Atomic Layer Deposition of Lithium–Nickel–Silicon Oxide Cathode Material for Thin-Film Lithium-Ion Batteries
Abstract
1. Introduction
- (1)
- Supercycle approach. An ALD supercycle is defined as the minimum sequence of individual binary cycles that are repeated over the course of the ALD process [55]. For example, one ALD supercycle for the deposition of AxByOz is composed of a linear combination ALD subcycles for binary compound deposition, i.e., (n × AxO + k × ByO), where n and k are the numbers of ALD deposition cycles of the binary compounds AxO and ByO, respectively. The ratio and the sequence of subcycles can be chosen, considering the growth rates of the binary compounds [33] and the appearance of layers in the crystal structure [42].
- (2)
- ALD process, which uses multiconstituent precursors, i.e., precursors containing two or more elements of the resulting films. This approach was successfully used for ALD of lithium phosphates using lithium tert-butoxide (LiOtBu) as a lithium source and trimethyl phosphate (TMPO) as phosphate source [15].
- (3)
- ALD of multilayered films of lithium oxides and metal oxides followed by annealing [45]. In some cases, lithiation can occur without annealing, but rather directly during the ALD of lithium oxide on the surface of already deposited β-MnO2.
2. Materials and Methods
3. Results and Discussion
3.1. ALD of LNO Thin Films
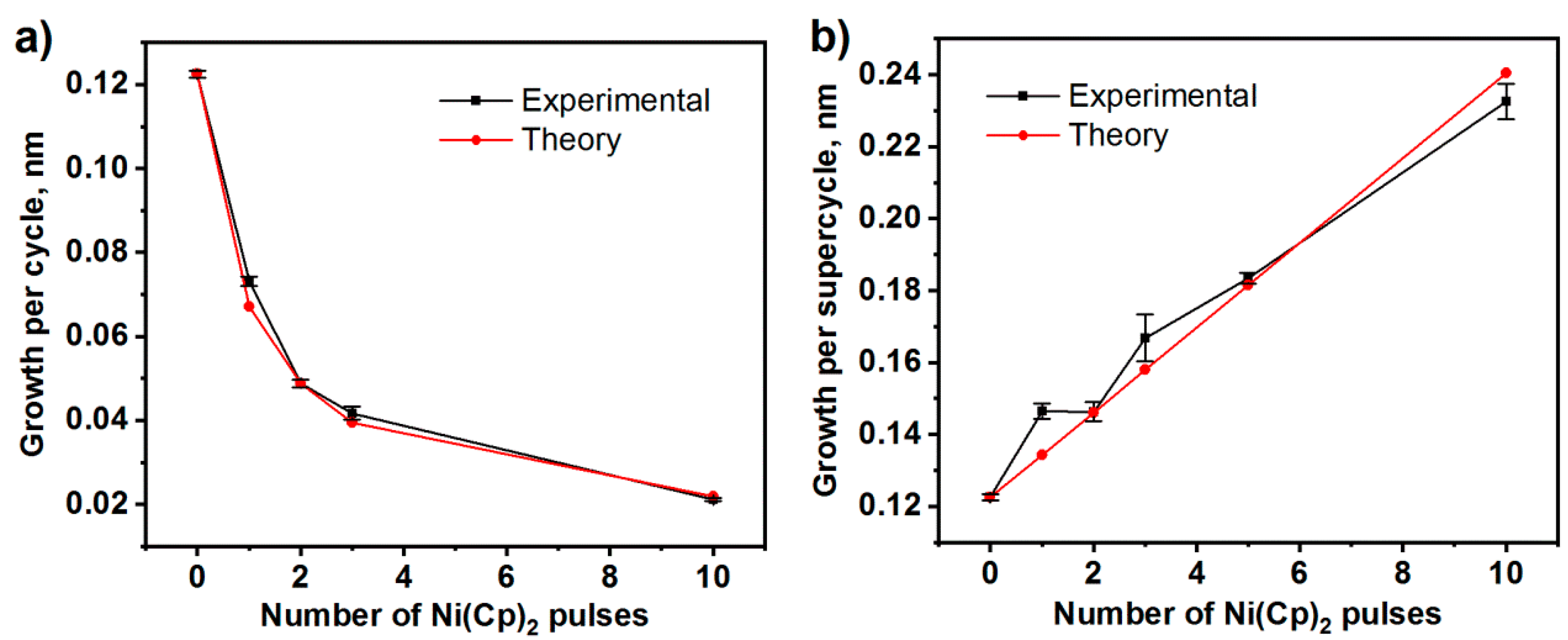
3.1.1. Atomic Layer Deposition and Growth Characteristics
3.1.2. Chemical Composition of the Films Determined Using XPS and SEM-EDX
3.1.3. Structure of the Films Determined Using XRD and XRR
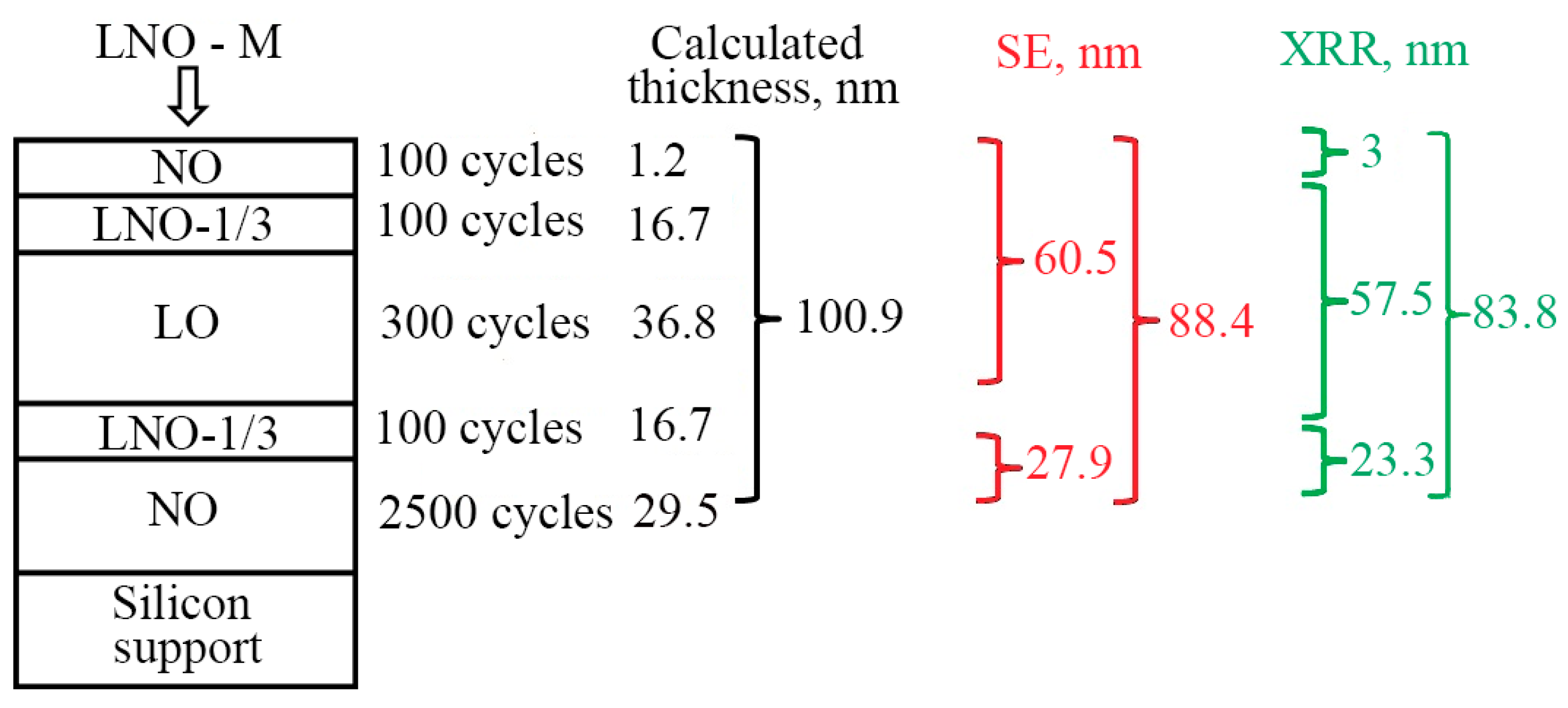
3.2. ALD and Growth Characteristics of Multilayered LNO Thin Films
- (1)
- ALD of nickel oxide layer-NO (2500 cycles) on the surface of silicon and stainless-steel substrate,
- (2)
- ALD of transition layer-LNO-1/3 (100 cycles),
- (3)
- ALD of lithium oxide layer-LO (300 cycles),
- (4)
- ALD of transition layer-LNO-1/3 (100 cycles),
- (5)
- ALD of nickel oxide layer-NO (100 cycles), which was supposed to serve as a protective layer against the oxidation of lithium oxide.
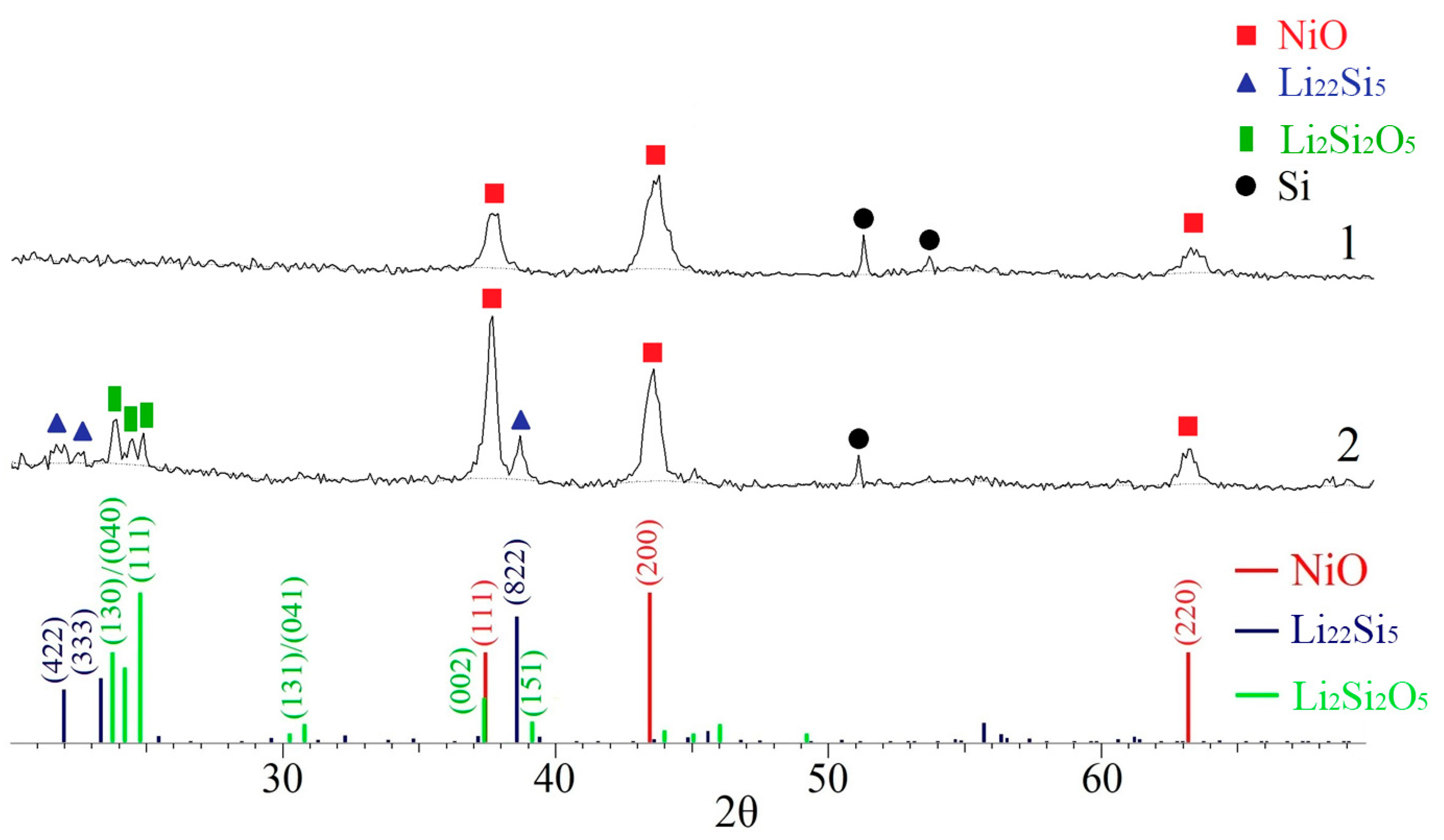
3.2.1. XRD of Multilayered LNO Thin Films
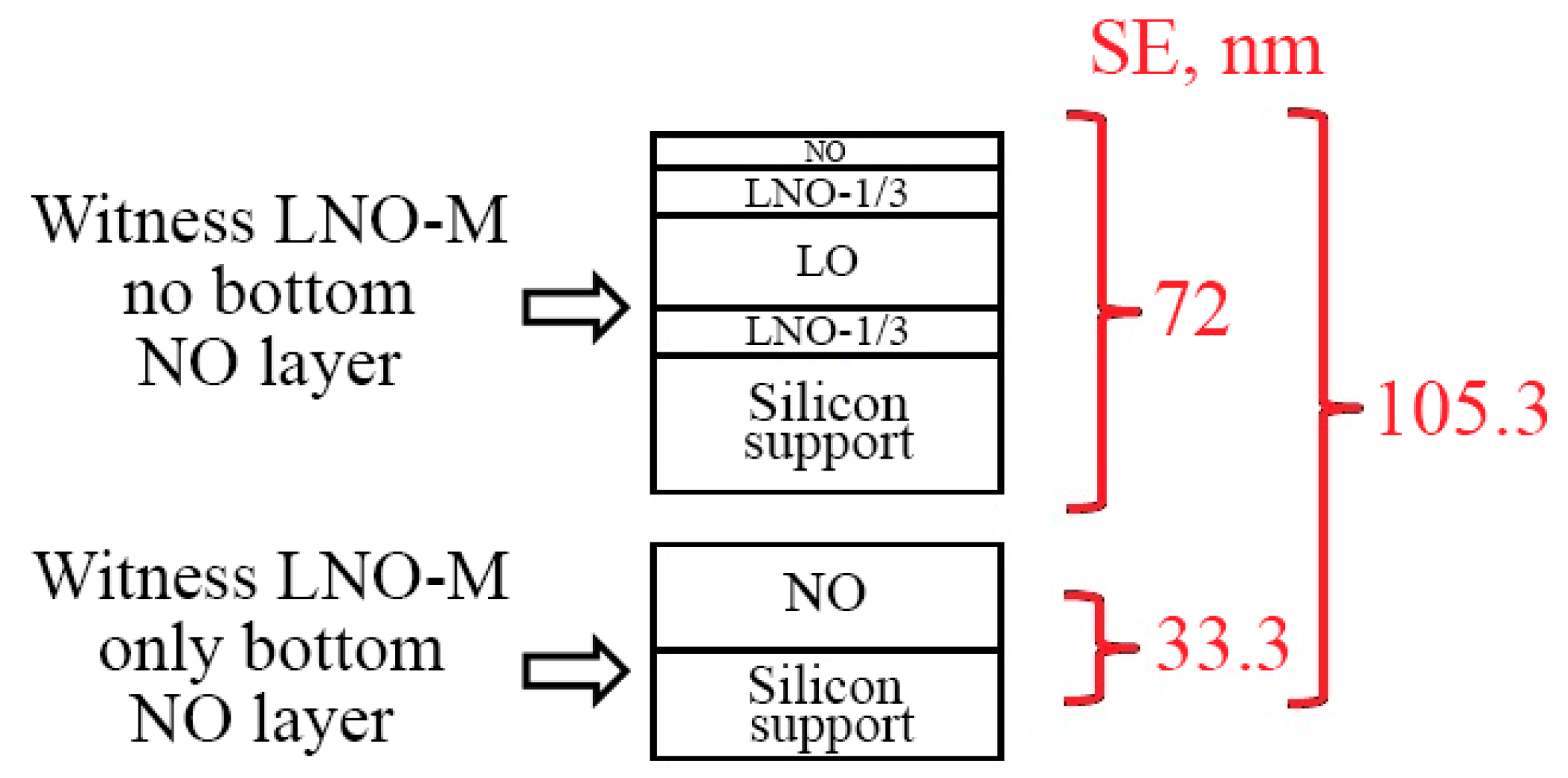
3.2.2. Spectral Ellipsometry and X-ray Reflectometry
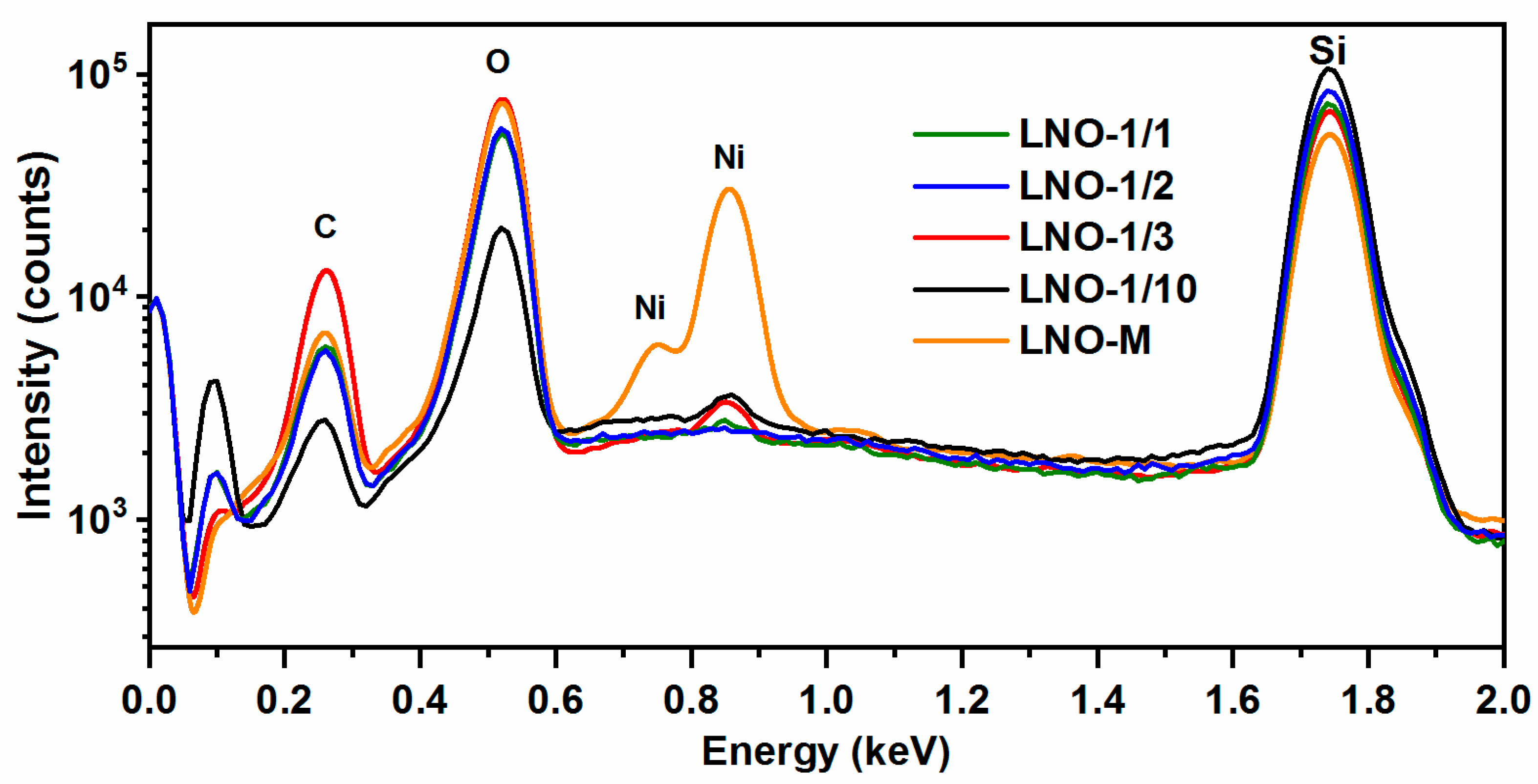
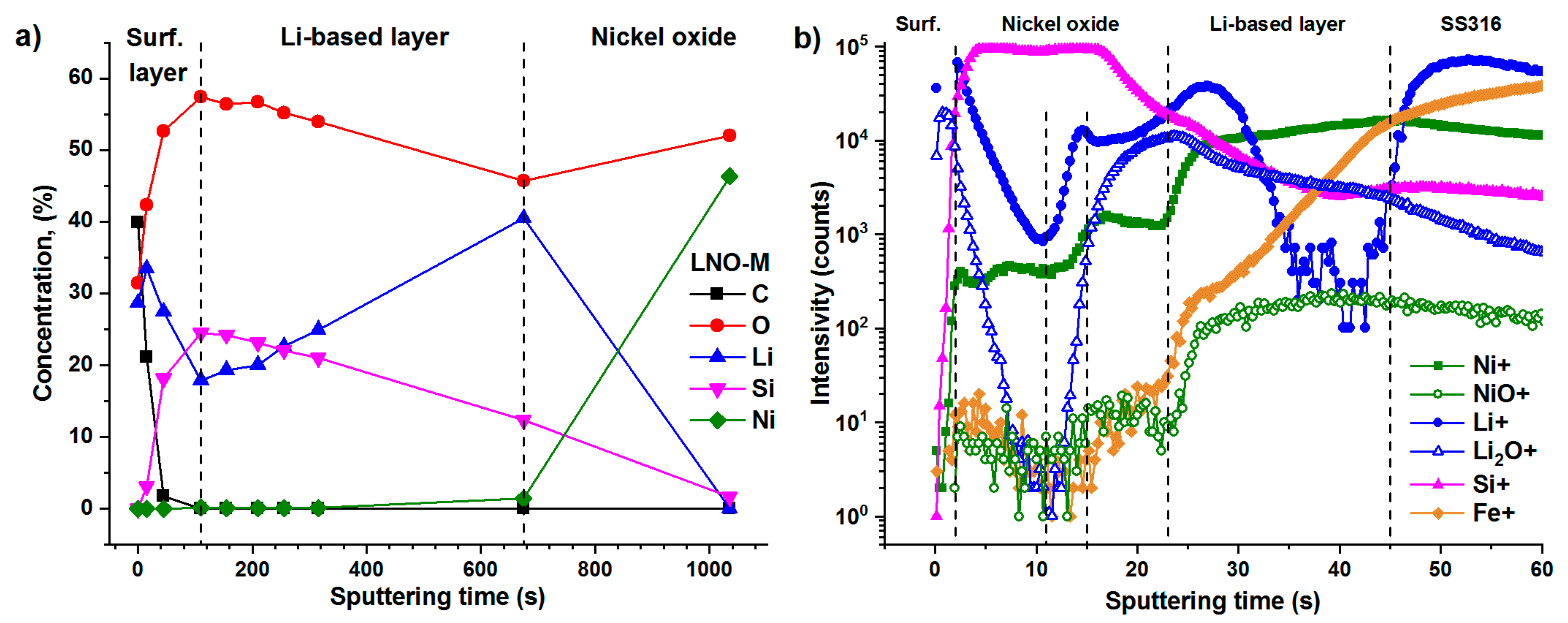
3.2.3. Chemical Composition of the Films. XPS and TOF-SIMS Depth Profiling
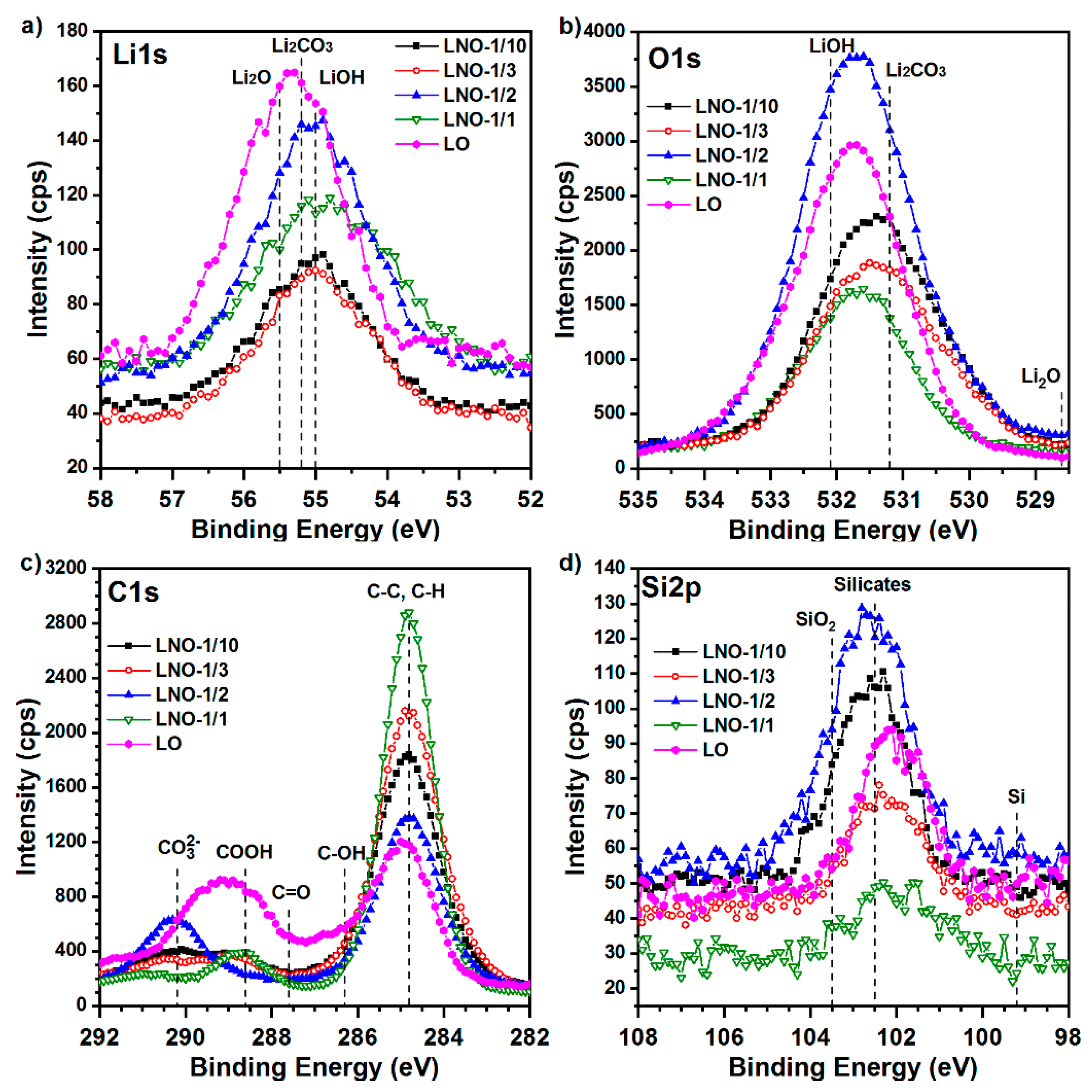
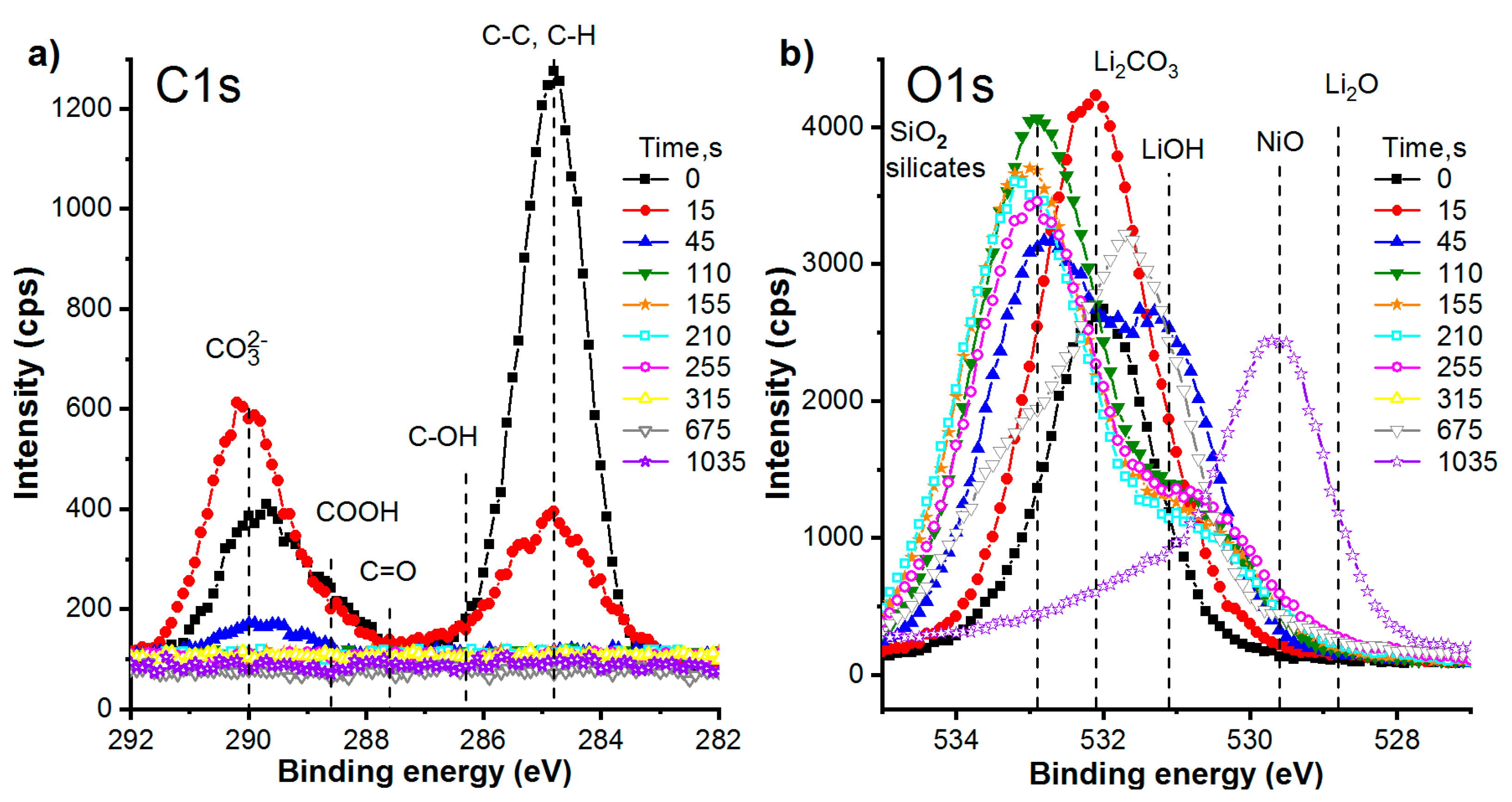
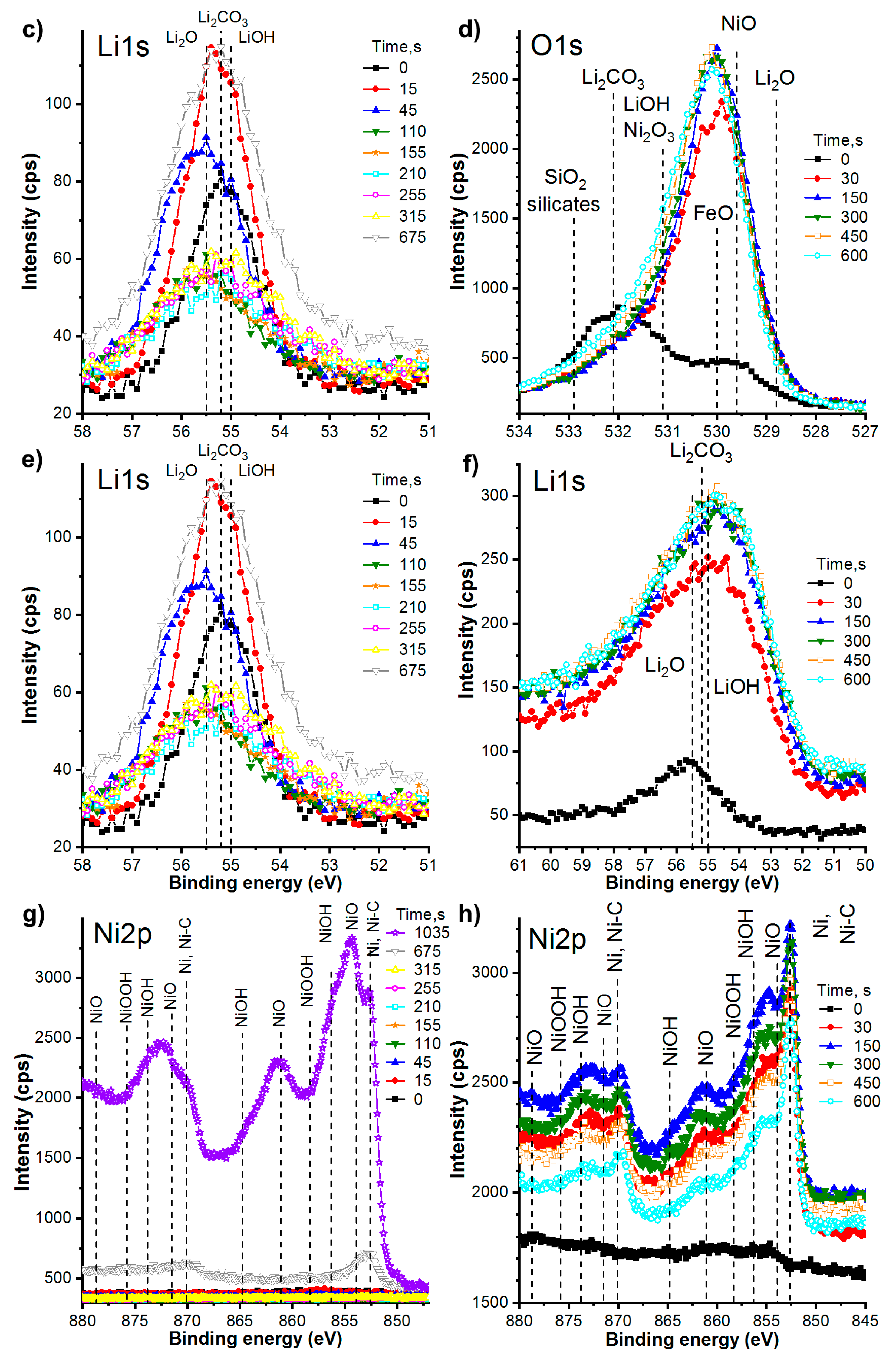
3.2.4. Chemical Composition of the Films. Detailed Study of XPS Spectra
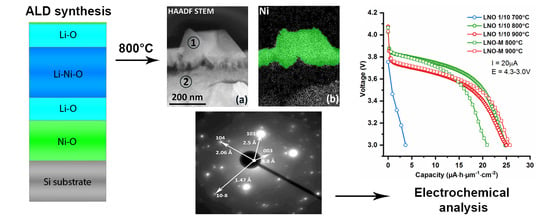
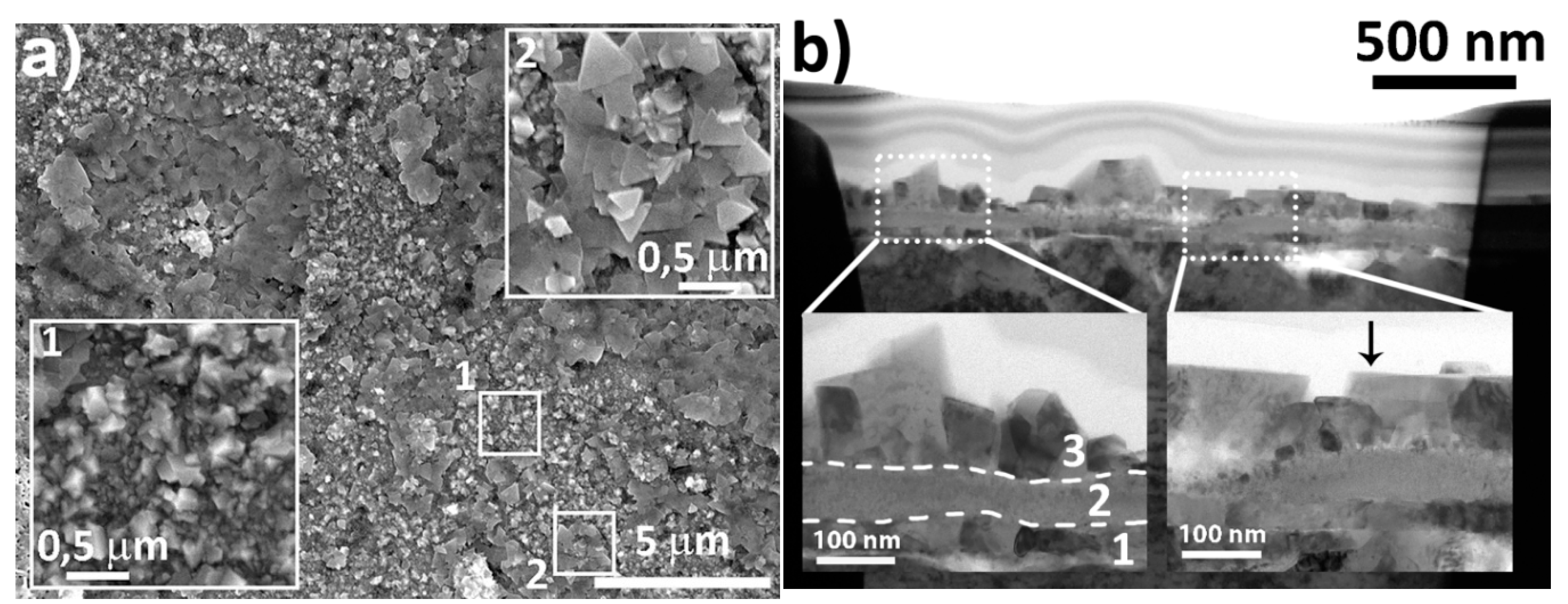
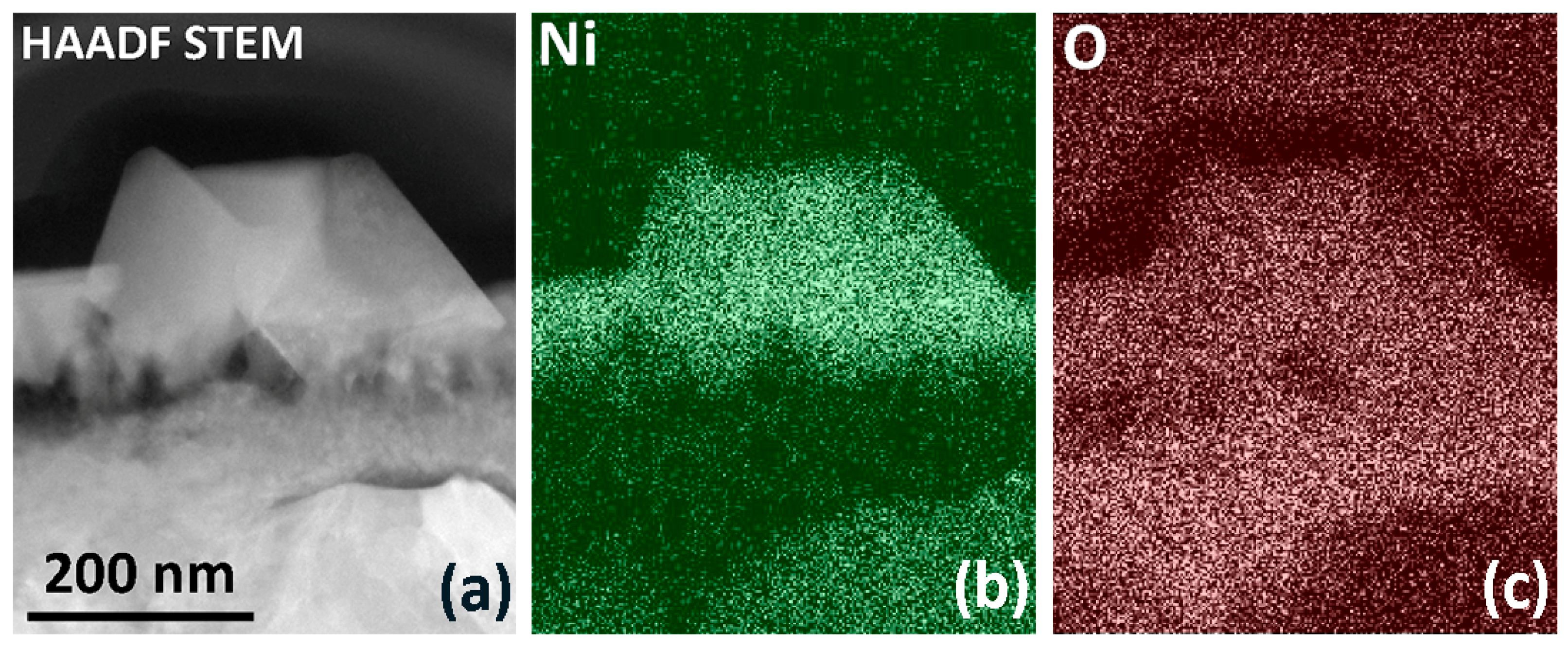
3.2.5. SEM and TEM. Morphology, Local Structure and Composition of LNO-M-800 Thin Film
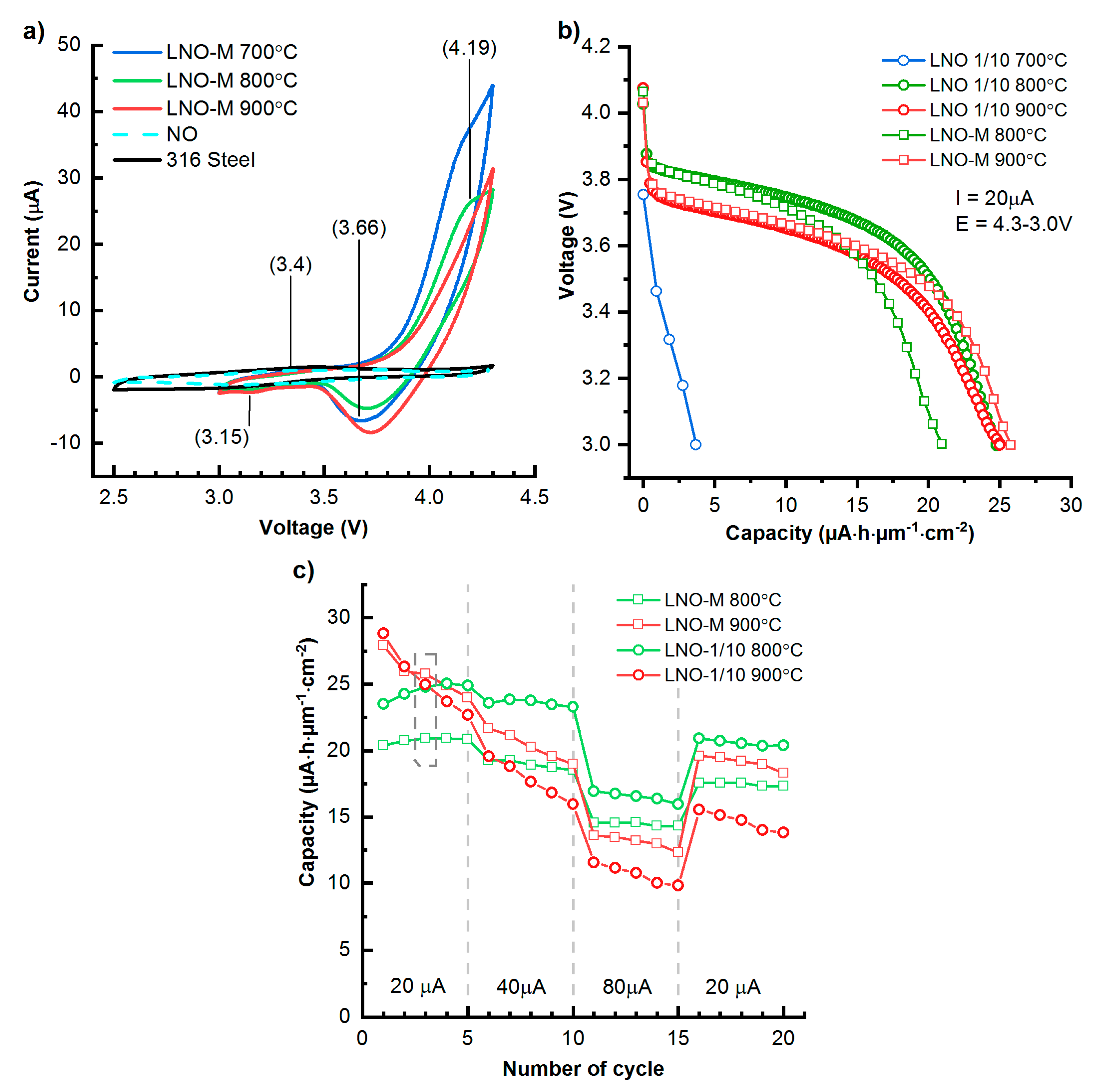
3.3. Electrochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Salot, R.; Martin, S.; Oukassi, S.; Bedjaoui, M.; Ubrig, J. Microbattery technology overview and associated multilayer encapsulation process. Appl. Surf. Sci. 2009, 256, S54–S57. [Google Scholar] [CrossRef]
- Wu, Q.; Zheng, J.P.; Hendriekson, M.; Plichta, E.J. Dry Process for Fabricating Low Cost and High Performance Electrode for Energy Storage Devices. Mrs Adv. 2019, 4, 857–863. [Google Scholar] [CrossRef]
- Micro Technology (Wyon). Available online: https://www.wyon.ch/en/home.html (accessed on 27 January 2020).
- Oudenhoven, J.F.M.; Baggetto, L.; Notten, P.H.L. All-Solid-State Lithium-Ion Microbatteries: A Review of Various Three-Dimensional Concepts. Adv. Energy Mater. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- De-Leon, S. Lithium Pouch Flexible Battery Market. In Proceedings of the 2019 München in parallel to the trade fair eMove360° Europe, München, Germany, 15–16 October 2019; p. 26. [Google Scholar]
- Moitzheim, S.; Put, B.; Vereecken, P.M. Advances in 3D Thin-Film Li-Ion Batteries. Adv. Mater. Interfaces 2019, 6, 17. [Google Scholar] [CrossRef]
- Pearse, A.; Schmitt, T.; Sahadeo, E.; Stewart, D.M.; Kozen, A.; Gerasopoulos, K.; Talin, A.A.; Lee, S.B.; Rubloff, G.W.; Gregorczyk, K.E. Three-Dimensional Solid-State Lithium-Ion Batteries Fabricated by Conformal Vapor-Phase Chemistry. ACS Nano 2018, 12, 4286–4294. [Google Scholar] [CrossRef]
- Choi, W.G.; Yoon, S.G. Structural and electrical properties of LiCoO2 thin-film cathodes deposited on planar and trench structures by liquid-delivery metalorganic chemical vapour deposition. J. Power Sources 2004, 125, 236–241. [Google Scholar] [CrossRef]
- Cho, S.I.; Yoon, S.G. Improvement of discharge capacity of LiCoO2 thin-film cathodes deposited in trench structure by liquid-delivery metalorganic chemical vapor deposition. Appl. Phys. Lett. 2003, 82, 3345–3347. [Google Scholar] [CrossRef]
- Long, J.W.; Dunn, B.; Rolison, D.R.; White, H.S. Three-dimensional battery architectures. Chem. Rev. 2004, 104, 4463–4492. [Google Scholar] [CrossRef]
- Baggetto, L.; Niessen, R.A.H.; Roozeboom, F.; Notten, P.H.L. High energy density all-solid-state batteries: A challenging concept towards 3D integration. Adv. Funct. Mater. 2008, 18, 1057–1066. [Google Scholar] [CrossRef]
- Notten, P.H.L.; Roozeboom, F.; Niessen, R.A.H.; Baggetto, L. 3-D integrated all-solid-state rechargeable batteries. Adv. Mater. 2007, 19, 4564–4567. [Google Scholar] [CrossRef]
- Kia, A.M.; Bonhardt, S.; Zybell, S.; Kuhnel, K.; Haufe, N.; Weinreich, W. Development of Rutile Titanium Oxide Thin Films as Battery Material Component Using Atomic Layer Deposition. Phys. Status Solidi A-Appl. Mater. Sci. 2018. [Google Scholar] [CrossRef]
- Wang, B.Q.; Liu, J.; Sun, Q.; Xiao, B.W.; Li, R.Y.; Sham, T.K.; Sun, X.L. Titanium Dioxide/Lithium Phosphate Nanocomposite Derived from Atomic Layer Deposition as a High-Performance Anode for Lithium Ion Batteries. Adv. Mater. Interfaces 2016, 3, 8. [Google Scholar] [CrossRef]
- Nazarov, D.V.; Maximov, M.Y.; Novikov, P.A.; Popovich, A.A.; Silin, A.O.; Smirnov, V.M.; Bobrysheva, N.P.; Osmolovskaya, O.M.; Osmolovsky, M.G.; Rumyantsev, A.M. Atomic layer deposition of tin oxide using tetraethyltin to produce high-capacity Li-ion batteries. J. Vac. Sci. Technol. A 2017, 35. [Google Scholar] [CrossRef]
- Maximov, M.Y.; Novikov, P.A.; Nazarov, D.V.; Rymyantsev, A.M.; Silin, A.O.; Zhang, Y.; Popovich, A.A. Characterization and Electrochemical Performance at High Discharge Rates of Tin Dioxide Thin Films Synthesized by Atomic Layer Deposition. J. Electron. Mater. 2017, 46, 6571–6577. [Google Scholar] [CrossRef]
- Zhao, B.; Mattelaer, F.; Kint, J.; Werbrouck, A.; Henderick, L.; Minjauw, M.; Dendooven, J.; Detavernier, C. Atomic layer deposition of ZnO-SnO2 composite thin film: The influence of structure, composition and crystallinity on lithium-ion battery performance. Electrochim. Acta 2019, 320, 13. [Google Scholar] [CrossRef]
- Kint, J.; Mattelaer, F.; Minjauw, M.; Zhao, B.; Detavernier, C. Atomic layer deposition of thin films as model electrodes: A case study of the synergistic effect in Fe2O3-SnO2. J. Vac. Sci. Technol. A 2019, 37. [Google Scholar] [CrossRef]
- Bonhardt, S.; Kuhnel, K.; Kia, A.M.; Weinreich, W. Formation of highly conformal spinel lithium titanate thin films based on a novel three-step atomic layer deposition process. J. Vac. Sci. Technol. A 2019, 37, 6. [Google Scholar] [CrossRef]
- Meng, X.B.; Liu, J.; Li, X.F.; Banis, M.N.; Yang, J.L.; Li, R.Y.; Sun, X.L. Atomic layer deposited Li4Ti5O12 on nitrogen-doped carbon nanotubes. RSC Adv. 2013, 3, 7285–7288. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Nilsen, O.; Laitinen, M.; Sajavaara, T.; Fjellvag, H. Atomic layer deposition of LixTiyOz thin films. RSC Adv. 2013, 3, 7537–7542. [Google Scholar] [CrossRef]
- Kazyak, E.; Chen, K.H.; Wood, K.N.; Davis, A.L.; Thompson, T.; Bielinski, A.R.; Sanchez, A.J.; Wang, X.; Wane, C.M.; Sakamoto, J.; et al. Atomic Layer Deposition of the Solid Electrolyte Garnet Li7La3Zr2O12. Chem. Mater. 2017, 29, 3785–3792. [Google Scholar] [CrossRef]
- Hofstetter, K.; Samson, A.J.; Dai, J.Q.; Gritton, J.E.; Hu, L.B.; Wachsman, E.D.; Thangadurai, V. Electrochemical Stability of Garnet-Type Li7La2.75Ca0.25Zr1.75Nb0.25O12 with and without Atomic Layer Deposited-Al2O3 under CO2 and Humidity. J. Electrochem. Soc. 2019, 166, A1844–A1852. [Google Scholar] [CrossRef]
- Nisula, M.; Shindo, Y.; Koga, H.; Karppinen, M. Atomic Layer Deposition of Lithium Phosphorus Oxynitride. Chem. Mater. 2015, 27, 6987–6993. [Google Scholar] [CrossRef]
- Shibata, S. Thermal Atomic Layer Deposition of Lithium Phosphorus Oxynitride as a Thin-Film Solid Electrolyte. J. Electrochem. Soc. 2016, 163, A2555–A2562. [Google Scholar] [CrossRef]
- Kozen, A.C.; Pearse, A.J.; Lin, C.F.; Noked, M.; Rubloff, G.W. Atomic Layer Deposition of the Solid Electrolyte LiPON. Chem. Mater. 2015, 27, 5324–5331. [Google Scholar] [CrossRef]
- Put, B.; Mees, M.J.; Hornsveld, N.; Hollevoet, S.; Sepulveda, A.; Vereecken, P.M.; Kessels, W.M.M.; Creatore, M. Plasma-Assisted ALD of LiPO(N) for Solid State Batteries. J. Electrochem. Soc. 2019, 166, A1239–A1242. [Google Scholar] [CrossRef]
- Wang, B.Q.; Zhao, Y.; Banis, M.N.; Sun, Q.; Adair, K.R.; Li, R.Y.; Sham, T.K.; Sun, X.L. Atomic Layer Deposition of Lithium Niobium Oxides as Potential Solid-State Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 1654–1661. [Google Scholar] [CrossRef]
- Ostreng, E.; Sonsteby, H.H.; Sajavaara, T.; Nilsen, O.; Fjellvag, H. Atomic layer deposition of ferroelectric LiNbO3. J. Mater. Chem. C 2013, 1, 4283–4290. [Google Scholar] [CrossRef]
- Atosuo, E.; Mantymaki, M.; Mizohata, K.; Heikkila, M.J.; Raisanen, J.; Ritala, M.; Leskela, M. Preparation of Lithium Containing Oxides by the Solid State Reaction of Atomic Layer Deposited Thin Films. Chem. Mater. 2017, 29, 998–1005. [Google Scholar] [CrossRef]
- Amirmaleki, M.; Cao, C.H.; Wang, B.Q.; Zhao, Y.; Cui, T.; Tam, J.; Sun, X.L.; Sun, Y.; Filleter, T. Nanomechanical elasticity and fracture studies of lithium phosphate (LPO) and lithium tantalate (LTO) solid-state electrolytes. Nanoscale 2019, 11, 18730–18738. [Google Scholar] [CrossRef]
- Liu, J.; Banis, M.N.; Li, X.F.; Lushington, A.; Cai, M.; Li, R.Y.; Sham, T.K.; Sun, X.L. Atomic Layer Deposition of Lithium Tantalate Solid-State Electrolytes. J. Phys. Chem. C 2013, 117, 20260–20267. [Google Scholar] [CrossRef]
- Li, X.F.; Liu, J.; Banis, M.N.; Lushington, A.; Li, R.Y.; Cai, M.; Sun, X.L. Atomic layer deposition of solid-state electrolyte coated cathode materials with superior high-voltage cycling behavior for lithium ion battery application. Energy Environ. Sci. 2014, 7, 768–778. [Google Scholar] [CrossRef]
- Comstock, D.J.; Elam, J.W. Mechanistic Study of Lithium Aluminum Oxide Atomic Layer Deposition. J. Phys. Chem. C 2013, 117, 1677–1683. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Nilsen, O.; Li, H.; King, S.W.; Laitinen, M.; Sajavaara, T.; Fjellvag, H. Atomic layer deposited lithium aluminum oxide: (In) dependency of film properties from pulsing sequence. J. Vac. Sci. Technol. A 2015, 33, 7. [Google Scholar] [CrossRef]
- Aaltonen, T.; Alnes, M.; Nilsen, O.; Costelle, L.; Fjellvag, H. Lanthanum titanate and lithium lanthanum titanate thin films grown by atomic layer deposition. J. Mater. Chem. 2010, 20, 2877–2881. [Google Scholar] [CrossRef]
- Wang, B.Q.; Liu, J.; Banis, M.N.; Sun, Q.; Zhao, Y.; Li, R.Y.; Sham, T.K.; Sun, X.L. Atomic Layer Deposited Lithium Silicates as Solid-State Electrolytes for All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2017, 9, 31786–31793. [Google Scholar] [CrossRef]
- Aaltonen, T.; Nilsen, O.; Magraso, A.; Fjellvag, H. Atomic Layer Deposition of Li2O-Al2O3 Thin Films. Chem. Mater. 2011, 23, 4669–4675. [Google Scholar] [CrossRef]
- Kazyak, E.; Chen, K.H.; Davis, A.L.; Yu, S.; Sanchez, A.J.; Lasso, J.; Bielinski, A.R.; Thompson, T.; Sakamoto, J.; Siegel, D.J.; et al. Atomic layer deposition and first principles modeling of glassy Li3BO3-Li2CO3 electrolytes for solid-state Li metal batteries. J. Mater. Chem. A 2018, 6, 19425–19437. [Google Scholar] [CrossRef]
- Chernyaeva, O.Y.; Kyashkin, V.M.; Ivleva, A.Y.; Yrova, V.Y.; Solovyova, E.O. The influence of process conditions on the phase composition of the LiFePO4 film obtained by the atomic layer method. Polyhedron 2019, 157, 297–300. [Google Scholar] [CrossRef]
- Liu, J.; Banis, M.N.; Sun, Q.; Lushington, A.; Li, R.Y.; Sham, T.K.; Sun, X.L. Rational Design of Atomic-Layer-Deposited LiFePO4 as a High-Performance Cathode for Lithium-Ion Batteries. Adv. Mater. 2014, 26, 6472–6477. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, B.W.; Banis, M.N.; Li, R.Y.; Sham, T.K.; Sun, X.L. Atomic layer deposition of amorphous iron phosphates on carbon nanotubes as cathode materials for lithium-ion batteries. Electrochim. Acta 2015, 162, 275–281. [Google Scholar] [CrossRef]
- Donders, M.E.; Knoops, H.C.M.; Kessels, W.M.M.; Notten, P.H.L. Remote Plasma Atomic Layer Deposition of Thin Films of Electrochemically Active LiCoO2. Available online: https://iopscience.iop.org/article/10.1149/1.3633683 (accessed on 10 February 2020).
- Donders, M.E.; Arnoldbik, W.M.; Knoops, H.C.M.; Kessels, W.M.M.; Notten, P.H.L. Atomic Layer Deposition of LiCoO2 Thin-Film Electrodes for All-Solid-State Li-Ion Micro-Batteries. J. Electrochem. Soc. 2013, 160, A3066–A3071. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Ruud, A.; Ostreng, E.; Nilsen, O.; Laitinen, M.; Sajavaara, T.; Fjellvag, H. Atomic Layer Deposition of Spinel Lithium Manganese Oxide by Film-Body-Controlled Lithium Incorporation for Thin-Film Lithium-Ion Batteries. J. Phys. Chem. C 2014, 118, 1258–1268. [Google Scholar] [CrossRef]
- Nieminen, H.E.; Miikkulainen, V.; Settipani, D.; Simonelli, L.; Honicke, P.; Zech, C.; Kayser, Y.; Beckhoff, B.; Honkanen, A.P.; Heikkila, M.J.; et al. Intercalation of Lithium Ions from Gaseous Precursors into beta-MnO2 Thin Films Deposited by Atomic Layer Deposition. J. Phys. Chem. C 2019, 123, 15802–15814. [Google Scholar] [CrossRef]
- Young, M.J.; Schnabel, H.D.; Holder, A.M.; George, S.M.; Musgrave, C.B. Band Diagram and Rate Analysis of Thin Film Spinel LiMn2O4 Formed by Electrochemical Conversion of ALD-Grown MnO. Adv. Funct. Mater. 2016, 26, 7895–7907. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Golabkan, V.; Pereira-Ramos, J.P.; Mantoux, A.; Lincot, D. A Raman study of the lithium insertion process in vanadium pentoxide thin films deposited by atomic layer deposition. J. Raman Spectrosc. 2002, 33, 631–638. [Google Scholar] [CrossRef]
- Badot, J.C.; Mantoux, A.; Baffier, N.; Dubrunfaut, O.; Lincot, D. Electrical properties of V2O5 thin films obtained by atomic layer deposition (ALD). J. Mater. Chem. 2004, 14, 3411–3415. [Google Scholar] [CrossRef]
- Badot, J.C.; Mantoux, A.; Baffier, N.; Dubrunfaut, O.; Lincot, D. Submicro- and nanostructural eff ects on electrical properties of Li0.2V2O5 thin films obtained by atomic layer deposition (ALD). J. Phys. Chem. Solids 2006, 67, 1270–1274. [Google Scholar] [CrossRef]
- Prasadam, V.P.; Bahlawane, N.; Mattelaer, F.; Rampelberg, G.; Detavernier, C.; Fang, L.; Jiang, Y.; Martens, K.; Parkin, I.P.; Papakonstantinou, I. Atomic layer deposition of vanadium oxides: Process and application review. Mater. Today Chem. 2019, 12, 396–423. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X.L. Elegant design of electrode and electrode/electrolyte interface in lithium-ion batteries by atomic layer deposition. Nanotechnology 2015, 26. [Google Scholar] [CrossRef]
- Nilsen, O.; Miikkulainen, V.; Gandrud, K.B.; Ostreng, E.; Ruud, A.; Fjellvag, H. Atomic layer deposition of functional films for Li-ion microbatteries. Phys. Status Solidi A-Appl. Mater. Sci. 2014, 211, 357–367. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; Schneider, J.R.; MacIsaac, C.; Baker, J.G.; Bent, S.F. Synthesis of Doped, Ternary, and Quaternary Materials by Atomic Layer Deposition: A Review. Chem. Mater. 2019, 31, 1142–1183. [Google Scholar] [CrossRef]
- Liu, C.F.; Neale, Z.G.; Cao, G.Z. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Manthiram, A.; Knight, J.C.; Myung, S.T.; Oh, S.M.; Sun, Y.K. Nickel-Rich and Lithium-Rich Layered Oxide Cathodes: Progress and Perspectives. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.T.; Son, D.Y.; Ono, L.K.; Qi, Y.B. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Nazarov, D.; Ezhov, I.; Mitrofanov, I.; Lyutakov, O.; Maximov, M.Y. The Use of the TMA as Stabilizing Reagent for the Li-O System Obtained by Atomic Layer Deposition. Key Eng. Mater. 2019, 822, 787–794. [Google Scholar] [CrossRef]
- Koshtyal, Y.; Nazarov, D.; Ezhov, I.; Mitrofanov, I.; Kim, A.; Rymyantsev, A.; Lyutakov, O.; Popovich, A.; Maximov, M. Atomic Layer Deposition of NiO to Produce Active Material for Thin-Film Lithium-Ion Batteries. Coatings 2019, 9, 301. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Chaistain, J., Ed.; Perkin-Elmer Corporation, Physical Electronics Devision: Eden Prairie, Minnesota, 1995. [Google Scholar]
- Yao, K.P.C.; Kwabi, D.G.; Quinlan, R.A.; Mansour, A.N.; Grimaud, A.; Lee, Y.L.; Lu, Y.C.; Shao-Horn, Y. Thermal Stability of Li2O2 and Li2O for Li-Air Batteries: In Situ XRD and XPS Studies. J. Electrochem. Soc. 2013, 160, A824–A831. [Google Scholar] [CrossRef]
- Gray, R.C.; Hercules, D.M. X-RAY PHOTOELECTRON SPECTROSCOPIC (ESCA) STUDY OF BONDING IN PENTACOORDINATE SILICON-COMPOUNDS. Inorg. Chem. 1977, 16, 1426–1427. [Google Scholar] [CrossRef]
- Richey, N.E.; Paula, C.D.; Bent, S.F. Understanding chemical and physical mechanisms in atomic layer deposition. J. Chem. Phys. 2020, 152. [Google Scholar] [CrossRef]
- Tomczak, Y.; Knapas, K.; Sundberg, M.; Leskelä, M.; Ritala, M. In Situ Reaction Mechanism Studies on Lithium Hexadimethyldisilazide and Ozone Atomic Layer Deposition Process for Lithium Silicate. J. Phys. Chem. C 2013, 117, 14241–14246. [Google Scholar] [CrossRef]
- Hämäläinen, J.; Munnik, F.; Hatanpää, T.; Holopainen, J.; Ritala, M.; Leskelä, M. Study of amorphous lithium silicate thin films grown by atomic layer deposition. J. Vac. Sci. Technol. A 2012, 30. [Google Scholar] [CrossRef]
- Mantymaki, M.; Atosuo, E.; Heikkila, M.J.; Vehkamaki, M.; Mattinen, M.; Mizohata, K.; Raisanen, J.; Ritala, M.; Leskela, M. Studies on solid state reactions of atomic layer deposited thin films of lithium carbonate with hafnia and zirconia. J. Vac. Sci. Technol. A 2019, 37. [Google Scholar] [CrossRef]
- Clarke, T.A.; Rizkalla, E.N. X-ray Photoelectron-Spectroscopy of Some Silicates. Chem. Phys. Lett. 1976, 37, 523–526. [Google Scholar] [CrossRef]
- Alexander, M.R.; Short, R.D.; Jones, F.R.; Stollenwerk, M.; Zabold, J.; Michaeli, W. An X-ray photoelectron spectroscopic investigation into the chemical structure of deposits formed from hexamethyldisiloxane/oxygen plasmas. J. Mater. Sci. 1996, 31, 1879–1885. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Hussin, R.; Choy, K.L.; Hou, X.H. The Effect of Substrate on TiO2 Thin Films Deposited by Atomic Layer Deposition (ALD). Adv. Mater. Res. 2015, 1087, 147–151. [Google Scholar] [CrossRef]
- 7Stephenson, T.; Kubis, A.; Derakhshesh, M.; Hazelton, M.; Holt, C.; Eaton, P.; Newman, B.; Hoff, A.; Gray, M.; Mitlin, D. Corrosion-Fouling of 316 Stainless Steel and Pure Iron by Hot Oil. Energy Fuels 2011, 25, 4540–4551. [Google Scholar] [CrossRef]
- Ren, D.; Yang, Y.; Shen, L.; Zeng, R.; Abruna, H.D. Ni-rich LiNi0.88Mn0.06Co0.06O2 cathode interwoven by carbon fiber with improved rate capability and stability. J. Power Sources 2020, 447, 227344. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Kalaiselvi, N.; Periasamy, P. A preliminary investigation into the new class of lithium intercalating LiNiSiO4 cathode material. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Julien, C.; Mauger, A.; Vijh, A.; Zaghib, K. Cathode Materials with Two-Dimensional Structure. In Lithium Batteries; Julien, C., Mauger, A., Vijh, A., Zaghib, K., Eds.; Springer: Cham, Switzerland, 2016; Volume 119. [Google Scholar]
- Chong, S.K.; Liu, Y.N.; Yan, W.W.; Chen, Y.Z. Effect of valence states of Ni and Mn on the structural and electrochemical properties of Li1.2NixMn0.8-xO2 cathode materials for lithium-ion batteries. RSC Adv. 2016, 6, 53662–53668. [Google Scholar] [CrossRef]
- Rempel, J.; Pullen, A.; Kaplan, D.; Barnett, B.; Sriramulu, S. High-nickel cathode/graphite anode cells for diverse DoD applications. In Proceedings of the 48th Power Sources Conference, Denver, CO, USA, 11–14 June 2018; pp. 404–407. [Google Scholar]
- Li, J.G.; Zhao, R.S.; He, X.M.; Liu, H.C. Preparation of LiCoO2 cathode materials from spent lithium-ion batteries. Ionics 2009, 15, 111–113. [Google Scholar] [CrossRef]
- Park, K.S.; Xiao, P.H.; Kim, S.Y.; Dylla, A.; Choi, Y.M.; Henkelman, G.; Stevenson, K.J.; Goodenough, J.B. Enhanced Charge-Transfer Kinetics by Anion Surface Modification of LiFePO4. Chem. Mater. 2012, 24, 3212–3218. [Google Scholar] [CrossRef]
- Xia, H.; Luo, Z.T.; Xie, J.P. Nanostructured LiMn2O4 and their composites as high-performance cathodes for lithium-ion batteries. Prog. Nat. Sci.-Mater. Int. 2012, 22, 572–584. [Google Scholar] [CrossRef]
- Dobbelaere, T.; Mattelaer, F.; Dendooven, J.; Vereecken, P.; Detavernier, C. Plasma-Enhanced Atomic Layer Deposition of Iron Phosphate as a Positive Electrode for 3D Lithium-Ion Microbatteries. Chem. Mater. 2016, 28, 3435–3445. [Google Scholar] [CrossRef]
- Gandrud, K.B.; Pettersen, A.; Nilsen, O.; Fjellvag, H. High-performing iron phosphate for enhanced lithium ion solid state batteries as grown by atomic layer deposition. J. Mater. Chem. A 2013, 1, 9054–9059. [Google Scholar] [CrossRef]
- Howard, W.F.; Spotnitz, R.M. Theoretical evaluation of high-energy lithium metal phosphate cathode materials in Li-ion batteries. J. Power Sources 2007, 165, 887–891. [Google Scholar] [CrossRef]
- Jin, Y.; Tang, X.C.; Wang, Y.; Dang, W.; Huang, J.; Fang, X. High-tap density LiFePO4 microsphere developed by combined computational and experimental approaches. Crystengcomm 2018, 20, 6695–6703. [Google Scholar] [CrossRef]



| Sample | Li/Ni Ratio | Number of Supercycles | Number of Cycles | The Thickness and Its Gradient, nm |
|---|---|---|---|---|
| NO | 0/1 | - | 2300 | 27.2 ± 2.3 |
| LO | 1/0 | - | 600 | 73.5 ± 0.5 |
| LNO-1/1 | 1/1 | 600 | 1200 | 87.9 ± 0.5 |
| LNO-1/2 | 1/2 | 600 | 1800 | 87.7 ± 0.6 |
| LNO-1/3 | 1/3 | 600 | 2400 | 98.1 ± 5.9 |
| LNO-1/10 | 1/10 | 250 | 2750 | 58.2 ± 1.3 |
| Sample | C, % | O, % | Li, % | Si, % | Ni, % | N, % |
|---|---|---|---|---|---|---|
| LO | 31.47 | 42.68 | 23.11 | 0.91 | 0 | 1.83 |
| NO | 12.44 | 40.97 | 0 | 0 | 46.59 | 0 |
| LNO-1/10 | 50.08 | 26.17 | 21.45 | 1.94 | 0.21 | 0.16 |
| LNO-1/3 | 55.23 | 21.34 | 22.01 | 0.94 | 0.38 | 0.09 |
| LNO-1/2 | 33.42 | 33.96 | 30.29 | 1.91 | 0 | 0.42 |
| LNO-1/1 | 54.67 | 14.28 | 29.82 | 0.80 | 0 | 0.40 |
| Sample | Layer | Thickness, nm | Roughness, nm | Density, g/cm3 | |
|---|---|---|---|---|---|
| Up | Bottom | ||||
| LNO-1/3 | Layer 2 | 6.40 | 1.35 | 1.67 | 0.53 |
| Layer 1 | 79.9 | 2.28 | 2.49 | 2.46 | |
| Si-substrate | - | - | 2.33 | 2.33 | |
| LNO-1/10 | Layer 2 | 5.19 | 2.39 | 2.26 | 1.20 |
| Layer 1 | 38.5 | 1.19 | 2.58 | 2.96 | |
| Si-substrate | - | - | 2.33 | 2.33 | |
| Layer | Thickness, nm | Roughness, nm | Density, g/cm3 | |
|---|---|---|---|---|
| Up | Bottom | |||
| Layer 3 | 2.96 | 1.14 | 2.55 | 2.55 |
| Layer 2 | 57.5 | 0.19 | 2.31 | 1.86 |
| Layer 1 | 23.3 | 0.97 | 6.6 | 6.0 |
| Si | - | - | 2.32 | 2.32 |
| Sample | Сabs, μAh | СО, μAh·μAm−1·cm−2 | Thickness, nm | Iр, μA/C-Rate |
|---|---|---|---|---|
| Powders | ||||
| LiCoO2 [78] | 15.30 | 78.3 | 100 | -/0.2 С at 3.0–4.3 V |
| LiFePO4 [79] | 10.58 | 54 | -/0.1 C | |
| LiMn2O4 [80] | 10.07 | 51.4 | -/1.0 C | |
| LiNiO2 [77] | 20.1 | 103 | -/0.5 С | |
| Thin Films | ||||
| LiCoO2 [45] | 3.2 | 27 | 60 | 0.5 μA/0.35 С |
| LiFePO4 [81] | 0.57 | 10.9 | 55 | 1 μA/- |
| FePO4 [82] | 9.35 | 47.7 | 46 | (181 μA/g)/1 C |
| LixMn2O4 [46] | 16.7 (230 μAh/g) 9.06 (125 μAh/g) | 98.9 53.75 | ≥86 | 50 μA/3 C at (2.5–4.5 V) 200 μA/14 C at (2.2–4.5 V) |
| LNO-1/10, 800 °C | 2.8 | 24.3 | 58 * | 20 μA/6.9 С |
| LNO-1/10, 900 °C | 3.0 | 26.3 | 58 * | 20 μA/6.3 C |
| LNO-M, 800 °C | 3.6 | 20.7 | 88 * | 20 μA/5.3 С |
| LNO-M, 900 °C | 4.5 | 26 | 88 * | 20 μA/5.5 С |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maximov, M.; Nazarov, D.; Rumyantsev, A.; Koshtyal, Y.; Ezhov, I.; Mitrofanov, I.; Kim, A.; Medvedev, O.; Popovich, A. Atomic Layer Deposition of Lithium–Nickel–Silicon Oxide Cathode Material for Thin-Film Lithium-Ion Batteries. Energies 2020, 13, 2345. https://doi.org/10.3390/en13092345
Maximov M, Nazarov D, Rumyantsev A, Koshtyal Y, Ezhov I, Mitrofanov I, Kim A, Medvedev O, Popovich A. Atomic Layer Deposition of Lithium–Nickel–Silicon Oxide Cathode Material for Thin-Film Lithium-Ion Batteries. Energies. 2020; 13(9):2345. https://doi.org/10.3390/en13092345
Chicago/Turabian StyleMaximov, Maxim, Denis Nazarov, Aleksander Rumyantsev, Yury Koshtyal, Ilya Ezhov, Ilya Mitrofanov, Artem Kim, Oleg Medvedev, and Anatoly Popovich. 2020. "Atomic Layer Deposition of Lithium–Nickel–Silicon Oxide Cathode Material for Thin-Film Lithium-Ion Batteries" Energies 13, no. 9: 2345. https://doi.org/10.3390/en13092345
APA StyleMaximov, M., Nazarov, D., Rumyantsev, A., Koshtyal, Y., Ezhov, I., Mitrofanov, I., Kim, A., Medvedev, O., & Popovich, A. (2020). Atomic Layer Deposition of Lithium–Nickel–Silicon Oxide Cathode Material for Thin-Film Lithium-Ion Batteries. Energies, 13(9), 2345. https://doi.org/10.3390/en13092345