Nickel Salicylaldoxime-Based Coordination Polymer as a Cathode for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. General Considerations
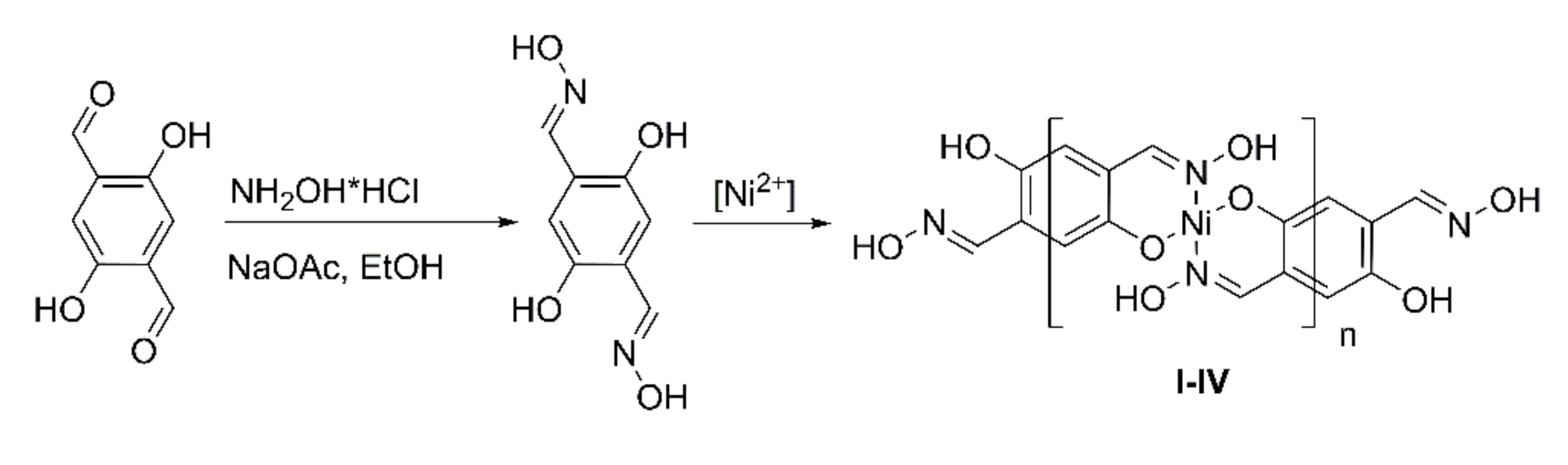
2.2. Synthesis
- I.
- Obtained according to the previously described protocol in aqueous 1,4-dioxane with Ni(OAc)2 as a nickel source and NaOAc to maintain the pH [24]. Yield 193.7 mg, 76.6%.
- II.
- Warm solutions of ligand (0.1962 g, 1 mmol) in glacial acetic acid (6 mL) and nickel acetate tetrahydrate (0.2490 g, 1 mmol) in the same solvent (4 mL) were mixed and stirred at 100 °C for 16 h. The brick-red precipitate was filtered, washed with acetic acid and 1,4-dioxane, and dried in vacuo at 100 °C for 4 h. Yield 166.3 mg, 65.8%.
- III.
- To the solution of ligand (0.1962 g, 1 mmol) in a mixture of dry acetonitrile (10 mL) and 1,4-dioxane (5 mL), the solution of nickel acetylacetonate (0.2569 g, 1 mmol) in acetonitrile (5 mL) was gradually introduced. The carrot-colored mixture was stirred in a sealed flask at 60 °C for 16 h. The product was filtered, washed thoroughly with acetonitrile, and dried in vacuo. Yield 178.0 mg, 70.4%.
- IV.
- To the solution of ligand (0.1962 g, 1 mmol) in freshly distilled water- and oxygen-free THF (10 mL), diisopropylethylamine (0.297 g 2.30 mmol) in THF (2 mL) was added under an argon atmosphere. The mixture turned deep-yellow and was stirred for 15 min. A solution of nickel acetylacetonate (0.2569 g, 1 mmol) in THF (8 mL) was added dropwise in 5 min, and the mixture was stirred for 1 h at r.t. Sienna-colored precipitate started to form. After being stirred at 60 °C for 16 h, the product was repeatedly washed with THF using centrifugation. Yield 219.7 mg, 86.9%. For further investigations, synthesis was reproduced without final drying. The “wet-clay” product obtained after centrifugation was resuspended in N-methylpyrrolidone (NMP) , and THF was removed from the suspension by stirring in vacuo at RT for 3 days, affording the ca. 10% dispersion of the product in NMP.
2.3. Half-Cell Fabrication
2.4. Electrochemical Studies
2.5. Computations
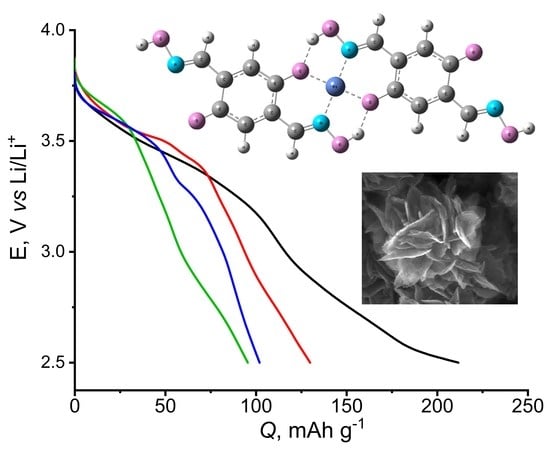
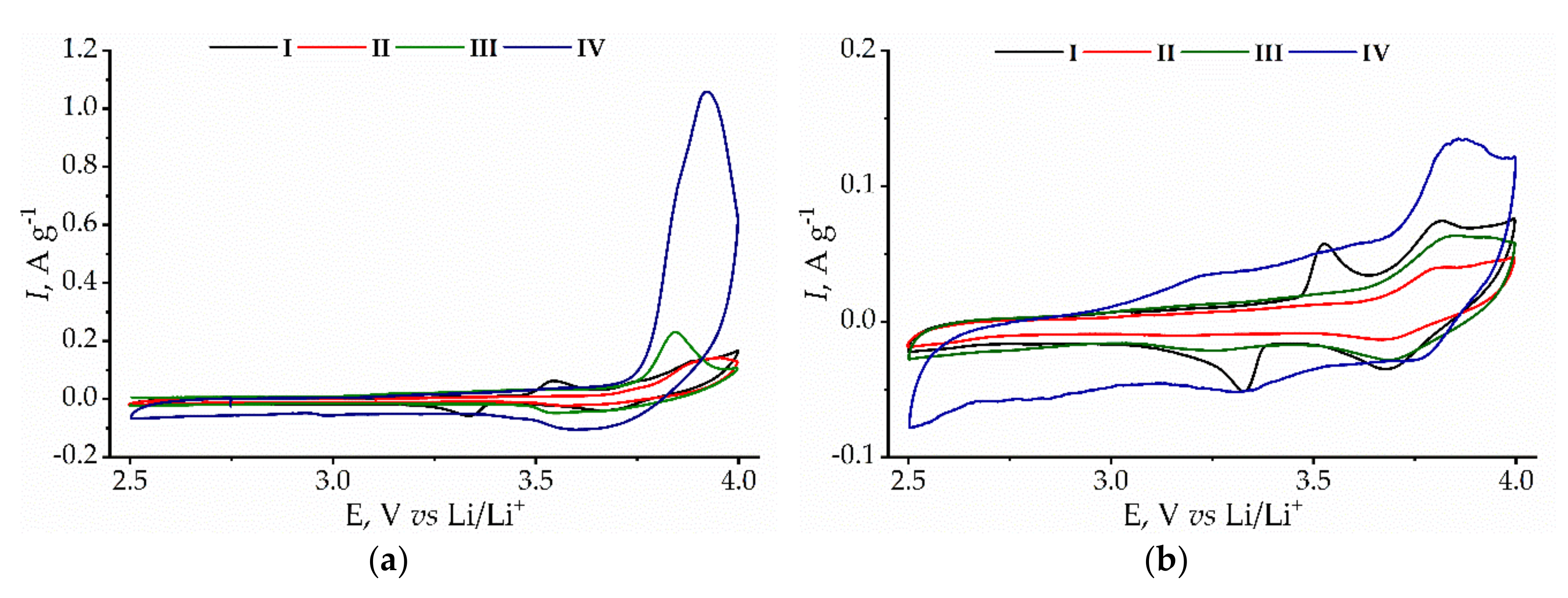
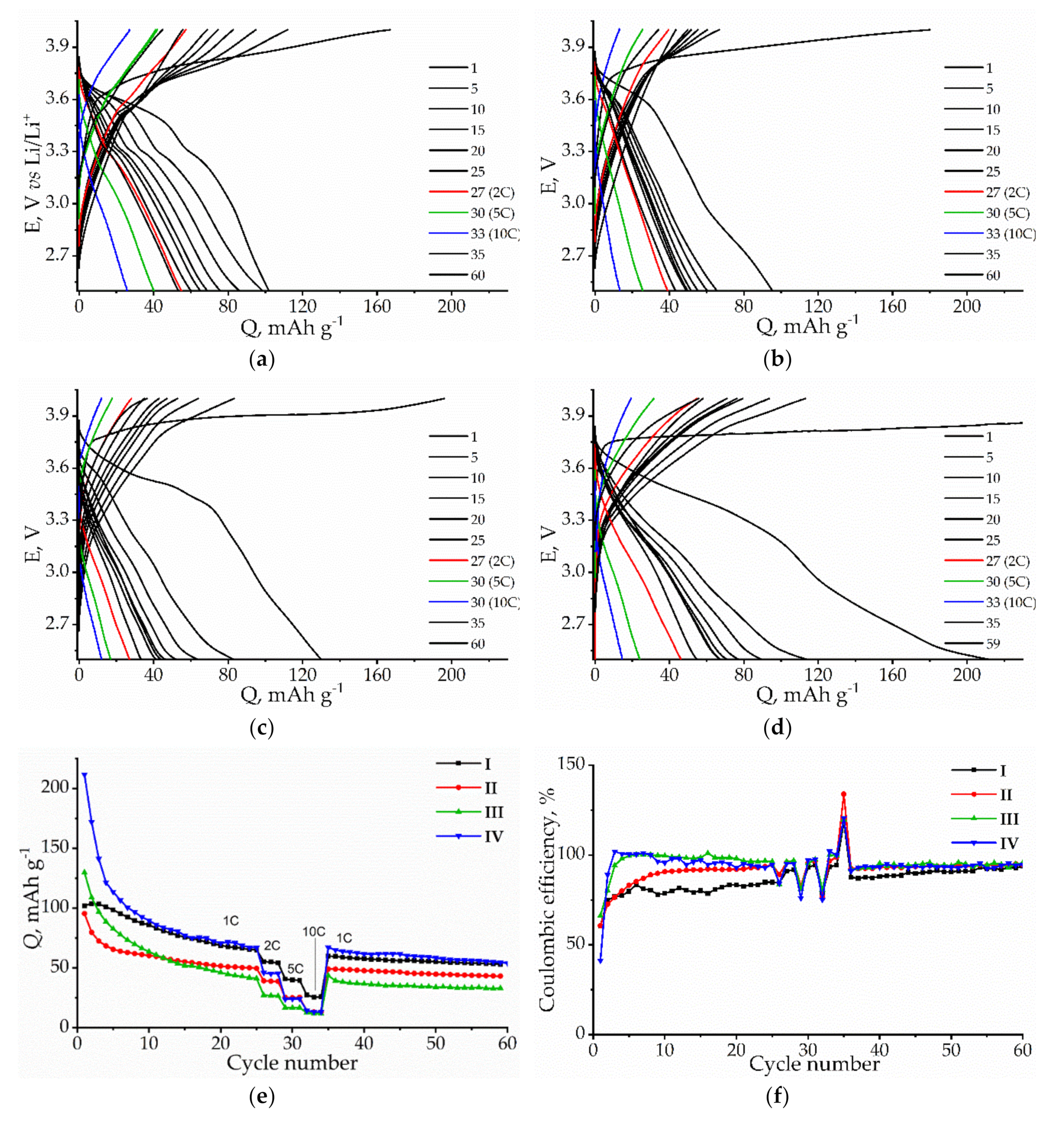
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243. [Google Scholar] [CrossRef]
- Ding, J.; Hu, W.; Paek, E.; Mitlin, D. Review of Hybrid Ion Capacitors: From Aqueous to Lithium to Sodium. Chem. Rev. 2018, 118, 6457–6498. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y.; Dunn, B. Materials science. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, C.; Chao, D.; Yan, Q.; Fan, H.J. Nonaqueous Hybrid Lithium-Ion and Sodium-Ion Capacitors. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Q.; Hu, J. Asymmetric capacitor based on superior porous Ni–Zn–Co oxide/hydroxide and carbon electrodes. J. Power Sources 2010, 195, 3017–3024. [Google Scholar] [CrossRef]
- Yi, H.; Wang, H.; Jing, Y.; Peng, T.; Wang, X. Asymmetric supercapacitors based on carbon nanotubes@NiO ultrathin nanosheets core-shell composites and MOF-derived porous carbon polyhedrons with super-long cycle life. J. Power Sources 2015, 285, 281–290. [Google Scholar] [CrossRef]
- Mao, M.L.; Sun, L.X.; Xu, F. Metal-Organic Frameworks/Carboxyl Graphene Derived Porous Carbon as a Promising Supercapacitor Electrode Material. Key Eng. Mater. 2017, 727, 756–763. [Google Scholar] [CrossRef]
- Pilon, L.; Wang, H.; d’Entremont, A. Recent Advances in Continuum Modeling of Interfacial and Transport Phenomena in Electric Double Layer Capacitors. J. Electrochem. Soc. 2015, 162, A5158–A5178. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.J.; Shao-Horn, Y.; Dinca, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, P.; Zheng, C.; Qiu, H.; Wei, M. Metal–organic frameworks: A new promising class of materials for a high performance supercapacitor electrode. J. Mater. Chem. A 2014, 2, 16640–16644. [Google Scholar] [CrossRef]
- Lai, F.; Huang, Y.; Miao, Y.-E.; Liu, T. Controllable preparation of multi-dimensional hybrid materials of nickel-cobalt layered double hydroxide nanorods/nanosheets on electrospun carbon nanofibers for high-performance supercapacitors. Electrochim. Acta 2015, 174, 456–463. [Google Scholar] [CrossRef]
- Jiao, Y.; Pei, J.; Yan, C.; Chen, D.; Hu, Y.; Chen, G. Layered nickel metal–organic framework for high performance alkaline battery-supercapacitor hybrid devices. J. Mater. Chem. A 2016, 4, 13344–13351. [Google Scholar] [CrossRef]
- Yan, Y.; Gu, P.; Zheng, S.; Zheng, M.; Pang, H.; Xue, H. Facile synthesis of an accordion-like Ni-MOF superstructure for high-performance flexible supercapacitors. J. Mater. Chem. A 2016, 4, 19078–19085. [Google Scholar] [CrossRef]
- Xu, J.; Yang, C.; Xue, Y.; Wang, C.; Cao, J.; Chen, Z. Facile synthesis of novel metal-organic nickel hydroxide nanorods for high performance supercapacitor. Electrochim. Acta 2016, 211, 595–602. [Google Scholar] [CrossRef]
- Wada, K.; Sakaushi, K.; Sasaki, S.; Nishihara, H. Multielectron-Transfer-based Rechargeable Energy Storage of Two-Dimensional Coordination Frameworks with Non-Innocent Ligands. Angew. Chem. Int. Ed. Engl. 2018, 57, 8886–8890. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G.; Millange, F.; Morcrette, M.; Serre, C.; Doublet, M.L.; Greneche, J.M.; Tarascon, J.M. Mixed-valence li/fe-based metal-organic frameworks with both reversible redox and sorption properties. Angew. Chem. Int. Ed. Engl. 2007, 46, 3259–3263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yoshikawa, H.; Awaga, K. Monitoring the solid-state electrochemistry of Cu(2,7-AQDC) (AQDC = anthraquinone dicarboxylate) in a lithium battery: Coexistence of metal and ligand redox activities in a metal-organic framework. J. Am. Chem. Soc. 2014, 136, 16112–16115. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.N.; Alekseeva, E.V.; Vereshchagin, A.A.; Volkov, A.I.; Vlasov, P.S.; Konev, A.S.; Levin, O.V. Nickel-Salen Type Polymers as Cathode Materials for Rechargeable Lithium Batteries. Macromol. Chem. Phys. 2017, 218. [Google Scholar] [CrossRef]
- Beletskii, E.V.; Volosatova, Y.A.; Eliseeva, S.N.; Levin, O.V. The Effect of Electrode Potential on the Conductivity of Polymer Complexes of Nickel with Salen Ligands. Russ. J. Electrochem. 2019, 55, 339–345. [Google Scholar] [CrossRef]
- Vereshchagin, A.A.; Vlasov, P.S.; Konev, A.S.; Yang, P.; Grechishnikova, G.A.; Levin, O.V. Novel highly conductive cathode material based on stable-radical organic framework and polymerized nickel complex for electrochemical energy storage devices. Electrochim. Acta 2019, 295, 1075–1084. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals; Elsevier/Butterworth-Heinemann: Burlington, MA, USA, 2009. [Google Scholar] [CrossRef]
- Worden, L.R.; Kaufman, K.D.; Smith, P.J.; Widiger, G.N. Synthesis of 4,6-dihydroxyisophthalaldehyde and the 5-methyl and 5-methoxy-derivatives. J. Chem. Soc. C Org. 1970, 227–230. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Okamura, H.; Nakamura, S. Photographisches aufzeichnungsmaterial und verfahren unter dessen verwendung. DE3219033A1, 19 May 1981. [Google Scholar]
- Manecke, G.; Wille, W.E.; Kossmehl, G. Preparation and properties of monomeric and polymeric Schiff bases derived from salicylaldehyde and 2,5-dihydroxyterephthalaldehyde. II. Electrical conductivity. Makromol. Chem. 1972, 160, 111–126. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Yoshio, M.; Noguchi, H. A Review of Positive Electrode Materials for Lithium-Ion Batteries. In Lithium-Ion Batteries: Science and Technologies; Yoshio, M., Brodd, R.J., Kozawa, A., Eds.; Springer: New York, NY, USA, 2009; pp. 9–48. [Google Scholar] [CrossRef]
- Sizov, V.V.; Novozhilova, M.V.; Alekseeva, E.V.; Karushev, M.P.; Timonov, A.M.; Eliseeva, S.N.; Vanin, A.A.; Malev, V.V.; Levin, O.V. Redox transformations in electroactive polymer films derived from complexes of nickel with SalEn-type ligands: Computational, EQCM, and spectroelectrochemical study. J. Solid State Electrochem. 2015, 19, 453–468. [Google Scholar] [CrossRef]
- Dmitrieva, E.; Rosenkranz, M.; Danilova, J.S.; Smirnova, E.A.; Karushev, M.P.; Chepurnaya, I.A.; Timonov, A.M. Radical formation in polymeric nickel complexes with N 2 O 2 Schiff base ligands: An in situ ESR and UV–vis–NIR spectroelectrochemical study. Electrochim. Acta 2018, 283, 1742–1752. [Google Scholar] [CrossRef]

| Atomic Ratio | C/Ni | N/Ni | O/Ni |
|---|---|---|---|
| Calculated | 8 | 2 | 4 |
| I | 8.0 ± 1.8 | 2.0 ± 0.6 | 4.5 ± 1.0 |
| II | 17.5 ± 4.4 | 4.5 ± 1.9 | 8.3 ±2.9 |
| III | 22.2 ± 3.5 | 7.9 ± 2.6 | 10.8 ± 2.6 |
| IV | 12.0 ± 0.3 | N/A 1 | 5.3 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beletskii, E.V.; Lukyanov, D.A.; Vlasov, P.S.; Yankin, A.N.; Atangulov, A.B.; Sizov, V.V.; Levin, O.V. Nickel Salicylaldoxime-Based Coordination Polymer as a Cathode for Lithium-Ion Batteries. Energies 2020, 13, 2480. https://doi.org/10.3390/en13102480
Beletskii EV, Lukyanov DA, Vlasov PS, Yankin AN, Atangulov AB, Sizov VV, Levin OV. Nickel Salicylaldoxime-Based Coordination Polymer as a Cathode for Lithium-Ion Batteries. Energies. 2020; 13(10):2480. https://doi.org/10.3390/en13102480
Chicago/Turabian StyleBeletskii, Evgenii V., Daniil A. Lukyanov, Petr S. Vlasov, Andrei N. Yankin, Arslan B. Atangulov, Vladimir V. Sizov, and Oleg V. Levin. 2020. "Nickel Salicylaldoxime-Based Coordination Polymer as a Cathode for Lithium-Ion Batteries" Energies 13, no. 10: 2480. https://doi.org/10.3390/en13102480
APA StyleBeletskii, E. V., Lukyanov, D. A., Vlasov, P. S., Yankin, A. N., Atangulov, A. B., Sizov, V. V., & Levin, O. V. (2020). Nickel Salicylaldoxime-Based Coordination Polymer as a Cathode for Lithium-Ion Batteries. Energies, 13(10), 2480. https://doi.org/10.3390/en13102480