1. Introduction
Lithium-ion batteries are widely used to power portable electronic devices, aerospace systems and more recently, electric vehicles and stationary energy storage systems, due to their high specific energy and power, their significant working resource and wide range of operating conditions. The main components of a lithium-ion battery are the active materials of electrodes, electrolyte and the separator. The function of the separator in the lithium-ion battery is very important: it prevents the direct electronic contact of the electrodes, ensuring the exchange of lithium ions between them. Significant efforts have been applied to improve separators: the goals are to reduce the thickness of the separator, to increase the wettability of the electrolyte solutions, to increase the mechanical strength and to provide a programmable behavior of the separator in the emergency operation of the battery, for example, blocking ion exchange at rapid heating. One of the significant characteristics of lithium-ion batteries is their ability for long-term cycling whilst maintaining a stable capacity and a shape of charge–discharge curves at various currents. The separator can play a noticeable role in this ability. The improved cycling behavior of a LiMn
2O
4-electrode [
1] in a half-cell with a metal Li counter electrode and a 1M LiPF
6 electrolyte in a mixture of organic solvents was demonstrated in which a separation material, based on electrospun polyvinylidene fluoride (PVDF)-hexafluoropropylen (HFP) and its modifications with silicon (IV) oxide, was used as a separator in comparison with a polypropylene-based separator. The authors [
2] presented a comparison of the electrochemical behavior of LiFePO
4 electrode in a half-cell with a metallic Li-electrode, where a polysulfone-amide/polyacrilonitril/polysulfone-amide multilayer material was used as a separator, which was of minimal resistance and demonstrated an improved performance during long-term cycling compared to a commercial PP separator. In other works, electroformed PVDF-based separation materials with ceramic nanoparticles based on SiO
2 [
3] and Al
2O
3 [
4] were obtained. Thus, the cycled capacity of the LiMn
2O
4 electrode after 100 cycles in a half cell with a metallic Li electrode and an experimental separator according to [
4] turned out to be 10% higher than under the same conditions with a commercial polypropylene (PP) separator. According to the data of [
5,
6], separators materials having high porosity values obtained by the electrospinning method markedly improved the electrochemical behavior of the studied electrodes. All the listed examples of separators relate to the capillary electrospinning method. In our previously published work [
7], it was shown that the capillary-less electrospinning method allowed to obtain a separator based on the PVDF | polytetrafluoroethylene (PTFE) polymer composition having a high porosity of 71.4% and an electrolyte wettability of 800% using 0.67 M LiClO
4 electrolyte in a mixture of propylene carbonate (PC) and dimethoxyethane (DME). This made it possible to obtain improved characteristics of the specific charge–discharge capacity of the Li
4Ti
5O
12 electrode in a cell with a metallic Li electrode at various currents from 0.1 C to 25 C compared to the similar cell where commercial PP-based separator was used.
Along with polymer separators, glass-fiber (GF) separators are also used in lithium-ion batteries. They are characterized by a high thermal stability, strength and chemical inertness, which determines their high working life in the battery [
8]. One of the disadvantages of such separators is the rather large pore size of more than 1 μm, which practically excludes protection against a short circuit due to the formation of dendritic lithium. Another disadvantage is the relatively large thickness of the material, which consequently increases the resistance to the transfer of lithium ions between the electrodes. The authors [
9] reported obtaining a glass fiber separator having fairly good physical characteristics: thermal stability at 150 °C, a relatively small thickness of about 45 μm and an electrolyte wettability of 200%–300%. As a result, the specific discharge capacity of the LiFePO
4 electrode in a half cell with a metallic Li electrode and the mentioned separator after the 100th cycle is 10% higher than the value obtained in a similar cell with a commercial Celgard2400 separator.
To summarize what has been said here, the main problems in lithium storage systems that the separator is designed to solve what follows. First of all, this is a possible growth of dendrites on the anode—needle-shaped lithium crystals, which can form at potentials close to the potential of a lithium metal electrode. Dendrites, as they grow, are able to puncture the separator and short-circuit the electrodes. Separator developers are trying to create a separator structure that can prevent dendrites from germinating. For this purpose, the separator must have a dense finely porous structure. However, such a structure creates significant resistance to electric current. On some anodes, the formation of dendrites is fundamentally impossible, for example, on Li4Ti5O12. In the absence of this problem, the structure of the separator can be optimized in order to increase the conductive properties. This especially concerns electrochemical systems in which power is a priority. These systems also include the Li4Ti5O12 anode. The fibrous electrospun structure of the separator on the basis of polymeric PVDF|PTFE composition is best suited to the characteristics of the Li4Ti5O12 anode.
This paper presented the results of a comparative study of the electrochemical properties of a Li
4Ti
5O
12 electrode in a half-cell with a metallic Li-electrode, an 0.67 M LiClO
4 electrolyte in the mixed solvent composed of PC and DME (7:3), as well as one of the separators: an electrospun separator based on the polymeric PVDF|PTFE composition described in [
7], commercial PP material and GF material. A difference in the change in the electrochemical properties during long-term cycling of the cell depending on the used separation material is shown. Among the analyzed electrochemical characteristics of the Li
4Ti
5O
12 electrode were the following: cycled capacity, average working voltage, and also parameters found as a result of an analysis of the electrochemical impedance spectra, including the diffusion coefficient
D of lithium ions in the electrode material.
2. Materials and Methods
Li4Ti5O12 active electrode material was synthesized by solid state reaction with preliminary mechanochemical activation pretreatment. The initial chemical precursors were as follows: titanium (IV) oxide TiO2 of crystalline modification rutile (purity qualification «pure», producer «Lenreaktiv», Russia), carbonate of lithium Li2CO3 (purity qualification «pure», producer «Neva-Reaktiv», Russia), used for the synthesis of the lithium titanate electrode material (Li4Ti5O12) in a stoichiometric ratio.
The reaction blend was mechanically activated in the AGO-2 planetary ball mill (Novits, Russia). Steel drums filled with 6 mm steel balls were used. Acetone was used as a dispersion medium. The mixture of reagents was treated during 20 min at a spin rate of 560 rpm.
The annealing of the ground reactant blend was conducted in a tube furnace under the argon atmosphere at the rate temperature increasing of 10 °C·min
−1. After turning off the heating the Li
4Ti
5O
12 product was cooled together with the furnace. Our approach to the producing of lithium pentatitanate was based on our earlier work [
10]. The use of an inert atmosphere during heat treatment to obtain Li
4Ti
5O
12 was an excessive measure, since this material was stable both in an inert or reducing atmosphere and in an oxidizing one. In the vast majority of works, it was the oxidizing (air) atmosphere that was used in the synthesis of this material, since such conditions were easier to create. However, there are examples of obtaining this material in an inert atmosphere [
11], when a carbon additive was introduced into the reaction mixture for synthesis, which subsequently increased the electronic conductivity of the electrode material. In our case, an inert atmosphere was preferable for the operation of the annealing furnace in order to exclude the possible ingress of unwanted impurities into the annealing zone. At the same time, this did not affect the composition and the characteristics of the resulting material.
The separator based on the nanofibers mix of PVDF|PTFE was produced using a capillary-less electrospinning process fed by the polymeric solution with the application of the NS LAB-200 equipment (producer «Elmarco», Czech Republic). The PVDF and PVDF|PTFE copolymers were dissolved in the solvent mix of dimethylformamide (DMF) and butylacetate (BA) in a proportion of 1:1 by weight at constant mixing during 2 h to obtain polymeric solutions of the concentrations of >8 wt.%. The polymeric solution was immersed in an electrospinning bath. The electrospinning process was conducted in accordance with the following optimal conditions: the collecting electrode consisted of one string, the supplying electrode consisted of four strings, the inter-electrodes distance approached 170 mm and the electrode spin rate was 16 rpm. Paper was used as a substrate to obtain a defective-less structure of the separator. The operation voltage was 70 kV, current was 0.02–0.03 mA, the humidity and the temperature during the process conducted in the NS LAB-200 were 14% ± 2% and 25 °C ± 2 °C, respectively. The thicknesses of the separators were measured using the thickness gauge Absolute Digimatic ID-S 543-790 (producer «Mitutoyo Corp.», Japan). The fiber thicknesses of the separators were measured by scanning electron microscopy (SEM) using a MIRA LMU microscope (producer «Tescan», Czech Republic).
The electrolyte uptake (EU) of the separator was measured by soaking the PVDF|PTFE, PP and glass fiber membranes in 0.67 M LiClO
4 electrolyte in the mixture of PC:DME = 7:3 (by vol.), which was calculated in accordance with Equation (1):
where
w0 and
w1 designate the separator weight preceding and following the soaking in the electrolyte for 1 h, respectively.
The working electrode was prepared by mixing 80 w% active material Li
4Ti
5O
12, 10 w% acetylene black conductive additive and 10 w% PVdF polymer binder. The electrolyte composition was a 0.67 M LiClO
4 solution in PC: DME (7:3, vol.). The electrode slurry was cast on the Al-foil with a coating density of 5 mg∙cm
−2 by «Dr. Blade» technique. The electrochemical tests were performed in pouch cells containing the electrode under testing, the lithium counter electrode and the lithium reference electrode. An Elins/P-45X potentiostat/galvanostat with FRA module (Elins, Russian Federation) and an Autolab/PGSTAT302N modular high-current potentiostat/galvanostat (ECO CHEMIE, Netherlands) with a FRA frequency response analyzer were used for the electrochemical impedance spectroscopy (EIS) measurements. The measurements were carried out in the frequency range 1 mHz–100 kHz and the electrode response was measured in two limiting states—completely lithiated and delithiated at different stages of cycling in the galvanostatic charge–discharge mode, similar to what was done in our earlier work [
7]. Neware/BTS10V10mA (Neware Technology Limited, China) and Booster/UZR 0.03–10 (Booster, Russian Federation) charge–discharge modules were utilized for the galvanostatic charge–discharge tests. C-rate tests of the cells with different samples of the separator were performed by galvanostatic charge and discharge in a series of subsequently applied normalized currents of 0.1C, 0,2C, 0.5C, 1C. The current value C was taken based on the theoretical capacity of the material 175 mAh·g
−1. The electrode potentials hereafter were given versus a Li reference electrode in the same solution. All the electrochemical measurements were carried out at under 25 °C.
4. Discussion
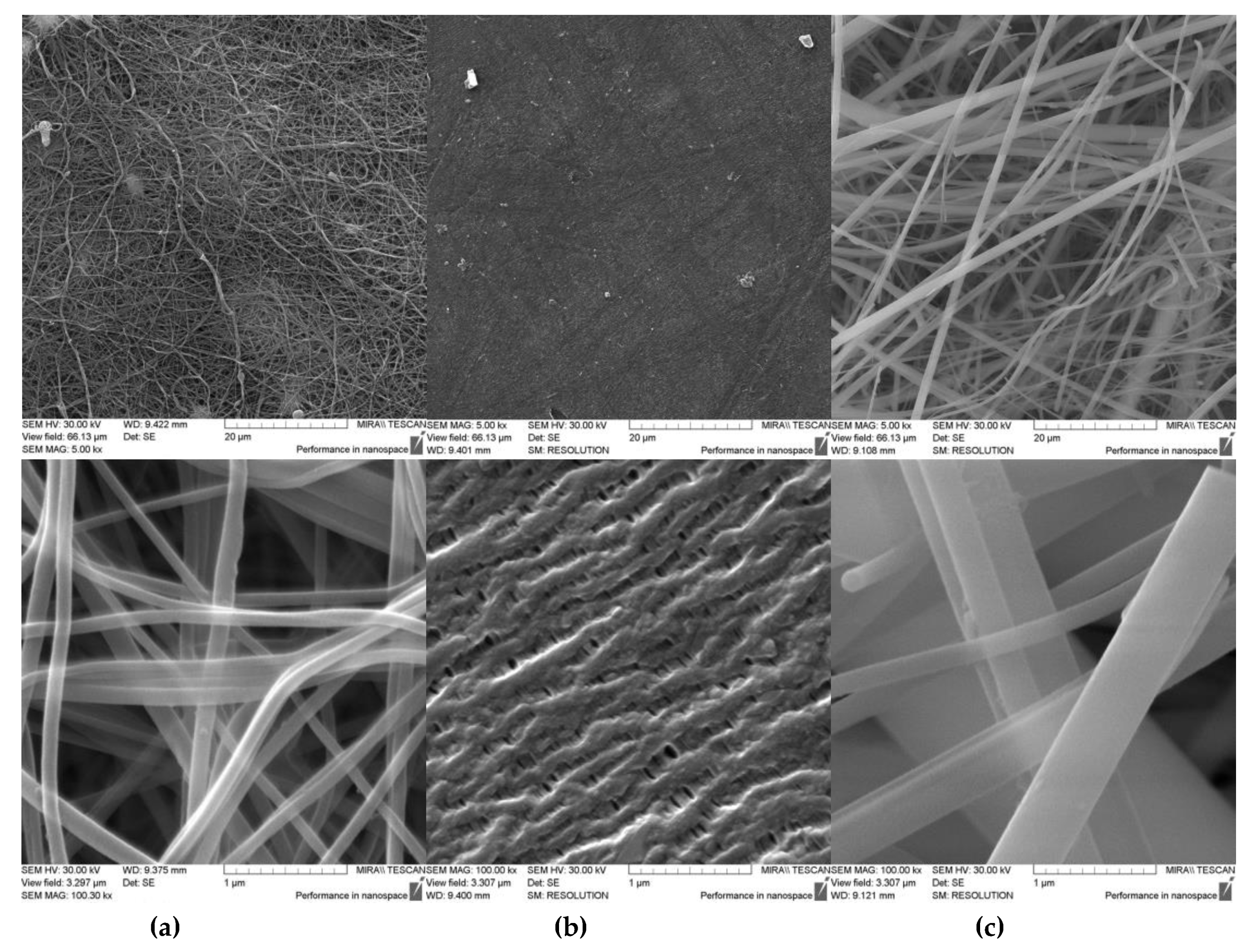
Nowadays, polymer separators with a non-fibrous structure, the basis of which, as a rule, is polypropylene, are widely distributed and characterized by their low porosity and wettability by electrolyte [
12]. The dense structure of such separators reduces the likelihood of the formation of dendrites from lithium metal, however, this factor reduces the electrochemical characteristics of the electrodes. Fibrous separators create a much lower resistance to ion flow due to their higher porosity and wettability with electrolyte, however, their strength is much lower and therefore they are not suitable for use with electrode materials on which lithium dendrites can grow. For materials on which dendritic formation is impossible, like Li
4Ti
5O
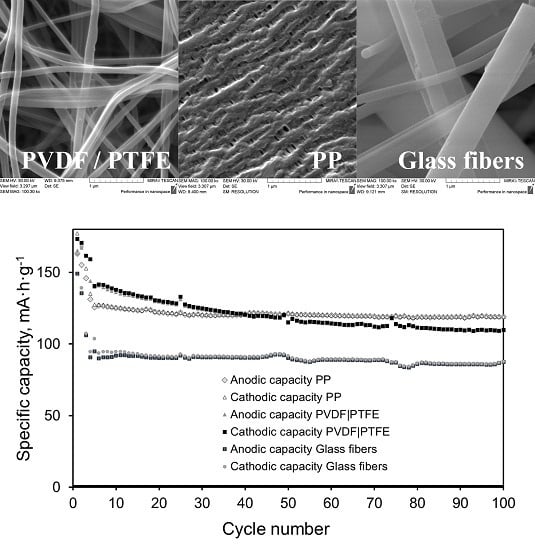
12, they are quite suitable. In addition to polymeric fiber separators like the electrospun PVDF|PTFE mix fiber-based separator, there are also inorganic materials such as the glass fiber separator discussed in this article. Their structures have common features (
Figure 1): the fibers are much thicker and less frequently located, but the space between the fibers is smaller, which explains the close values of porosity. The rheological properties of the fibers are also different: the glass fibers are more fragile, therefore to give the material strength it is necessary to increase its thickness, which we observed in the case of the glass fiber separator. Obviously, the structure of the separator will also affect the electrochemical characteristics of the electrodes. Earlier in [
7], we compared the electrochemical characteristics of the electrodes in combination with the separators on the basis of the electrospun PVDF|PTFE fibers and on the basis of PP in terms of their ability to maintain high charge–discharge rates of electrodes. However, the effect of the separator type on the characteristics of the electrodes during the long-term cycling remained unexamined, which was the subject of this work.
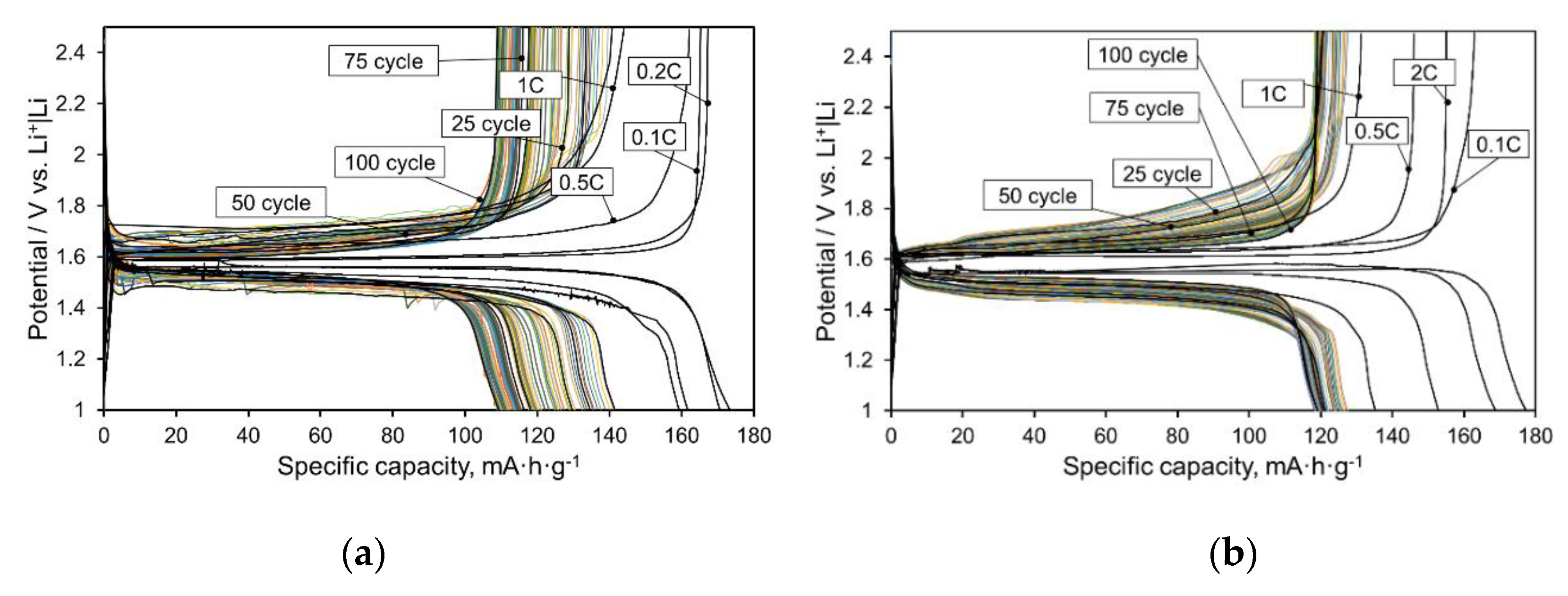
Figure 2 and
Figure 3, as well as
Table 1, show the data of galvanostatic cycling of half cells with all the tested samples of separators. The test mode consisted of two stages: 1) cycling at the current varying in the range 0.1 C–1 C; 2) long-term cycling at the current 1 C. The electrospun PVDF|PTFE mix fiber-based separator demonstrated superior capacity in the first stage of cycling during current increase. The capacity of the PP-based separator and the glass fiber separator significantly dropped when the current increased. This indicates a significantly lower ion flow resistance, which creates a PVDF|PTFE separator in comparison with the PP and especially with the GF separators. The observed sharp decrease in the capacity of the Li
4Ti
5O
12 electrode with an increasing current in the series 0.1C–1C in cells with the above two types of separator indicates a high ionic resistance of the electrolyte in the pores of the separator. This was due to several factors, including a small pore size and consequently, a relatively small effective cross-section of the electrolyte layer between the electrodes; as well as the poor wettability by electrolyte in the case of the PP separator, and therefore a small amount of retained electrolyte in the pores and possibly part of the broken conduction channels that were not completely filled with electrolyte; and finally, a greater thickness in the case of a GF separator, which increased the length of the conduction channels. At the same time, the higher stability of capacity was during the long-term cycling, the lower was this capacity. This phenomenon is associated with the different redistribution of polarization in the cell: in the series of PVDF|PTFE → PP → GF separators, the fraction of polarization per the electrolyte in the separator increased and the fraction per electrode decreased. Because of this, the concentration gradient decreased over the depth of the particles of the electrode material and the capacity extracted from the electrode was smaller. However, the smaller the fraction of the extracted lithium capacity, the softer the mode of operation of the electrode material was and the less degradation effects occurred.
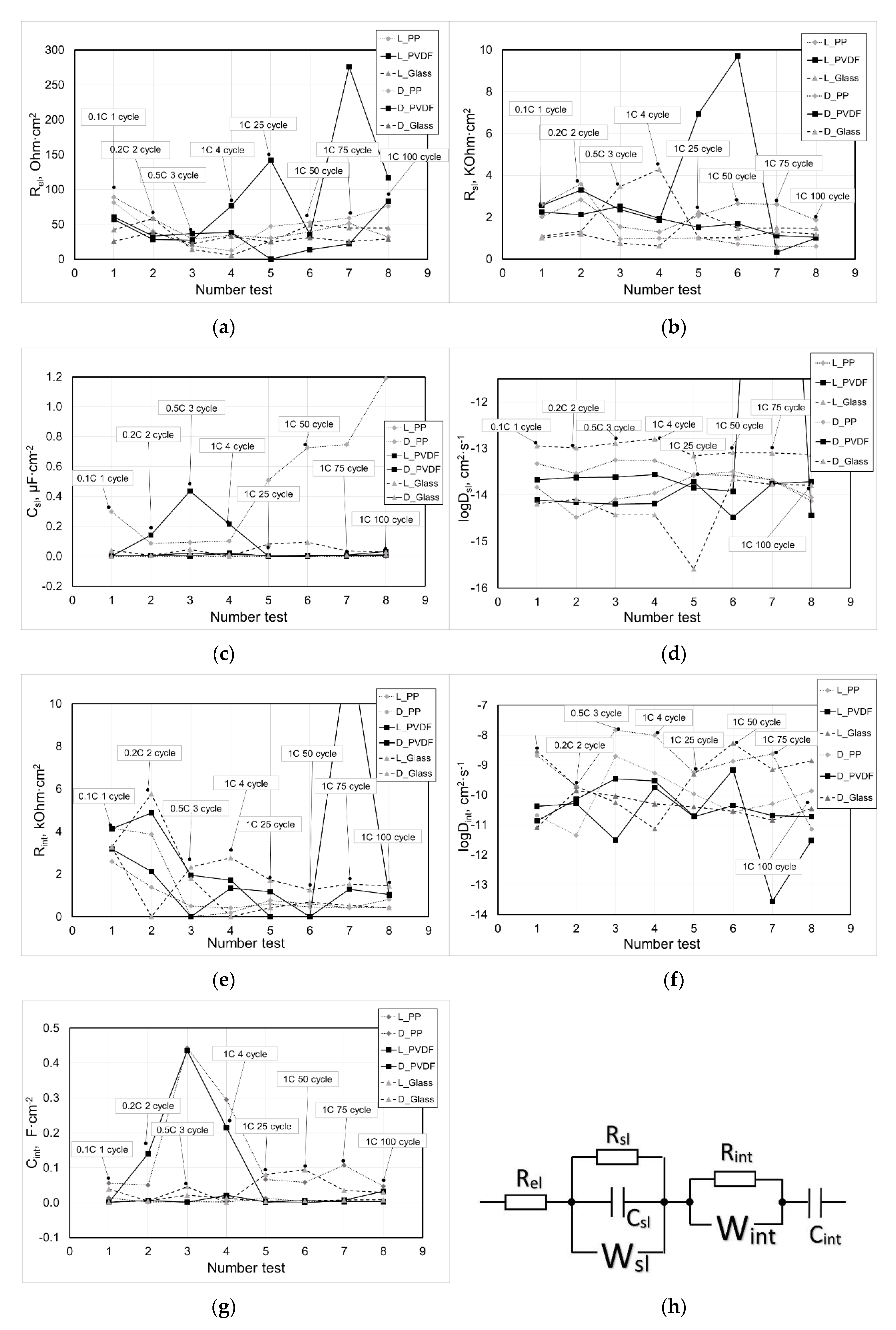
To diagnose the state of the electrode material, the EIS method was used. The approach that was previously developed by us [
7,
13,
14] was applied to the analysis of the impedance spectra (
Figure 4). For the different initial values of the resistance of the electrolyte and the separator
Rel for most of the samples of the separators, the tendency for its change during cycling was similar: at the first stage (current change 0.1 C–1 C), the values decreased, after which over 100 cycles the values returned to the previous level. Only the cell with the PVDF|PTFE sample measured after lithiation showed periodic sharp resistance jumps. Perhaps this was due to the strong nonequilibrium conditions at the interface of the electrode, electrolyte and separator, since the main fraction of the polarization fell on the electrode. The resistance of the surface layer of the electrode
Rsl monotonically decreased throughout the cycling. This was probably due to the development of ion conduction paths in the surface layer during cycling. The geometric capacitance of the surface layer of the electrode
Csl for most samples varied slightly, except in cases in which nonequilibrium conditions arose at the interface of the electrode, electrolyte and the separator, which apparently affected the dielectric constant of the surface layer. The diffusion coefficient of lithium ions in the surface layer
Dsl also practically did not undergo changes during cycling, with the exception of sharp jumps in nonequilibrium conditions. The resistance of the bulk of the intercalation material
Rint related to electron transfer tended to decrease during cycling, probably due to an increase in the defectiveness of the structure of the Li
4Ti
5O
12 compound. The diffusion coefficient of the lithium ions in the intercalation material
Dint and intercalation capacity
Cint, as the most sensitive parameters to the state of the electrode material, showed a noticeable scatter of values due to the nonequilibrium conditions. However, the general trend of their change was practically not observed.
Thus, long-term cycling at a moderate current (1 C) does not significantly affect the transport properties of an Li
4Ti
5O
12 electrode, in comparison with the effect of cycling in extreme current modes [
7]. A possible reason for the dependence of the degradation rate of the electrode capacity on the type of separator material used is a different degree of nonequilibrium of cycling conditions due to the redistribution of polarization in the cell between the electrode and electrolyte in the pores of the separator.