Orthogonal Reference Surrogate Fuels for Operability Testing
Abstract
1. Introduction
2. Materials and Methods
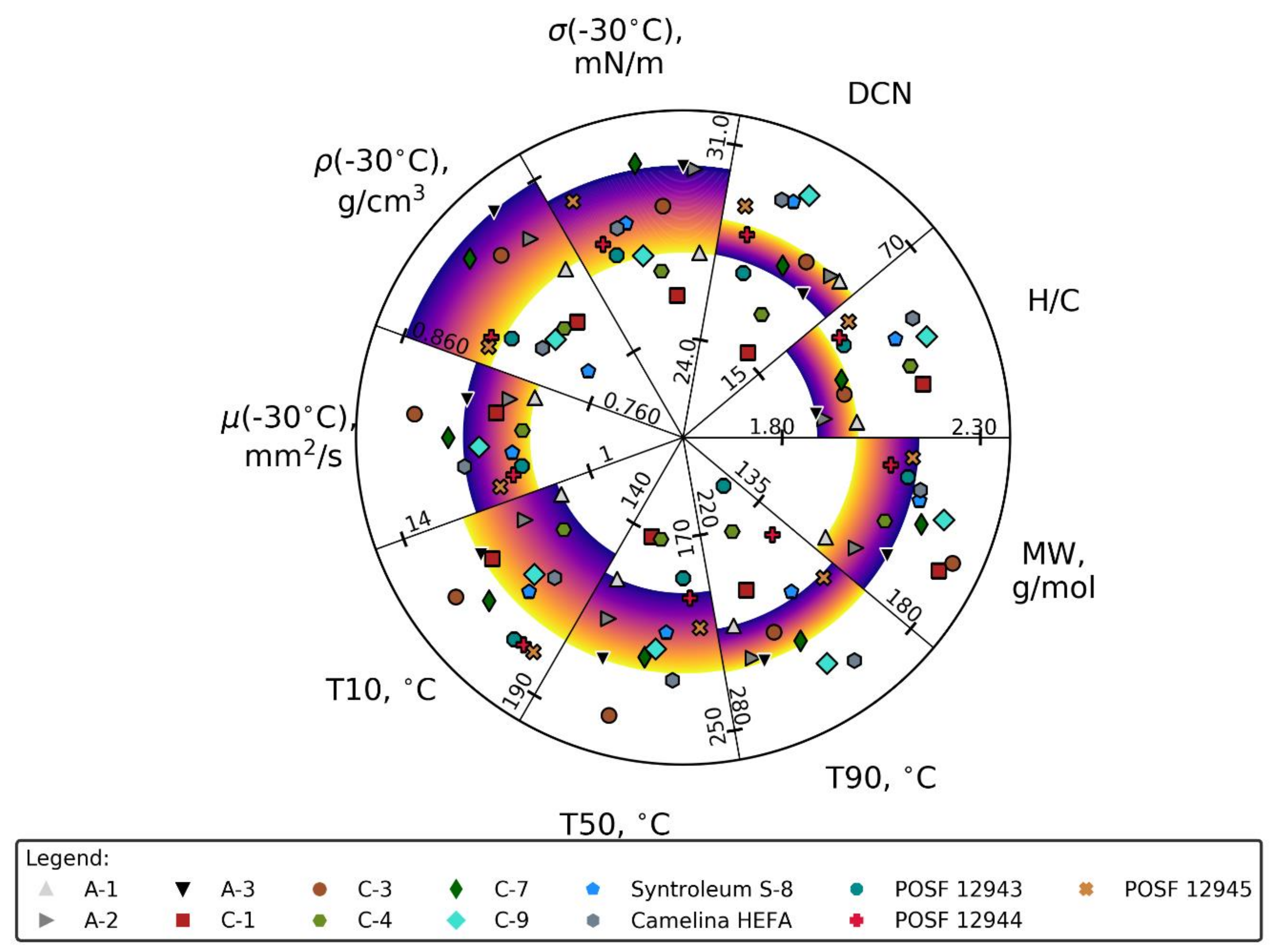
2.1. Materials
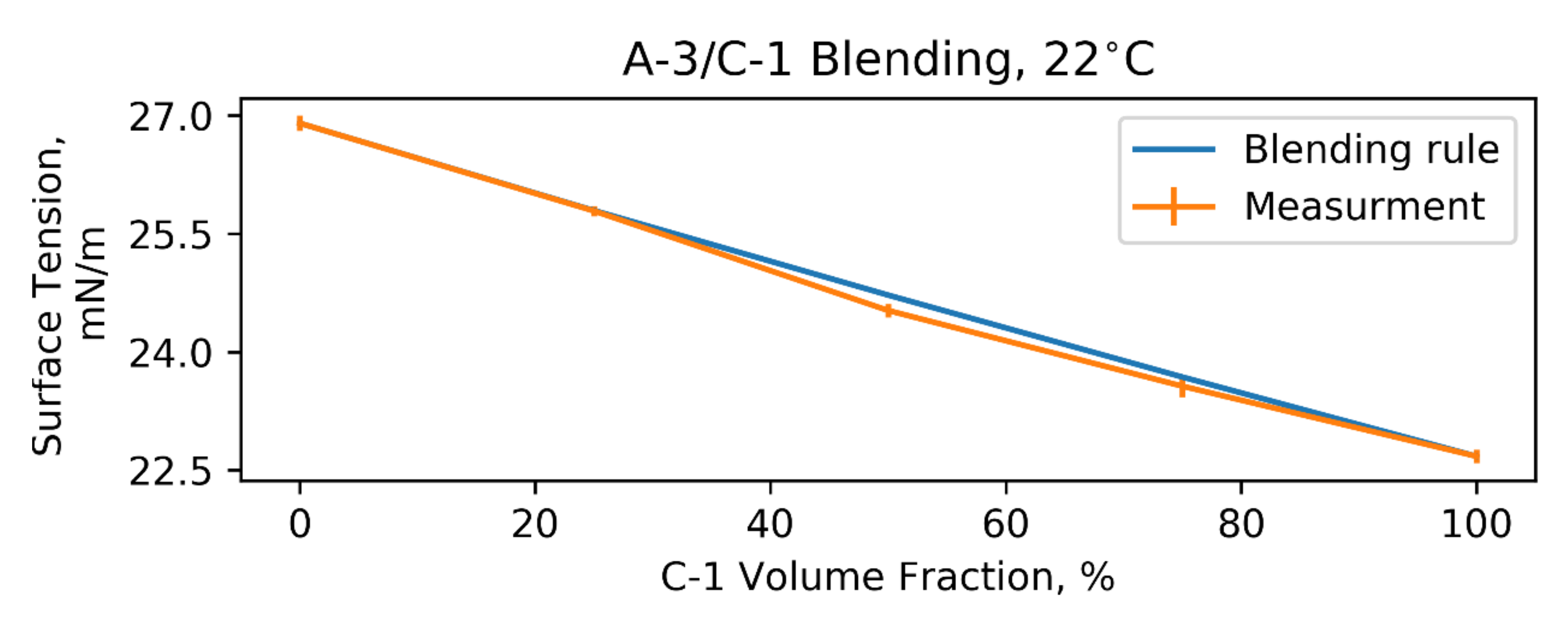
2.2. Blending Rule
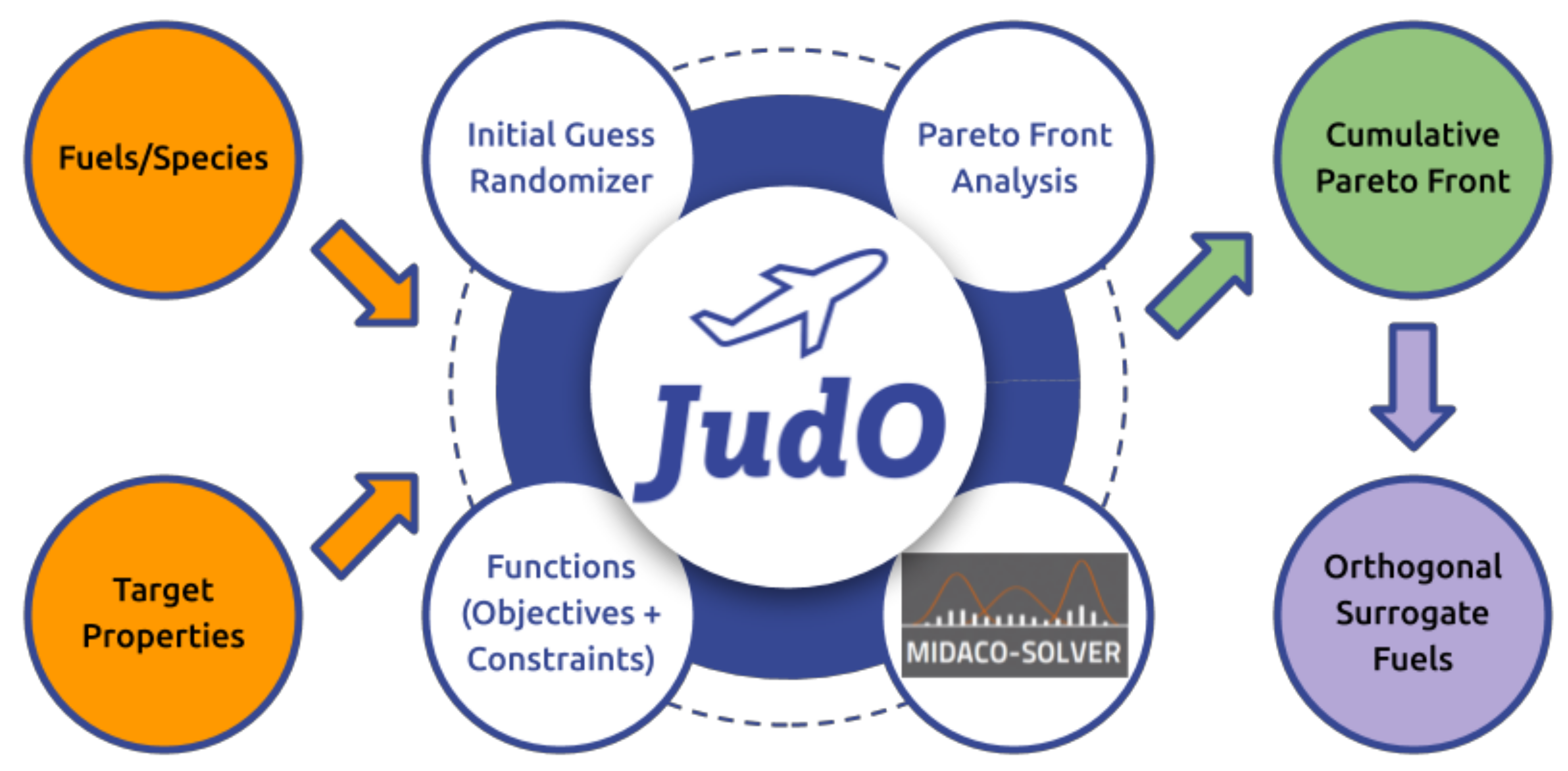
2.3. Jet Fuel Blend Optimizer (JudO)
2.4. Orthogonal Array Testing
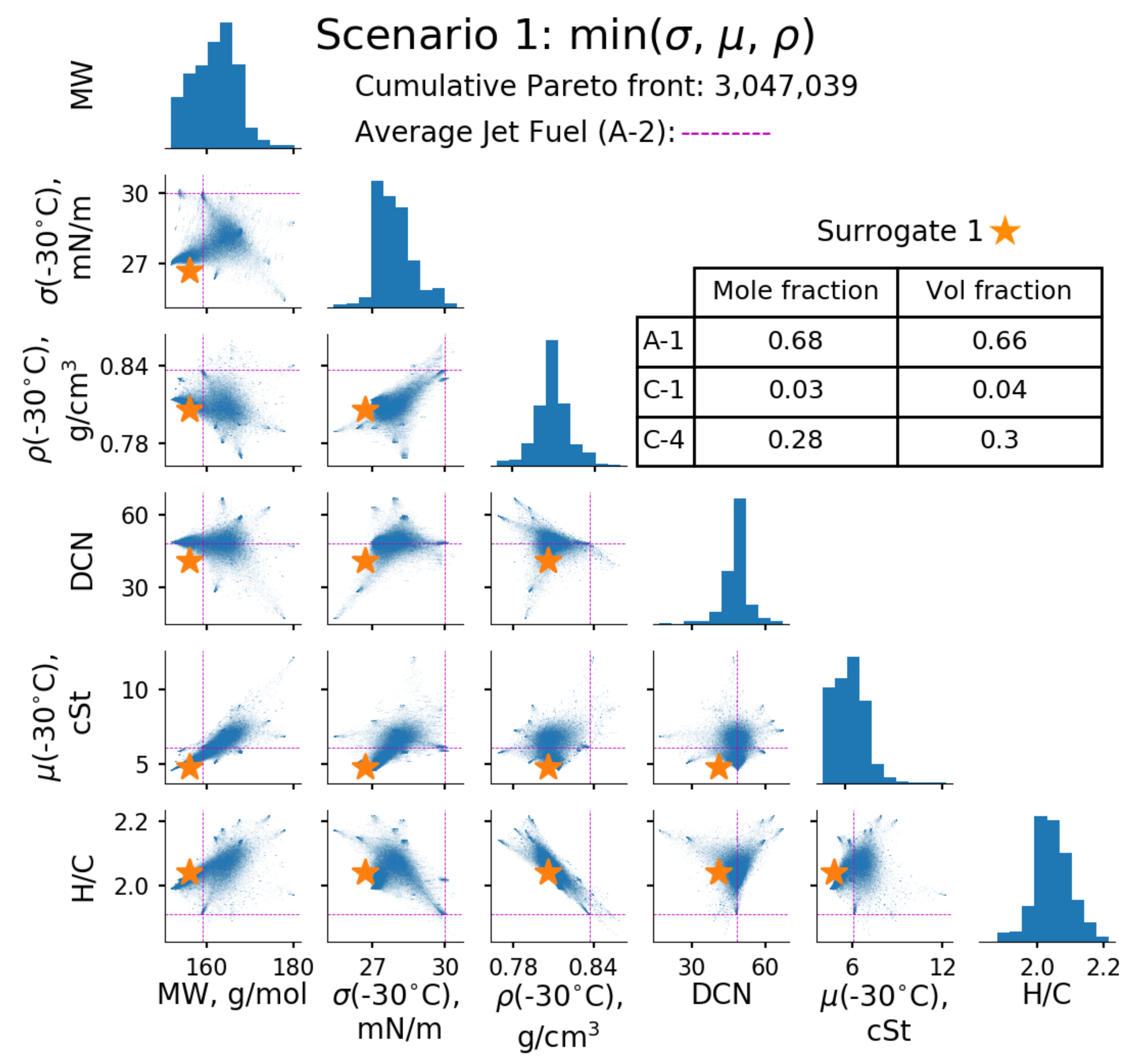
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APU | Auxiliary Power Unit |
| CORSIA | Carbon Offsetting and Reduction Scheme for International Aviation |
| DCN | Derived Cetane Number (ASTM D6890) |
| FOM | Figure of Merit |
| GHG | Greenhouse Gas |
| H/C | Atomic Hydrogen-to-Carbon Ratio |
| ICAO | International Civil Aviation Organization |
| JudO | Jet Fuel Blend Optimizer |
| LBO | Lean Blowout |
| MIDACO | Mixed Integer Distributed Ant Colony Optimization |
| MW | Molecular Weight, g/mol |
| NJFCP | National Jet Fuels Combustion Program |
| RFR | Random Forest Regression |
| SAF | Sustainable Aviation Fuel |
Appendix A
| Fuel, POSF | MW | H/C | DCN | Surface Tension −30 °C * (), mN/m | Viscosity −30 °C * (), | Density −30 °C * (), kg/m3 | T10, °C | T50, °C | T90, °C |
|---|---|---|---|---|---|---|---|---|---|
| A-1, 10264 | 152 | 1.99 | 48.8 | 27.1 | 4.6 | 0.814 | 149 | 193 | 249 |
| A-2, 10325 | 159 | 1.91 | 48.3 | 30.0 | 6.1 | 0.837 | 160 | 209 | 260 |
| A-3, 10289 | 166 | 1.89 | 39.2 | 30.1 | 8.9 | 0.859 | 174 | 225 | 262 |
| C-1, 11498 | 178 | 2.17 | 17.1 | 25.5 | 6.9 | 0.789 | 172 | 172 | 240 |
| C-3, 12341 | 180 | 1.97 | 47.0 | 28.7 | 12.2 | 0.840 | 185 | 246 | 255 |
| C-4, 12344 | 162 | 2.15 | 28.0 | 26.4 | 5.1 | 0.792 | 153 | 172 | 222 |
| C-7, 12973 | 170 | 1.98 | 42.6 | 30.3 | 9.9 | 0.851 | 179 | 220 | 261 |
| C-9, 12968 | 174 | 2.22 | 63.3 | 27.1 | 7.9 | 0.791 | 166 | 216 | 271 |
| Syntroleum S-8, 5018 | 168 | 2.14 | 59.7 | 28.3 | 5.7 | 0.769 | 170 | 209 | 247 |
| Camelina HEFA, 7720 | 168 | 2.20 | 58.9 | 28.3 | 9.0 | 0.794 | 163 | 228 | 275 |
| 12943 | 164 | 2.02 | 36.1 | 27.4 | 5.3 | 0.810 | 181 | 187 | 209 |
| 12944 | 160 | 2.02 | 46.4 | 27.9 | 5.9 | 0.819 | 181 | 195 | 230 |
| 12945 | 165 | 2.01 | 54.0 | 29.7 | 6.9 | 0.818 | 181 | 207 | 250 |
References
- Neumann, B.; Vafeidis, A.T.; Zimmermann, J.; Nicholls, R.J. Future Coastal Population Growth and Exposure to Sea-Level Rise and Coastal Flooding—A Global Assessment. PLoS ONE 2015, 10, e0118571. [Google Scholar] [CrossRef] [PubMed]
- Office of Transportation and Air Quality Fast Facts, U.S. Transportation Sector Greenhouse Gas Emissions 1990–2017; Office of Transportation and Air Quality Fast Facts U.S.: Washington, DC, USA, 2019. [Google Scholar]
- NASA. NASA Update FAA ASCENT Meeting; NASA: Washington, DC, USA, 2018. [Google Scholar]
- IATA. Carbon Offsetting and Reduction Scheme for International Aviation (CORSIA): Fact Sheet; IATA: Montreal, QC, Canada, 2016. [Google Scholar]
- Chao, H.; Buyung, D.; Delaurentis, D.; Stechel, E.B. Carbon off setting and reduction scheme with sustainable aviation fuel options: Fleet-level carbon emissions impacts for U.S. airlines. Transp. Res. Part D 2020, 75, 42–56. [Google Scholar] [CrossRef]
- Rock, N.; Emerson, B.; Seitzman, J.; Lieuwen, T. Liquid Fuel Property Effects on Lean Blowout in an Aircraft Relevant Combustor. J. Eng. Gas Turbines Power 2019, 141, 1–13. [Google Scholar] [CrossRef]
- Lefebvre, A.H. Fuel Effects On Gas Turbine Combustion. Int. J. Turbo Jet Engines 2004, 3, 3. [Google Scholar] [CrossRef]
- Stachler, R.; Heyne, J.; Stouffer, S.; Miller, J. Lean Blowoff in a Toroidal Jet-Stirred Reactor: Implications for Alternative Fuel Approval and Potential Mechanisms for Autoignition and Extinction. Energy Fuels 2020. [Google Scholar] [CrossRef]
- Rumizen, M. D4054 Clearinghouse 2018; CAAFI(Commercial Aviation Alternative Fuels Initiative): Washington, DC, USA, 2018. [Google Scholar]
- Colket, M.; Heyne, J.; Rumizen, M.; Gupta, M.; Edwards, T.; Roquemore, W.M.; Andac, G.; Boehm, R.; Lovett, J.; Williams, R.; et al. Overview of the National Jet Fuels Combustion Program. AIAA J. 2017, 55, 1087–1104. [Google Scholar] [CrossRef]
- Heyne, J.S.; Colket, M.B.; Gupta, M.; Jardines, A.; Moder, J.P.; Edwards, J.T.; Roquemore, M.; Li, C.; Rumizen, M. Year 2 of the National Jet Fuels Combustion Program: Towards a Streamlined Alternative Jet Fuels Certification Process. In Proceedings of the 55th AIAA Aerospace Sciences Meeting, Grapevine, TX, USA, 9–13 January 2017; pp. 1–14. [Google Scholar]
- Heyne, J.S.; Peiffer, E.; Colket, M.B.; Jardines, A.; Shaw, C.; Moder, J.P.; Roquemore, W.M.; Edwards, J.T.; Li, C.; Rumizen, M.; et al. Year 3 of the National Jet Fuels Combustion Program: Practical and Scientific Impacts of Alternative Jet Fuel Research. In Proceedings of the 2018 AIAA Aerospace Sciences Meeting, Kissimmee, FL, USA, 8–10 January 2018. [Google Scholar]
- Peiffer, E.E.; Heyne, J.S. Characteristic Timescales for Lean Blowout of Alternative Jet Fuels in Four Combustor Rigs. In Proceedings of the 2018 Joint Propulsion Conference, Cincinnati, OH, USA, 9–11 July 2018. [Google Scholar]
- Peiffer, E.E.; Heyne, J.S.; Colket, M. Sustainable Aviation Fuels Approval Streamlining: Auxiliary Power Unit Lean Blowout Testing. AIAA J. 2019, 57, 4854–4862. [Google Scholar] [CrossRef]
- Opacich, K.C.; Heyne, J.S.; Peiffer, E.; Stouffer, S.D. Analyzing the Relative Impact of Spray and Volatile Fuel Properties on Gas Turbine Combustor Ignition in Multiple Rig Geometries. AIAA Sci. Technol. Forum 2019, 1, 1434. [Google Scholar]
- Dooley, S.; Won, S.H.; Chaos, M.; Heyne, J.; Ju, Y.; Dryer, F.L.; Kumar, K.; Sung, C.J.; Wang, H.; Oehlschlaeger, M.A.; et al. A jet fuel surrogate formulated by real fuel properties. Combust. Flame 2010, 157, 2333–2339. [Google Scholar] [CrossRef]
- Kim, D.; Martz, J.; Violi, A. A surrogate for emulating the physical and chemical properties of conventional jet fuel. Combust. Flame 2014, 161, 1489–1498. [Google Scholar] [CrossRef]
- Kim, D.; Martz, J.; Abdul-Nour, A.; Yu, X.; Jansons, M.; Violi, A. A six-component surrogate for emulating the physical and chemical characteristics of conventional and alternative jet fuels and their blends. Combust. Flame 2017, 179, 86–94. [Google Scholar] [CrossRef]
- Bell, D.; Heyne, J.S.; Won, S.H.; Dryer, F.; Haas, F.M.; Dooley, S. On the Development of General Surrogate Composition Calculations for Chemical and Physical Properties. In Proceedings of the 55th AIAA Aerospace Sciences Meeting, Grapevine, TX, USA, 9–13 January 2017; pp. 1–5. [Google Scholar]
- Ruan, H.; Qin, Y.; Heyne, J.; Gieleciak, R.; Feng, M.; Yang, B. Chemical compositions and properties of lignin-based jet fuel range hydrocarbons. Fuel 2019, 256, 115947. [Google Scholar] [CrossRef]
- Edwards, J.T.; Shafer, L.M.; Klein, J.K. U.S. Air Force Hydroprocessed Renewable Jet (HRJ) Fuel Research; Air Force Research Laboratory: Dayton, OH, USA, 2013. [Google Scholar]
- Edwards, T. Reference Jet Fuels for Combustion Testing. In Proceedings of the 55th AIAA Aerospace Sciences Meeting, Grapevine, TX, USA, 9–13 January 2017; pp. 1–58. [Google Scholar]
- Poling, B.; Prausnitz, J.; O’Connell, J. The Properties of Gases and Gas Mixtures; McGraw-Hill: New York, NY, USA, 2000; ISBN 0071499997. [Google Scholar]
- Weinaug, C.F.; Katz, D.L. Surface Tensions of Methane-Propane Mixtures. Ind. Eng. Chem. 1943, 35, 239–246. [Google Scholar] [CrossRef]
- Kosir, S.T.; Behnke, L.; Heyne, J.S.; Zabarnick, S.; Flora, G.; Denney, R.K.; Tai, J.; Gupta, M. Improvement in Jet Aircraft Operation with the Use of High-Performance Alternative Drop-in Fuels in Conventional Fuels. AIAA SciTech Forum 2019, 1, 0993. [Google Scholar]
- Flora, G.; Kosir, S.; Heyne, J.; Zabarnik, S.; Grupta, M. Properties Calculator and Optimization for Drop-in Alternative Jet Fuel Blends. AIAA SciTech Forum 2019, 1, 2368. [Google Scholar]
- Schlueter, M.; Erb, S.O.; Gerdts, M.; Kemble, S. MIDACO on MINLP Space Applications. Adv. Space Res. 2013, 51, 1116–1131. [Google Scholar] [CrossRef]
- Lagergren, E.S.; Filliben, J.J. TaguchVs Orthogonal Arrays Are Classical Designs of Experiments. J. Res. Natl. Inst. Stand. Technol. 1991, 96, 577. [Google Scholar]
- TSUI, K.-L. An Overview of Taguchi Method and Newly Developed Statistical Methods for Robust Design. IIE Trans. 1992, 24, 44–57. [Google Scholar] [CrossRef]
- Heyne, J.S.; Opacich, K.C.; Peiffer, E.E.; Colket, M. The effect of chemical and physical fuel properties on the approval and evaluation of alternative jet fuels. In Proceedings of the 11th U.S. National Combustion Meeting, Combustion Institute, Pasadena, CA, USA, 24–27 March 2019. [Google Scholar]
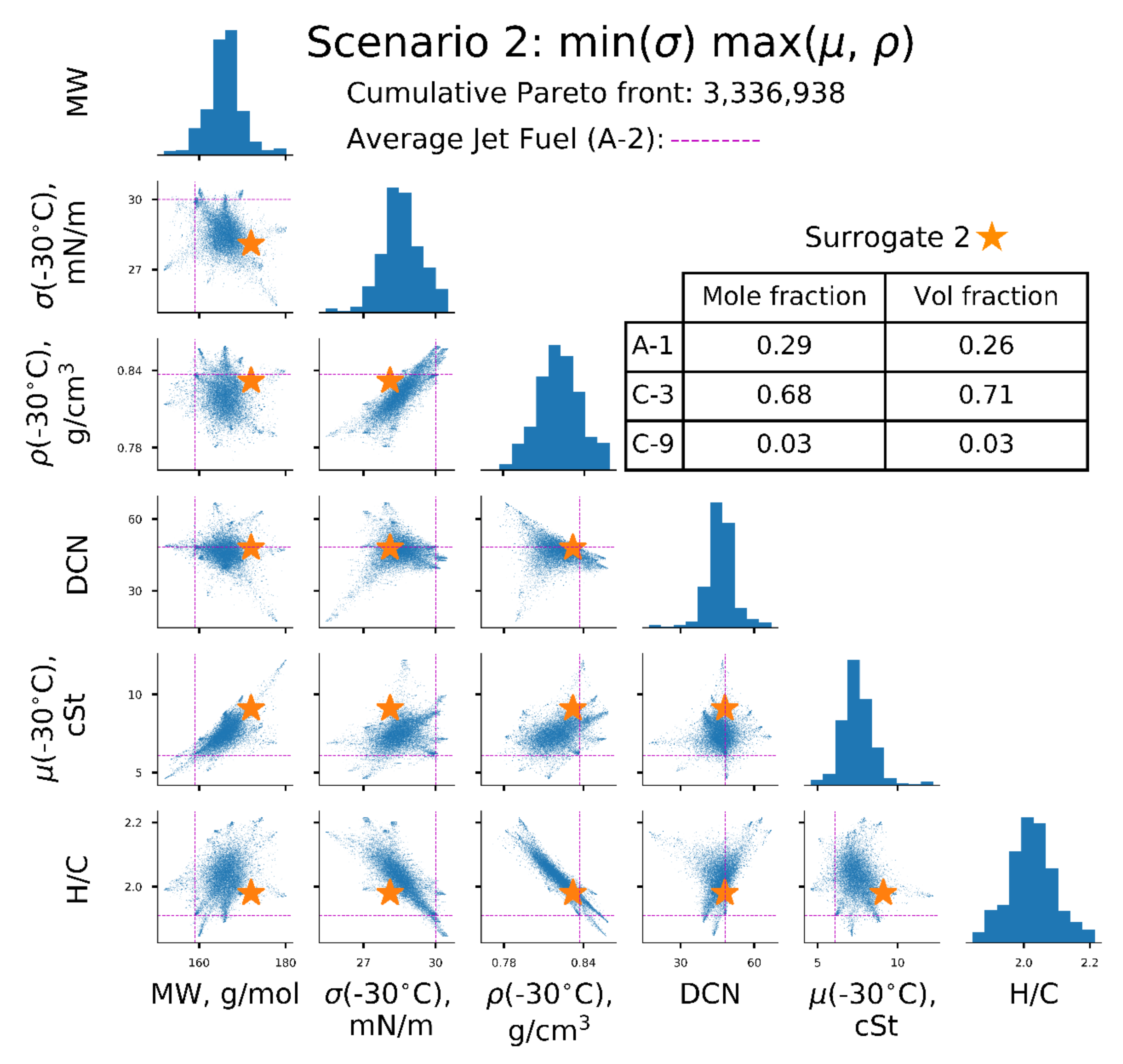
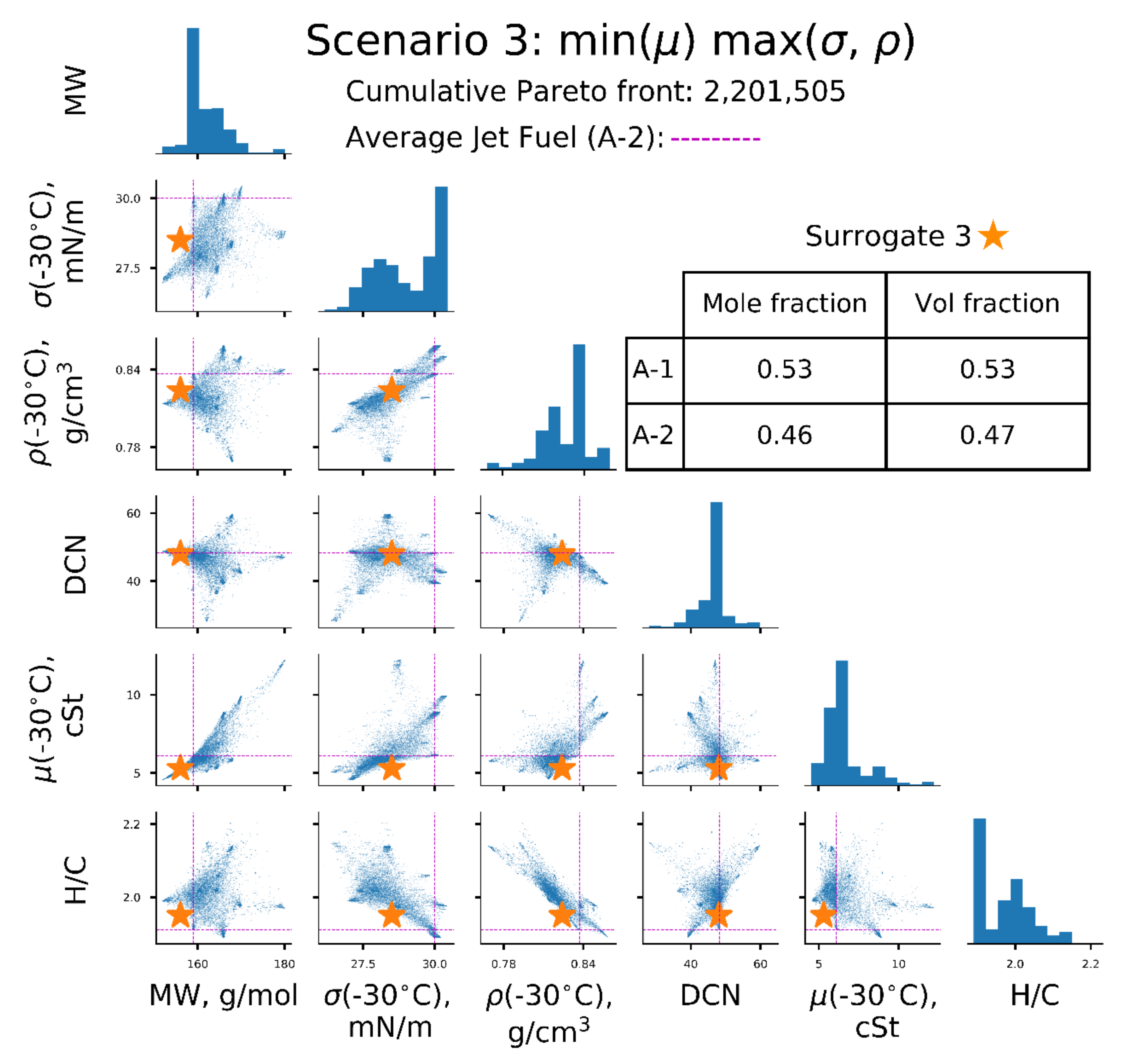
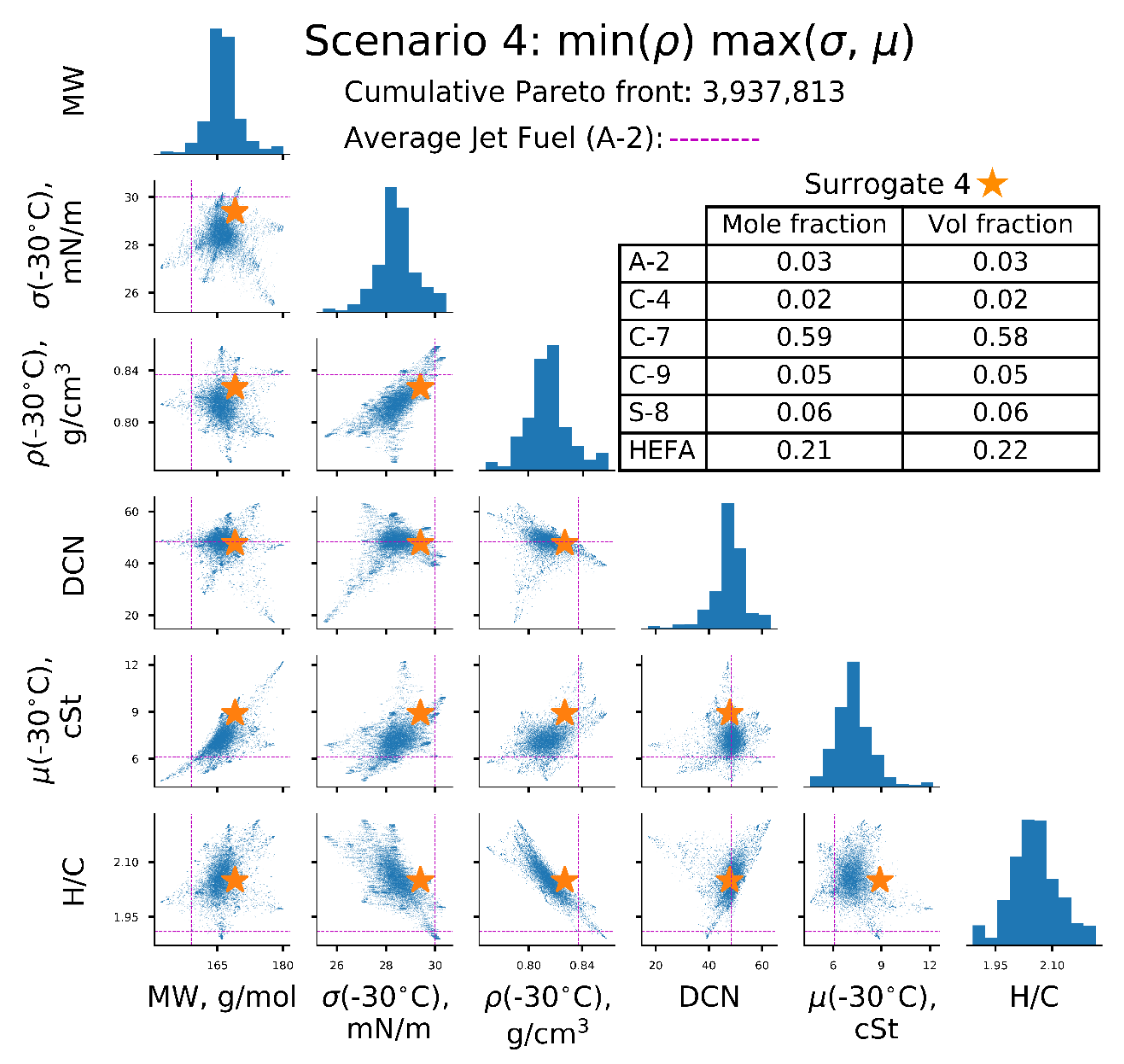
| Scenarios | σ (−30 °C), mN/m | µ (−30 °C), | ρ(−30 °C), kg/L | Surrogate |
|---|---|---|---|---|
| Reference | 30.0 | 6.1 | 0.837 | A-2 |
| [1] | ↓ | ↓ | ↓ | 1 |
| [2] | ↓ | ↑ | ↑ | 2 |
| [3] | ↑ | ↓ | ↑ | 3 |
| [4] | ↑ | ↑ | ↓ | 4 |
| Scenario | |||||
|---|---|---|---|---|---|
| Property | A-2 Reference | Surrogate 1 min (σ, µ, ρ) | Surrogate 2 min (σ), max (µ, ρ) | Surrogate 3 min (µ), max (σ, ρ) | Surrogate 4 min(ρ), max (σ, µ) |
| MW, g/mol | 159 | 156 | 172 | 156 | 169 |
| σ (−30 °C), mN/m | 30.0 | 26.7 | 28.1 | 28.5 | 29.4 |
| ρ (−30 °C), kg/L | 0.837 | 0.806/ 0.806 * | 0.832/ 0.832 * | 0.824/ 0.824 * | 0.827/ 0.826 * |
| µ (−30 °C), | 6.1 | 4.8/ 4.5 * | 9.1/ 10.3 * | 5.3/ 5.2 * | 8.9/ 8.5 * |
| DCN | 48 | 41 | 48 | 48 | 48 |
| H/C | 1.91 | 2.04 | 1.98 | 1.95 | 2.05 |
| T10, °C | 160 | 151 | 175 | 154 | 173 |
| T50, °C | 209 | 186 | 231 | 201 | 218 |
| T90, °C | 260 | 241 | 254 | 254 | 262 |
| Mole fraction | |||||
| A-1 | 0.68 | 0.29 | 0.53 | ||
| A-2 | 1.00 | 0.46 | 0.03 | ||
| C-1 | 0.03 | ||||
| C-3 | 0.68 | ||||
| C-4 | 0.28 | 0.02 | |||
| C-7 | 0.59 | ||||
| C-9 | 0.03 | 0.05 | |||
| S-8 | 0.06 | ||||
| HEFA | 0.21 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Stachler, R.; Heyne, J.S. Orthogonal Reference Surrogate Fuels for Operability Testing. Energies 2020, 13, 1948. https://doi.org/10.3390/en13081948
Yang Z, Stachler R, Heyne JS. Orthogonal Reference Surrogate Fuels for Operability Testing. Energies. 2020; 13(8):1948. https://doi.org/10.3390/en13081948
Chicago/Turabian StyleYang, Zhibin, Robert Stachler, and Joshua S. Heyne. 2020. "Orthogonal Reference Surrogate Fuels for Operability Testing" Energies 13, no. 8: 1948. https://doi.org/10.3390/en13081948
APA StyleYang, Z., Stachler, R., & Heyne, J. S. (2020). Orthogonal Reference Surrogate Fuels for Operability Testing. Energies, 13(8), 1948. https://doi.org/10.3390/en13081948






