1. Introduction
By definition a renewable resource, biomass remains an important aspect of the search for sustainable energy sources [
1]. This includes not only refuse and energetic wood plantations, but also specially grown algae [
2]. Algae-derived biomass may be used for energy production, whether by simple firing or co-firing with refuse-derived fuels, other biomass, or non-renewable fuels, or through production of biofuels [
3]. The algae-derived biofuels may take the form of pyrolytic solid fuels [
4], burnable gases such as hydrogen and methane [
5], or liquid hydrocarbons and biodiesel [
6,
7]. Other chemical components, such as ammonia, may as well be produced through algae cultivation [
8]. As photosynthetic organisms consume carbon dioxide in the process of photosynthesis, algae breeding also presents a desirable side-effect of carbon sequestration [
9,
10].
Like other autotrophic organisms, algae grow best when certain optimal conditions are reached. This makes outdoor algae farming impractical in colder climates, as the conditions for growth occur only through a part of the year at best; in addition to this, the natural conditions are subject to modification to a highly limited extent. However, unlike most multicellular plants, algae do not require soil and have a limited need for space. As a result, algae can be grown in specially prepared vessels or photobioreactors. This arrangement not only allows for year-round breeding of algae, but also for the adjustment of conditions such as, e.g., light wavelength [
11] or light intensity, or even vessel geometry [
12], in search for the optimal growth rate. Moreover, industrial photobioreactors must also allow for practical removal of algal matter without interrupting their operation. Therefore, research into algal growth conditions and photobioreactor construction remain an important and promising segment of renewable energy research.
As mentioned above, light is one of the most important factors affecting the rate of growth of photosynthetic organisms. The influence of light energy varies for different processes and different species. Both excessive lighting and lack of light adversely affect the production of biomass. Within a photobioreactor, the conversion of available light depends on the concentration of biomass, and thus on the optimal distribution of radiant energy, so that all cells receive the appropriate amount. It is assumed that the optimal value of the density of the photon flux for algae is in the range of 100–600 μmol·m
−2·s
−1 and is characteristic not only for a given species, but even for a given type [
13]. In addition to the above, the microalgae cells reduce the intensity of light within the photobioreactor. Located farther from the source of light, they will receive less radiation as a result of mutual occlusion, which results in a decrease in the rate of growth. There is a process of absorption and dispersion called the effect of shading [
14]. As the cell density increases, the intensity of light in the reactor is suppressed. Excessive lighting also acts as a brake causing the so-called photoinhibition phenomenon, i.e., a situation in which a photosynthetic microorganism is exposed to a greater amount of radiant energy than the one it is able to process. Thus, the second-order photositemator is damaged [
15,
16,
17]. The situation usually takes place in the early stages, just after the establishment of the breeding.
Numerous constructional solutions of photobioreactors, such as columnary photobioreactors or cylindrical bubble columns made of light-transparent materials, with aeration culture of aerated algae in different gas mixtures mounted in them [
18,
19], can be found in the literature. Such solutions due to the large area of exposure and the price are ideal for outdoor farming, used on an industrial scale for the production of algae [
20]. Tubular photobioreactors combined in a series are also used in outdoor cultures. The bundles of pipes directed towards the sun maximize the effect of solar radiation [
21]. In artificial lighting cultures for illuminating photobioreactors, LED diodes are most often used due to the emission of radiation with narrow wavelength ranges and the ability to easily select the color of light. The research suggests that the growth of algae is largely influenced by the spectrum of red light, favoring their faster multiplication and growth. On the other hand, the spectrum of blue light causes cell growth [
22].
Commonly used types of PBRs (photobioreactors) are flat panels used outdoors in conditions of natural surroundings. Due to their design and location, they are characterized by a perpendicular arrangement of the longest side of the bioreactor in relation to lighting, or a constant incapable of changing the inclination angle in relation to the radiation incidence plane [
23]. These reactors do not allow for optimal exposure conditions for the photoautotrophic biomass located in them and thus do not allow control of the intensity of light falling on the surface of the photobioreactor. In the case of excessive intensity of lighting, the known solutions are used to inhibit the growth of biomass, which directly translates into the result of productivity. In the above systems it is also difficult to control temperature or gas removal [
24,
25]. There is also an example of a Polish photobioreactor solution contained in patent specification PL 224183 regarding a flat panel intended for growing microorganisms in the natural environment. The invention relates to the construction of a system following natural light. In respect to the method of gas introduction and the shape of the vessel, the air lift type of PBRs has seen interest. The reactors are divided into the light and the dark zone, while the driving force is provided by the air-lift module responsible for the circulation of the biomass and therefore the uniform exposition of algae cells to the light [
26,
27].
Another solution are photobioreactors made of a transparent film forming cultured reservoirs permeable to light [
28]. Devices of this type are economically advantageous due to the possibility of easy assembly in places where it is possible to use, for example, waste carbon dioxide [
14,
29,
30].
Laboratory solutions for photobioreactors of various constructions, fully automated, performing the task of breeding, research and optimization in multiplication processes of photoautotrophic microorganisms, despite the fact that they meet the tasks set before them, show a number of imperfections, including those related to the distribution of light. A number of solutions implement the radiant energy in an artificial way, hence the light structures are arranged both inside photobioreactors and outside the units [
13,
24,
31]. The above solutions, however, do not ensure that the breeding facilities are illuminated from below. They also do not allow symmetrical and homogeneous illumination of cultures in a cylindrical bioreactor and thus do not provide a steady and uniform intensity of photosynthetically active radiation (PAR) reaching the cylindrical reactor, which can provide a cylindrical light jacket. They do not measure the radiation inside the reactor during real-time cultivation and do not compensate for changes related to the uneven distribution of radiation inside the reactor or the phytotron chamber. The aim of the article is to present an innovative concept of a photobioreactor prototype for the cultivation of photosynthetic microorganisms, in particular, the integrated follow-up lighting system. The model has been extended with additional elements by installing a circulating pump forcing the circulation of cultures so that all cells receive the same amount of light energy that directly affects their growth.
Taking the above into account, as part of research on photosynthetic microorganisms, a simplified photobioreactor model for studying the influence of light and other parameters on microalgae cultures was designed and built at the Department of Mechanical Engineering and Agrophysics. The photobioreactor prototype with the follow-up lighting system was submitted to the Patent Office and is currently assigned a number (P.429507) [
32]. The invention relates to a specialized device for cultivating microorganisms, including microalgae, both in a continuous and periodic system. The solution for continuous production is intended for industrial needs, whereas the solution for periodic farming is for the needs of scientific research and to pilot optimization of breeding.
In order to ensure uniform and sufficient distribution of light along with dynamically changing conditions during cultivation, a photobioreactor prototype was designed with a follow-up, or lagging, lighting system. The key role in the lagging lighting system is played by a fully controlled and programmed unit, coupled with a measurement of the productivity of the farm and PAR (photosynthetically active radiation, a range of wavelengths of light in the range of 400–700 nm) [
33] in the medium, enabling change of the lighting parameters relative to the changing culture conditions during the process. The entire lighting system is implemented by using LED RBGW and LED RBG lamps, selected in terms of wavelengths so that they best match the natural needs of photosynthetic microorganisms. LED lighting during operation generates less heat and is energy-saving. The controllers enable independent, smooth control of the intensity of colored LED panels, in individual modules, in the full power range together with the measurement of PAR radiation. The use of this type of lighting allows for smooth adjustment of light intensity and tunability of color intensity. In addition, the unit allows you to set independent brightening or blanking speeds of individual lighting modules with different time increments. The intensity of radiation is coupled with PAR measurement inside the reactor.
Functionally, the system has been divided into three basic lighting modules. A characteristic feature of the lighting system is a cylindrical light jacket ensuring an even illumination of the medium around the perimeter of the reactor. In addition, the system includes lighting for the bottom of the tank and lid. Thanks to the multidirectional illumination of cylindrical reactors, a large, developed light surface for breeding is obtained.
2. Materials and Methods
2.1. Construction of the System
A glass cylindrical reaction vessel (photobioreactor), transparent to solar and artificial radiation, ensures the transmission of as much energy as possible to the absorbing surface. The reactor consists of three lighting modules with control and registration system, feeding, mixing and sampling. The glass cylinder of the photobioreactor is placed in the light jacket, covered from the top with an airtight cover together with holes for taking samples, through which the feeding, mixing, venting, and transporting systems of the culture medium pass. The photobioreactor’s lighting consists of the following lighting modules:
module I—the cylindrical jacket panel inside which the reactor is placed,
module II—lower lighting panel, implemented by lighting from the bottom of the reaction tank —the light source is lighting,
module III—the panel of the illuminated reactor tank cover.
All light modules are coupled together and allow for full regulation and control of both color and intensity, duration of lighting time, and the possibility of setting independent speeds of simulating sunrises and sunsets or applying pulsed radiation of the required color. The lighting system is intended for cylindrical units with various L/d modules (aspect; height divided by diameter). The lighting is controlled by a computer (MCU), which selects the optimal parameters based on the sensor of the photosynthetic active PAR radiation intensity and the time of day and night. Knowledge of the distribution of radiation energy density and degradation in the PBR and energy absorption by algal biomass is necessary for the analysis and optimization of cultures in photobioreactors with artificial lighting. The control parameters are selected empirically and saved to the MCU in the form of patterns. The sensor of the intensity of photosynthetic PAR radiation can be moved additionally along the radius of the cylinder, which allows the measurement of the intensity of PAR radiation in the reactor space.
The upper light panel, which at the same time constitutes the reactor cover, ensures the supply and drainage of media necessary for culturing. In addition, the entire photobioreactor unit is equipped with:
a sensor for measuring PAR (linear, point),
a real-time optical density meter for breeding,
a diffuser for the CO2 feeding system and feeding of other process gases, equipped with an inlet nozzle with a shut-off valve and a meter to measure the amount of gas fed,
a biomass mixing and sampling system—a suction and delivery pump with a discharge connector for removing biomass with a shut-off valve,
a temperature measurement sensor,
a system for measuring filtration and UV disinfection and nutrient solution,
an inoculum delivery and nutrient replenishment system, and
a cooling and heating system—a thermostabilization system for breeding.
The photobioreactor according to the invention works in strictly controlled and programmed conditions, suitable for use at a semi-industrial and industrial scale. The scheme of the photobioreactor is shown in
Figure 1.
The photobioreactor (1) is a glass, cylindrical reaction vessel with a dm3·d−1 module (0.3–2.0), transparent in the visible and invisible spectrum for photosynthetically active radiation and embedded inside a lighting system that includes a cylindrical light jacket (2), lower light panel (3), and upper light panel (4) (constituting the reactor cover). The photobioreactor system is equipped with a suction and force pump (5), quantum immersion sensor for measuring photosynthetic radiation PAR (6), temperature sensor (7), CO2 feeding system, and the introduction of other process gases (8). Moreover, the entire photobioreactor system also includes a biomass pickup system (9), UV measurement and disinfection unit of the filtrate (10), nutrient solution and inoculum (11), and heating-cooling system (12).
All systems, meters, and sensors are connected to the microcomputer controller (13), to which the lighting controls of the three light panels are also connected.
A simplified prototype was made at the University of Agriculture in Krakow. During the experimentation of cultivation, the Chlorella Vulgaris strain BA0002a obtained from the Collection of Baltic Sea Cultures of the University of Gdańsk (CCBA) was used. The first experiments were carried out in a prototype glass photobioreactor with an active volume of 4 dm³. Before the experiments were started, the photobioreactor was sterilized and inoculated with 2 ml of microalgae in the medium. The experiments were carried out at a temperature of 23–25 °C and at a pH of 6.5–7.0. The culture was fed with carbon dioxide fed to the interior of the photobioreactor by means of a diffuser. Circulation of the culture medium was ensured using a circulating pump with an average capacity of 80.0 dm3·h−1. The lighting was a follow-up lighting system using LED RBGW diodes, with the number of lighting points being not less than 1500 RGBW LEDs and RGB LED per m2, in the form of a cylindrical light jacket and a lower panel lighting system and illuminated cover.
2.2. Methodology for Measuring the Intensity of Lighting
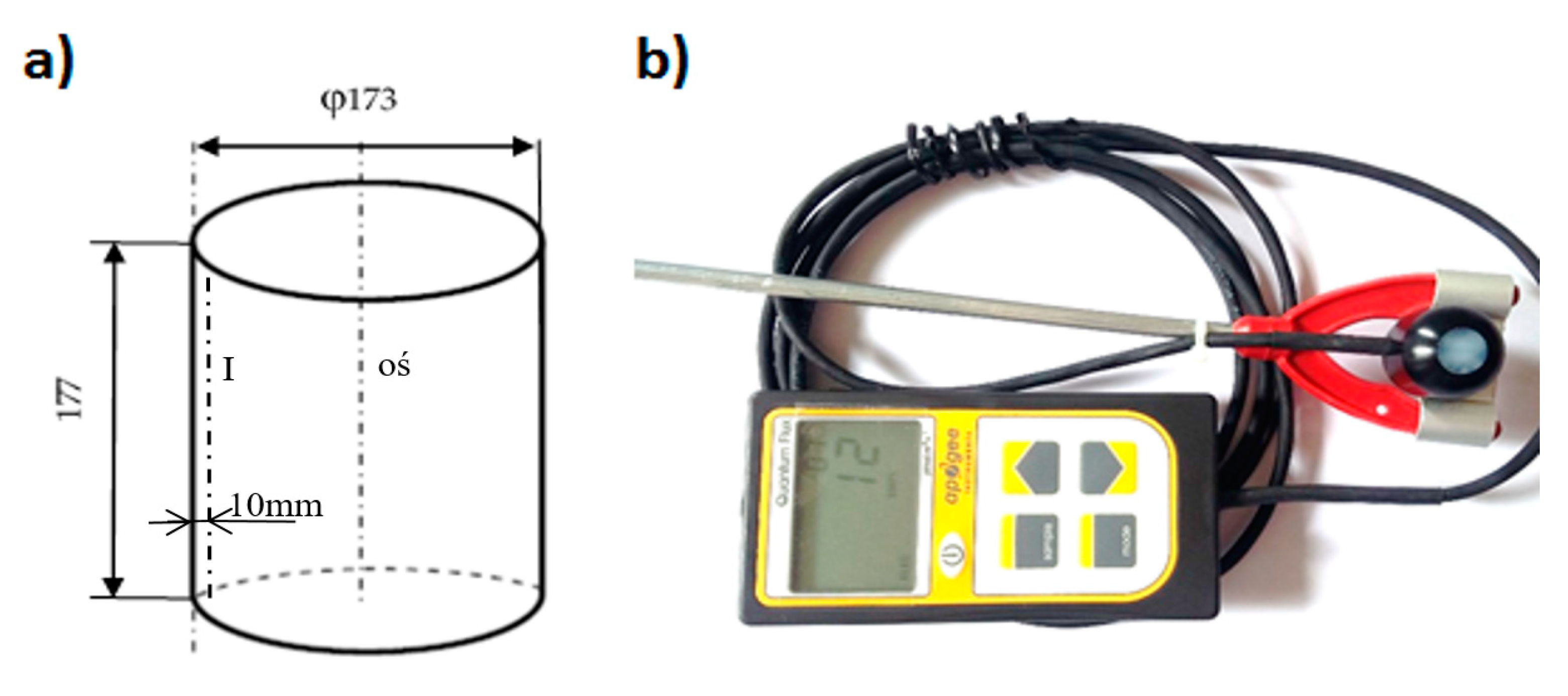
Preliminary pilot studies were carried out on the designed prototype of the photobioreactor. Measurement of the PAR radiation intensity inside the culture medium was carried out with the Quantum Meter MQ-100 Apogee Instruments measuring probe (
Figure 2b) for measurements in an aqueous environment with a spectral range of 410–655 nm. The measuring device consisted of a digital meter with a quantum sensor. The tests were carried out during breeding, inside the culture medium with a directional probe set to the light source. Measurements were made along measurement path I marked on the diagram and on the axis of the photobioreactor (WWTP). The meter sensor was mounted on a tripod rail and moved vertically every 10 mm ± 1.0 mm along the marked measurement lines (
Figure 2a). The measurements made will ultimately form the measurement grid of the PAR radiation intensity in the active volume of the reactors with a different lighting system.
The tests were carried out for the dual cylindrical light jacket switched on and the low light panel also switched on. LED directional lighting placed from the bottom contained 30 points of light of about 18 W, 1100 lm, 3000 K (warm white color), and 230 V, while the double light jacket consisted of: module I—150 LED RBG type LED lights with 36 W, 12 V, controller, white on; module II—300 points of light—18 W 950 lm, with a color temperature of 3100 K (warm white), 12 V power supply. The lighting worked in the day/night 14/10 system. The geometrical dimensions of the cylindrical light jacket were: diameter (24.5 ± 1.0) mm, height (19.5 ± 1.0) mm.
Growth of microalgae Chlorella vulgaris BA0002a from the collection of Baltic algae cultures at the CCBA (Culture Collection of Baltic Algae) was carried out in a sterilized cylindrical glass reactor with an dm
3·d
−1 module equal to 0.985 and 4.16 dm
3, including an active capacity of 4 dm
3 on nutrient medium about the composition of
Table 1.
The medium of 4 dm
3 was inoculated with 2 ml of microalgae inoculum.
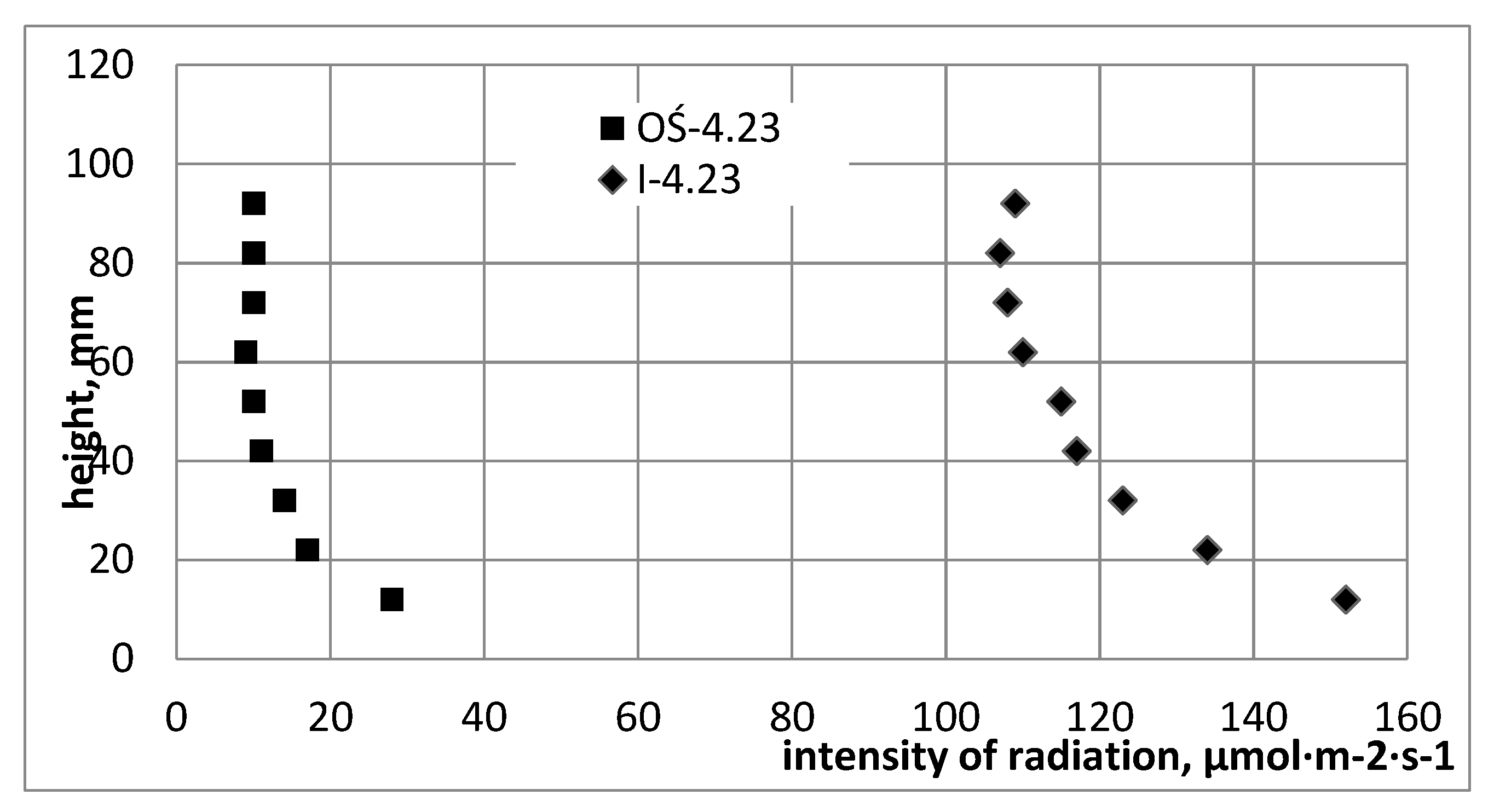
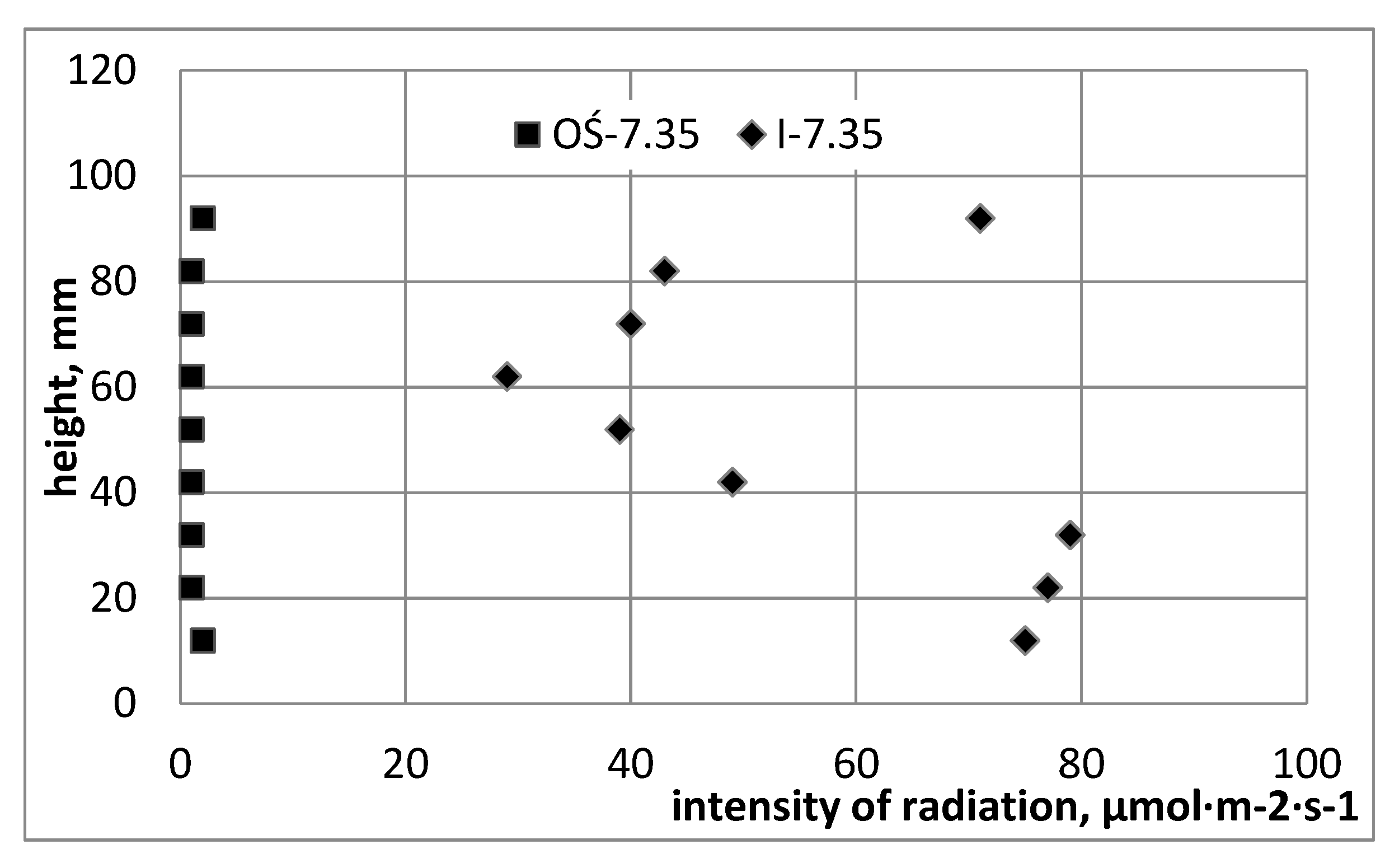
Figure 3 presents graphs from pilot measurements of the intensity of photosynthetically active radiation, using a measuring probe whose head was directed towards the light jacket and was moved along measuring path I and on the axis of the reactor. Measurements of PAR radiation intensity were made for two different optical densities of the cultured 4.23 McF and 7.35 McF media, determined using a DEN-1B densitometer, in the McF degrees (0–180·10
4 cell·dm
−3).