Wet Chemical Oxidation to Improve Interfacial Properties of Al2O3/Si and Interface Analysis of Al2O3/SiOx/Si Structure Using Surface Carrier Lifetime Simulation and Capacitance–Voltage Measurement
Abstract
1. Introduction
2. Experimental
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- King, R.R.; Sinton, R.A. Studies of diffused phosphorus emitters: Saturation current, surface recombination velocity, and quantum efficiency. IEEE Trans. Electron. Dev. 1990, 37, 365–371. [Google Scholar] [CrossRef]
- Cuevas, A.; Basore, P.A.; Giroult-Matlakowski, G.; Dubois, C. Surface recombination velocity of highly doped n-type silicon. J. Appl. Phys. 1996, 80, 3370–3375. [Google Scholar] [CrossRef]
- Blakers, A.W.; Wang, A.; Milne, A.M.; Zhao, J.; Green, M.A. 22.8% efficient silicon solar cell. Appl. Phys. Lett. 1989, 55, 1363–1365. [Google Scholar] [CrossRef]
- Sterk, S.; Glunz, S.W.; Knobloch, J.; Wettling, W. High efficiency (>22%) Si-solar cells with optimized emitter. In Proceedings of the IEEE 1st World Conference on Photovoltaic Energy Conversion, Waikoloa, HI, USA, 5–9 December 1994. [Google Scholar]
- Zhang, S.; Yao, Y.; Hu, D.; Lian, W.; Qian, H.; Jie, J.; Wei, Q.; Ni, Z.; Zhang, X.; Xie, L. Application of silicon oxide on high efficiency monocrystalline silicon PERC solar cells. Energies 2019, 12, 1168. [Google Scholar] [CrossRef]
- Gong, L.; Zhou, C.; Zhu, J.; Wang, W. Passivation characteristics of new silicon oxide. IEEE J. Photovolt. 2019, 9, 1873–1879. [Google Scholar] [CrossRef]
- Aberle, A.G.; Lauinger, T.; Schmidt, J.; Hezel, R. Injection-level dependent surface recombination velocities at the silicon-plasma silicon nitride interface. Appl. Phys. Lett. 1995, 66, 2828–2830. [Google Scholar] [CrossRef]
- Schmidt, J.; Aberle, A.G. Carrier recombination at silicon–silicon nitride interfaces fabricated by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 1999, 85, 3626–3633. [Google Scholar] [CrossRef]
- Lelièvre, J.-F.; Kafle, B.; Saint-Cast, P.; Brunet, P.; Magnan, R.; Hernandez, E.; Pouliquen, S.; Massines, F. Efficient silicon nitride SiNx:H antireflective and passivation layers deposited by atmospheric pressure PECVD for silicon solar cells. Prog. Photovolt. Res. Appl. 2020, 27, 1007–1019. [Google Scholar] [CrossRef]
- Hoex, B.; Schmidt, J.; Bock, R.; Altermatt, P.P.; Van de Sanden, M.C.M.; Kessels, W.M.M. Excellent passivation of highly doped p-type Si surfaces by the negative-charge-dielectric Al2O3. Appl. Phys. Lett. 2007, 91, 112107. [Google Scholar] [CrossRef]
- Richter, A.; Benick, J.; Hermle, M. Boron emitter passivation with Al2O3 and Al2O3/SiNx stacks using ALD Al2O. IEEE J. Photovolt. 2013, 3, 236–245. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wu, Y.-C.; You, H.-C.; Chen, C.-H.; Chen, P.-H.; Tsai, Y.-H.; Yang, Y.-Y.; Chang-Liao, K.S. Silicon heterojunction solar cells using AlOx and plasma-immersion ion implantation. Energies 2014, 7, 3653–3663. [Google Scholar] [CrossRef]
- Dicks, O.A.; Cottom, J.; Shluger, A.L.; Afamas’ev, V.V. The origin of negative charging in amorphous Al2O3 films: The role of native defects. Nanotechnology 2019, 30, 205201. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Weber, K.; Hameiri, Z.; De Wolf, S. Industrially feasible, dopant-free, carrier-selective contacts for high-efficiency silicon solar cells. Prog. Photovolt. Res. Appl. 2017, 25, 896–904. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Lin, F.-R.; Lin, T.-C.; Chen, C.-H.; Pei, Z. Low-temperature, chemically grown titanium oxide thin films with a high hole tunneling rate for Si solar cells. Energies 2016, 9, 402. [Google Scholar] [CrossRef]
- Chen, T.-C.; Yang, T.-C.; Cheng, H.-E.; Yu, I.-S.; Yang, Z.-P. Single material TiO2 thin film by atomic layer deposition for antireflection and surface passivation applications on p-type c-Si. Appl. Surf. Sci. 2018, 451, 121–127. [Google Scholar] [CrossRef]
- Geissbühler, J.; Werner, J.; Martin de Nicolas, S.; Barraud, L.; Hessler-Wyser, A.; Despeisse, M.; Nicolay, S.; Tomasi, A.; Niesen, B.; De Wolf, S.; et al. 22.5% efficient silicon heterojunction solar cell with molybdenum oxide hole collector. Appl. Phys. Lett. 2015, 107, 081601. [Google Scholar] [CrossRef]
- Bivour, M.; Temmler, J.; Steinkemper, H.; Hermle, M. Molybdenum and tungsten oxide: High work function wide band gap contact materials for hole selective contacts of silicon solar cells. Sol. Energy. Mater. Sol. Cells 2015, 142, 34–41. [Google Scholar] [CrossRef]
- Römer, U.; Peibst, R.; Ohrdes, T.; Lim, B.; Krügener, J.; Wietler, T.; Brendel, R. Ion implantation for poly-Si passivated back-junction back-contacted solar cells. IEEE J. Photovolt. 2015, 5, 507–514. [Google Scholar] [CrossRef]
- Moldovan, A.; Feldmann, F.; Zimmer, M.; Rentsch, J.; Benick, J.; Hermle, M. Tunnel oxide passivated carrier-selective contacts based on ultra-thin SiO2 layers. Sol. Energy Mater. Sol. Cells 2015, 142, 123–127. [Google Scholar] [CrossRef]
- Choi, S.; Min, K.H.; Jeon, M.S.; Lee, J.I.; Kang, M.G.; Song, H.-E.; Kang, Y.; Lee, H.-S.; Kim, D.; Kim, K.-H. Structural evolution of tunneling oxide passivating contact upon thermal annealing. Sci. Rep. 2017, 7, 12853. [Google Scholar] [CrossRef]
- Feldmann, F.; Reichel, C.; Müller, R.; Hermle, M. The application of poly-Si/SiOx contacts as passivated top/rear contacts in Si solar cells. Sol. Energy. Mater. Sol. Cells 2017, 159, 256–271. [Google Scholar] [CrossRef]
- Hoex, B.; Gielis, J.J.H.; van de Sanden, M.C.M.; Kessels, W.M.M. On the c-Si surface passivation mechanism by the negative-charge-dielectric Al2O3. J. Appl. Phys. 2008, 104, 1113703. [Google Scholar] [CrossRef]
- Naumann, V.; Otto, M.; Wehrspohn, R.B.; Werner, M.; Hagendorf, C. Interface and material characterization of thin ALD-Al2O3 layers on crystalline silicon. Energy Procedia 2012, 27, 312–318. [Google Scholar] [CrossRef]
- Kersten, F.; Schmid, A.; Bordihn, S.; Müller, J.W.; Heitmann, J. Role of annealing conditions on surface passivation properties of ALD Al2O3 films. Energy Procedia 2013, 38, 843–848. [Google Scholar] [CrossRef]
- Veith, B.; Werner, F.; Zielke, D.; Brendel, R.; Schmidt, J. Comparison of the thermal stability of single Al2O3 layers and Al2O3/SiNx stacks for the surface passivation of silicon. Energy Procedia 2011, 8, 307–312. [Google Scholar] [CrossRef]
- Dingemans, G.; Terlinden, N.M.; Verheijen, M.A.; van de Sanden, M.C.M.; Kessels, W.M.M. Controlling the fixed charge and passivation properties of Si(100)/Al2O3 interfaces using ultrathin SiO2 interlayers synthesized by atomic layer deposition. J. Appl. Phys. 2011, 110, 093715. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamashita, Y.; Nakato, Y.; Komeda, T.; Nishioka, Y. Interface states at ultrathin oxide/Si(111) interfaces obtained from x-ray photoelectron spectroscopy measurements under biases. Appl. Phys. Lett. 1996, 69, 2276–2278. [Google Scholar] [CrossRef]
- Imamura, K.; Takahashi, M.; Asuha; Hirayama, Y.; Imai, S.; Kobayashi, H. Nitric acid oxidation of Si method at 120 °C: HNO3 concentration dependence. J. Appl. Phys. 2010, 107, 054503. [Google Scholar] [CrossRef]
- Kobayashi Asuha, H.; Maida, O.; Takahashi, M.; Iwasa, H. Nitric acid oxidation of Si to form ultrathin silicon dioxide layers with a low leakage current density. J. Appl. Phys. 2003, 94, 7328–7335. [Google Scholar] [CrossRef]
- Laades, A.; Angermann, H.; Sperlich, H.-P.; Stürzebecher, U.; Álvarez, C.A.D.; Bähr, M.; Lawerenz, A. Wet chemical oxidation of silicon surfaces prior to the deposition of all-PECVD AlOx/a-SiNx passivation stacks for silicon solar cells. Solid State Phenom. 2013, 195, 310–313. [Google Scholar] [CrossRef]
- Bordihn, S.; Engelhart, P.; Mertens, V.; Kesser, G.; Köhn, D.; Dingemans, G.; Mandoc, M.M.; Müller, J.W.; Kessels, W.M.M. High surface passivation quality and thermal stability of ALD Al2O3 on wet chemical grown ultra-thin SiO2 on silicon. Energy Procedia 2011, 8, 654–659. [Google Scholar] [CrossRef]
- Kern, W. The evolution of silicon wafer cleaning technology. J. Elecrochem. Soc. 1990, 137, 1887–1892. [Google Scholar] [CrossRef]
- Sinton, R.A.; Cuevas, A. Contactless determination of current-voltage characteristics and minority carrier lifetimes in semiconductors from quasi-steady-state photoconductance data. Appl. Phys. Lett. 1996, 69, 2510–2512. [Google Scholar] [CrossRef]
- Aberle, A.G. Crystalline Silicon Solar Cells, Advanced Surface Passivation and Analysis; UNSW: Sydney, Australia, 2009. [Google Scholar]
- Bonilla, R.S.; Hoex, B.; Hamer, P.; Wilshaw, P.R. Dielectric surface passivation for silicon solar cells: A review. Phys. Status Solidi A 2017, 214, 1700293. [Google Scholar] [CrossRef]
- Schmidt, J.; Moschner, J.D.; Henze, J.; Dauwe, S.; Hezel, R. Recent progress in the surface passivation of silicon solar cells using silicon nitride. In Proceedings of the 19th European Photovoltaic Solar Energy Conference, Paris, France, 7–11 June 2004; pp. 391–396. [Google Scholar]
- McIntosh, K.R.; Black, L.E. On effective surface recombination parameters. J. Appl. Phys. 2014, 116, 014503. [Google Scholar] [CrossRef]
- Ma, F.-J.; Hoex, B.; Samudra, G.S.; Aberle, A.G. Modelling and simulation of field-effect surface passivation of crystalline silicon-based solar cells. Energy Procedia 2012, 15, 155–161. [Google Scholar] [CrossRef]
- Terman, L.M. An investigation of surface states at a silicon/silicon oxide interface employing metal-oxide-silicon diodes. Solid State Electron. 1962, 5, 285–299. [Google Scholar] [CrossRef]
- Nicollian, E.H.; Brews, J.R. MOS (Metal Oxide Semiconductor) Physics and Technology; Wiley: New York, NY, USA, 1982. [Google Scholar]
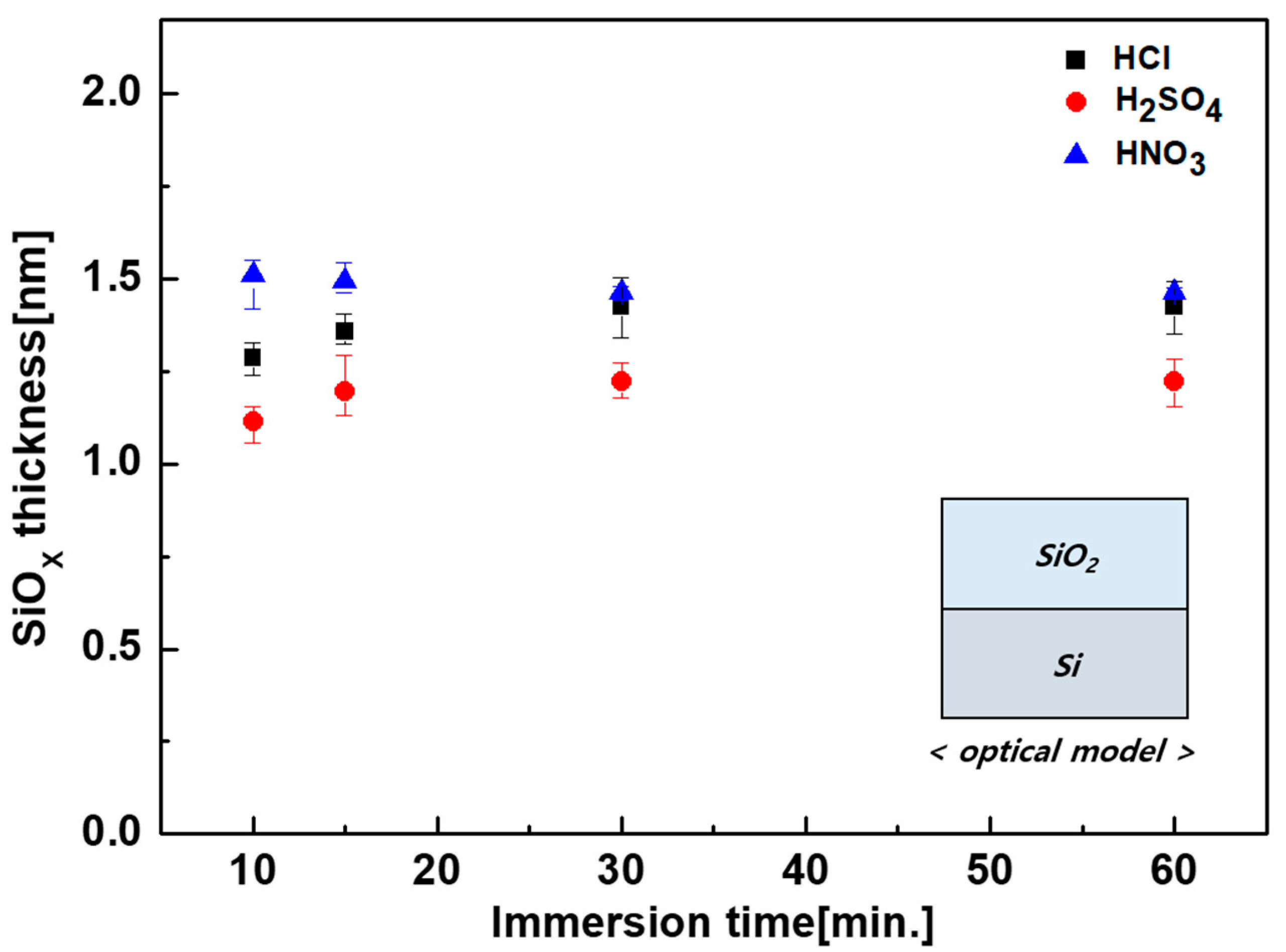
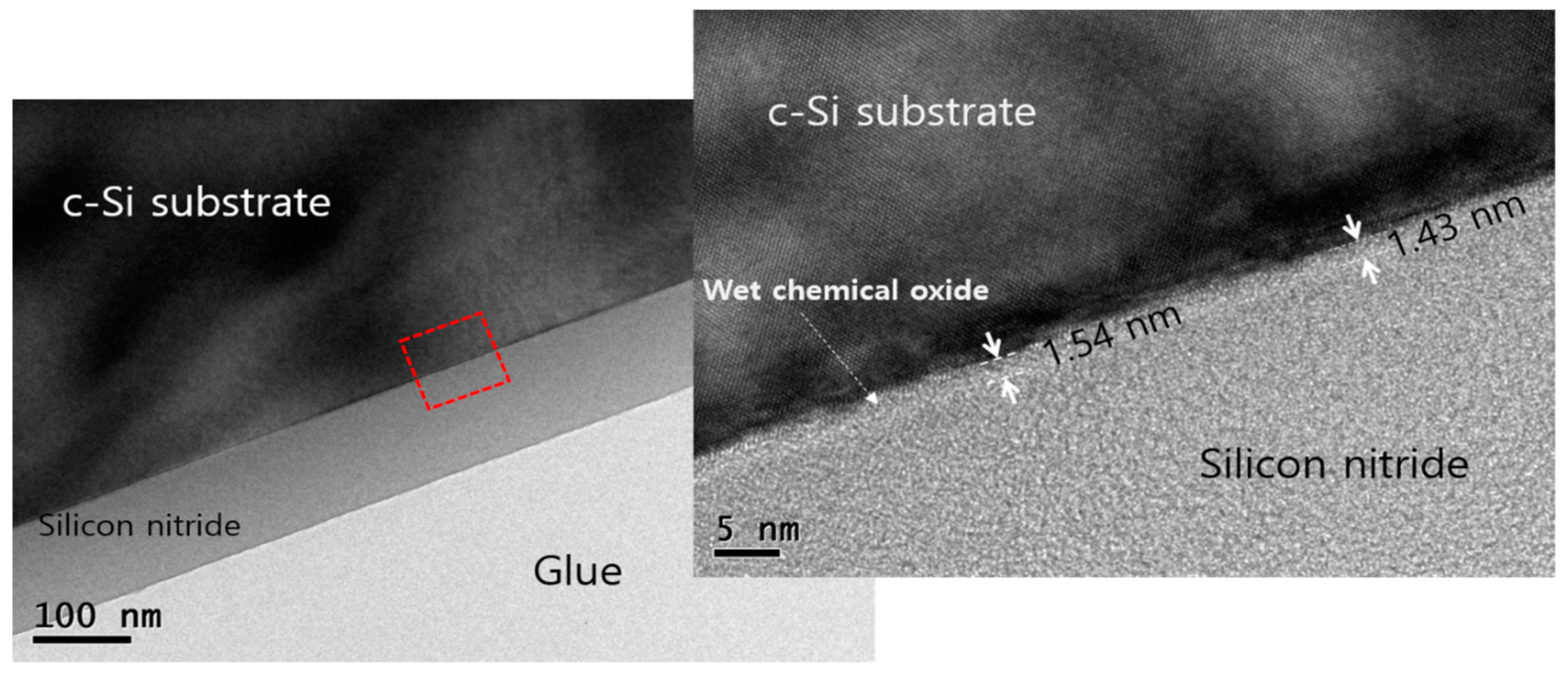
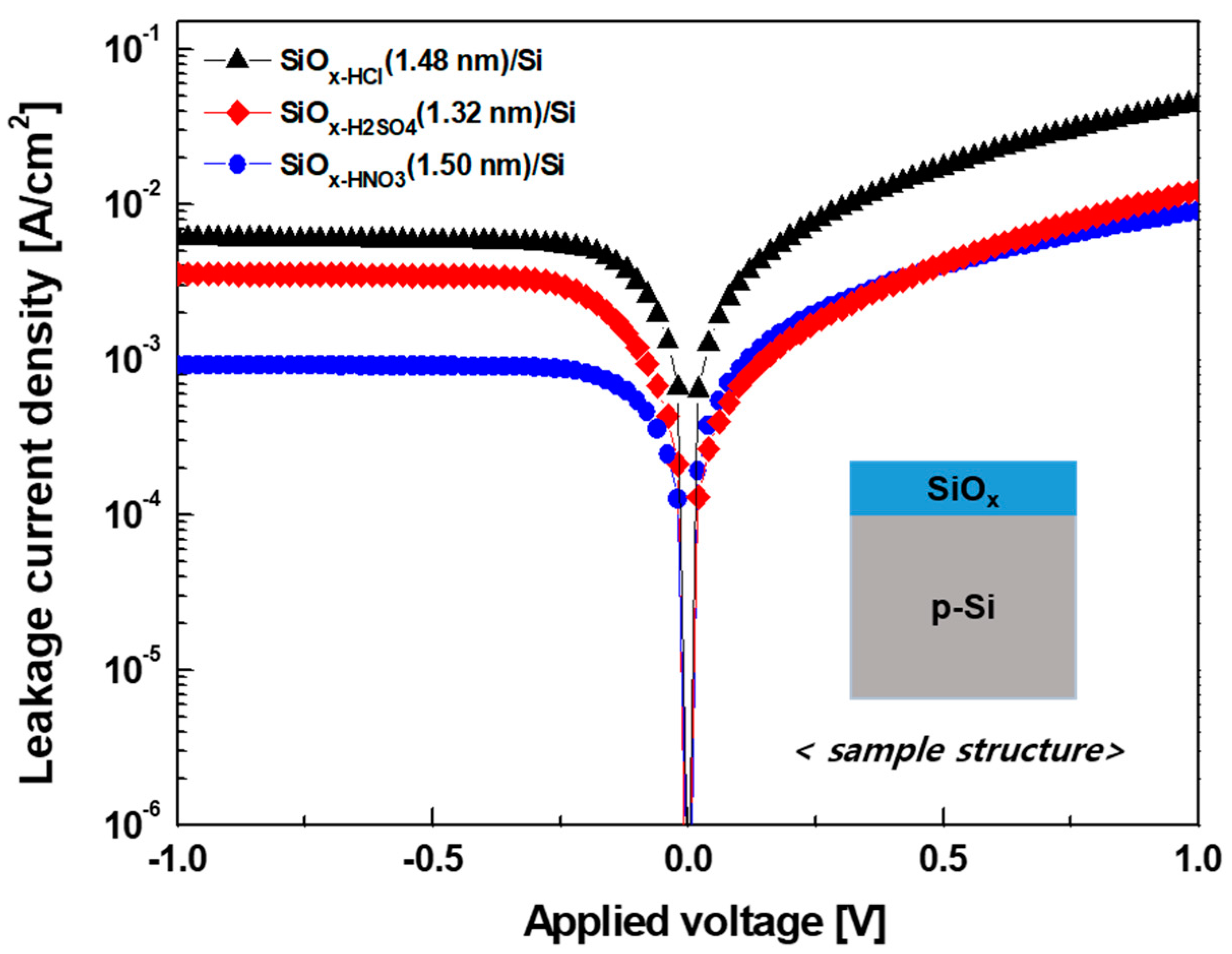
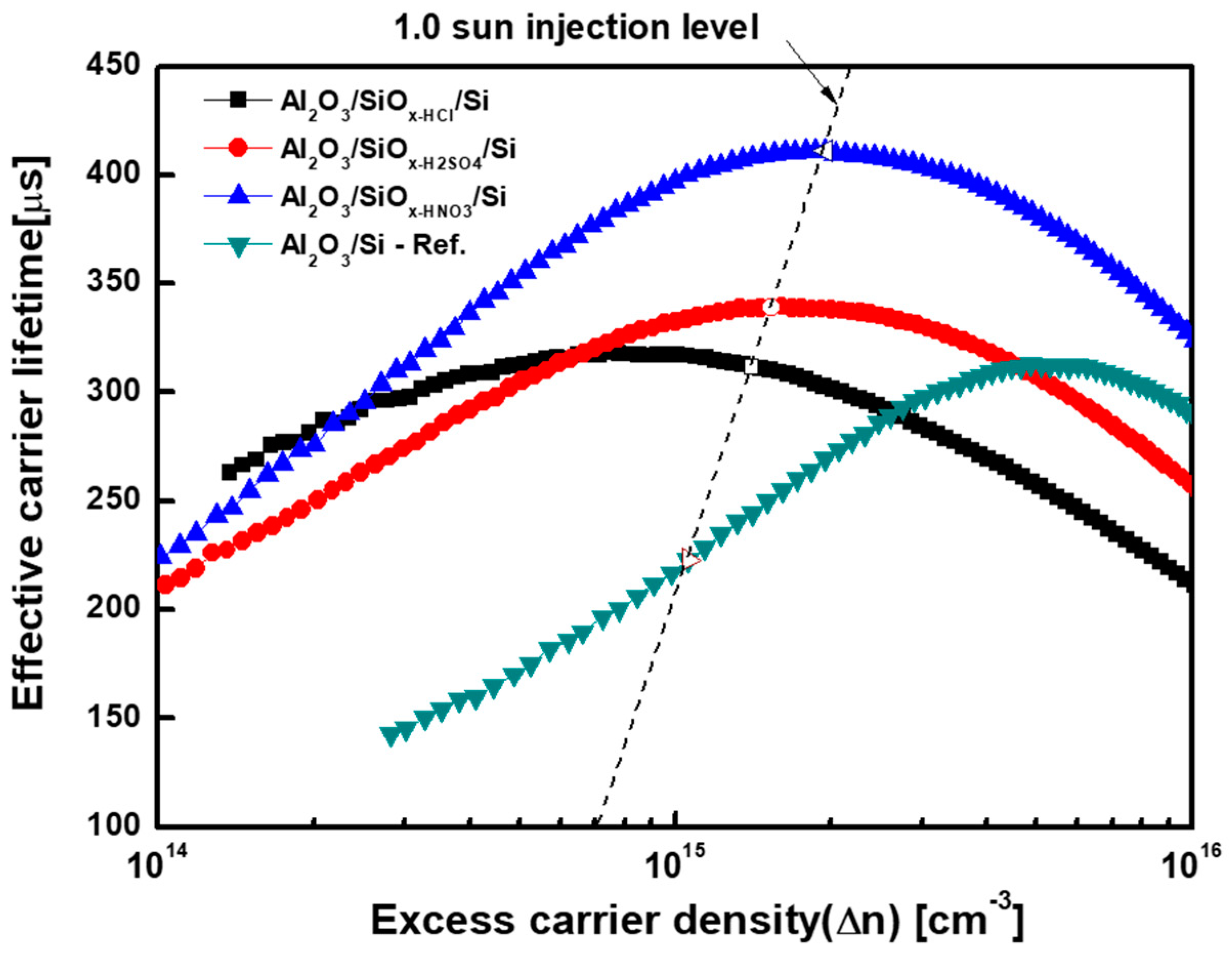
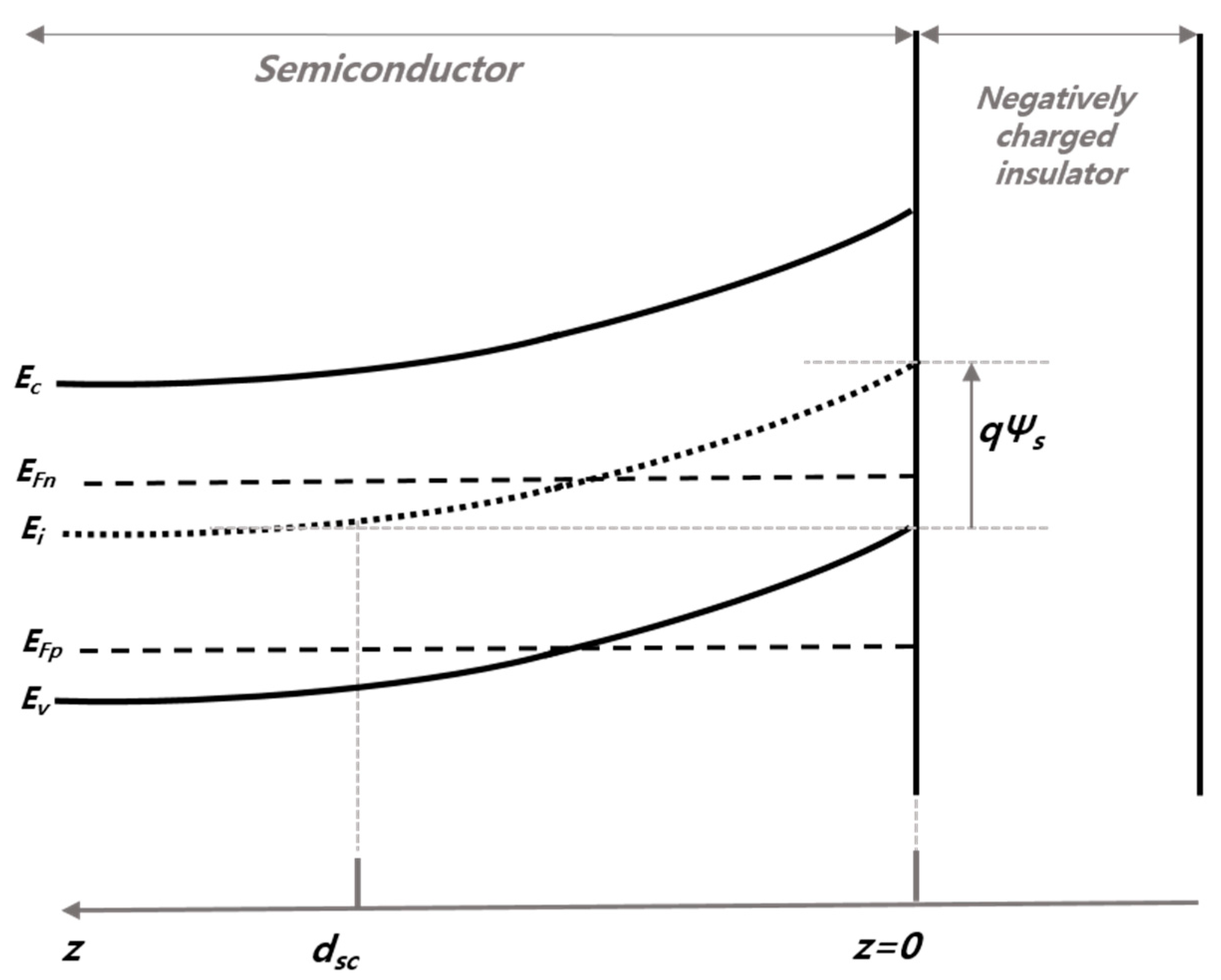
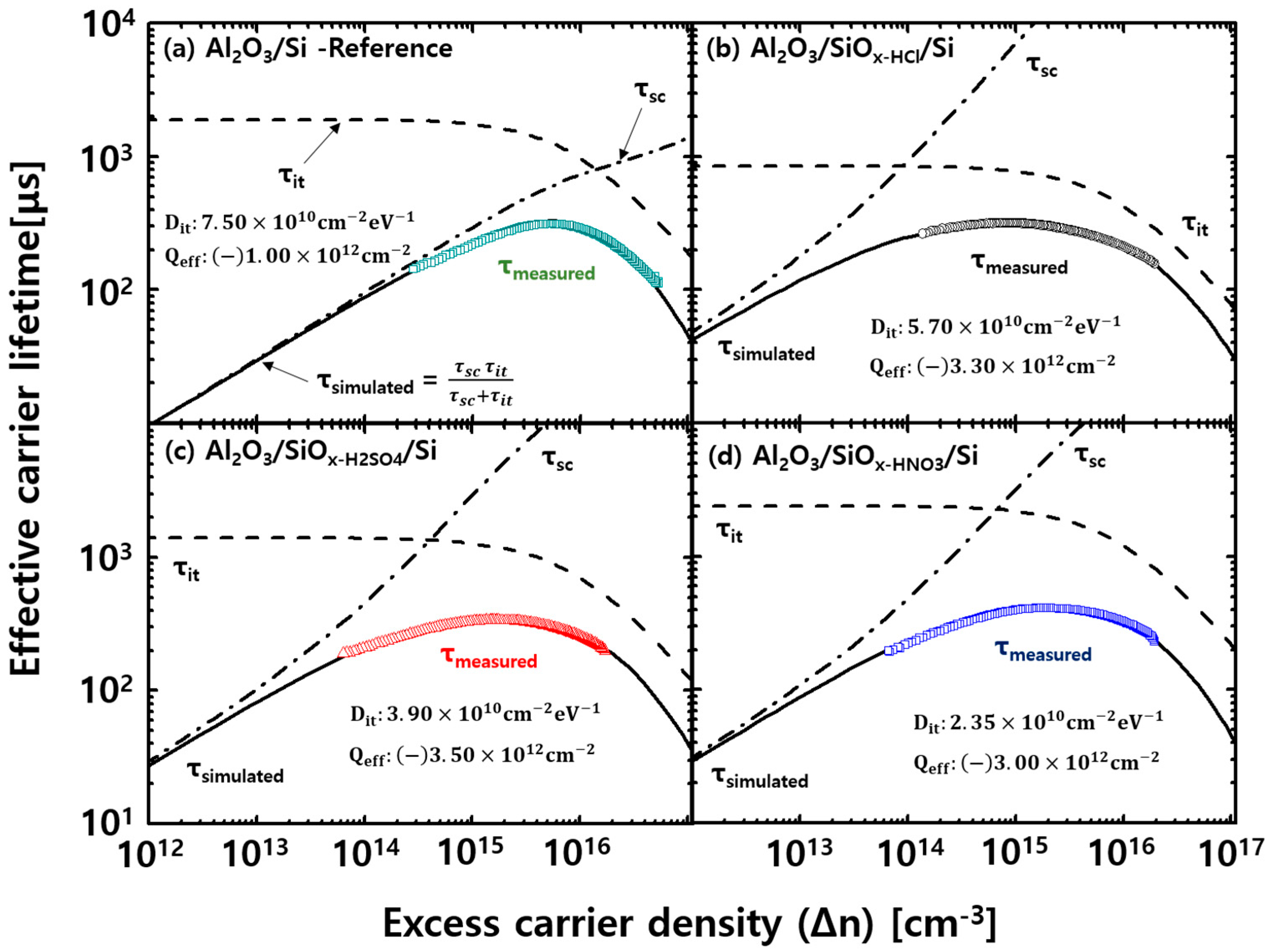
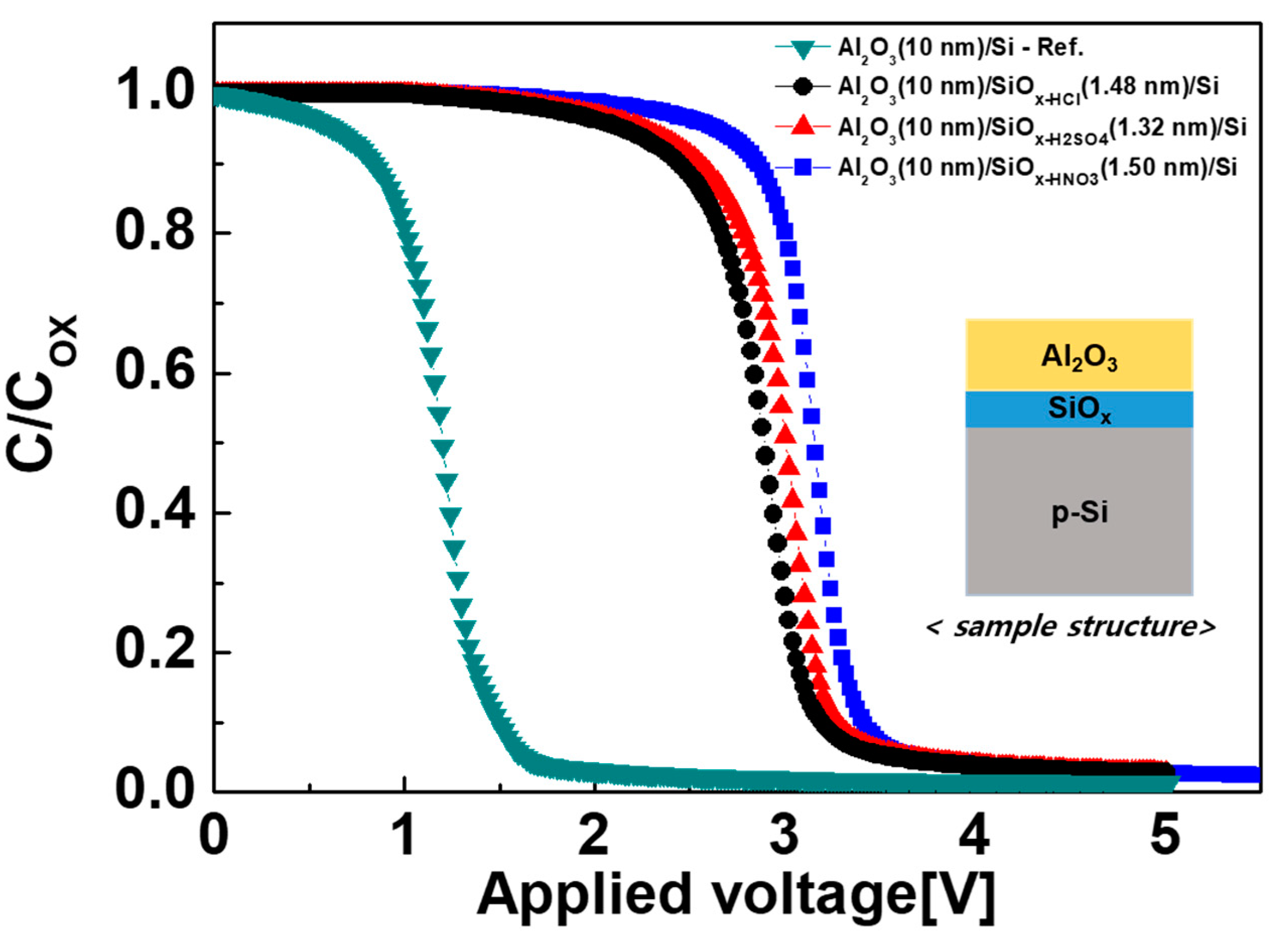
| Solution | Thickness (nm) | Refractive Index (n) at 630 nm |
|---|---|---|
| HCl | 1.49 | 1.421 |
| H2SO4 | 1.32 | 1.417 |
| HNO3 | 1.50 | 1.430 |
| Sample (Average of Five Samples) | Capacitance–Voltage (C–V) Analysis | Surface Carrier Lifetime Analysis | |||
|---|---|---|---|---|---|
| Qf (1012 cm−2) | Dit (1010 cm−2eV−1) | VFB (V) | Qf (1012 cm−2) | Dit (1010 cm−2eV−1) | |
| Al2O3/Si | −1.53 | 7.01 | 1.20 | −1.00 | 7.50 |
| Al2O3/SiOx-HCl/Si | −3.03 | 4.94 | 2.97 | −3.30 | 5.70 |
| Al2O3/SiOx-H2SO4/Si | −3.12 | 4.60 | 3.12 | −3.50 | 3.90 |
| Al2O3/SiOx-HNO3/Si | −3.24 | 2.88 | 3.28 | −3.52 | 2.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, K.H.; Choi, S.; Jeong, M.S.; Park, S.; Kang, M.G.; Lee, J.I.; Kang, Y.; Kim, D.; Lee, H.-S.; Song, H.-e. Wet Chemical Oxidation to Improve Interfacial Properties of Al2O3/Si and Interface Analysis of Al2O3/SiOx/Si Structure Using Surface Carrier Lifetime Simulation and Capacitance–Voltage Measurement. Energies 2020, 13, 1803. https://doi.org/10.3390/en13071803
Min KH, Choi S, Jeong MS, Park S, Kang MG, Lee JI, Kang Y, Kim D, Lee H-S, Song H-e. Wet Chemical Oxidation to Improve Interfacial Properties of Al2O3/Si and Interface Analysis of Al2O3/SiOx/Si Structure Using Surface Carrier Lifetime Simulation and Capacitance–Voltage Measurement. Energies. 2020; 13(7):1803. https://doi.org/10.3390/en13071803
Chicago/Turabian StyleMin, Kwan Hong, Sungjin Choi, Myeong Sang Jeong, Sungeun Park, Min Gu Kang, Jeong In Lee, Yoonmook Kang, Donghwan Kim, Hae-Seok Lee, and Hee-eun Song. 2020. "Wet Chemical Oxidation to Improve Interfacial Properties of Al2O3/Si and Interface Analysis of Al2O3/SiOx/Si Structure Using Surface Carrier Lifetime Simulation and Capacitance–Voltage Measurement" Energies 13, no. 7: 1803. https://doi.org/10.3390/en13071803
APA StyleMin, K. H., Choi, S., Jeong, M. S., Park, S., Kang, M. G., Lee, J. I., Kang, Y., Kim, D., Lee, H.-S., & Song, H.-e. (2020). Wet Chemical Oxidation to Improve Interfacial Properties of Al2O3/Si and Interface Analysis of Al2O3/SiOx/Si Structure Using Surface Carrier Lifetime Simulation and Capacitance–Voltage Measurement. Energies, 13(7), 1803. https://doi.org/10.3390/en13071803







