Ethanol Production from High Solid Loading of Rice Straw by Simultaneous Saccharification and Fermentation in a Non-Conventional Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Preparation
2.2. Simultaneous Saccharification and Fermentation (SSF)
2.2.1. Enzymes and Microorganism
2.2.2. Effect of Process Variables
2.2.3. Effect of Solid Loading in Batch SSF
2.2.4. Fed-Batch Strategies
2.3. Parameters Calculation
2.4. Analysis
2.5. Statistical Analysis
3. Results and Discussion
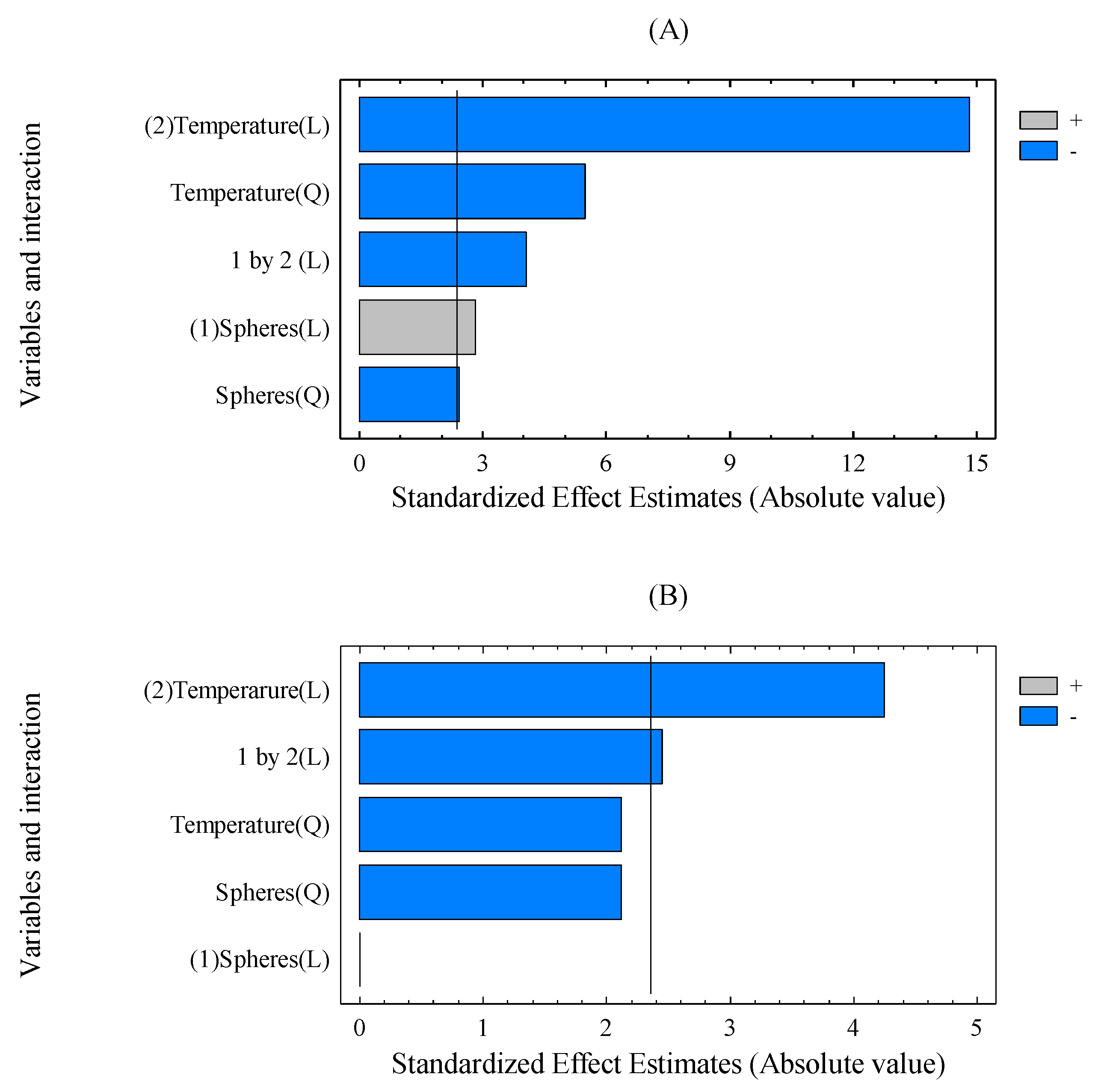
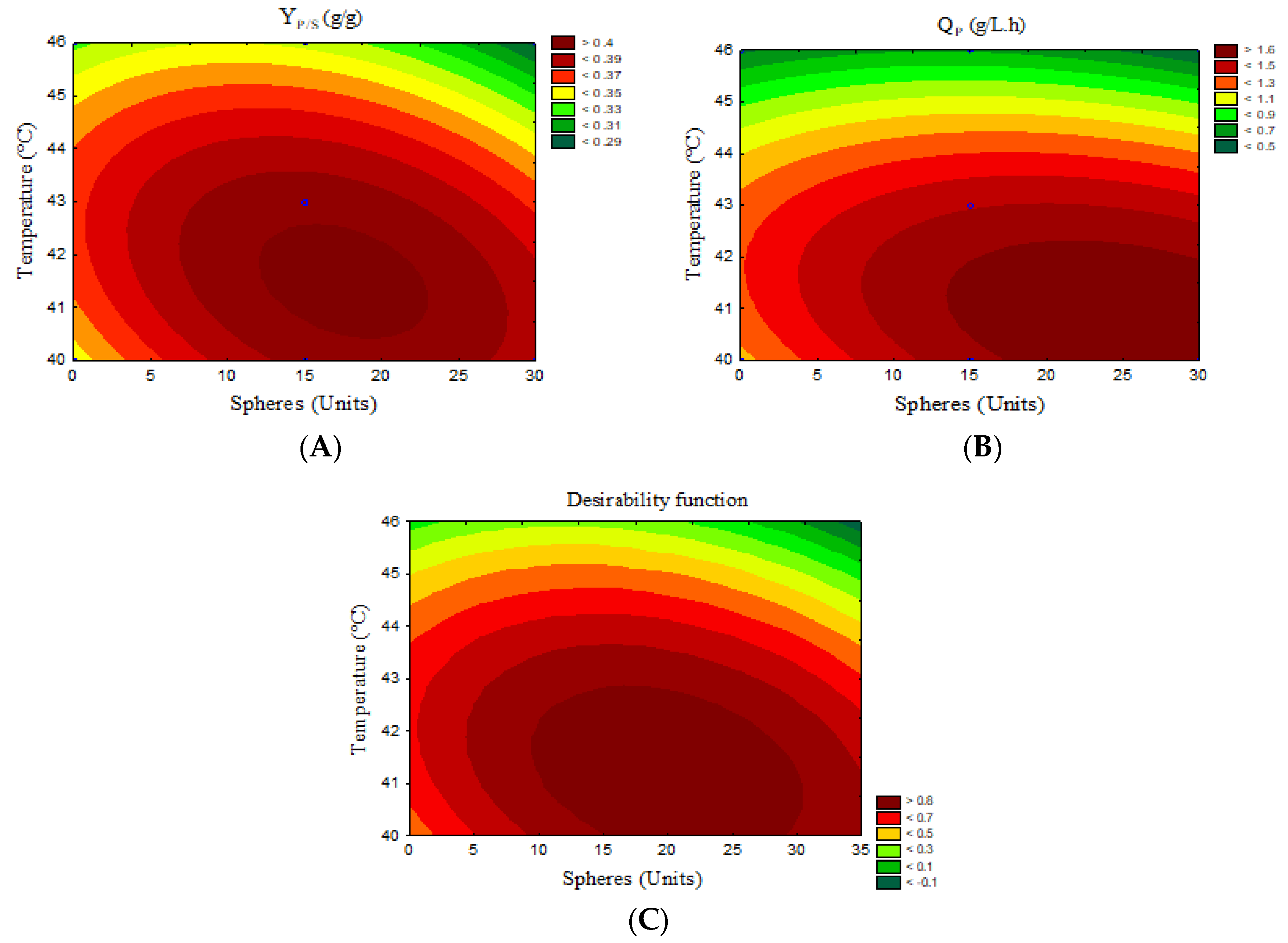
3.1. Effect of Operational Variables on SSF Process in the VBM Reactor
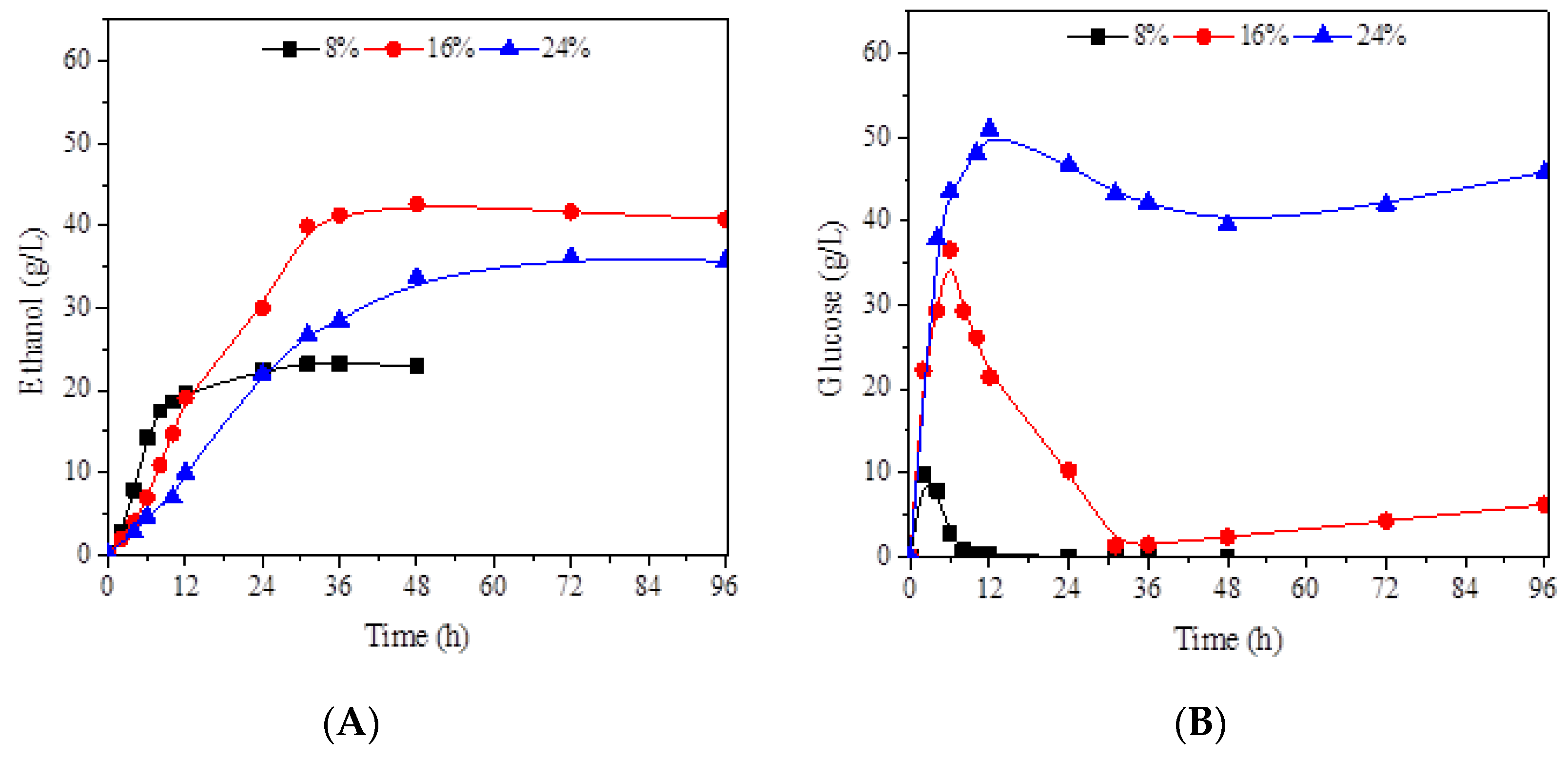
3.2. Effect of Solid Loading in Batch SSF
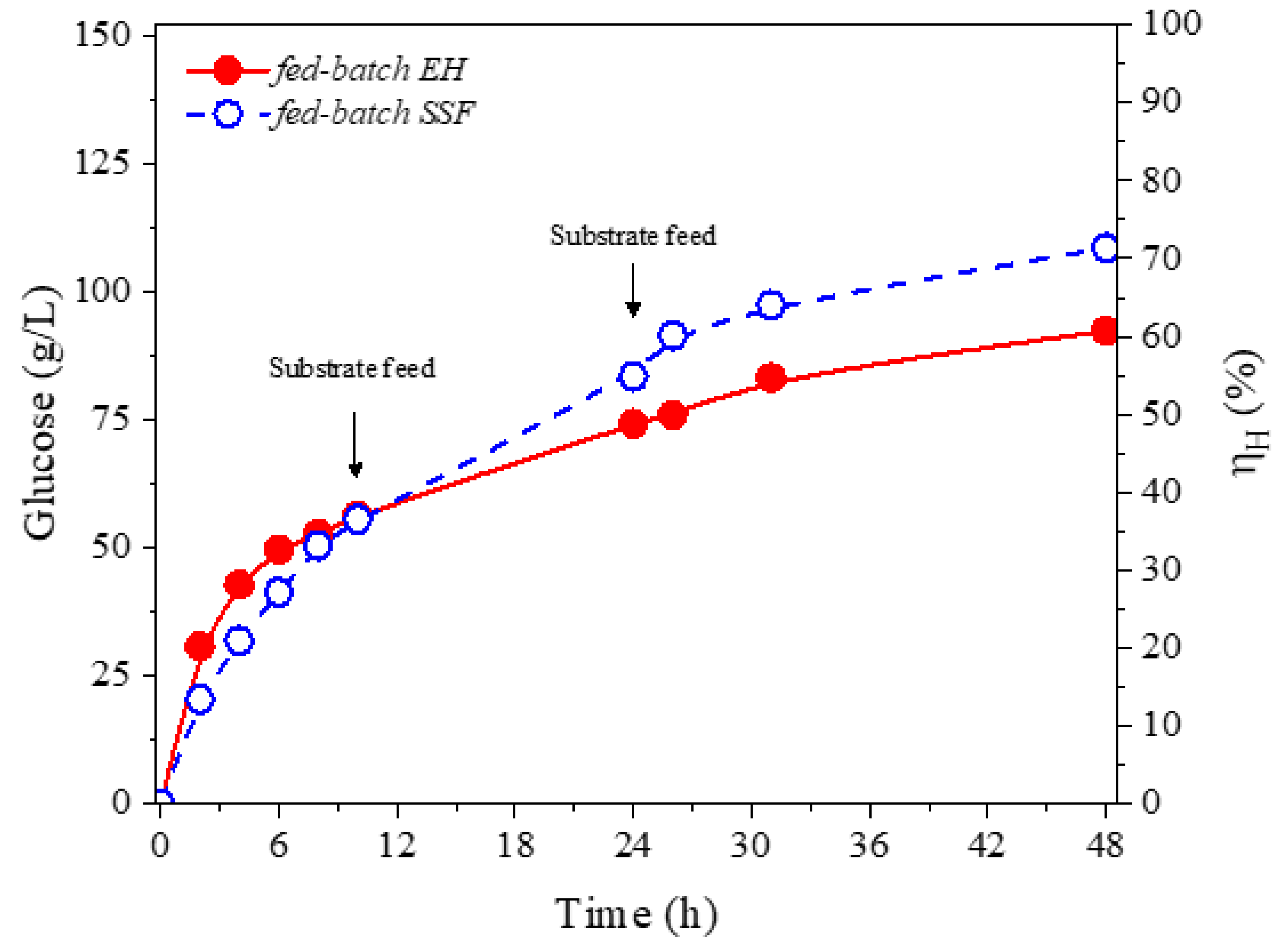
3.3. Evaluation of Fed-Batch Strategies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hughes, S.R.; Qureshi, N. Biofuel demand realization. In Biomass to Biofuels: Strategies for Global Industries; Vertès, A.A., Qureshi, N., Blaschek, H.P., Yukawa, H., Eds.; Wiley: Hoboken, NJ, USA, 2010; pp. 55–69. [Google Scholar]
- Saini, J.K.; Agrawal, R.; Satlewal, A.; Saini, R.; Gupta, R.; Mathur, A.; Tuli, D. Second generation bioethanol production at high gravity of pilot-Scale pretreated wheat straw employing newly isolated thermotolerant yeast Kluyveromyces marxianus DBTIOC-35. RSC Adv. 2015, 5, 37485–37494. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United States. FAO Cereal Supply and Demand Brief. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 7 April 2020).
- Mussatto, S.I. Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier Inc.: Waltham, MA, USA, 2016; p. 674. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Guimarães, P.M.R.; Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C.; Vicente, A.; Domingues, L.; Teixeira, J.A. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 2010, 28, 817–830. [Google Scholar] [CrossRef]
- Li, J.; Zhou, P.; Liu, H.; Xiong, C.; Lin, J.; Xiao, W.; Gong, Y.; Liu, Z. Synergism of cellulase, xylanase, and pectinase on hydrolyzing sugarcane bagasse resulting from different pretreatment technologies. Bioresour. Technol. 2014, 155, 258–265. [Google Scholar] [CrossRef]
- Di Risio, S.; Hu, C.S.; Saville, B.A.; Liao, D.; Lortie, J. Large-Scale, high-Solids enzymatic hydrolysis of steam-Exploded poplar. Biofuels Bioprod. Bioref. 2011, 5, 609–620. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.M. Biomass pretreatment, biorefineries and potential products for a bioeconomy development. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier Inc.: Waltham, MA, USA, 2016; pp. 1–22. [Google Scholar]
- Horn, S.J.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V.G.H. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 45. [Google Scholar] [CrossRef]
- Ha, S.-J.; Galazka, J.M.; Kim, S.R.; Choi, J.-H.; Yang, X.; Seo, J.-H.; Glass, N.L.; Cate, J.H.D.; Jin, Y.-S. Engineered Saccharomyces cerevisiae capable of simultaneous cellobiose and xylose fermentation. Proc. Natl. Acad. Sci. USA 2010, 108, 504–509. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Liu, Z.-H. Enzymatic hydrolysis of lignocellulosic biomass from low to high solids loading. Eng. Life Sci. 2017, 17, 489–499. [Google Scholar] [CrossRef]
- Du, J.; Cao, Y.; Liu, G.; Zhao, J.; Li, X.; Qu, Y. Identifying and overcoming the effect of mass transfer limitation on decreased yield in enzymatic hydrolysis of lignocellulose at high solid concentrations. Bioresour. Technol. 2017, 229, 88–95. [Google Scholar] [CrossRef]
- Kang, K.E.; Chung, D.-P.; Kim, Y.; Chung, B.-W.; Choi, G.-W. High-Titer ethanol production from simultaneous saccharification and fermentation using a continuous feeding system. Fuel 2015, 145, 18–24. [Google Scholar] [CrossRef]
- Nguyen, T.Y.; Cai, C.M.; Kumar, R.; Wyman, C.E. Overcoming factors limiting high-solids fermentation of lignocellulosic biomass to ethanol. Proc. Natl. Acad. Sci. USA 2017, 114, 11673–11678. [Google Scholar] [CrossRef]
- Jørgensen, H.; Vibe-Pedersen, J.; Larsen, J.; Felby, C. Liquefaction of lignocellulose at high-Solids concentrations. Biotechnol. Bioeng. 2007, 96, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Liguori, R.; Ventorino, V.; Pepe, O.; Faraco, V. Bioreactors for lignocellulose conversion into fermentable sugars for production of high added value products. Appl. Microbiol. Biotechnol. 2016, 100, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, F.; Su, R.; Qi, W.; He, Z. Ethanol production from high dry matter corncob using fed-Batch simultaneous saccharification and fermentation after combined pretreatment. Bioresour. Technol. 2010, 101, 4959–4964. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, L.; Zhang, J.; Bao, J. Helically agitated mixing in dry dilute acid pretreatment enhances the bioconversion of corn stover into ethanol. Biotechnol. Biofuels 2014, 7, 1. [Google Scholar] [CrossRef]
- Chen, M.L.; Wang, F.S. Optimization of a fed-Batch simultaneous saccharification and cofermentation process from lignocellulose to ethanol. Ind. Eng. Chem. Res. 2010, 49, 5775–5785. [Google Scholar] [CrossRef]
- Alfani, F.; Gallifuoco, A.; Saporosi, A.; Spera, A.; Cantarella, M. Comparison of SHF and SSF processes for the bioconversion of steam-Exploded wheat straw. J. Ind. Microbiol. Biotechnol. 2000, 25, 184–192. [Google Scholar] [CrossRef]
- Ballesteros, M.; Oliva, J.M.M.; Negro, M.J.J.; Manzanares, P.; Ballesteros, I. Ethanol from lignocellulosic materials by a simultaneous saccharification and fermentation process (SFS) with Kluyveromyces marxianus CECT 10875. Process Biochem. 2004, 39, 1843–1848. [Google Scholar] [CrossRef]
- Cannella, D.; Jørgensen, H. Do new cellulolytic enzyme preparations affect the industrial strategies for high solids lignocellulosic ethanol production? Biotechnol. Bioeng. 2014, 111, 59–68. [Google Scholar] [CrossRef]
- Castro, R.C.A.; Mussatto, S.I.; Roberto, I.C. A vertical ball mill as a new reactor design for biomass hydrolysis and fermentation process. Renew. Energy 2017, 114, 775–780. [Google Scholar] [CrossRef]
- Castro, R.C.A.; Fonseca, B.G.; Santos, H.T.L.; Ferreira, I.S.; Mussatto, S.I.; Roberto, I.C. Alkaline deacetylation as a strategy to improve sugars recovery and ethanol production from rice straw hemicellulose and cellulose. Ind. Crops Prod. 2017, 106, 65–73. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Adney, B.; Baker, J. Measurement of Cellulase Activities. Technical Report NREL/TP-510-42628; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Wood, T.M.; Bhat, K.M. Methods for measuring cellulase activities. In Methods Enzymology; Wood, W.A., Kellogg, S.T., Eds.; Academic Press: London, UK, 1998; pp. 87–112. [Google Scholar]
- Castro, R.C.A.; Roberto, I.C. Effect of nutrient supplementation on ethanol production in different strategies of saccharification and fermentation from acid pretreated rice straw. Biomass Bioenerg. 2015, 78, 156–163. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-Acid lignocellulosic hydrolyzates for use in fermentative processes-A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef]
- Varga, E.; Klinke, H.B.; Reczey, K.; Thomsen, A.B. High solid simultaneous saccharification and fermentation of wet oxidized corn stover to ethanol. Biotechnol. Bioeng. 2004, 88, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Koppram, R.; Olsson, L. Combined substrate, enzyme and yeast feed in simultaneous saccharification and fermentation allow bioethanol production from pretreated spruce biomass at high solids loadings. Biotechnol. Biofuels 2014, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.M.; Lavenson, D.M.; Tozzi, E.J.; McCarthy, M.J.; Jeoh, T. The effects of water interactions in cellulose suspensions on mass transfer and saccharification efficiency at high solids loadings. Cellulose 2011, 18, 759–773. [Google Scholar] [CrossRef]
- Unrean, P.; Khajeeram, S.; Laoteng, K. Systematic optimization of fed-Batch simultaneous saccharification and fermentation at high-Solid loading based on enzymatic hydrolysis and dynamic metabolic modeling of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2016, 100, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, W.; Bao, J. Reactors for high solid loading pretreatment of lignocellulosic biomass. Adv. Biochem. Eng. Biotechnol. 2015, 152, 75–90. [Google Scholar]
- Koppram, R.; Tomás-Pejó, E.; Xiros, C.; Olsson, L. Lignocellulosic ethanol production at high-Gravity: Challenges and perspectives. Trends Biotechnol. 2014, 32, 46–53. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, L.; Chen, L.; Liu, D. Batch and multi-Step fed-Batch enzymatic saccharification of Formiline-pretreated sugarcane bagasse at high solid loadings for high sugar and ethanol titers. Bioresour. Technol. 2013, 135, 350–356. [Google Scholar] [CrossRef]
- Wang, R.; Unrean, P.; Franzén, C.J. Model-Based optimization and scale-Up of multi-Feed simultaneous saccharification and co-Fermentation of steam pre-treated lignocellulose enables high gravity ethanol production. Biotechnol. Biofuels 2016, 9, 88. [Google Scholar] [CrossRef][Green Version]
- Gladis, A.; Bondesson, P.-M.; Galbe, M.; Zacchi, G. Influence of different SSF conditions on ethanol production from corn stover at high solids loadings. Energy Sci. Eng. 2015, 3, 481–489. [Google Scholar] [CrossRef]
- Vargas, F.; Domínguez, E.; Vila, C.; Rodríguez, A.; Garrote, G. Agricultural residue valorization using a hydrothermal process for second generation bioethanol and oligosaccharides production. Bioresour. Technol. 2015, 191, 263–270. [Google Scholar] [CrossRef] [PubMed]

| Component | Rice Straw in natura | Deacetylated Rice Straw | Cellulignin |
|---|---|---|---|
| Cellulose | 35.3 ± 0.2 | 43.5 ± 0.2 | 61.8 ± 0.7 |
| Hemicellulose | 23.8 ± 0.4 | 27.8 ± 0.5 | 11.1 ± 0.1 |
| Acetyl groups | 2.6 ± 0.4 | 0.07 ± 0.02 | 0.06 ± 0.01 |
| Total lignin | 17.5 ± 0.7 | 11.5 ± 1.1 | 17.1 ± 0.3 |
| Insoluble lignin | 13.1 ± 0.7 | 9.1 ± 0.4 | 16.2 ± 0.6 |
| Soluble lignin | 4.4 ± 0.2 | 2.3 ± 0.3 | 0.9 ± 0.1 |
| Ashes | 11.3 ± 0.1 | 3.8 ± 0.04 | 6.0 ± 0.1 |
| Independent Variable | Symbol | Range and Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Number of spheres (units) | X1 | 0 | 15 | 30 |
| Temperature (°C) | X2 | 40 | 43 | 46 |
| Parameters | Fed-Batch Strategies | ||
|---|---|---|---|
| FBS-1 | FBS-2 | FBS-3 | |
| Initial solid loading (%, w/v) | 8 | 16 | 16 |
| Feeding number (load %, w/v) | 2 (8) | 1 (8) | 2 (4) |
| Feeding time (h) | 16 and 36 | 24 | 10 and 24 |
| Total solids (%, w/v) | 24 | 24 | 24 |
| Cellubrix loading (FPU/gglucan) | 21.5 | 14.3 | 14.3 |
| Novozyme 188 loading (IU/gglucan) | 26.5 | 17.6 | 17.6 |
| Initial cell concentration (g/L) | 1.0 | 1.0 | 3.0 |
| Parameter | Symbol | Unit | Equation |
|---|---|---|---|
| Ethanol yield (g ethanol/g glucose available in biomass) | YP/S | g/g | |
| Fermentation efficiency (ethanol yield/theoretical ethanol yield) | ηF | % | |
| Ethanol volumetric productivity (ethanol concentration/time) | QP | g/L.h | |
| Hydrolysis efficiency (g glucose/g glucose available in biomass) | ηH | % |
| Run | Independent Variable | Responses | ||||
|---|---|---|---|---|---|---|
| Number of Spheres (Units) | Temperature (°C) | Maximum Ethanol (g/L) | QP (g/L.h) | YP/S (g/g) | Residual Glucose (g/L) | |
| 1 | 0 | 40 | 19.45 | 1.14 | 0.35 | 1.14 |
| 2 | 30 | 40 | 21.29 | 1.64 | 0.39 | 1.64 |
| 3 | 0 | 46 | 17.31 | 0.60 | 0.32 | 0.60 |
| 4 | 30 | 46 | 15.40 | 0.51 | 0.28 | 0.51 |
| 5 | 15 | 43 | 21.27 | 1.52 | 0.41 | 1.52 |
| 6 | 15 | 43 | 21.16 | 1.41 | 0.39 | 1.41 |
| 7 | 15 | 43 | 21.57 | 1.54 | 0.39 | 1.54 |
| 8 | 15 | 40 | 20.93 | 1.61 | 0.38 | 1.61 |
| 9 | 15 | 46 | 19.28 | 0.64 | 0.35 | 0.64 |
| Source | Ethanol Yield (YP/S) | Ethanol Volumetric Productivity (QP) | ||||||
|---|---|---|---|---|---|---|---|---|
| SS | df | F-Ratio | p-Value | SS | df | F-Ratio | p-Value | |
| Model | 0.0130 | 5 | 9.72 | 0.0452 a | 1.7600 | 5 | 66.41 | 0.0029 a |
| X1 | 0.0000 | 1 | 0.00 | 1.0000 | 0.0420 | 1 | 7.94 | 0.0668 |
| X2 | 0.0048 | 1 | 18.06 | 0.0239 a | 1.1616 | 1 | 219.5 | 0.0007 |
| X12 | 0.0012 | 1 | 4.50 | 0.1240 | 0.0310 | 1 | 5.86 | 0.0941 |
| X22 | 0.0012 | 1 | 4.51 | 0.1237 | 0.1599 | 1 | 30.21 | 0.0119 |
| X1·X2 | 0.0016 | 1 | 6.00 | 0.0917 | 0.0870 | 1 | 16.45 | 0.0270 |
| Residual | 0.0008 | 3 | 0.0159 | 3 | ||||
| Lack of Fit | 0.0005 | 1 | 4.00 | 0.1835 b | 0.0061 | 1 | 1.24 | 0.3814 b |
| Pure Error | 0.0003 | 2 | 0.0098 | 2 | ||||
| Total (Corr) | 0.0140 | 8 | 1.7730 | 8 | ||||
| R2 = 0.94 | R2 = 0.99 | |||||||
| Solid loading * (%) | EM (g/L) | QP (g/L.h) | GR (g/L) | |||
|---|---|---|---|---|---|---|
| 8 | 21.0 | 1.50 | 0.00 | 75 | 75 | 1.00 |
| 16 | 41.3 | 1.14 | 1.40 | 75 | 74 | 1.01 |
| 24 | 33.7 | 0.70 | 39.6 | 64 | 40 | 1.60 |
| SSF Experiments | EM (g/L) | QP (g/L.h) | GR (g/L) | |||
|---|---|---|---|---|---|---|
| Batch * | 33.7 | 0.70 | 39.6 | 64 | 40 | 1.60 |
| FBS-1 * | 36.7 | 0.80 | 32.2 | 63 | 44 | 1.43 |
| FBS-2 ** | 45.3 | 0.90 | 12.9 | 67 | 58 | 1.15 |
| FBS-3 ** | 52.3 | 1.10 | 6.0 | 71 | 67 | 1.06 |
| Feedstock | Pre-Treatment | Solids (% w/w) | Enzyme Load | Yeast Strain and Inoculum | Reactor Type | Operational Conditions | Fermentation Parameters | Ref. |
|---|---|---|---|---|---|---|---|---|
| Rice straw | Alkali/Acid | 19 | 14.3 FPU Cellubrix and 17.6 IU β-glucosidase Novozyme 188/g glucan | K. marxianus NRRL Y-6860 (3 g/L) | 1.7-L vertical ball mill reactor | 41.5 °C, 200 rpm, 48 h | EM = 52.3 g/L QP = 1.1 g/L.h ηF = 67% | This work |
| Spruce | Not informed | 20 | 7.5 FPU CellicCTec2/g solid | S. cerevisiae Thermosacc® (5 g/L) | 3.6-L reactor (Infors HT-Labfors) | 35 °C, 400 rpm, 96 h | EM = 40.0 g/L QP = 0.42 g/L.h ηF = 53% | [31] |
| Sugarcane bagasse | Acid-catalyzed steam | 22 | 15 FPU CellicCTec2/g solid | S. cerevisiae Thermosacc® (0.02 g cell/g solid) | 2-L reactor (Biostat B. Braum Biotech) | 35 °C, 500 rpm, 120 h | EM = 65.4 g/L QP = 0.45 g/L.h ηF = 85% | [33] |
| Sugarcane bagasse | Formalin | 20 | 10 FPU /g solid | S. cerevisiae CICC 31014 (10% v/v) | 150-mL Erlenmeyer flasks | 37–38 °C, 150 rpm, 120 h | EM = 80.0 g/L QP = 0.67 g/L.h ηF = 83% | [36] |
| Wheat straw | Acid-catalyzed steam | 20 | 10 FPU CellicCTec2/g solid | S. cerevisiae KE-6-12A (0.02 g/g solid) | 3.6-L reactor (Infors HT-Labfors) | 35 °C, 400 rpm, 72 h | EM = 57.3 g/L QP = 0.79 g/L.h ηF = 66% | [37] |
| Corn stover | Acid-catalyzed steam | 20 | 7.5 FPU CellicCTec2/g solid | S. cerevisiae dry baker yeas T(3 g/L) | 2-L reactor (Infors AG) | 35 °C, 144 h | EM = 53.4 g/L QP = 0.74 g/L.h ηF = 75% | [38] |
| Barley straw | Hydrothermal | 25 | 10 FPU Celluclast 1.5L/g solid and 5 IU Novozyme 188/FPU | S. cerevisiae CECT-1170 (10 mL) | 250-mL Erlenmeyer flasks | 35 °C, 120 rpm, 96 h | EM = 51.7 g/L QP = 0.54 g/L.h ηF = 77% | [39] |
| Corncob | Acid/Alkali | 25 | 22.8 FPU GC220/g glucan | S. cerevisiae (5.0 g/L) | 6-L reactor (Biostat B. Braum Biotech) | Pre-hydrolysis (50 °C, 24 h) Fermentation (37 °C, 96 h) | EM = 84.7 g/L QP = 0.70 g/L.h ηF = 79% | [17] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberto, I.C.; Castro, R.C.A.; Silva, J.P.A.; Mussatto, S.I. Ethanol Production from High Solid Loading of Rice Straw by Simultaneous Saccharification and Fermentation in a Non-Conventional Reactor. Energies 2020, 13, 2090. https://doi.org/10.3390/en13082090
Roberto IC, Castro RCA, Silva JPA, Mussatto SI. Ethanol Production from High Solid Loading of Rice Straw by Simultaneous Saccharification and Fermentation in a Non-Conventional Reactor. Energies. 2020; 13(8):2090. https://doi.org/10.3390/en13082090
Chicago/Turabian StyleRoberto, Inês C., Rafael C. A. Castro, João Paulo A. Silva, and Solange I. Mussatto. 2020. "Ethanol Production from High Solid Loading of Rice Straw by Simultaneous Saccharification and Fermentation in a Non-Conventional Reactor" Energies 13, no. 8: 2090. https://doi.org/10.3390/en13082090
APA StyleRoberto, I. C., Castro, R. C. A., Silva, J. P. A., & Mussatto, S. I. (2020). Ethanol Production from High Solid Loading of Rice Straw by Simultaneous Saccharification and Fermentation in a Non-Conventional Reactor. Energies, 13(8), 2090. https://doi.org/10.3390/en13082090







