Application of Upgraded Drop-In Fuel Obtained from Biomass Pyrolysis in a Spark Ignition Engine
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Bio-Oil
2.2. Gasoline and Ethanol
2.3. Spark Ignition Engine and Generator
2.4. Characterisation of Organic Bio-Oil Fraction
2.5. Gas Emissions and Polycyclic Aromatic Hydrocarbons (PAHs) Trapping System
2.6. Blending Step
2.7. Fuel Consumption and Electrical Efficiency
3. Results and Discussion
3.1. Properties of the Bio-Oil, Ethanol and Gasoline
3.2. Performance of Engine Tests
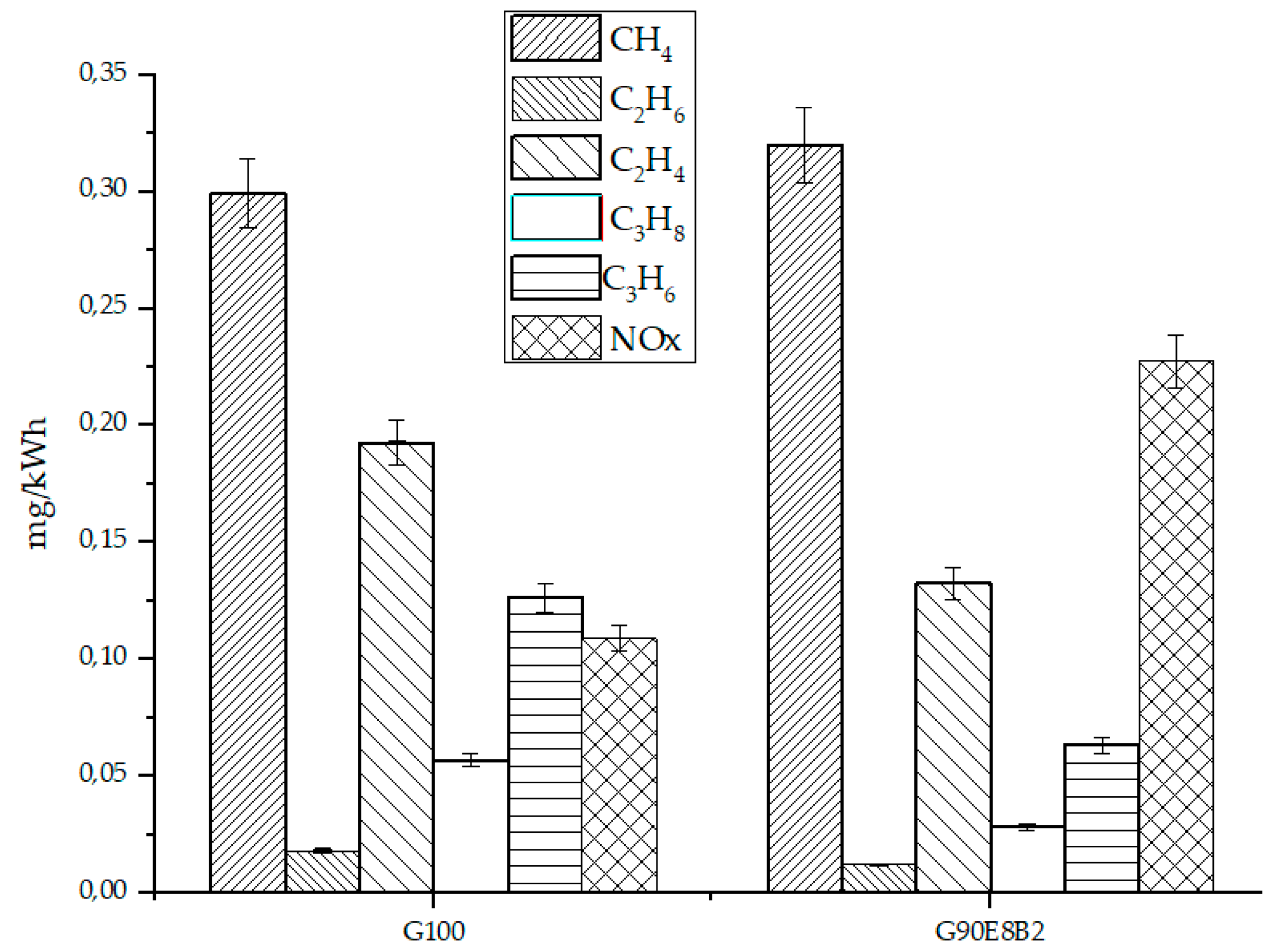
3.3. Exhaust Gaseous Emissions
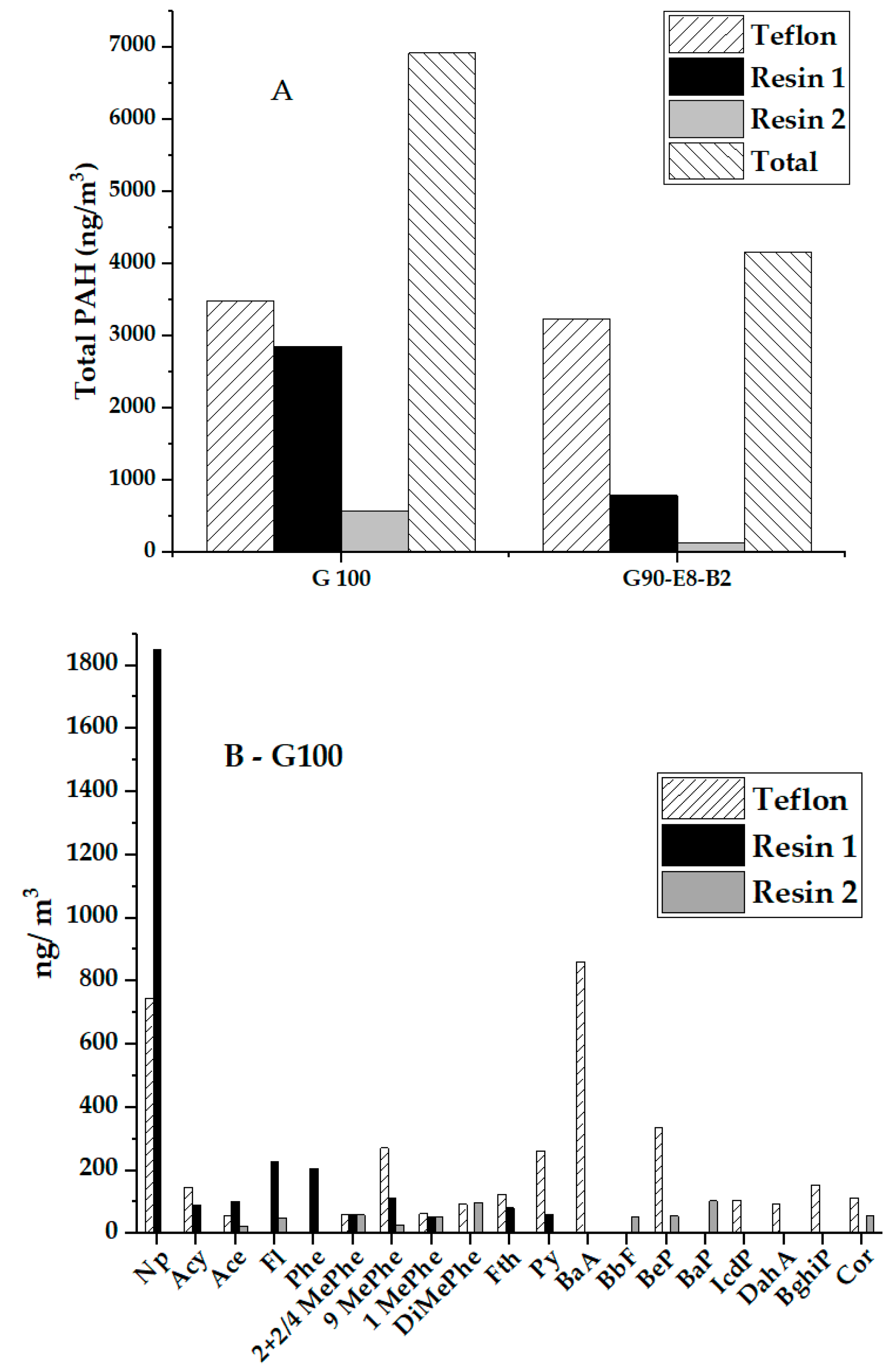
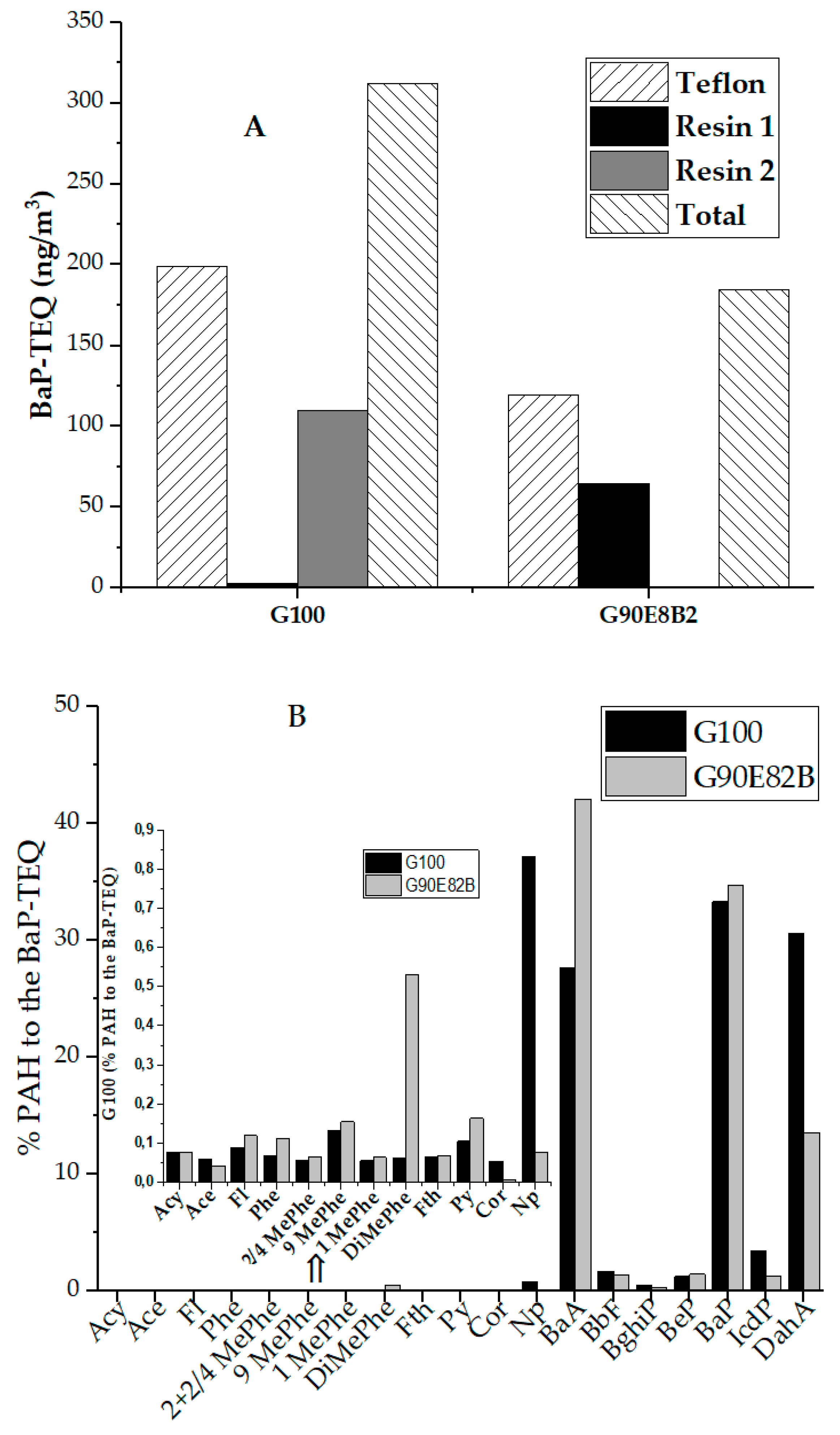
3.4. PAH Concentrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, S.I.; Wu, M.S.; Wu, C.Y. Application of biomass fast pyrolysis part I: Pyrolysis characteristics and products. Energy 2014, 66, 162–171. [Google Scholar] [CrossRef]
- Peters, J.F.; Iribarren, D.; Dufour, J. Biomass Pyrolysis for Biochar or Energy Applications? A Life Cycle Assessment. Environ. Sci. Technol. 2015, 49, 5195–5202. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Hossain, A.K.; Davies, P.A. Pyrolysis liquids and gases as alternative fuels in internal combustion engines—A review. Renew. Sustain. Energy Rev. 2013, 21, 165–189. [Google Scholar] [CrossRef]
- Cheng, F.; Bayat, H.; Jena, U.; Brewer, C.E. Impact of feedstock composition on pyrolysis of low-cost, protein- and lignin-rich biomass: A review. J. Anal. Appl. Pyrolysis 2020, 147, 104780. [Google Scholar] [CrossRef]
- Fermoso, J.; Pizarro, P.; Coronado, J.M.; Serrano, D.P. Advanced biofuels production by upgrading of pyrolysis bio-oil. Wiley Interdiscip. Rev. Energy Environ. 2017, 6, e245. [Google Scholar] [CrossRef]
- Krutof, A.; Hawboldt, K. Blends of pyrolysis oil, petroleum, and other bio-based fuels: A review. Renew. Sustain. Energy Rev. 2016, 59, 406–419. [Google Scholar] [CrossRef]
- No, S.-Y. Application of bio-oils from lignocellulosic biomass to transportation, heat and power generation—A review. Renew. Sustain. Energy Rev. 2014, 40, 1108–1125. [Google Scholar] [CrossRef]
- Van de Beld, B.; Holle, E.; Florijn, J. The use of pyrolysis oil and pyrolysis oil derived fuels in diesel engines for CHP applications. Appl. Energy 2013, 102, 190–197. [Google Scholar] [CrossRef]
- Hossain, A.K.; Ouadi, M.; Siddiqui, S.U.; Yang, Y.; Brammer, J.; Hornung, A.; Kay, M.; Davies, P.A. Experimental investigation of performance, emission and combustion characteristics of an indirect injection multi-cylinder CI engine fuelled by blends of de-inking sludge pyrolysis oil with biodiesel. Fuel 2013, 105, 135–142. [Google Scholar] [CrossRef]
- Hansen, S.; Mirkouei, A.; Diaz, L.A. A comprehensive state-of-technology review for upgrading bio-oil to renewable or blended hydrocarbon fuels. Renew. Sustain. Energy Rev. 2020, 118, 109548. [Google Scholar] [CrossRef]
- Pradhan, D.; Bendu, H.; Singh, R.K.; Murugan, S. Mahua seed pyrolysis oil blends as an alternative fuel for light-duty diesel engines. Energy 2017, 118, 600–612. [Google Scholar] [CrossRef]
- Van de Beld, B.; Holle, E.; Florijn, J. The use of a fast pyrolysis oil—Ethanol blend in diesel engines for chp applications. Biomass Bioenergy 2018, 110, 114–122. [Google Scholar] [CrossRef]
- Lee, S.; Kim, T.Y. Feasibility study of using wood pyrolysis oil–ethanol blended fuel with diesel pilot injection in a diesel engine. Fuel 2015, 162, 65–73. [Google Scholar] [CrossRef]
- Rezaei, P.S.; Shafaghat, H.; Daud, W.M.A.W. Production of green aromatics and olefins by catalytic cracking of oxygenate compounds derived from biomass pyrolysis: A review. Appl. Catal. A Gen. 2014, 469, 490–511. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—A review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Nguyen, D.; Honnery, D. Combustion of bio-oil ethanol blends at elevated pressure. Fuel 2008, 87, 232–243. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Mesa-Pérez, J.; Cortez, L.A.B.; Rocha, J.D.; Sanchez, C.G.; Marín, H. Use of blends of gasoline with biomass pyrolysis-oil derived fractions as fuels in an Otto engine. Energy Sustain. Dev. 2011, 15, 376–381. [Google Scholar] [CrossRef]
- Dupuis, D.P.; Grim, R.G.; Nelson, E.; Tan, E.C.D.; Ruddy, D.A.; Hernandez, S.; Westover, T.; Hensley, J.E.; Carpenter, D. High-Octane Gasoline from Biomass: Experimental, Economic, and Environmental Assessment. Appl. Energy 2019, 241, 25–33. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; Martínez, I.; Martínez, J.D.; López, J.M.; Navarro, M.V.; Callén, M.S.; Murillo, R.; García, T. Catalytic pyrolysis of wood biomass in an auger reactor using calcium-based catalysts. Bioresour. Technol. 2014, 162, 250–258. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; Callén, M.S.; Murillo, R.; García, T. An integrated process for the production of lignocellulosic biomass pyrolysis oils using calcined limestone as a heat carrier with catalytic properties. Fuel 2016, 181, 430–437. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; López, J.M.; Callén, M.S.; Murillo, R.; García, T. Production of upgraded bio-oils by biomass catalytic pyrolysis in an auger reactor using low cost materials. Fuel 2015, 141, 17–22. [Google Scholar] [CrossRef]
- Puértolas, B.; Veses, A.; Callén, M.S.; Mitchell, S.; García, T.; Pérez-Ramírez, J. Porosity-Acidity Interplay in Hierarchical ZSM-5 Zeolites for Pyrolysis Oil Valorization to Aromatics. ChemSusChem 2015, 8, 3283–3293. [Google Scholar] [CrossRef]
- Veses, A.; Puértolas, B.; López, J.M.; Callén, M.S.; Solsona, B.; García, T. Promoting Deoxygenation of Bio-Oil by Metal-Loaded Hierarchical ZSM-5 Zeolites. ACS Sustain. Chem. Eng. 2016, 4, 1653–1660. [Google Scholar] [CrossRef]
- Veses, A.; Puértolas, B.; Callén, M.S.; García, T. Catalytic upgrading of biomass derived pyrolysis vapors over metal-loaded ZSM-5 zeolites: Effect of different metal cations on the bio-oil final properties. Microporous Mesoporous Mater. 2015, 209, 189–196. [Google Scholar] [CrossRef]
- García, T.; Veses, A.; López, J.M.; Puértolas, B.; Pérez-Ramírez, J.; Callén, M.S. Determining Bio-Oil Composition via Chemometric Tools Based on Infrared Spectroscopy. ACS Sustain. Chem. Eng. 2017, 5, 8710–8719. [Google Scholar] [CrossRef]
- Veses, A.; López, J.M.; García, T.; Callén, M.S. Prediction of elemental composition, water content and heating value of upgraded biofuel from the catalytic cracking of pyrolysis bio-oil vapors by infrared spectroscopy and partial least square regression models. J. Anal. Appl. Pyrolysis 2018, 132, 102–110. [Google Scholar] [CrossRef]
- AENOR. UNE-EN ISO 16948:2015. Solid Biofuels-Determination of Total Content of Carbon, Hydrogen and Nitrogen; AENOR: Madrid, Spain, 2015. [Google Scholar]
- AENOR. UNE-EN ISO 18125:2018. Solid Biofuels-Determination of Calorific Value; AENOR: Madrid, Spain, 2018. [Google Scholar]
- ASTM. ASTM E203-96. Standard Test Method for Water Using Volumetric Karl Fischer Titration; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM. ASTM D445-19. Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity); ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Callén, M.S.; de la Cruz, M.T.; López, J.M.; Murillo, R.; Navarro, M.V.; Mastral, A.M. Some inferences on the mechanism of atmospheric gas/particle partitioning of polycyclic aromatic hydrocarbons (PAH) at Zaragoza (Spain). Chemosphere 2008, 73, 1357–1365. [Google Scholar] [CrossRef]
- Callén, M.S.; Iturmendi, A.; López, J.M. Source apportionment of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons by a PMF receptor model. Assessment of potential risk for human health. Environ. Pollut. 2014, 195, 167–177. [Google Scholar] [CrossRef]
- Patel, M.; Zhang, X.; Kumar, A. Techno-economic and life cycle assessment on lignocellulosic biomass thermochemical conversion technologies: A review. Renew. Sustain. Energy Rev. 2016, 53, 1486–1499. [Google Scholar] [CrossRef]
- European Commission. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources. Off. J. Eur. Union 2009, L140, 16–62. [Google Scholar]
- Oasmaa, A.; Peacocke, C. A Guide to Physical Property Characterisation of Biomass-Derived Fast Pyrolysis Liquids; VTT Publications; Technical Research Centre of Finland: Espoo, Finland, 2010; pp. 2–65. [Google Scholar]
- Chiaramonti, D.; Oasmaa, A.; Solantausta, Y. Power generation using fast pyrolysis liquids from biomass. Renew. Sustain. Energy Rev. 2007, 11, 1056–1086. [Google Scholar] [CrossRef]
- Meier, D.; Van De Beld, B.; Bridgwater, A.V.; Elliott, D.C.; Oasmaa, A.; Preto, F. State-of-the-art of fast pyrolysis in IEA bioenergy member countries. Renew. Sustain. Energy Rev. 2013, 20, 619–641. [Google Scholar] [CrossRef]
- Harnisch, F.; Blei, I.; dos Santos, T.R.; Möller, M.; Nilges, P.; Eilts, P.; Schröder, U. From the test-tube to the test-engine: Assessing the suitability of prospective liquid biofuel compounds. RSC Adv. 2013, 3, 9594–9605. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Garcìa-Pérez, M.; Chaala, A.; Pakdel, H.; Kretschmer, D.; Rodrigue, D.; Roy, C. Multiphase Structure of Bio-oils. Energy Fuels 2006, 20, 364–375. [Google Scholar] [CrossRef]
- Makarfi Isa, Y.; Ganda, E.T. Bio-oil as a potential source of petroleum range fuels. Renew. Sustain. Energy Rev. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent developments and key barriers to advanced biofuels: A short review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef]
- Cepsa Global Energy Company Home Page. Available online: http://www.cepsa.com (accessed on 24 March 2020).
- Sanahuja-Parejo, O.; Veses, A.; López, J.M.; Murillo, R.; Callén, M.S.; García, T. Ca-based Catalysts for the Production of High-Quality Bio-Oils from the Catalytic Co-Pyrolysis of Grape Seeds and Waste Tyres. Catalysts 2019, 9, 992. [Google Scholar] [CrossRef]
- Martínez, J.D.; Rodríguez-Fernández, J.; Sánchez-Valdepeñas, J.; Murillo, R.; García, T. Performance and emissions of an automotive diesel engine using a tire pyrolysis liquid blend. Fuel 2014, 115, 490–499. [Google Scholar] [CrossRef]
- Bergthorson, J.M.; Thomson, M.J. A review of the combustion and emissions properties of advanced transportation biofuels and their impact on existing and future engines. Renew. Sustain. Energy Rev. 2015, 42, 1393–1417. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Jia, M.; Wei, Q.; Meng, X.; Shu, G. Combustion and particle number emissions of a direct injection spark ignition engine operating on ethanol/gasoline and n-butanol/gasoline blends with exhaust gas recirculation. Fuel 2014, 130, 177–188. [Google Scholar] [CrossRef]
- Deng, X.; Chen, Z.; Wang, X.; Zhen, H.; Xie, R. Exhaust noise, performance and emission characteristics of spark ignition engine fuelled with pure gasoline and hydrous ethanol gasoline blends. Case Stud. Therm. Eng. 2018, 12, 55–63. [Google Scholar] [CrossRef]
- Sakthivel, P.; Subramanian, K.A.; Mathai, R. Experimental study on unregulated emission characteristics of a two-wheeler with ethanol-gasoline blends (E0 to E50). Fuel 2020, 262, 116504. [Google Scholar] [CrossRef]
- World Health Organization. Environmental health criteria 229: Nitrogenated polycyclic aromatic hydrocarbons. In WHO Report Number. International Program on Chemical Safety; WHO, Ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- [ATSDR] Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs) (Update); US Department of Health and Human Services: Atlanta, GA, USA, 1995.
- Song, J.; Cheenkachorn, K.; Wang, J.; Perez, J.; Boehman, A.L.; Young, P.J.; Waller, F.J. Effect of Oxygenated Fuel on Combustion and Emissions in a Light-Duty Turbo Diesel Engine. Energy Fuels 2002, 16, 294–301. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Pitz, W.J.; Curran, H.J. Chemical Kinetic Modeling Study of the Effects of Oxygenated Hydrocarbons on Soot Emissions from Diesel Engines. J. Phys. Chem. A 2006, 110, 6912–6922. [Google Scholar] [CrossRef]
- Yinhui, W.; Rong, Z.; Yanhong, Q.; Jianfei, P.; Mengren, L.; Jianrong, L.; Yusheng, W.; Min, H.; Shijin, S. The impact of fuel compositions on the particulate emissions of direct injection gasoline engine. Fuel 2016, 166, 543–552. [Google Scholar] [CrossRef]
- European Commission. Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air. Off. J. Eur. Union 2004, L23, 3–16. [Google Scholar]
- Nisbet, I.C.T.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]



| Engine Type | Air-Cooled, 4-Stroke, OHV, 90 L V-twin |
|---|---|
| Bore × Stroke | 77 × 66 mm |
| Displacement | 614 cm3 |
| Compression Ratio | 8.3:1 |
| Net Power Output | 13.5 kW at 3600 rpm |
| Net Torque | 40.6 Nm at 2500 rpm |
| Number of cylinders | 2 |
| Nominal fuel consumption | 4.1 L/h |
| Dimensions (L × W × H) | 388 × 457 × 452 mm |
| Net Weight | 42 kg |
| Start Type | Electric, Automatic |
|---|---|
| Number of phases | 3 (380/220 V/V) |
| Type of generator | Synchronic |
| Active power | 9.6 kW |
| Total power | 12 kVA |
| Noise level | 77 dB |
| Dimensions (L × W × H) | 900 × 600 × 580 mm |
| Weight | 107 kg |
| Fuel | Ultimate Analysis (%.wt) | Calorific Value (MJ/kg) | Water Content (%.wt) | pH–25 °C | TAN (mgKOH/g) | Viscosity (cP)–40 °C | Density (kg/L)–25 °C | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | H | N | S | |||||||
| Crude bio-oils | 30–40 | 35–55 | 6–7 | 0.2–0.5 | 0.0 | 16–17 | 15–30 | 2–3 | 60–90 | 10–15 | 1.1–1.4 |
| Upgraded bio-oil | 75 | 16.4 | 8.4 | 0.2 | 0.0 | 32.5 | 4.7 | 4.0 | 21.3 | 9.4 | 1.15 |
| G100 | 87.2 | 0.0 | 12.9 | 0.0 | 0.01 | 43.6 | <0.1 | 2.9 | <5 | 0.12 | 0.75 |
| EtOH | 52.2 | 34.7 | 13.1 | 0.0 | 0.0 | 29.6 | 3.8 | 7.3 | <5 | 1.9 | 0.78 |
| G90E8B2 | 84.2 | 3.1 | 12.8 | 0.004 | 0.009 | 42.3 | 0.4 | 3.2 | <5 | 0.45 | 0.77 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veses, A.; Martínez, J.D.; Callén, M.S.; Murillo, R.; García, T. Application of Upgraded Drop-In Fuel Obtained from Biomass Pyrolysis in a Spark Ignition Engine. Energies 2020, 13, 2089. https://doi.org/10.3390/en13082089
Veses A, Martínez JD, Callén MS, Murillo R, García T. Application of Upgraded Drop-In Fuel Obtained from Biomass Pyrolysis in a Spark Ignition Engine. Energies. 2020; 13(8):2089. https://doi.org/10.3390/en13082089
Chicago/Turabian StyleVeses, Alberto, Juan Daniel Martínez, María Soledad Callén, Ramón Murillo, and Tomás García. 2020. "Application of Upgraded Drop-In Fuel Obtained from Biomass Pyrolysis in a Spark Ignition Engine" Energies 13, no. 8: 2089. https://doi.org/10.3390/en13082089
APA StyleVeses, A., Martínez, J. D., Callén, M. S., Murillo, R., & García, T. (2020). Application of Upgraded Drop-In Fuel Obtained from Biomass Pyrolysis in a Spark Ignition Engine. Energies, 13(8), 2089. https://doi.org/10.3390/en13082089






