Effect of UV-Light Treatment on Efficiency of Perovskite Solar Cells (PSCs)
Abstract
1. Introduction
2. Experimental Details and Measurements
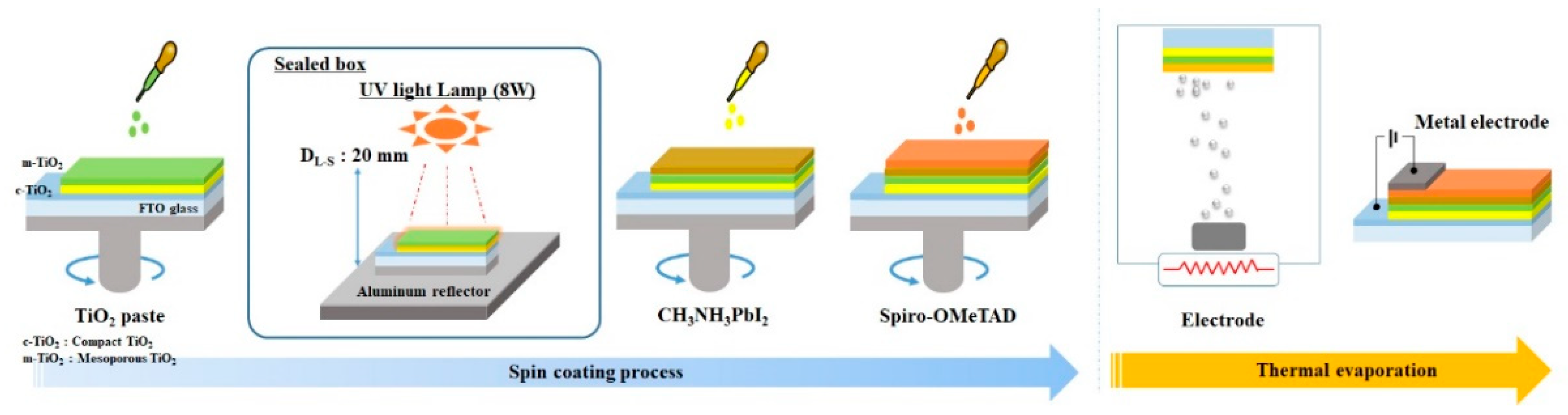
2.1. Materials and Preparation of Solar Cells
2.2. UV Surface Treatment of Samples
2.3. Measurements
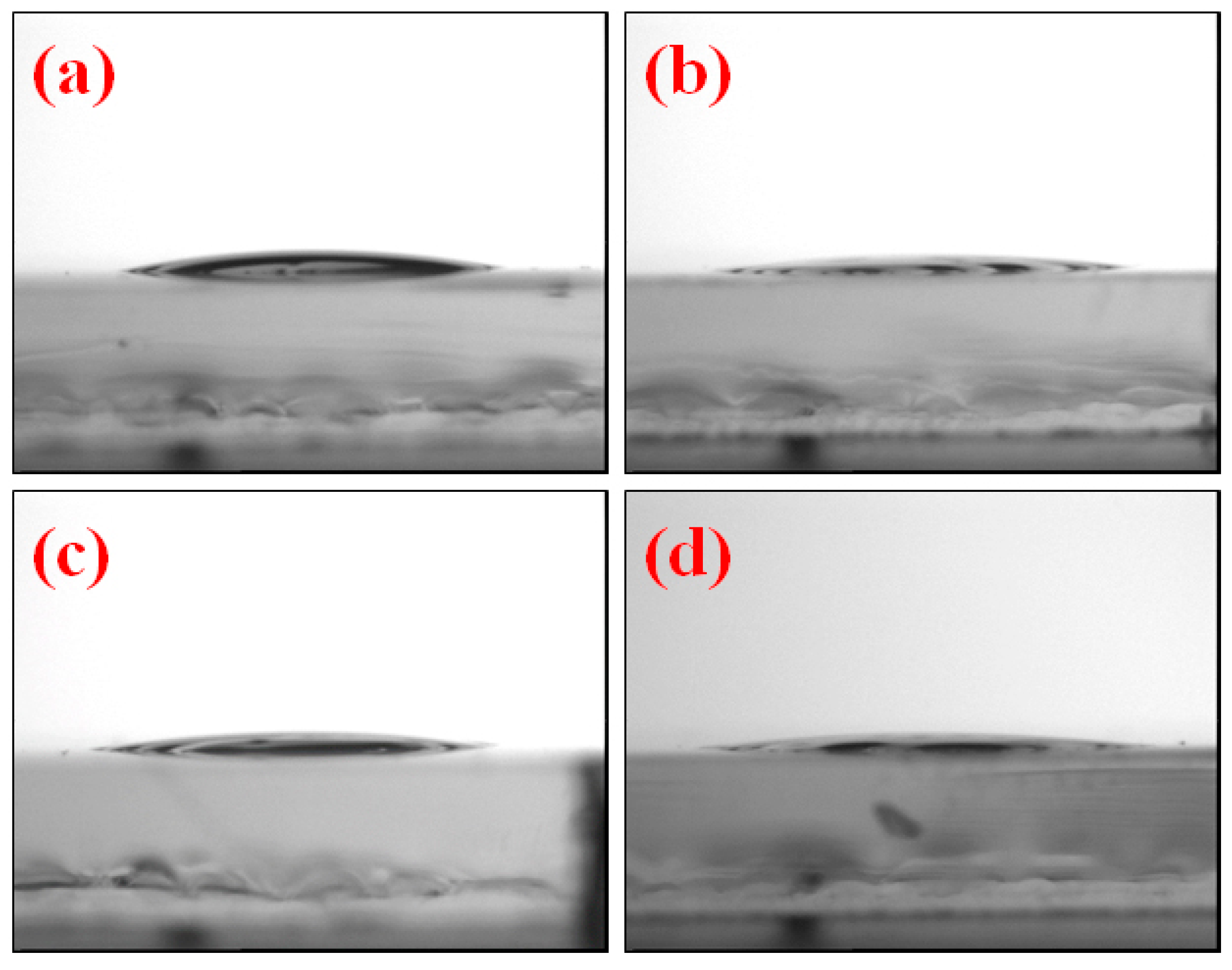
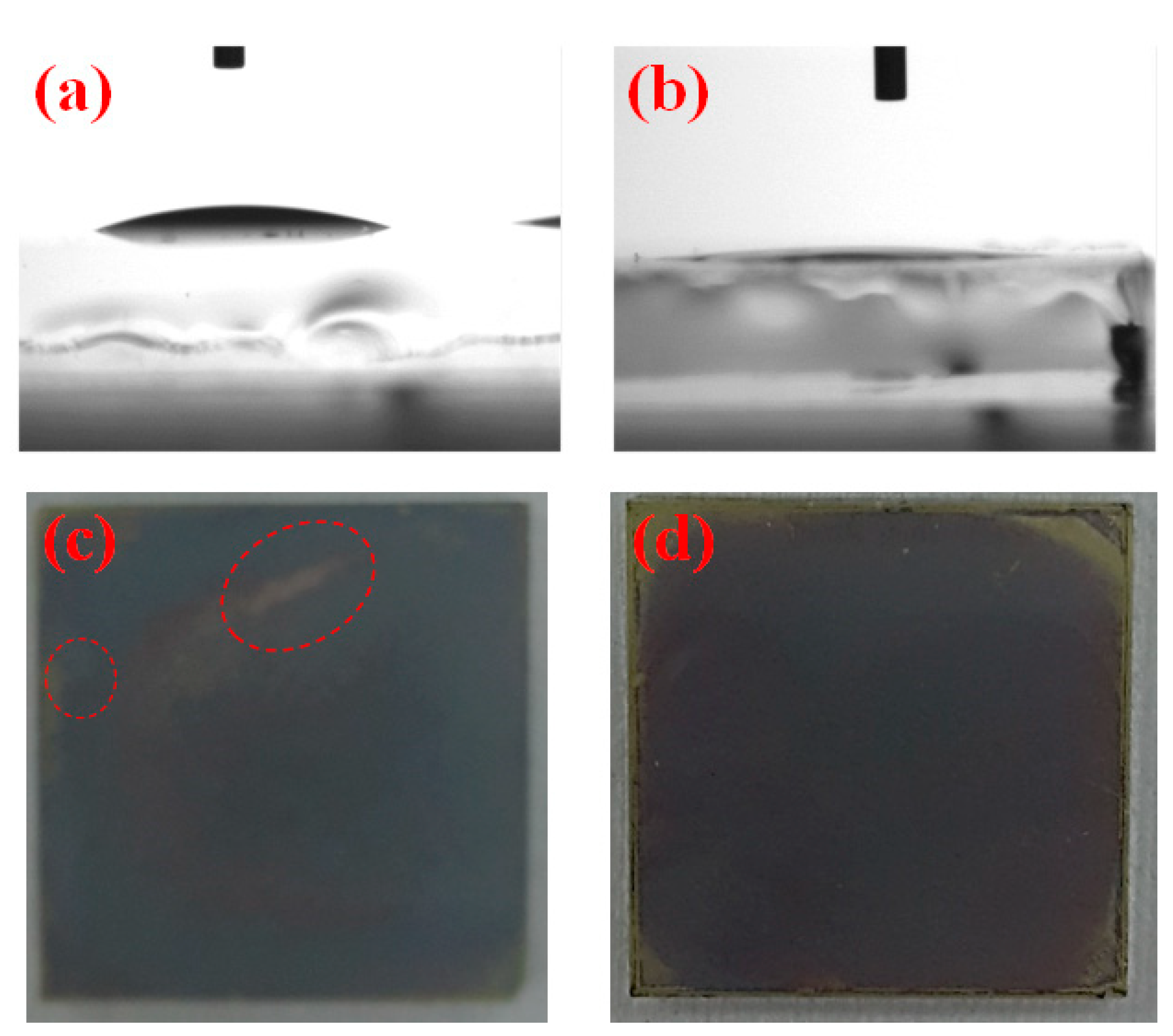
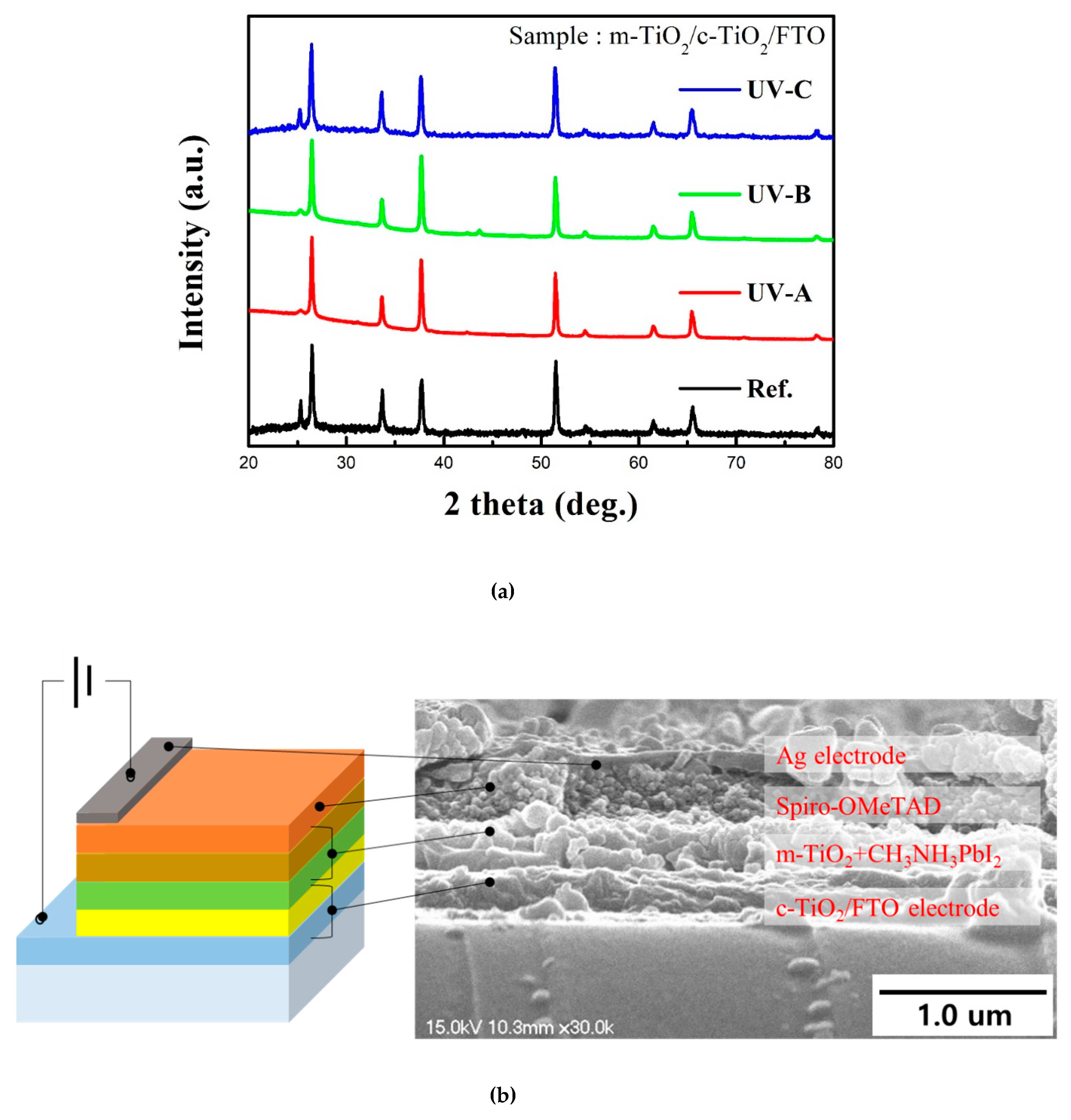
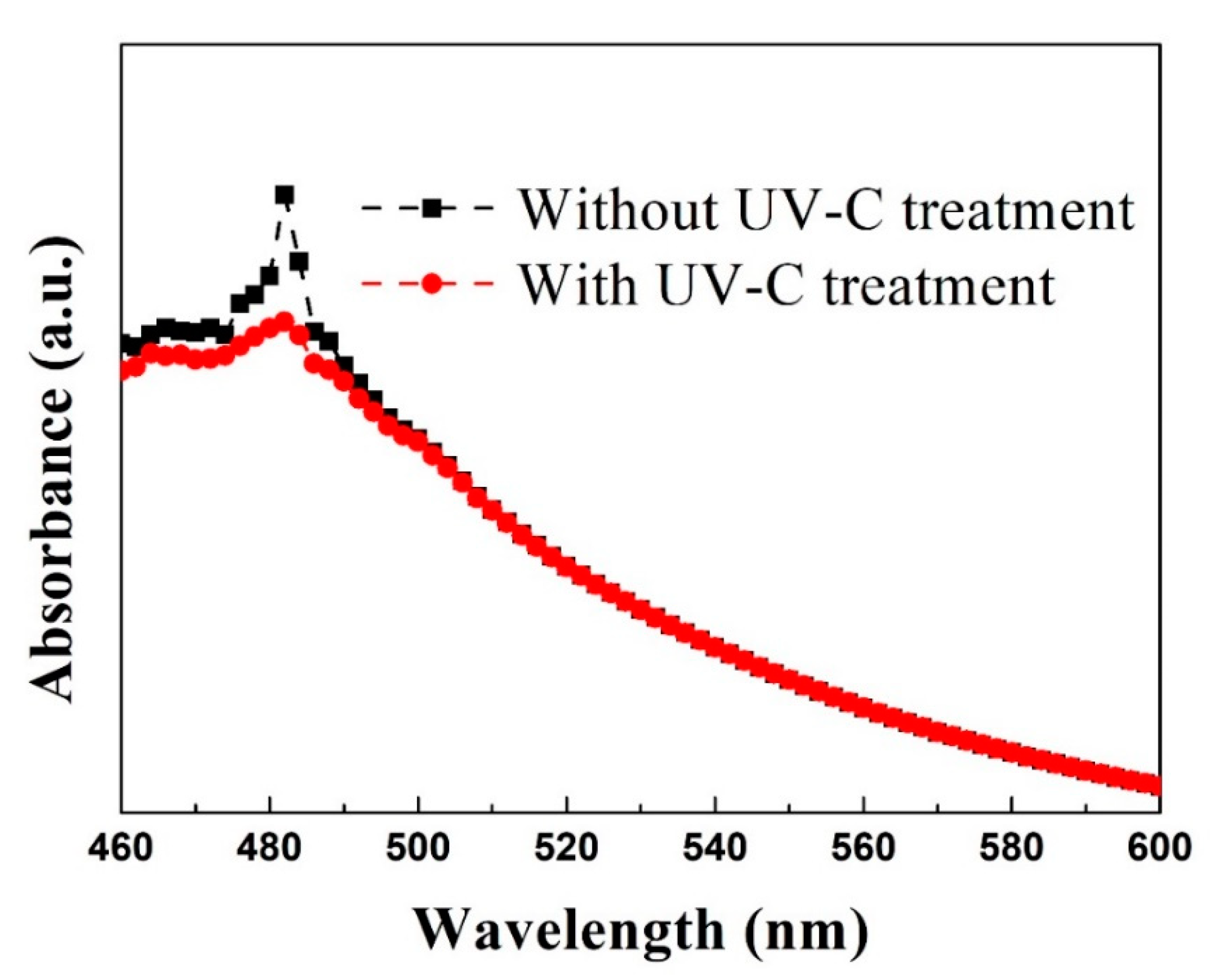
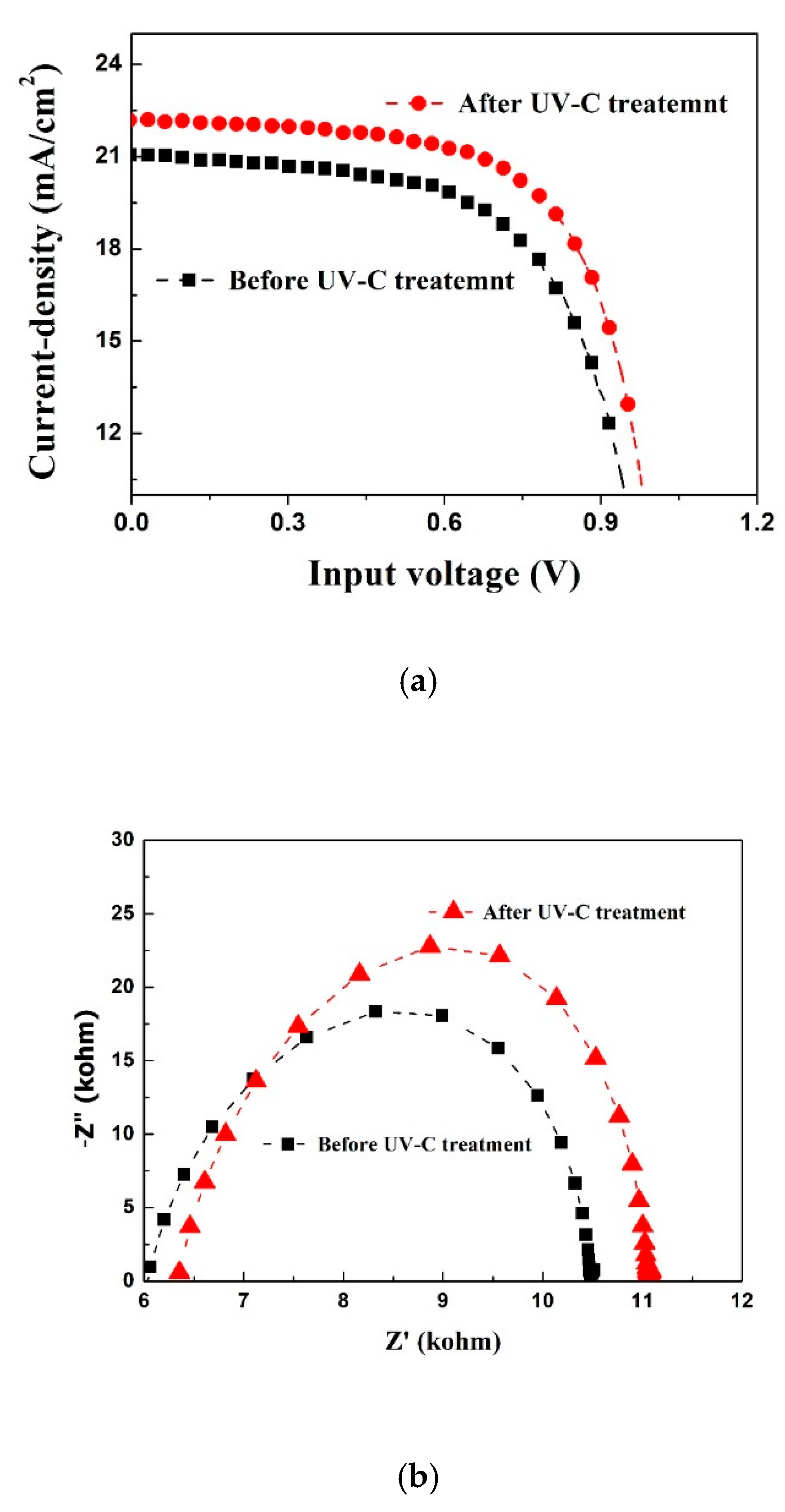
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Battaglia, C.; Cuevas, A.; De Wolf, S. High-efficiency crystalline silicon solar cells: Status and perspectives. Energy Environ. Sci. 2016, 9, 1552–1576. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.; Xiao, S.; Gu, X. Review of status developments of high-efficiency crystalline silicon solar cells. J. Phys. D Appl. Phys. 2018, 51, 123001. [Google Scholar] [CrossRef]
- Assadi, M.K.; Bakhodaa, S.; Saidur, R.; Hanaei, H. Recent progress in perovskite solar cells. Renew. Sustain. Energy Rev. 2018, 81, 2812–2822. [Google Scholar] [CrossRef]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275. [Google Scholar] [CrossRef]
- Ishikawa, R.; Ueno, K.; Shirai, H. Improved efficiency of methylammonium-free perovskite thin film solar cells by fluorinated ammonium iodide treatment. Org. Electron. 2020, 78, 1–7. [Google Scholar] [CrossRef]
- Bella, F. Polymer electrolytes and perovskites: Lights and shadows in photovoltaic devices. Electrochim. Acta 2015, 175, 151–161. [Google Scholar] [CrossRef]
- Noh, J.H.; Jeon, N.J.; Choi, Y.C.; Nazeeruddin, M.K.; Grätzelb, M.; Seok, S.I. Nanostructured TiO2/CH3NH3PbI3 heterojunction solar cells employing spiro-OMeTAD/Co-complex as hole-transporting material. J. Mater. Chem. A 2013, 1, 11842–11847. [Google Scholar] [CrossRef]
- Boix, P.P.; Nonomura, K.; Mathews, N.; Mhaisalkar, S.G. Current progress and future perspectives for organic/inorganic perovskite solar cells. Mater. Today 2014, 17, 16–23. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Liu, F.Z.; Tam, H.W.; Wong, M.K.; Ng, A.; Surya, C.; Chen, W.; He, Z.B. Perovskite solar cells —An overview of critical issues. Prog. Quantum Electron. 2017, 53, 1–37. [Google Scholar] [CrossRef]
- Yeh, N.; Yeh, P. Organic solar cells: Their developments and potentials. Renew. Sustain. Energy Rev. 2013, 21, 421–431. [Google Scholar] [CrossRef]
- Park, N.G. Crystal growth engineering for high efficiency perovskite solar cells. CrystEngComm 2016, 18, 5977–5985. [Google Scholar] [CrossRef]
- Ko, H.S.; Lee, J.W.; Park, H.H. 5.76% efficiency perovskite solar cells prepared under high relative humidity: Importance of PbI2 morphology in two-step deposition of CH3NH3PbI3. J. Mater. Chem. A 2015, 3, 8808–8815. [Google Scholar] [CrossRef]
- Fagiolari, L.; Bella, F. Carbon-based materials for stable, cheaper and large-scale processable perovskite solar cells. Energy Environ. Sci. 2019, 12, 3437–3472. [Google Scholar] [CrossRef]
- Gilbertz, K.-P.; Meineke, V.; Placzek, M. Ultraviolet Exposure: Health Effects Encyclopedia of Environmental Health, 2nd ed.; Elsevier: Cambridge, MA, USA, 2019; pp. 185–194. [Google Scholar]
- Lu, H.; He, B.; Ji, Y.; Shan, Y.; Zhong, C.; Xu, J.; LiuYang, J.; Wu, F.; Zhu, L. Dopant-free hole transport materials processed with green solvent for efficient perovskite solar cells. Chem. Eng. J. 2020, 385, 1–7. [Google Scholar] [CrossRef]
- Huan, T.N.; Corte, D.A.D.; Lamaison, S.; Karapinar, D.; Lutz, L.; Menguy, N.; Foldyna, M.; Turren-Cruz, S.-H.; Hagfeldt, A.; Bella, F.; et al. Low-cost high-efficiency system for solar-driven conversion of CO2 to hydrocarbons. PNAS 2019, 116, 9735–9740. [Google Scholar] [CrossRef] [PubMed]
- Menariya, B.K.; Ameta, R.; Ameta, S.C. A Comparative Study on Photocatalytic Activity of ZnO, SnO2 and ZnO-SnO2 Composites. Int. J. Chem. Sci. 2017, 15, 174. [Google Scholar]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Song, M.G.; Bark, C.W. Synthesis and characterization of UV-treated Fe-doped bismuth lanthanum titanate-doped TiO2 layers in dye-sensitized solar cells. J. Korean Phys. Soc. 2016, 68, 1399–1402. [Google Scholar] [CrossRef]
- Shin, S.G.; Bark, C.W.; Kim, S.; Choi, H.W. Properties of perovskite solar cells by the sputtered compact TiO2 Layer. Sci. Adv. Mater. 2017, 9, 1517–1521. [Google Scholar] [CrossRef]
- Im, J.H.; Kim, H.S.; Park, N.G. Morphology-photovoltaic property correlation in perovskite solar cells: One-step versus two-step deposition of CH3NH3PbI3. APL Mater. 2014, 2, 081510. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.Y.; Jang, I.H.; Kang, S.M.; Choi, M.; Park, N.G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via lewis base adduct of lead (II) iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Taherian, F.; Marcon, V.; van der Vegt, N.F.A. What Is the Contact Angle of Water on Graphene. Langmuir 2013, 29, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, C.; Park, J.Y.; Yamada, Y.; Lee, H.S.; Tsung, C.K.; Yang, P.; Somorjai, G.A. Sum frequency generation and catalytic reaction studies of the removal of organic capping agents from Pt nanoparticles by UV-ozone treatment. J. Phys. Chem. C 2009, 113, 6150–6155. [Google Scholar] [CrossRef]
- Chen, J.; Subramani, T.; Sun, T.; Jevasuwan, W.; Fukata, N. Efficiency enhancement of silicon nanowire solar cells by using UV/Ozone treatments and micro-grid electrodes. Appl. Surf. Sci. 2018, 439, 1057–1064. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Duan, J.; Gong, L.; Wu, J.; Jang, L.; Zhou, C.; Xie, H.; Gao, Y.; He, H.; et al. All-inorganic, hole-transporting-layer-free, carbon-based CsPbIBr2 planar perovskite solar cells by a two-step temperature-control annealing process. Mater. Sci. Semicond. Process. 2020, 108, 104870. [Google Scholar] [CrossRef]
- Roghabadi, F.A.; Fumani, N.M.R.; Alidaei, M.; Ahmadi, V.; Sadrameli, S.M. High Power UV-Light Irradiation as a New Method for Defect Passivation in Degraded Perovskite Solar Cells to Recover and Enhance the Performance. Sci. Rep. 2019, 9, 9448. [Google Scholar] [CrossRef]
- Saekow, S.; Maiakgree, W.; Jarenboon, W.; Pimanpang, S.; Amornkitbamrung, W. High intensity UV radiation ozone treatment of nanocrystalline TiO2 layers for high efficiency of dye-sensitized solar cells. J. Non-Cryst. Solids 2012, 358, 2496–2500. [Google Scholar] [CrossRef]
- Lee, H.; Bark, C.W.; Choi, H.W. Fabrication and characterization of perovskite solar cells with ZnGa2O4 mixed TiO2 photoelectrode. Jpn. J. Appl. Phys. 2019, 58, SDDE15. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Visal, S.; Kuniyoshi, M.; Kaneko, T.; Umezu, S.; Katsumata, T.; Iwamori, S.; Kakihana, M.; Taima, T.; Isomura, M.; et al. Low-Temperature-Processed Brookite-Based TiO2 Heterophase Junction Enhances Performance of Planar Perovskite Solar Cells. Nano Lett. 2019, 19, 598–604. [Google Scholar] [CrossRef]

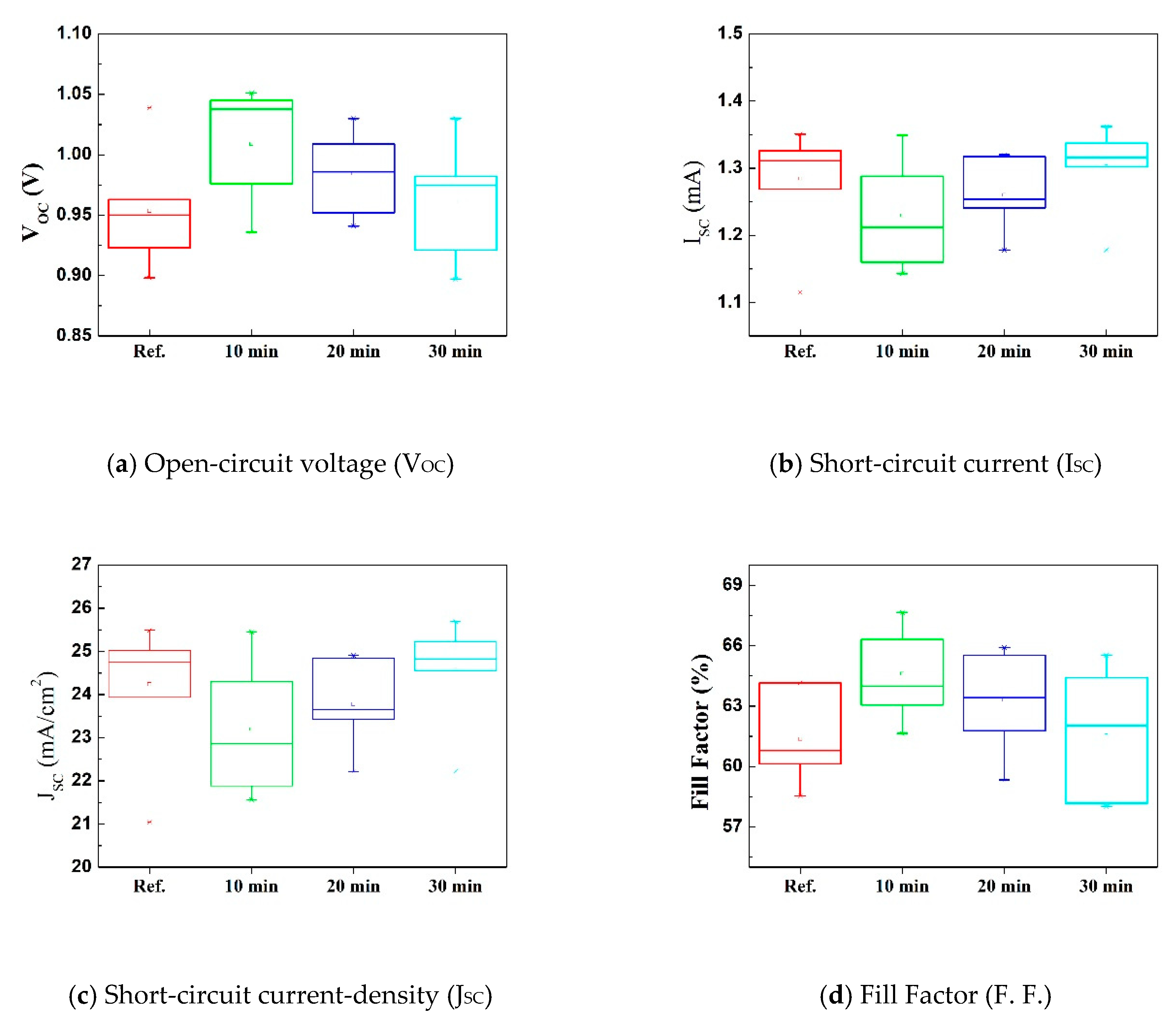
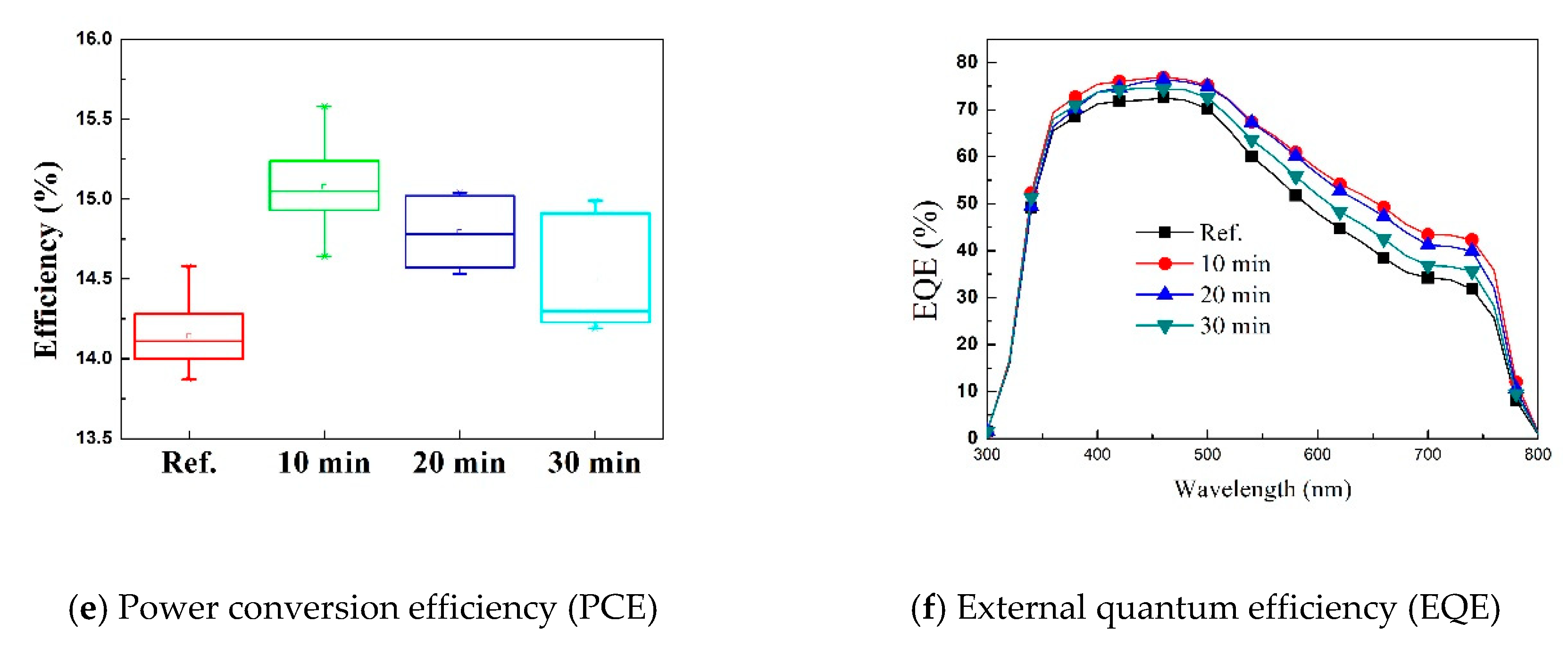
| Parameters | aVOC | bISC | cJSC | dF.F. | ePCE | |
|---|---|---|---|---|---|---|
| (V) | (mA) | (mA/cm2) | (%) | (%) | ||
| Without UV-C treatment | 0.953 | 1.284 | 24.239 | 61.37 | 14.142 | |
| (0.0436) | (0.0789) | (1.489) | (2.080) | (0.232) | ||
| With UV-C treatment | 10 min | 1.008 | 1.229 | 23.198 | 64.602 | 15.076 |
| (0.045) | (0.077) | (1.457) | (2.165) | (0.288) | ||
| 20 min | 0.942 | 1.260 | 23.774 | 63.312 | 14.794 | |
| (0.030) | (0.048) | (0.921) | (2.325) | (0.223) | ||
| 30 min | 0.961 | 1.302 | 24.573 | 61.537 | 14.493 | |
| (0.044) | (0.058) | (1.110) | (3.169) | (10.745) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Quy, H.V.; Choi, H.W.; Bark, C.W. Effect of UV-Light Treatment on Efficiency of Perovskite Solar Cells (PSCs). Energies 2020, 13, 1069. https://doi.org/10.3390/en13051069
Kim S, Quy HV, Choi HW, Bark CW. Effect of UV-Light Treatment on Efficiency of Perovskite Solar Cells (PSCs). Energies. 2020; 13(5):1069. https://doi.org/10.3390/en13051069
Chicago/Turabian StyleKim, Sangmo, Hoang Van Quy, Hyung Wook Choi, and Chung Wung Bark. 2020. "Effect of UV-Light Treatment on Efficiency of Perovskite Solar Cells (PSCs)" Energies 13, no. 5: 1069. https://doi.org/10.3390/en13051069
APA StyleKim, S., Quy, H. V., Choi, H. W., & Bark, C. W. (2020). Effect of UV-Light Treatment on Efficiency of Perovskite Solar Cells (PSCs). Energies, 13(5), 1069. https://doi.org/10.3390/en13051069






