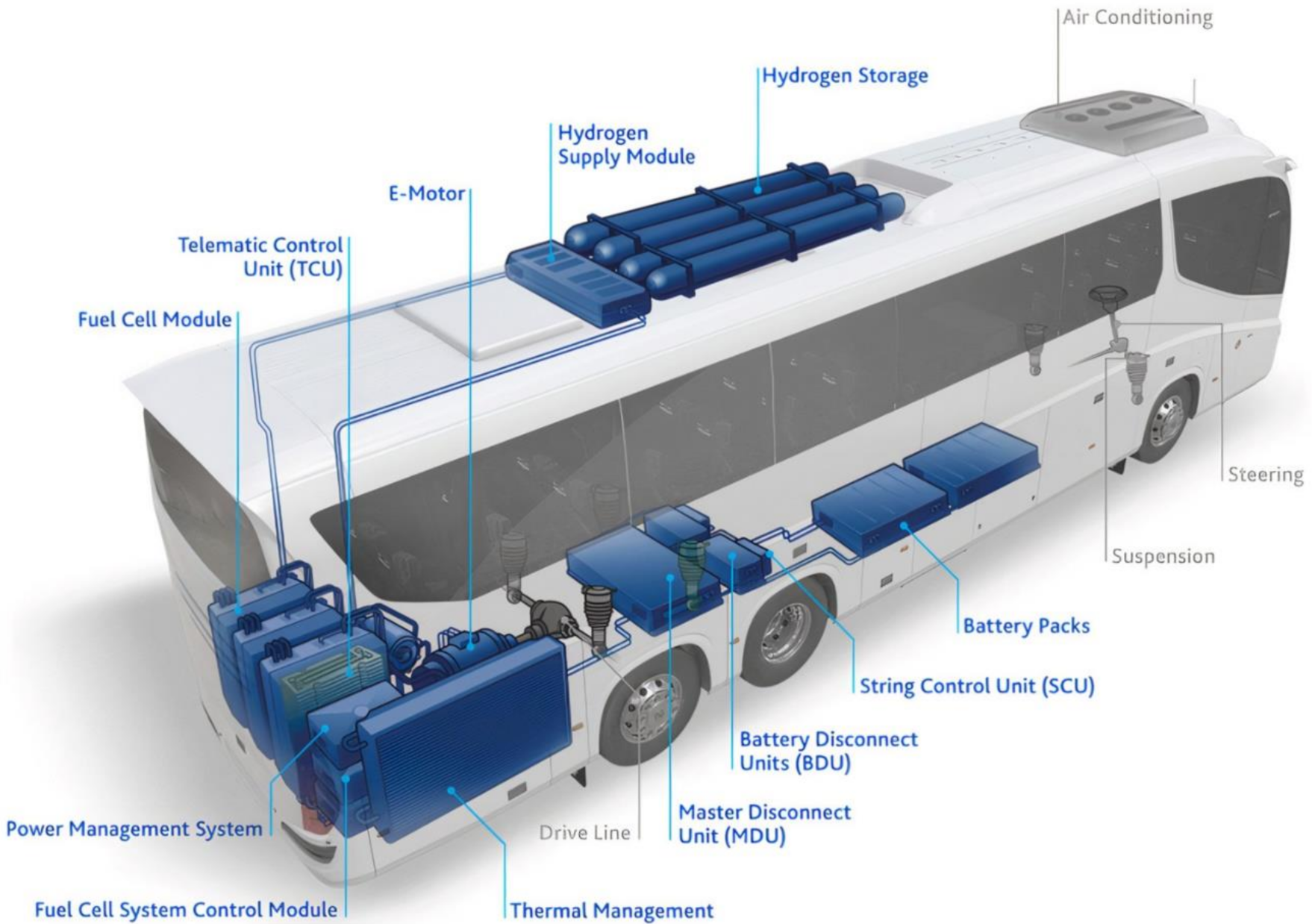
4.3. Heavy Duty Fuel Cell Electric Vehicle
5. Fuel Cell Optimization Criteria
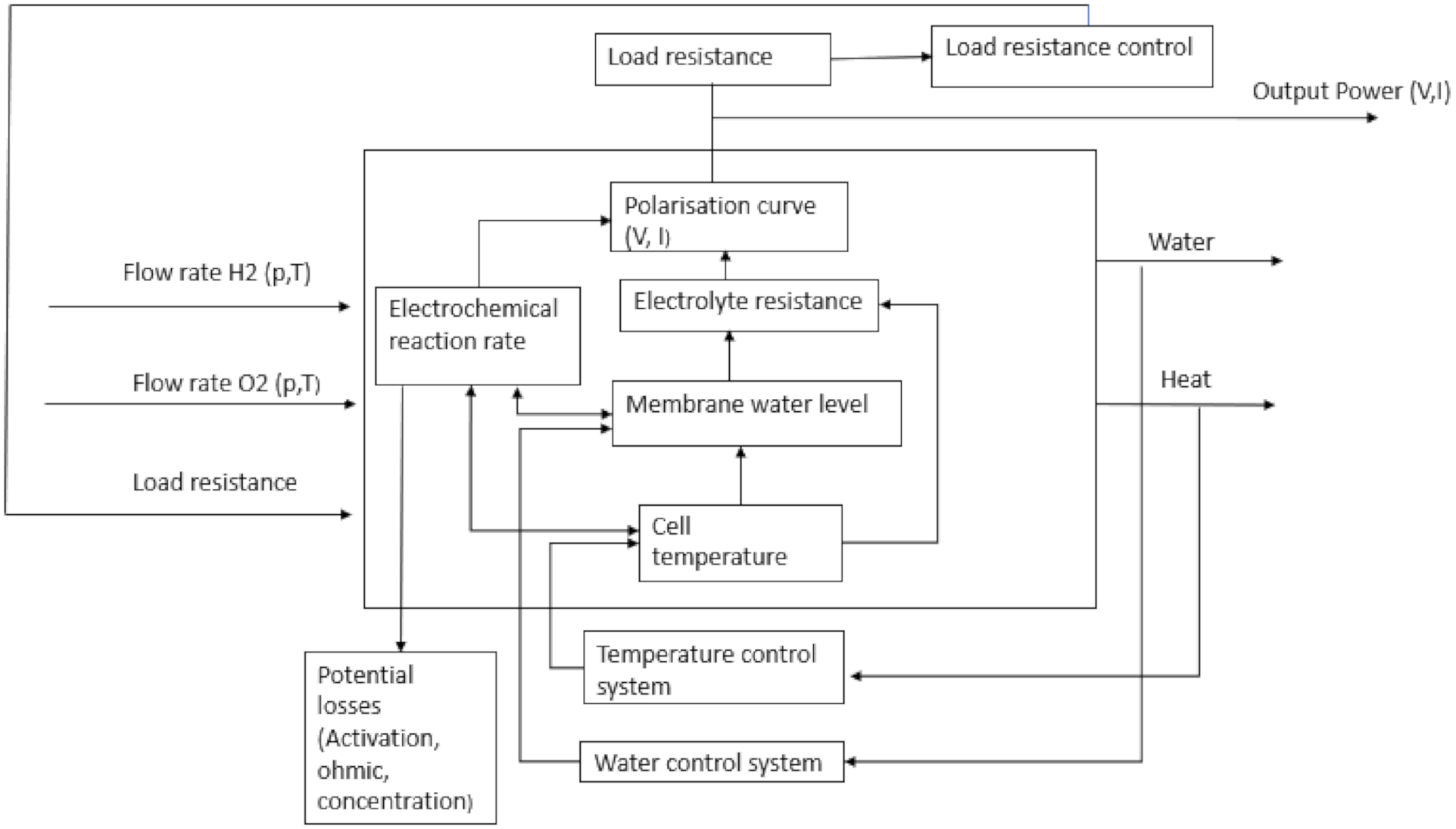
5.1. Operating Parameters of Fuel Cell
- Partial pressure of the reactants (hydrogen and oxygen, for a PEMFC)
- Stack temperature
- Membrane humidity
5.2. Techniques to Adopt to Improve Fuel Cell Performance
- Increase the cell temperature
- Use a catalyst that improves the reaction kinetics
- Increase the electrode roughness to increase the catalyst surface on the electrode and thus increase the reaction sites
- Increase the concentration of reagents as the density of the current exchange increases
- Increase reagent pressure as it increases concentration.
- Use electrodes with the highest conductivity
- Optimisation of design and choice of materials for bipolar plates
- Make the membrane as thin as possible
- Increase the membrane humidity.
- The speed with which the reagent gases are supplied
- The geometry of the reagent delivery channels
- The diffusive characteristics of gas diffusion layers and electrodes
- The air circulation in the cathode to avoid the accumulation of water produced by the reaction.
5.3. Fuel Cell Hybrid System
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilberforce, T.; Sayed, E.T.; Abdelkareem, M.A.; Elsaid, K.; Olabi, A. Value added products from wastewater using bioelectrochemical systems: Current trends and perspectives. J. Water Process. Eng. 2020, 101737. [Google Scholar] [CrossRef]
- Baroutaji, A.; Arjunan, A.; Alaswad, A.; Praveen, A.S.; Wilberforce, T.; Abdelkareem, M.A.; Olabi, A.-G. Materials for Fuel Cell Membranes. Ref. Module Mater. Sci. Mater. Eng. 2020. [Google Scholar] [CrossRef]
- Olabi, A.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Wilberforce, T.; Ijaodola, O.; Thompson, J.; Olabi, A. Selection of proton exchange membrane fuel cell for transportation. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Lootah, M.A.; Sayed, E.T.; Wilberforce, T.; Alawadhi, H.; Yousef, B.A.; Olabi, A.G. Fuel Cells for Carbon Capture Applications. Sci. Total Environ. 2020, 144243. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Wilberforce, T.; Ijaodola, O.; Thompson, J.; Olabi, A. Review of operating condition, design parameters and material properties for proton exchange membrane fuel cells. Int. J. Energy Res. 2020. [Google Scholar] [CrossRef]
- Rabaia, M.K.H.; Abdelkareem, M.A.; Sayed, E.T.; Elsaid, K.; Chae, K.J.; Wilberforce, T.; Olabi, A. Environmental impacts of solar energy systems: A review. Sci. Total Environ. 2021, 754, 141989. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, K.; Olabi, A.; Wilberforce, T.; Abdelkareem, M.A.; Sayed, E.T. Environmental Impacts of Nanofluids: A Review. Sci. Total Environ. 2020, 144202. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; AbdelGhani, E.A.; Olabi, A. Transition metal carbides and nitrides as oxygen reduction reaction catalyst or catalyst support in proton exchange membrane fuel cells (PEMFCs). Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Olabi, A.; Wilberforce, T.; Abdelkareem, M.A. Fuel cell application in the automotive industry and future perspective. Energy 2021, 214, 118955. [Google Scholar] [CrossRef]
- Olabi, A.; Onumaegbu, C.; Wilberforce, T.; Ramadan, M.; Abdelkareem, M.A.; Al-Alami, A.H. Critical review of energy storage systems. Energy 2021, 214, 118987. [Google Scholar] [CrossRef]
- Sayed, E.T.; Wilberforce, T.; Elsaid, K.; Rabaia MK, H.; Abdelkareem, M.A.; Chae, K.J.; Olabi, A.G. A critical review on Environmental Impacts of Renewable Energy Systems and Mitigation Strategies: Wind, Hydro, Biomass and Geothermal. Sci. Total Environ. 2020, 144505. [Google Scholar] [CrossRef]
- Elsaid, K.; Olabi, A.; Wilberforce, T.; Abdelkareem, M.A.; Sayed, E.T. DeNOx removal techniques for automotive applications—A review. Environ. Adv. 2020, 2, 100021. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Khatib, F.; Ogungbemi, E.; El Hassan, Z.; Thompson, J.; Olabi, A. Effect of humidification of reactive gases on the performance of a proton exchange membrane fuel cell. Sci. Total Environ. 2019, 688, 1016–1035. [Google Scholar] [CrossRef] [PubMed]
- Wilberforce, T.; El Hassan, Z.; Ogungbemi, E.; Ijaodola, O.; Khatib, F.; Durrant, A.; Thompson, J.; Baroutaji, A.; Olabi, A. A comprehensive study of the effect of bipolar plate (BP) geometry design on the performance of proton exchange membrane (PEM) fuel cells. Renew. Sustain. Energy Rev. 2019, 111, 236–260. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; Khatib, F.; Leslie, T.; El-Hassan, Z.; Thomposon, J.; Olabi, A. Technical evaluation of proton exchange membrane (PEM) fuel cell performance—A review of the effects of bipolar plates coating. Renew. Sustain. Energy Rev. 2019, 113. [Google Scholar] [CrossRef]
- Alawadhi, H.; Abdelkareem, M.A.; Hussain, N.; Wilberforce, T.; Sayed, E.T. A composite of graphitic carbon nitride and Vulcan carbon as an effective catalyst support for Ni in direct urea fuel cells. J. Taiwan Inst. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Elsaid, K.; Kamil, M.; Sayed, E.T.; Abdelkareem, M.A.; Wilberforce, T.; Olabi, A. Environmental impact of desalination technologies: A review. Sci. Total Environ. 2020, 748, 141528. [Google Scholar] [CrossRef]
- Al-Anazi, A.; Wilberforce, T.; Khatib, F.; Vichare, P.; Olabi, A. Performance evaluation of an air breathing polymer electrolyte membrane (PEM) fuel cell in harsh environments—A case study under Saudi Arabia’s ambient condition. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2020, 143203. [Google Scholar] [CrossRef]
- Olabi, A.; Wilberforce, T.; Ramadan, M.; Abdelkareem, M.A.; Alami, A.H. Compressed air energy storage systems: Components and operating parameters—A review. J. Energy Storage 2020, 102000. [Google Scholar] [CrossRef]
- Khatib, F.; Wilberforce, T.; Thompson, J.; Olabi, A. A comparison on the dynamical performance of a proton exchange membrane fuel cell (PEMFC) with traditional serpentine and an open pore cellular foam material flow channel. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental aspects of fuel cells: A review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.; Mahmoud, M.; Soudan, B.; Wilberforce, T.; Ramadan, M. Geothermal based hybrid energy systems, toward eco-friendly energy approaches. Renew. Energy 2020, 147, 2003–2012. [Google Scholar] [CrossRef]
- Wilberforce, T.; Baroutaji, A.; El Hassan, Z.; Thompson, J.; Soudan, B.; Olabi, A. Prospects and challenges of concentrated solar photovoltaics and enhanced geothermal energy technologies. Sci. Total Environ. 2019, 659, 851–861. [Google Scholar] [CrossRef]
- Wilberforce, T.; El Hassan, Z.; Durrant, A.; Thompson, J.; Soudan, B.; Olabi, A. Overview of ocean power technology. Energy 2019, 175, 165–181. [Google Scholar] [CrossRef]
- Wilberforce, T.; Baroutaji, A.; Soudan, B.; Al-Alami, A.H.; Olabi, A. Outlook of carbon capture technology and challenges. Sci. Total Environ. 2019, 657, 56–72. [Google Scholar] [CrossRef]
- Ijaodola, O.; Hassan, Z.E.-; Ogungbemi, E.; Khatib, F.; Wilberforce, T.; Thompson, J.; Olabi, A. Energy efficiency improvements by investigating the water flooding management on proton exchange membrane fuel cell (PEMFC). Energy 2019, 179, 246–267. [Google Scholar] [CrossRef]
- Olabi, A.; Wilberforce, T.; Sayed, E.T.; Elsaid, K.; Rezk, H.; Abdelkareem, M.A. Recent progress of graphene based nanomaterials in bioelectrochemical systems. Sci. Total Environ. 2020, 749, 141225. [Google Scholar] [CrossRef]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive investigation on hydrogen and fuel cell technology in the aviation and aerospace sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef]
- Ijaodola, O.; Ogungbemi, E.; Khatib, F.; Wilberforce, T.; Ramadan, M.; El Hassan, Z.; Thompson, J.; Olabi, A. Evaluating the Effect of Metal Bipolar Plate Coating on the Performance of Proton Exchange Membrane Fuel Cells. Energies 2018, 11, 3203. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.; Wilberforce, T.; El Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A. Fuel cell membranes–Pros and cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef]
- Thomas, C. Fuel cell and battery electric vehicles compared. Int. J. Hydrogen Energy 2009, 34, 6005–6020. [Google Scholar] [CrossRef]
- Alaswad, A.; Dassisti, M.; Palumbo, A.; Olabi, A.G. Fuel Cell Technologies, Applications, and State of the Art. A Reference Guide; Elsevier Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Wilberforce, T.; Olabi, A. Performance Prediction of Proton Exchange Membrane Fuel Cells (PEMFC) Using Adaptive Neuro Inference System (ANFIS). Sustainbility 2020, 12, 4952. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A. Design of Experiment (DOE) Analysis of 5-Cell Stack Fuel Cell Using Three Bipolar Plate Geometry Designs. Sustainbility 2020, 12, 4488. [Google Scholar] [CrossRef]
- Wilberforce, T.; Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A. Advances in stationary and portable fuel cell applications. Int. J. Hydrogen Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef]
- Wilberforce, T.; Khatib, F.N.; Ogungbemi, E.; Olabi, A.G. Water Electrolysis Technology. Ref. Module Mater. Sci. Mater. Eng. 2018. [Google Scholar] [CrossRef]
- David, M.-S. A Review of Developments in Electrical Battery, Fuel Cell and Energy Recovery Systems for Railway Applications; A Report for the Scottish Association for Public Transport; Scottish Association for Public Transport: Glasgow, UK, 2019. [Google Scholar] [CrossRef]
- Collen, S. Designing and Building Fuel Cells; Mc Graw Hill: Pennsylvania, PA, USA, 2007; ISBN 0-07-148977-0. [Google Scholar]
- Wilberforce, T.; El Hassan, Z.; Khatib, F.; Al Makky, A.; Mooney, J.; Barouaji, A.; Carton, J.G.; Olabi, A.-G. Development of Bi-polar plate design of PEM fuel cell using CFD techniques. Int. J. Hydrogen Energy 2017, 42, 25663–25685. [Google Scholar] [CrossRef]
- Kirubakaran, A.; Jain, S.; Nema, R. A review on fuel cell technologies and power electronic interface. Renew. Sustain. Energy Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Ali, D.M.; Salman, S. A Comprehensive Review of the Fuel Cells Technology and Hydrogen Economy. In Proceedings of the 41st International Universities Power Engineering Conference, Newcastle-upon-Tyne, UK, 6–8 September 2006; Volume 1, pp. 98–102. [Google Scholar]
- Ziaur, R.; Mashrur, I. Comparative Study of Different Fuel Cell Technologies. Asian J. Con. Sci. Technol. 2019, 1, 29–32. [Google Scholar] [CrossRef]
- Ellamla, H.R.; Staffell, I.; Bujlo, P.; Pollet, B.G.; Pasupathi, S. Current status of fuel cell based combined heat and power systems for residential sector. J. Power Sources 2015, 293, 312–328. [Google Scholar] [CrossRef]
- Appleby, A. Fuel cell technology: Status and future prospects. Energy 1996, 21, 521–653. [Google Scholar] [CrossRef]
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.; Al Makky, A.; Baroutaji, A.; Carton, J.G.; Olabi, A.G. Developments of electric cars and fuel cell hydrogen electric cars. Int. J. Hydrogen Energy 2017, 42, 25695–25734. [Google Scholar] [CrossRef]
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.; Al Makky, A.; Baroutaji, A.; Carton, J.G.; Thompson, J.; Olabi, A.G. Modelling and simulation of Proton Exchange Membrane fuel cell with serpentine bipolar plate using MATLAB. Int. J. Hydrogen Energy 2017, 42, 25639–25662. [Google Scholar] [CrossRef]
- Khatib, F.; Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; El-Hassan, Z.; Durrant, A.; Thompson, J.; Olabi, A. Material degradation of components in polymer electrolyte membrane (PEM) electrolytic cell and mitigation mechanisms: A review. Renew. Sustain. Energy Rev. 2019, 111, 1–14. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; El Hassan, Z.; Thompson, J.; Olabi, A.G. Effect of Bipolar Plate Materials on Performance of Fuel Cells. Ref. Module Mater. Sci. Mater. Eng. 2018. [Google Scholar] [CrossRef]
- Erdinç, O.; Uzunoglu, M. Recent trends in PEM fuel cell-powered hybrid systems: Investigation of application areas, design architectures and energy management approaches. Renew. Sustain. Energy Rev. 2010, 14, 2874–2884. [Google Scholar] [CrossRef]
- Islam, M.; Shabani, B.; Rosengarten, G.; Andrews, J. The potential of using nanofluids in PEM fuel cell cooling systems: A review. Renew. Sustain. Energy Rev. 2015, 48, 523–539. [Google Scholar] [CrossRef]
- Alizadeh, E.; Rahgoshay, S.; Rahimi-Esbo, M.; Khorshidian, M.; Saadat, S. A novel cooling flow field design for polymer electrolyte membrane fuel cell stack. Int. J. Hydrogen Energy 2016, 41, 8525–8532. [Google Scholar] [CrossRef]
- Hossain, A.K.; Serrano, C.; Brammer, J.; Omran, A.; Ahmed, F.; Smith, D.; Davies, P.A. Combustion of fuel blends containing digestate pyrolysis oil in a multi-cylinder compression ignition engine. Fuel 2016, 171, 18–28. [Google Scholar] [CrossRef]
- Ehsani, M.; Gao, Y.; Gay, S.E.; Emadi, A. Modern Electric, Hybrid Electric, and Fuel Cell Vehicles: Fundamentals, Theory, and Design, 2nd ed.; CRC Press: Florida, FL, USA, 2004. [Google Scholar]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Javaid, Z.S.M.; Matsuura, T. Polymer membranes for fuel cells. In Polymer Membranes for Fuel Cells; Springer: New York, NY, USA, 2009; pp. 1–431. [Google Scholar]
- Van Vliet, O.; Brouwer, A.S.; Kuramochi, T.; Broek, M.V.D.; Faaij, A. Energy use, cost and CO2 emissions of electric cars. J. Power Sources 2011, 196, 2298–2310. [Google Scholar] [CrossRef]
- Amjad, S.; Neelakrishnan, S.; Rudramoorthy, R. Review of design considerations and technological challenges for successful development and deployment of plug-in hybrid electric vehicles. Renew. Sustain. Energy Rev. 2010, 14, 1104–1110. [Google Scholar] [CrossRef]
- UK Government. 2010 to 2015 Government Policy: Transport Emissions–GOV.UK. 2015. Available online: https://www.gov.uk/government/publications/2010-to-2015-government-policy-transport-emissions/2010-to-2015-government-policy-transport-emissions (accessed on 17 March 2020).
- Olabi, A.; Wilberforce, T.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Prospects of Fuel Cell Combined Heat and Power Systems. Energies 2020, 13, 4104. [Google Scholar] [CrossRef]
- UKH2Mobility. UK H2Mobility|Hydrogen: Fuelling Cleaner Motoring. 2012. Available online: http://www.ukh2mobility.co.uk/ (accessed on 17 March 2020).
- Mokrani, Z.; Rekioua, D.; Mebarki, N.; Bacha, S. Proposed energy management strategy in electric vehicle for recovering power excess produced by fuel cells. Int. J. Hydrogen Energy 2017, 42, 19556–19575. [Google Scholar] [CrossRef]
- Kumar, A.; Sehgal, M. Hydrogen Fuel Cell Technology for a Sustainable Future: A Review. SAE Techn. Pap. Ser. 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Qi, Z. Application: Transportation: Light traction: Fuel cells. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Dicks, A.L. PEM fuel cells: Applications. In Comprehensive Renewable Energy; Sayigh, A., Ed.; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Colella, W.G. Market prospects, design features, and performance of a fuel cell-powered scooter. J. Power Sources 2000, 86, 255–260. [Google Scholar] [CrossRef]
- Lin, B. Conceptual design and modeling of a fuel cell scooter for urban Asia. J. Power Sources 2000, 86, 202–213. [Google Scholar] [CrossRef]
- Hwang, J.; Wang, D.; Shih, N.; Lai, D.; Chen, C. Development of fuel-cell-powered electric bicycle. J. Power Sources 2004, 133, 223–228. [Google Scholar] [CrossRef]
- Hwang, J.; Wang, D.; Shih, N. Development of a light weight fuel cell vehicle. J Power Sources 2005, 141, 108–115. [Google Scholar] [CrossRef]
- Hochgraf, C. Application: transportation: Electric vehicles: Fuel cells. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Bill Thomas. Hyundai Celebrates World’s First Assembly Line Production of Zero-Emissions Fuel Cell Vehicles. Available online: https://www.prweb.com/releases/hyundai-ix35-fuel-cell/03/prweb10475471.htm (accessed on 10 October 2020).
- Igbinovia, F.O.; Fandi, G.; Mahmoud, R.; Tlusty, J. A Review of Electric Vehicles Emissions and its Smart Charging Techniques Influence on Power Distribution Grid. J. Eng. Sci. Technol. Rev. 2016, 2016, 80–85. [Google Scholar] [CrossRef]
- Gabe, B.K. Audi Loses Faith In Batteries, Restarts Hydrogen Powertrain Development. Available online: https://carbuzz.com/news/audi-loses-faith-in-batteries-restarts-hydrogen-powertrain-development (accessed on 30 July 2020).
- Zorpette, G. Supercharged [ultracapacitors]. IEEESpectr 2005, 42, 32–37. [Google Scholar]
- Pollet, B.G.; Staffell, I.; Shang, J.L. Current status of hybrid, battery and fuel cell electric vehicles: From electrochemistry to market prospects. Electrochim. Acta 2012, 84, 235–249. [Google Scholar] [CrossRef]
- Campanari, S.; Manzolini, G.; De La Iglesia, F.G. Energy analysis of electric vehicles using batteries or fuel cells through well-to-wheel driving cycle simulations. J. Power Sources 2009, 186, 464–477. [Google Scholar] [CrossRef]
- Josh Goldman. Comparing Electric Vehicles: Hybrid vs. BEV vs. PHEV vs. FCEV. 2014. Available online: https://blog.ucsusa.org/josh-goldman/comparing-electric-vehicles-hybrid-vs-bev-vs-phev-vs-fcev-411 (accessed on 28 November 2020).
- Nguyen, T.; Ward, J. Well-to-Wheel Green House Gas Emissions and Petroleum Use Formid-Size Light-Duty Vehicles; Record#1001; US Department of Energy: Washington, DC, USA, 2010.
- Ahluwalia, R.; Hua, T.Q.; Peng, J. On-board and Off-board performance of hydrogen storage options for light-duty vehicles. Int. J. Hydrogen Energy 2012, 37, 2891–2910. [Google Scholar] [CrossRef]
- Fuel Cells and Hydrogen (Joint Undertaking). Final Report Of The Bus Study: Urban Buses: Alternative Power Trains For Europe. Available online: https://www.fch.europa.eu/node/790 (accessed on 26 December 2020).
- Eudy, L.; Chandler, K.; Gikakis, C. Fuel Cell Buses in U.S.Transit Fleets: Current Status 2012; NREL/TP-5600–56406; National Renewable Energy Laboratory: Golden, CO, USA, 2012.
- Ally, J.; Pryor, T. Life-cycle assessment of diesel, natural gas and hydrogen fuel cell bus transportation systems. J. Power Sources 2007, 170, 401–411. [Google Scholar] [CrossRef]
- Saxe, M.; Folkesson, A.; Alvfors, P. Energy system analysis of the fuel cell buses operated in the project: Clean Urban Transport for Europe. Energy 2008, 33, 689–711. [Google Scholar] [CrossRef]
- Canada gets first fuel cell bus for 2010 Winter Olympics fleet. Fuel Cells Bull. 2009, 2009, 2. [CrossRef]
- Li, X.; Li, J.; Xu, L.; Yang, F.; Hua, J.; Ouyang, M. Performance analysis of proton-exchange membrane fuel cell stacks used in Beijing urban-route buses trial project. Int. J. Hydrogen Energy 2010, 35, 3841–3847. [Google Scholar] [CrossRef]
- Green Car Congress. FLixBus and Freudenberg Sealing Technologies Partner on Fuel Cell Long Distance Buses. Available online: https://www.greencarcongress.com/2019/09/20190903-flixb.html (accessed on 2 September 2020).
- Benziger, J.; Satterfield, M.B.; Hogarth, W.H.; Nehlsen, J.P.; Kevrekidis, I.G. The power performance curve for engineering analysis of fuel cells. J. Power Sources 2006, 155, 272–285. [Google Scholar] [CrossRef]
- Andrew, L.D.; David, A.J.R. Fuel Cell Systems Explained, 3rd ed.; John Wiley & Sons Ltd: Chichester, UK, 2018; ISBN 978-1-118-61352-8. [Google Scholar]
- Ibrahim, M.M. Fuel Cells for High Altitude UAS Modelling, Simulation and Performance Evaluation: PEM Fuel Cells for High Altitude UAS. 2015. Available online: http://shura.shu.ac.uk/13602/ (accessed on 26 September 2020).
- Shih, N.-C.; Weng, B.-J.; Lee, J.-Y.; Hsiao, Y.-C. Development of a small fuel cell underwater vehicle. Int. J. Hydrogen Energy 2013, 38, 11138–11143. [Google Scholar] [CrossRef]
- Amirinejad, M.; Rowshanzamir, S.; Eikani, M.H. Effects of operating parameters on performance of a proton exchange membrane fuel cell. J. Power Sources 2006, 161, 872–875. [Google Scholar] [CrossRef]
- Chan, S.; Goh, S.; Jiang, S. A mathematical model of polymer electrolyte fuel cell with anode CO kinetics. Electrochim. Acta 2003, 48, 1905–1919. [Google Scholar] [CrossRef]
- Bernardi, D.M. Water—Balance Calculations for Solid-Polymer-Electrolyte Fuel Cells. J. Electrochem. Soc. 1990, 137, 3344. [Google Scholar] [CrossRef]
- Jung, S.Y.; Nguyen, T.V. An along-the-channel model for proton exchange membrane fuel cells. J. Electrochem. Soc. 1998, 145, 1149–1159. [Google Scholar]
- Nguyen, T.V.; White, R.E. White.A Water and Heat Management Model for Proton-Exchange Membrane Fuel Cells. J. Electrochem. Soc. 1993, 140, 2178. [Google Scholar] [CrossRef]
- Büchi, F.N.; Srinivasan, S. Operating Proton Exchange Membrane Fuel Cells Without External Humidification of the Reactant Gases: Fundamental Aspects. J. Electrochem. Soc. 1997, 144, 2767. [Google Scholar] [CrossRef]
- Williams, M.V.; Kunz, H.; Fenton, J.M. Operation of Nafion®-based PEM fuel cells with no external humidification: influence of operating conditions and gas diffusion layers. J. Power Sources 2004, 135, 122–134. [Google Scholar] [CrossRef]
- Tesfahunegn, S.; Vie, P.; Undeland, T.; Ulleberg, E. A combined steady state and dynamic model of a proton exchange membrane fuel cell for use in dg system simulation. In Proceedings of the IPEC 2010, International Power Electronics Conference (IEEE) 2010, Sapporo, Japan, 21–24 June 2010; pp. 2457–2464. [Google Scholar]


| PEMFC | AFC | PAFC | DMFC | MCFC | SOFC | Source | |
|---|---|---|---|---|---|---|---|
| Catalyst layer | Pt | Pt or Ni | Pt | Pt and Ru (1:1) | Ni | Ni | [41] |
| Electrolyte | NafionTM | KOH | H3PO4 | NafionTM | Molten Carbonate | YSZ | [42] |
| Anodic Reactant | H2 | H2 | H2/CH3OH | CH3OH/H2 | H2/CO | H2/CO | [43] |
| Cell temperature (oC) | 60–85 | 20–80 | Greater than 170 | Less than 70 | 500–750 | 650–1100 | [44] |
| Merits | 1- Wider range of power operation. 2- Scaling is simple. 3- Starting it can be done within a limited period of time. 4- Increased power density compared to other types of fuel cells | 1- Pt can be substituted with other materials. 2- Cost effective 3- Faster rate of reaction. 4- Can be started quickly. | 1- Lesser impact of carbon monoxide on the cell. 2- Cost effective because less pt is required. 3- Can be utilised with combine heat and power systems 4- Very stable | 1- Not toxic emissions. 2- Energy density from cell high. 3- Storage of methanol can be done easily. 4- Methanol is less expensive. | 1- Higher cell performance 2- Different type of fuel can be used. 3- Can be integrated on gas turbines. 4- Less expensive. | 1- Less expensive 2- Less expensive. 3- Different type of fuel can be used. | [45] |
| Demerits | 1- Reduction reaction can be slow. 2- Management of heat in cell. 3- Excess water in cell. 4- Carbon monoxide poisoning. 5- The purity of the fuel used must be high. | 1- Cell performance reduce in the presence of carbon dioxide. 2- The purity of the oxidant at the cathode region must also be very high. | 1- Takes longer time to start. 2- Materials used in the manufacture of the cells are limited. 3- Ionic conductivity in the cell is low. 4- Power density is lower compared to that obtained from other cell. | 1- Possibility of the reactants mixing. 2- The concentration of the fuel has direct effect on the cell performance. 3- Catalyst used is expensive. 4- Cathodic electrode can easily be poisoned. | 1- Some of the materials used in building the cell are susceptible to corrosion. 2- Power density is lower. 3- Takes quite sometime for it to start. 4- Materials suitable for the cell development are few. | 1- Takes time for it to start. 2- Materials suitable for the cell development are few 3- Electrolytes are susceptible to high resistance. 4- Application is limited. | [46] |
| Electrical Efficiency (%) | 40–80 | 50–75 | 50 | 20–40 | 50% | 55–60 | [46] |
| Range of power operation | Less than 550 kW | 5W–250 kW | 45 kW–1.5 MW | 80 MW–1.5 kW | Less than 1 kW–1.5 mW | 4 kW–3.5 MW | [45] |
| Uses | 1- Power backup like UPS. 2- Portable applications like charging laptops 3- Automotive industry | 1- Unmanned vehicles. 2- Automotive applications. 3- Portable applications | 1- Distributed generation | 1- Portable applications | 1- Portable applications 2- Distributed generation | 1- Back up power 2- Distributed generation | [44] |
| Price ($/W) | 40–150 | - | 3–5 | 130 | - | - | [43,46] |
| Advantages | Disadvantages | Reference |
|---|---|---|
| Higher energy density | Limited hydrogen infrastructure | [51] |
| No toxic emissions | Requirement of continuous stream of fuel and air | [52] |
| No noise produced during operation | Need of auxiliaries to run, thus requiring power | [53] |
| Simple design and operation | Still lavish due to lack of bulk productions | [54] |
| Quick start-up | Need of a proper control system, which adds to the production cost | [55] |
| Multiple applications | Desiccation of membrane and submerging of the cathode layer is also a challenge | [56] |
| Operate under stop-start driving circumstances | [57] |
| Equations | Comments |
|---|---|
| Reaction at the anode side | |
| Reaction at the cathode | |
| Overall reaction without the side reactions | |
| The reaction is an exothermic activity | |
| The enthalpy is the variance of the thermal energy of formation of water (at 25 °C and 1 atm) and H2 & O2. In the case of vapour: | |
| The quantity of hydrogen enthalpy that can be altered to electricity in FC matches Gibbs free energy. Some of the power in the hydrogen enthalpy is transformed to heat as the equation shows. | |
| The entropy is the variance of entropies of water (at 25 °C and 1 atm) and H2 & O2. | |
| Manipulative Parameters | Parameters Depending on Operating Conditions |
|---|---|
Parameters controlled during operation:
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaswad, A.; Omran, A.; Sodre, J.R.; Wilberforce, T.; Pignatelli, G.; Dassisti, M.; Baroutaji, A.; Olabi, A.G. Technical and Commercial Challenges of Proton-Exchange Membrane (PEM) Fuel Cells. Energies 2021, 14, 144. https://doi.org/10.3390/en14010144
Alaswad A, Omran A, Sodre JR, Wilberforce T, Pignatelli G, Dassisti M, Baroutaji A, Olabi AG. Technical and Commercial Challenges of Proton-Exchange Membrane (PEM) Fuel Cells. Energies. 2021; 14(1):144. https://doi.org/10.3390/en14010144
Chicago/Turabian StyleAlaswad, Abed, Abdelnasir Omran, Jose Ricardo Sodre, Tabbi Wilberforce, Gianmichelle Pignatelli, Michele Dassisti, Ahmad Baroutaji, and Abdul Ghani Olabi. 2021. "Technical and Commercial Challenges of Proton-Exchange Membrane (PEM) Fuel Cells" Energies 14, no. 1: 144. https://doi.org/10.3390/en14010144
APA StyleAlaswad, A., Omran, A., Sodre, J. R., Wilberforce, T., Pignatelli, G., Dassisti, M., Baroutaji, A., & Olabi, A. G. (2021). Technical and Commercial Challenges of Proton-Exchange Membrane (PEM) Fuel Cells. Energies, 14(1), 144. https://doi.org/10.3390/en14010144











