Light-Emitting Diode Power Conversion Capability and CO2 Fixation Rate of Microalgae Biofilm Cultured Under Different Light Spectra
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Biofilm Culture
2.2. Determining the Biomass and Chlorophyll Contents
2.3. Determining Cellular Composition
2.4. LED Power Conversion Capability
2.5. Determining CO2 Fixation Rate
3. Results and Discussion
3.1. Growth of Microalgae Biofilms Cultured Under Different LEDs
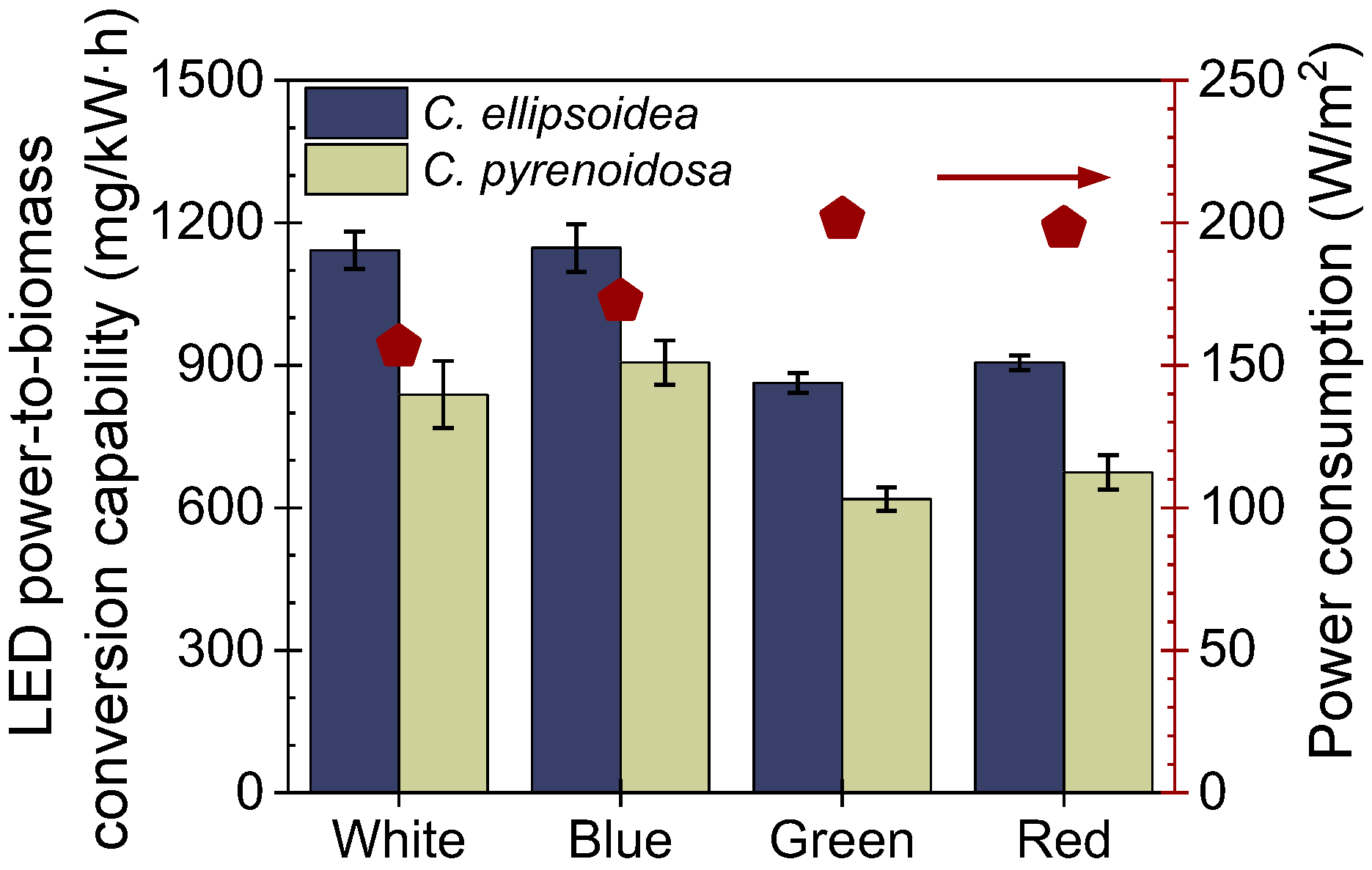
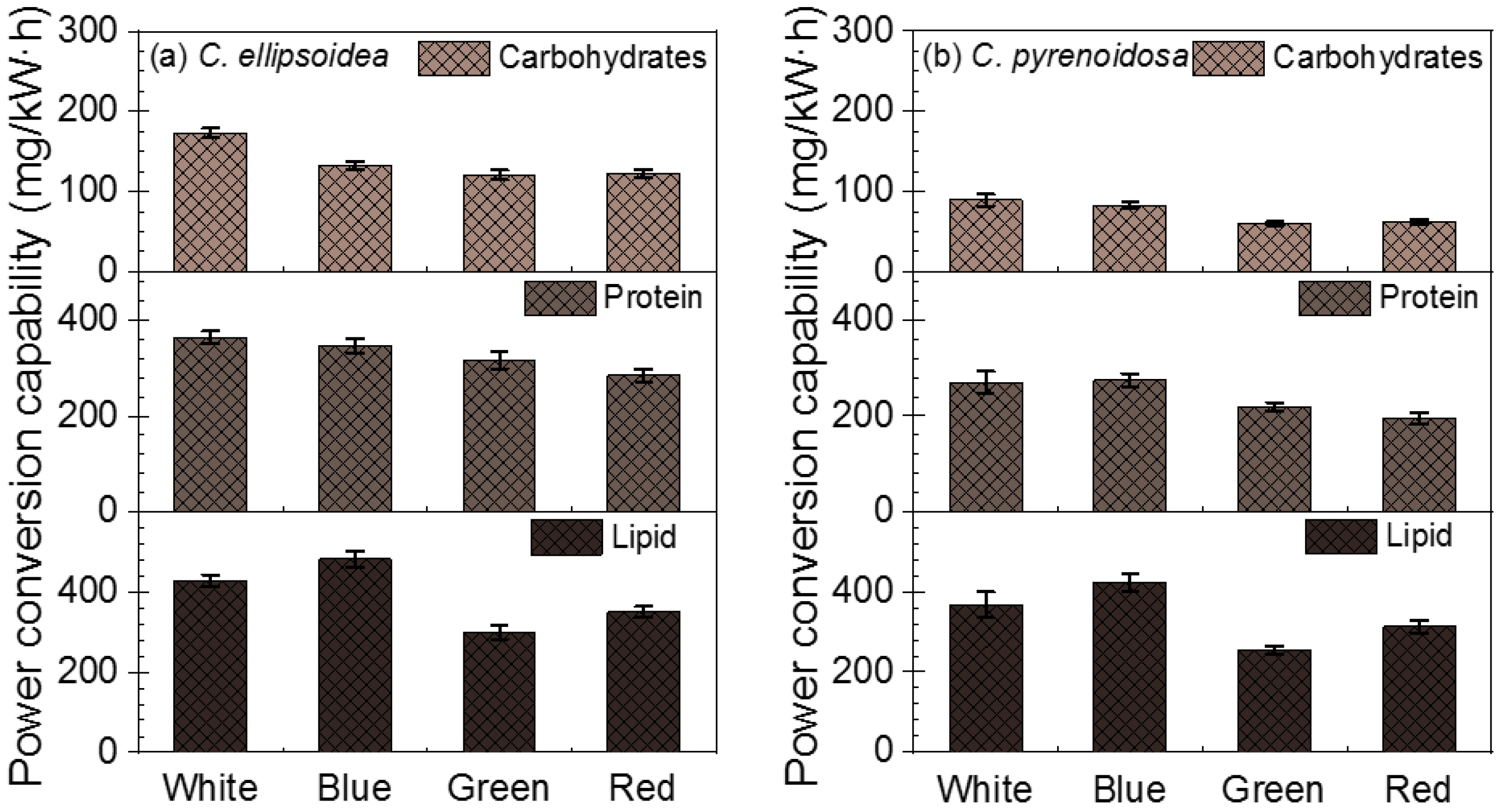
3.2. The LED Power Conversion Capability of Microalgae Biofilm
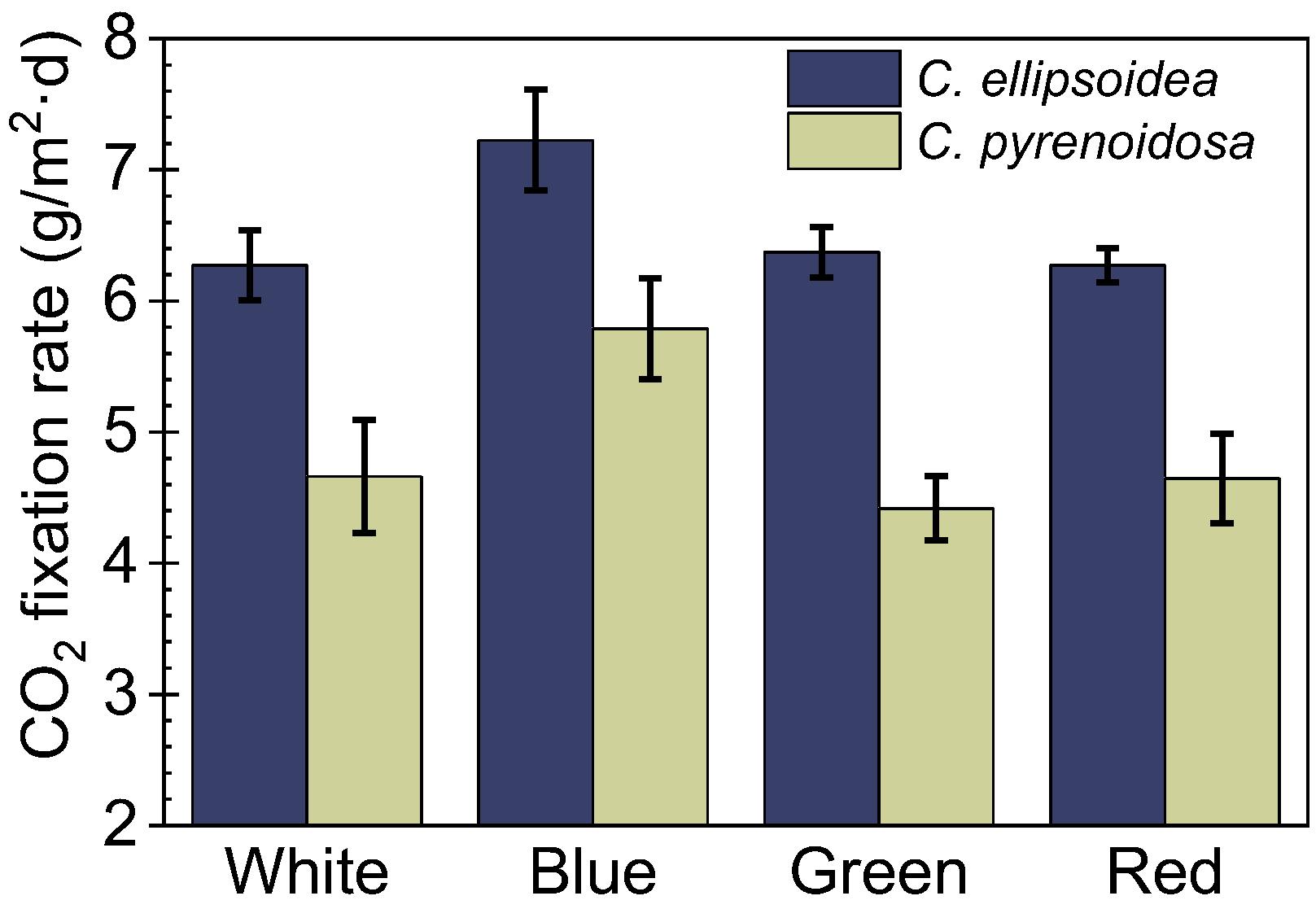
3.3. The CO2 Fixation Rate of Microalgae Biofilm
3.4. Implications of LED Selection in Biofilm-Based Microalgae Cultivation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maeda, Y.; Yoshino, T.; Matsunaga, T.; Matsumoto, M.; Tanaka, T. Marine microalgae for production of biofuels and chemicals. Curr. Opin. Biotechnol. 2018, 50, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal biofuels: Current status and key challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef] [Green Version]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.M.; Ling, T.C.; Nagarajan, D.; Lee, D.J.; Chang, J.S. Sustainable approaches for algae utilisation in bioenergy production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Cui, B.; Wang, H.; Liu, T. Evaluation of filamentous green algae as feedstocks for biofuel production. Bioresour. Technol. 2016, 220, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clippinger, J.N.; Davis, R.E. Techno-Economic Analysis for the Production of Algal Biomass via Closed Photobioreactors: Future Cost Potential Evaluated Across a Range of Cultivation System Designs; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2019.
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Maneein, S.; Harvey, P.J. A brief review of anaerobic digestion of algae for bioenergy. Energies 2019, 12, 1166. [Google Scholar] [CrossRef] [Green Version]
- Berner, F.; Heimann, K.; Sheehan, M. Microalgal biofilms for biomass production. J. Appl. Psychol. 2015, 27, 1793–1804. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 23–571. [Google Scholar] [CrossRef]
- Zhuang, L.L.; Yu, D.; Zhang, J.; Liu, F.F.; Wu, Y.H.; Zhang, T.Y.; Dao, G.H.; Hu, H.Y. The characteristics and influencing factors of the attached microalgae cultivation: A review. Renew. Sustain. Energy Rev. 2018, 94, 1110–1119. [Google Scholar] [CrossRef]
- Chang, H.; Quan, X.; Zhong, N.; Zhang, Z.; Lu, C.; Li, G.; Cheng, Z.; Yang, L. High-efficiency nutrients reclamation from landfill leachate by microalgae Chlorella vulgaris in membrane photobioreactor for bio-lipid production. Bioresour. Technol. 2018, 266, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Mantzorou, A.; Ververidis, F. Microalgal biofilms: A further step over current microalgal cultivation techniques. Sci. Total Environ. 2019, 651, 3187–3201. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Podola, B.; Schultze, L.K.P.; Melkonian, M. Design scenario analysis for porous substrate photobioreactor assemblies. J. Appl. Psychol. 2019, 31, 1623–1636. [Google Scholar] [CrossRef]
- Posadas, E.; García-Encina, P.A.; Soltau, A.; Domínguez, A.; Díaz, I.; Muñoz, R. Carbon and nutrient removal from centrates and domestic wastewater using algal–bacterial biofilm bioreactors. Bioresour. Technol. 2013, 139, 50–58. [Google Scholar] [CrossRef]
- Guzzon, A.; Bohn, A.; Diociaiuti, M.; Albertano, P. Cultured phototrophic biofilms for phosphorus removal in wastewater treatment. Water Res. 2008, 42, 4357. [Google Scholar] [CrossRef]
- Shi, J.; Podola, B.; Melkonian, M. Application of a prototype-scale Twin-Layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresour. Technol. 2014, 154, 260–266. [Google Scholar] [CrossRef]
- Blanken, W.; Janssen, M.; Cuaresma, M.; Libor, Z.; Bhaiji, T.; Wijffels, R.H. Biofilm growth of Chlorella sorokiniana in a rotating biological contactor based photobioreactor. Biotechnol. Bioeng. 2014, 111, 2436–2445. [Google Scholar] [CrossRef]
- Schnurr, P.J.; Allen, D.G. Factors affecting algae biofilm growth and lipid production: A review. Renew. Sustain. Energy Rev. 2015, 52, 418–429. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Liu, T. Biofilm based attached cultivation technology for microalgal biorefineries—A review. Bioresour. Technol. 2017, 244, 1245–1253. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Parlevliet, D.A.; Laird, D.W.; Alameh, K.; Moheimani, N.R. Light management technologies for increasing algal photobioreactor efficiency. Algal Res. 2019, 39, 101433. [Google Scholar] [CrossRef]
- Liao, Q.; Chang, H.X.; Fu, Q.; Huang, Y.; Xia, A.; Zhu, X.; Zhong, N. Physiological-phased kinetic characteristics of microalgae Chlorella vulgaris growth and lipid synthesis considering synergistic effects of light, carbon and nutrients. Bioresour. Technol. 2018, 250, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Stiles, A.R.; Guo, C.; Liu, C.Z. Microalgae cultivation in photobioreactors: An overview of light characteristics. Eng. Life Sci. 2014, 14, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Ramanna, L.; Rawat, I.; Bux, F. Light enhancement strategies improve microalgal biomass productivity. Renew. Sustain. Energy Rev. 2017, 80, 765–773. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Liu, T.; Zhong, Y.; Ji, C.; Lu, Q.; Fan, L.; Li, J.; Leng, L.; Li, K.; et al. Microalgae biotechnology as an attempt for bioregenerative life support systems: Problems and prospects. J. Chem. Technol. Biotechnol. 2019, 94, 3039–3048. [Google Scholar] [CrossRef]
- Abomohra, A.E.F.; Shang, H.; El-Sheekh, M.; Eladel, H.; Ebaid, R.; Wang, S.; Wang, Q. Night illumination using monochromatic light-emitting diodes for enhanced microalgal growth and biodiesel production. Bioresour. Technol. 2019, 288, 121514. [Google Scholar] [CrossRef]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef]
- Glemser, M.; Heining, M.; Schmidt, J.; Becker, A.; Garbe, D.; Buchholz, R.; Brück, T. Application of light-emitting diodes (LEDs) in cultivation of phototrophic microalgae: Current state and perspectives. Appl. Microbiol. Biotechnol. 2016, 100, 1077–1088. [Google Scholar] [CrossRef]
- Ma, R.; Thomas-Hall, S.R.; Chua, E.T.; Eltanahy, E.; Netzel, M.E.; Netzel, G.; Lu, Y.; Schenk, P.M. LED power efficiency of biomass, fatty acid, and carotenoid production in Nannochloropsis microalgae. Bioresour. Technol. 2018, 252, 118–126. [Google Scholar] [CrossRef]
- Ajayan, K.V.; Anjula, K.; Syamasuruya, A.P.; Harilal, C.C. Energy efficient technology for enhanced growth and lipid production in Chlamydomonas reinhardtii through additional reflector coated LED photo-bioreactor. Biochem. Eng. J. 2019, 144, 81–88. [Google Scholar] [CrossRef]
- Maroneze, M.M.; Siqueira, S.F.; Vendruscolo, R.G.; Wagner, R.; De Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. The role of photoperiods on photobioreactors—A potential strategy to reduce costs. Bioresour. Technol. 2016, 219, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiong, W.; Liao, Q.; Fu, Q.; Xia, A.; Zhu, X.; Sun, Y. Comparison of Chlorella vulgaris biomass productivity cultivated in biofilm and suspension from the aspect of light transmission and microalgae affinity to carbon dioxide. Bioresour. Technol. 2016, 222, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Zhang, X.; Jiang, Z.; Chen, X.; Zhang, X. Quantitative criterion to predict cell adhesion by identifying dominant interaction between microorganisms and abiotic surfaces. Langmuir 2019, 35, 3524–3533. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, H.; Guan, L.; Wang, X.; Wang, Y.; Jiang, Z.; Cao, L.; Zhang, X. Influence of photoperiods on microalgae biofilm: Photosynthetic performance, biomass yield, and cellular composition. Energies 2019, 12, 3724. [Google Scholar] [CrossRef] [Green Version]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Dorsey, T.E.; McDonald, P.; Roels, O.A. Measurements of phytoplankton-protein content with the heated biuret-folin assay. J. Phycol. 1978, 14, 167–171. [Google Scholar] [CrossRef]
- Hellebust, J.A.; Stein, J.R.; Craigie, J. Handbook of Phycological Methods: Physiological and Biochemical Methods; Cambridge University Press: Cambridge, UK, 1978; Volume 2. [Google Scholar]
- Mercz, T.I. A Study of High Lipid Yielding Microalgae with Potential for Large-Scale Production of Lipids and Polyunsaturated Fatty Acids. Ph.D. Thesis, Murdoch University, Perth, Australia, 1994. [Google Scholar]
- Zhou, W.; Wang, J.; Chen, P.; Ji, C.; Kang, Q.; Lu, B.; Li, K.; Liu, J.; Ruan, R. Bio-mitigation of carbon dioxide using microalgal systems: Advances and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1163–1175. [Google Scholar] [CrossRef]
- Szabó, M.; Parker, K.; Guruprasad, S.; Kuzhiumparambil, U.; Lilley, R.M.; Tamburic, B.; Schliep, M.; Larkum, A.W.; Schreiber, U.; Raven, J.A. Photosynthetic acclimation of Nannochloropsis oculata investigated by multi-wavelength chlorophyll fluorescence analysis. Bioresour. Technol. 2014, 167, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Ra, C.H.; Kang, C.H.; Jung, J.H.; Jeong, G.T.; Kim, S.K. Enhanced biomass production and lipid accumulation of Picochlorum atomus using light-emitting diodes (LEDs). Bioresour. Technol. 2016, 218, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Kim, B.H.; Ramanan, R.; Choi, J.E.; Yang, J.W.; Oh, H.M.; Kim, H.S. A cost analysis of microalgal biomass and biodiesel production in open raceways treating municipal wastewater and under optimum light wavelength. J. Microbiol. Biotechnol. 2015, 25, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadiveloo, A.; Moheimani, N.R.; Cosgrove, J.J.; Bahri, P.A.; Parlevliet, D. Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res. 2015, 8, 121–127. [Google Scholar] [CrossRef]
- Roscher, E.; Zetsche, K. The effects of light quality and intensity on the synthesis of ribulose-1,5-bisphosphate carboxylase and its mRNAs in the green alga Chlorogonium elongatum. Planta 1986, 167, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chai, X.; Liu, G.; Hao, Y.; Zhao, Y. Characteristics of light regime on biofixation of carbon dioxide and growth of Scenedesmus obliquus with light-emitting diodes. J. Renew. Sustain. Energy 2014, 6, 033104. [Google Scholar] [CrossRef]
- Ra, C.H.; Sirisuk, P.; Jung, J.-H.; Jeong, G.-T.; Kim, S.K. Effects of light-emitting diode (LED) with a mixture of wavelengths on the growth and lipid content of microalgae. Bioprocess Biosyst. Eng. 2018, 41, 457–465. [Google Scholar] [CrossRef]
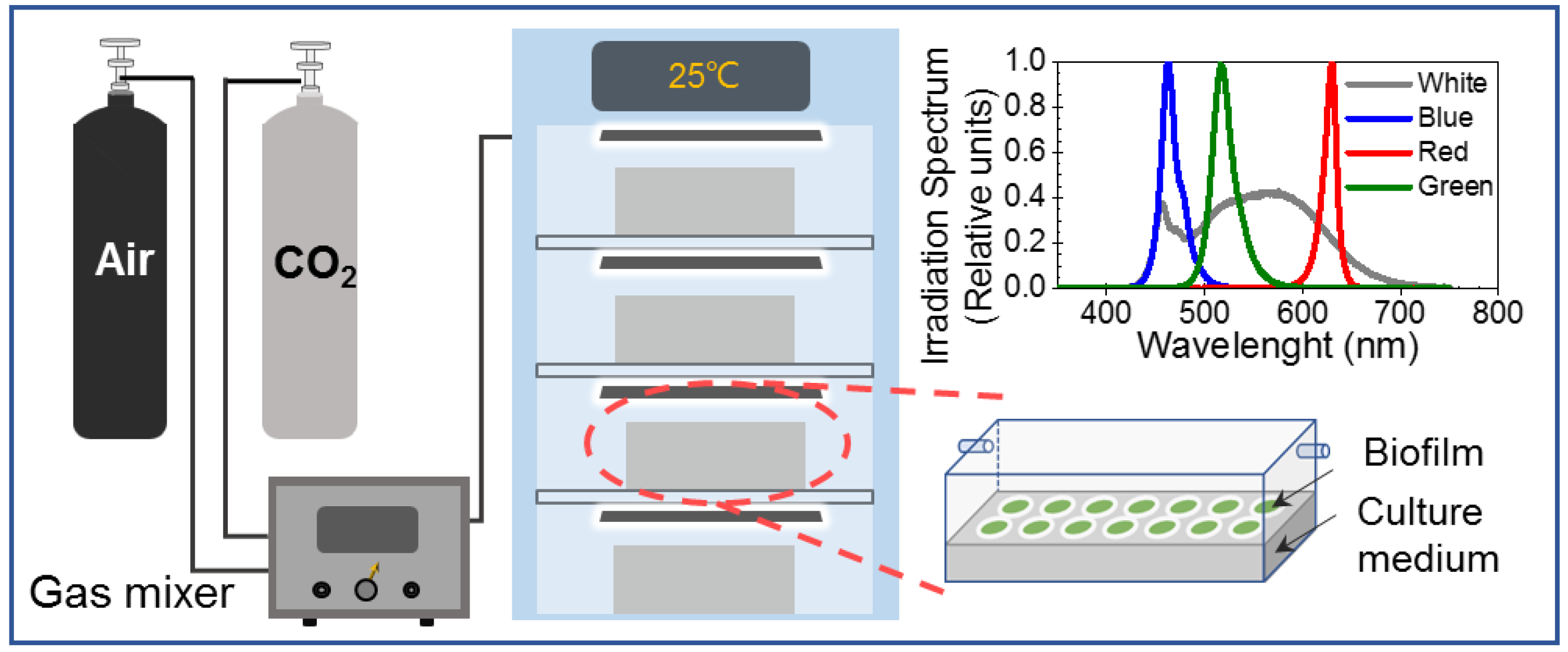
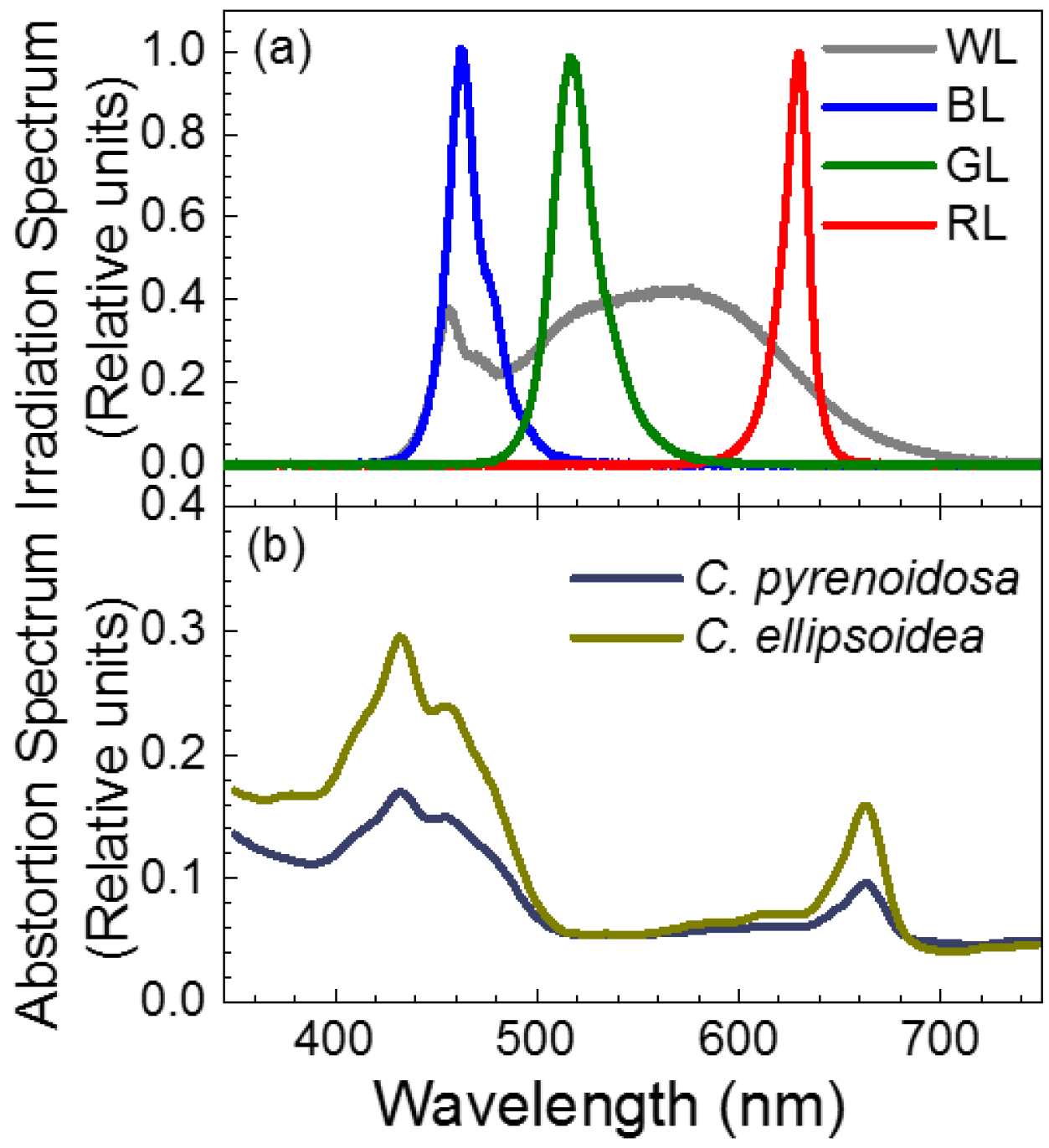
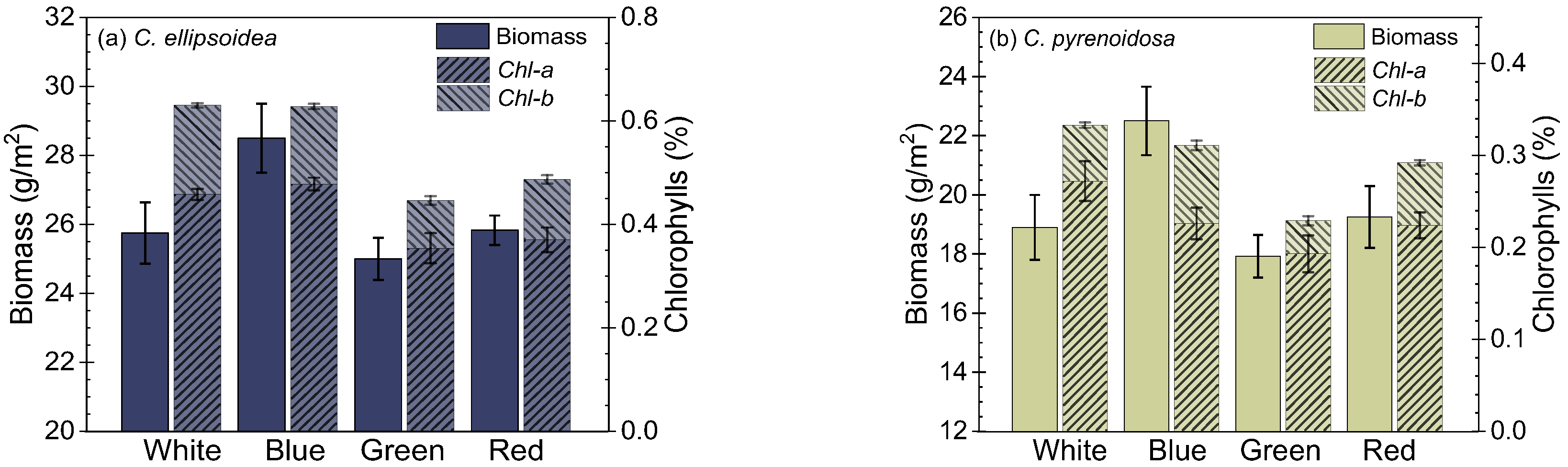
 ).
).
 ).
).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Wang, Y.; Xi, Y.; Jiang, Z.; Zhang, X.; Wang, X.; Zhang, X. Light-Emitting Diode Power Conversion Capability and CO2 Fixation Rate of Microalgae Biofilm Cultured Under Different Light Spectra. Energies 2020, 13, 1536. https://doi.org/10.3390/en13071536
Yuan H, Wang Y, Xi Y, Jiang Z, Zhang X, Wang X, Zhang X. Light-Emitting Diode Power Conversion Capability and CO2 Fixation Rate of Microalgae Biofilm Cultured Under Different Light Spectra. Energies. 2020; 13(7):1536. https://doi.org/10.3390/en13071536
Chicago/Turabian StyleYuan, Hao, Yi Wang, Yanaoming Xi, Zeyi Jiang, Xinru Zhang, Xinyu Wang, and Xinxin Zhang. 2020. "Light-Emitting Diode Power Conversion Capability and CO2 Fixation Rate of Microalgae Biofilm Cultured Under Different Light Spectra" Energies 13, no. 7: 1536. https://doi.org/10.3390/en13071536
APA StyleYuan, H., Wang, Y., Xi, Y., Jiang, Z., Zhang, X., Wang, X., & Zhang, X. (2020). Light-Emitting Diode Power Conversion Capability and CO2 Fixation Rate of Microalgae Biofilm Cultured Under Different Light Spectra. Energies, 13(7), 1536. https://doi.org/10.3390/en13071536





