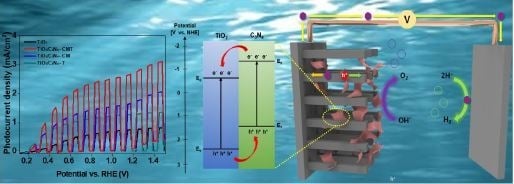
Influence of C3N4 Precursors on Photoelectrochemical Behavior of TiO2/C3N4 Photoanode for Solar Water Oxidation
Abstract
1. Introduction
2. Experimental
2.1. TiO2 Nanorods Synthesis
2.2. Graphitic C3N4 Synthesis
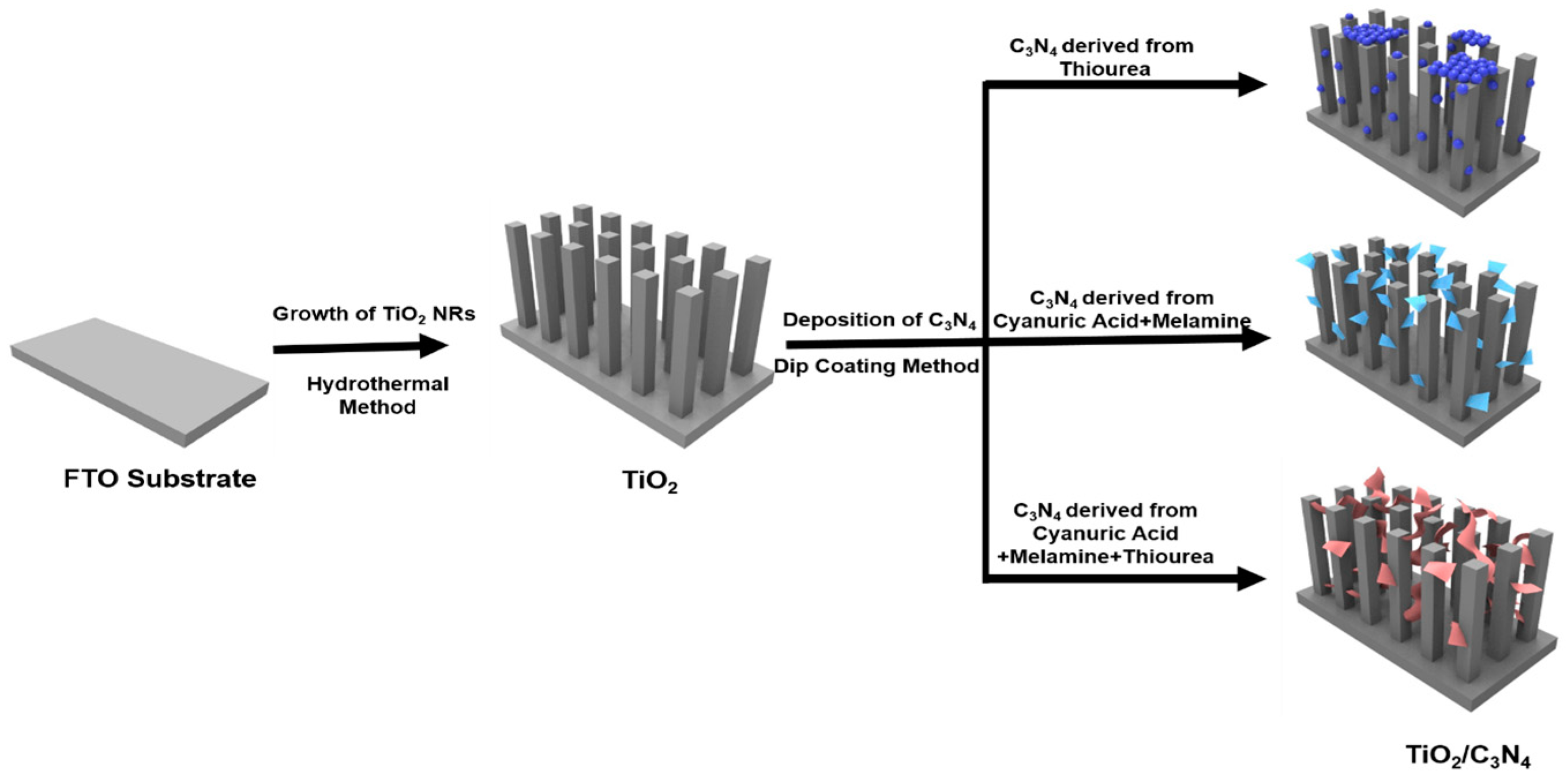
2.3. TiO2/C3N4 Fabrication
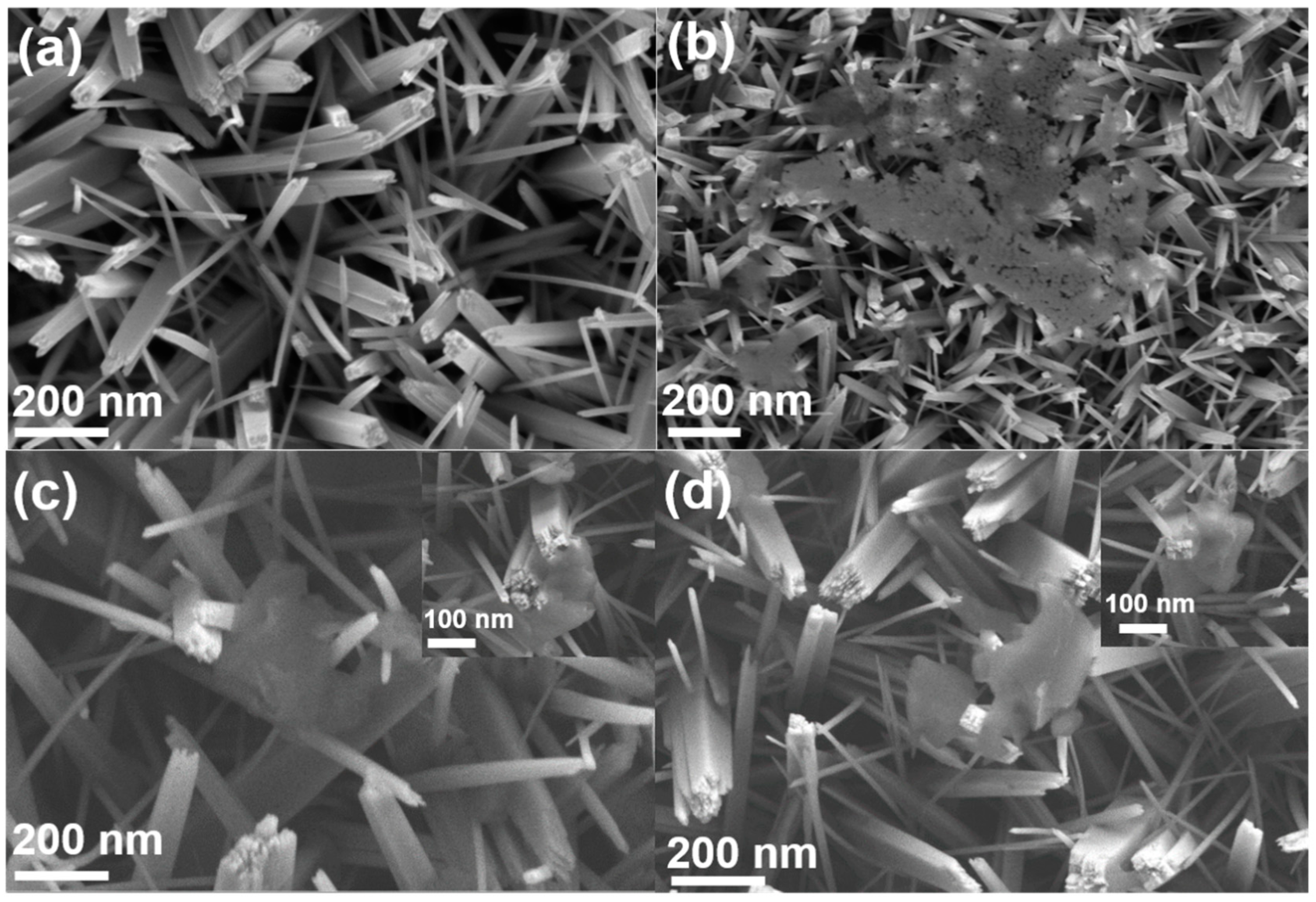
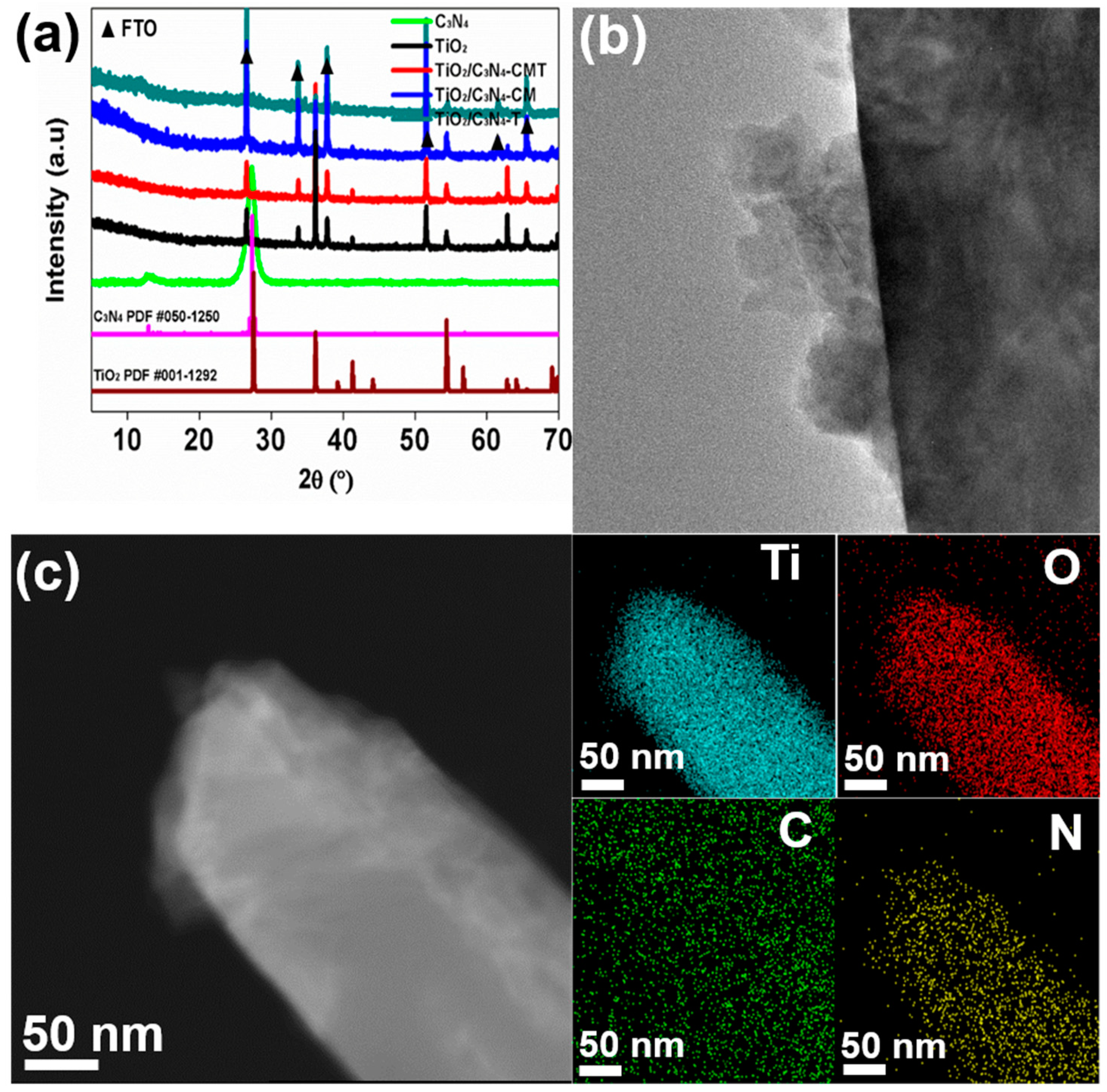
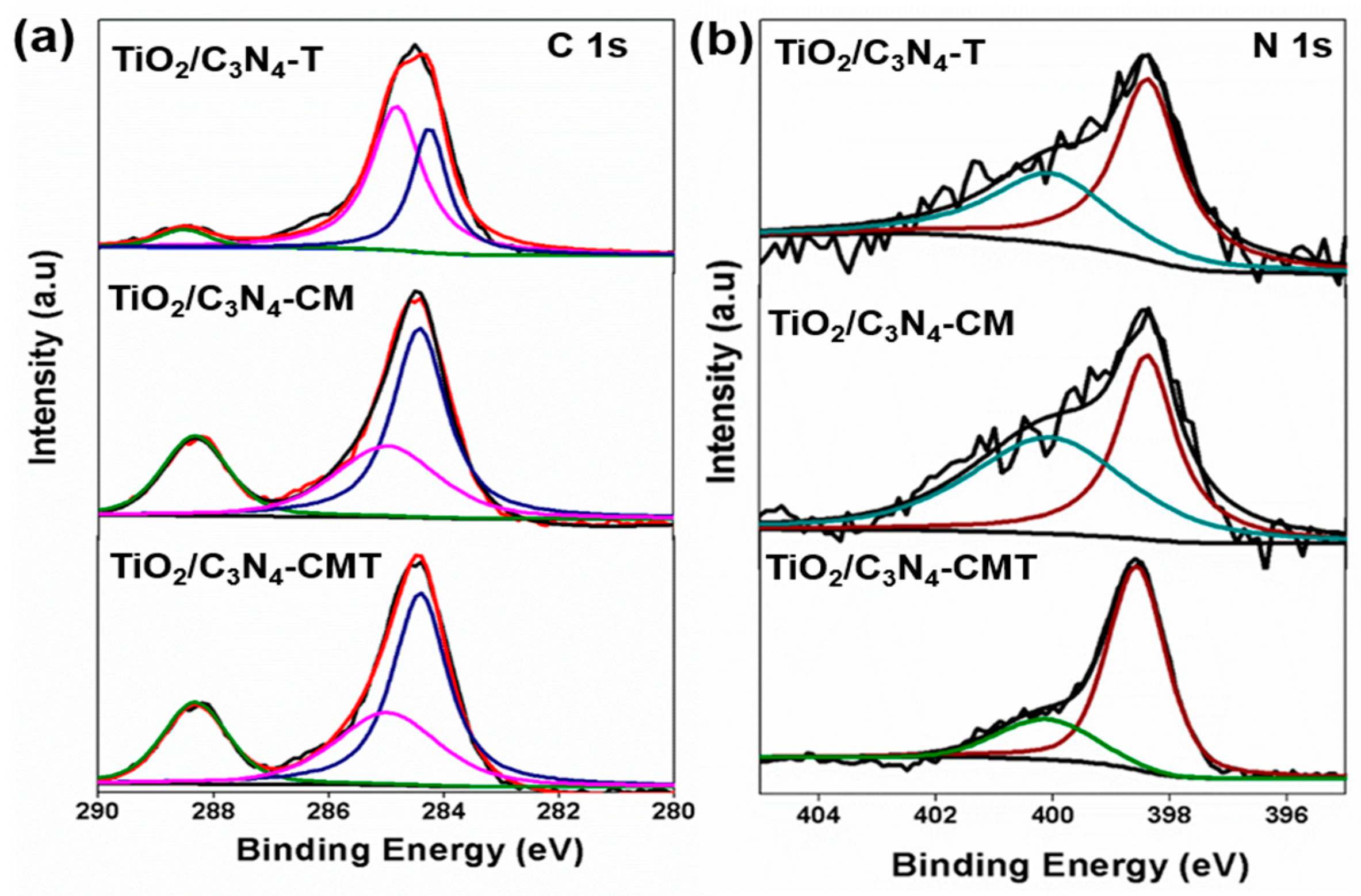
3. Materials Characterization
4. Photoelectrochemical Characterization
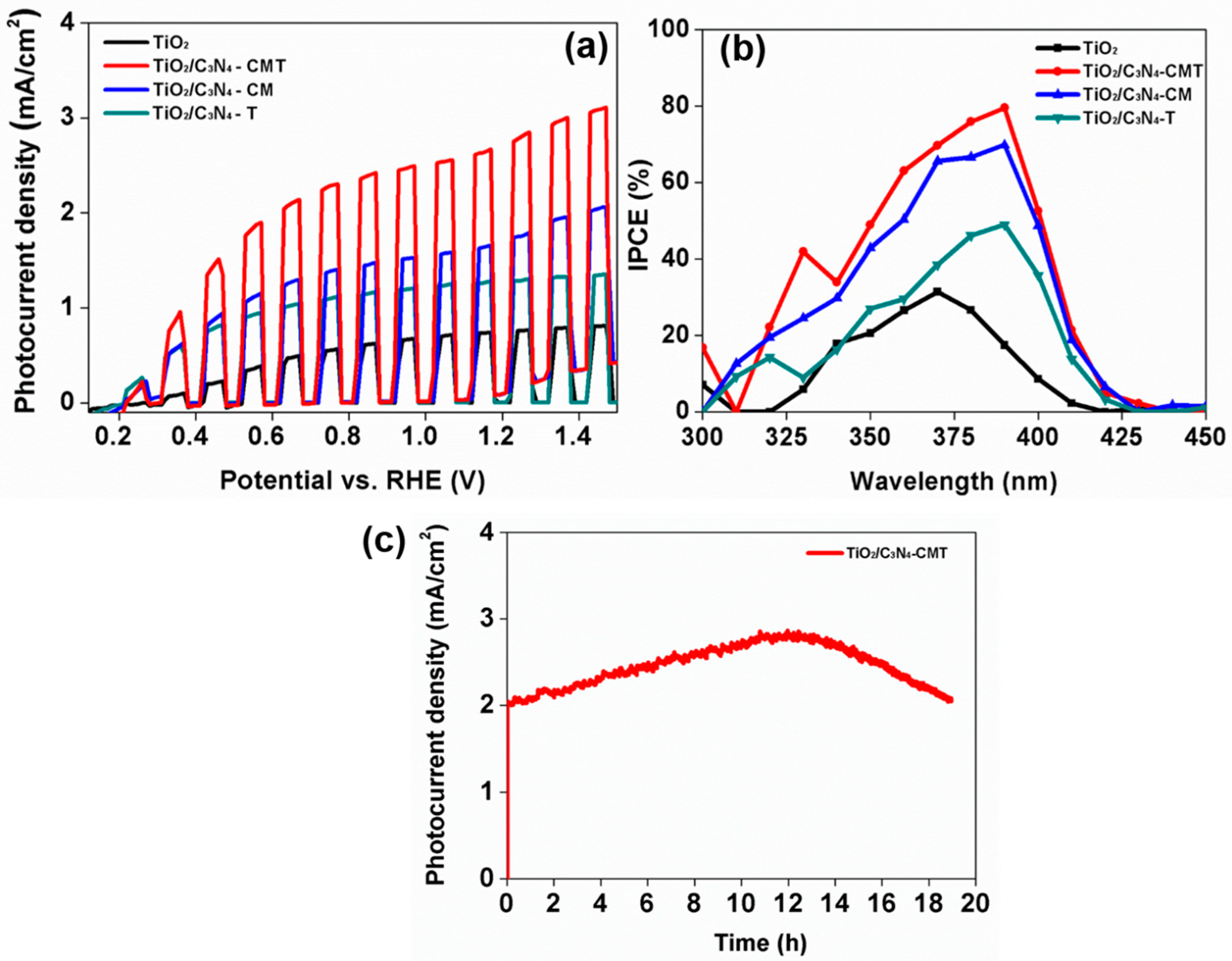
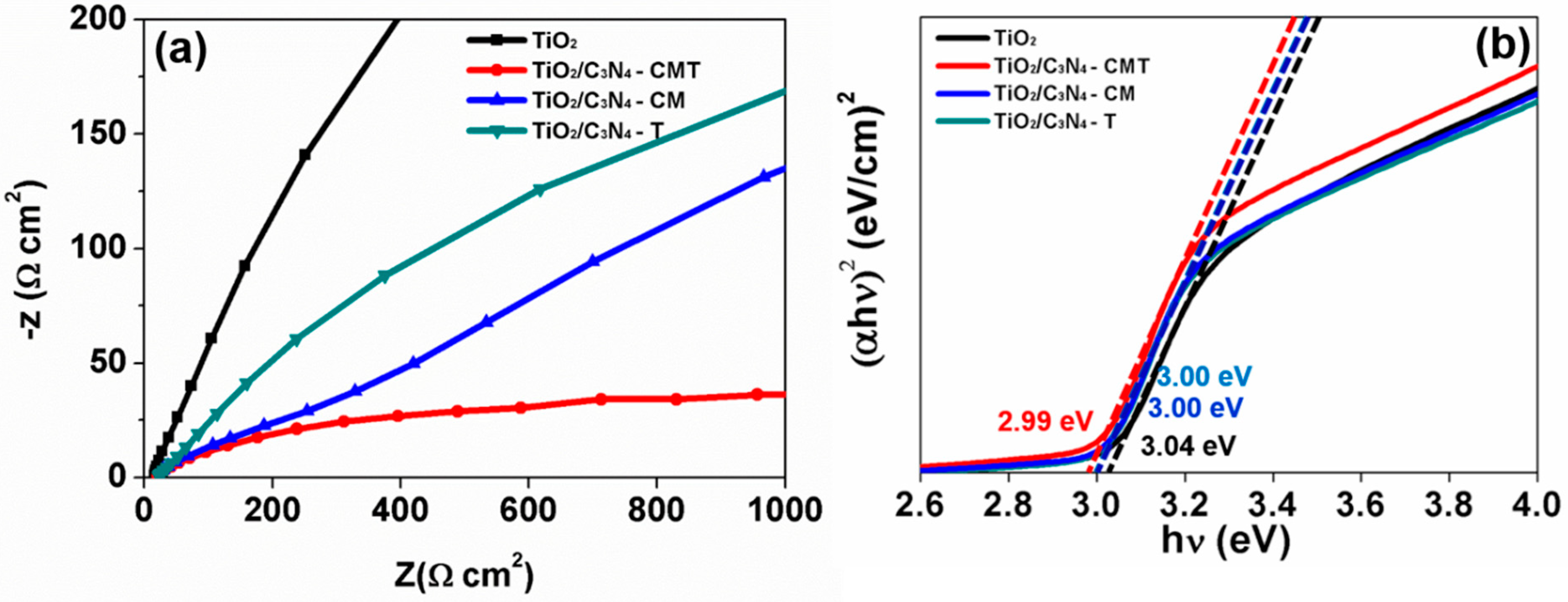
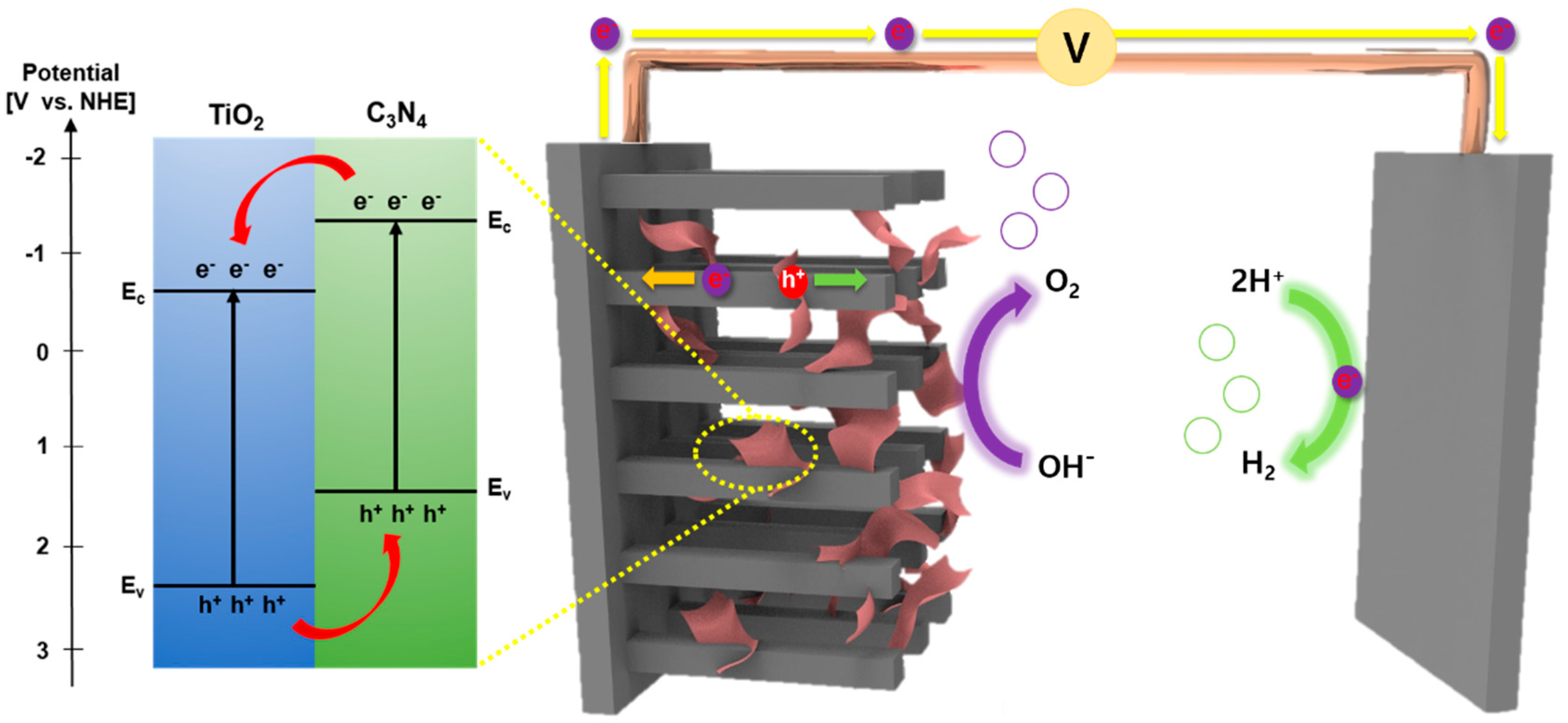
5. Results and Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bhat, S.S.M.; Pawar, S.A.; Potphode, D.; Moon, C.K.; Suh, J.M.; Kim, C.; Choi, S.; Patil, D.S.; Kim, J.J.; Shin, J.C.; et al. Substantially enhanced photoelectrochemical performance of TiO2 nanorods/CdS nanocrystals heterojunction photoanode decorated with MoS2 nanosheets. Appl. Catal. B Environ. 2019, 259, 118102. [Google Scholar] [CrossRef]
- Lee, M.G.; Park, J.S.; Jang, H.W. Review Solution-Processed Metal Oxide Thin Film Nanostructures for Water Splitting Photoelectrodes: A Review. J. Korean Ceram. Soc. 2018, 55, 185–202. [Google Scholar] [CrossRef]
- Andoshe, D.M.; Jeon, J.M.; Kim, S.Y.; Jang, H.W. Two-dimensional transition metal dichalcogenide nanomaterials for solar water splitting. Electron. Mater. Lett. 2015, 11, 323–335. [Google Scholar] [CrossRef]
- Choi, S.; Hwang, J.; Lee, T.H.; Kim, H.H.; Hong, S.P.; Kim, C.; Choi, M.J.; Park, H.K.; Bhat, S.S.M.; Suh, J.M.; et al. Photoelectrochemical hydrogen production at neutral pH phosphate buffer solution using TiO2 passivated InAs Nanowire/p-Si heterostructure photocathode. Chem. Eng. J. 2019, 123688. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Lee, S.A.; Suh, J.M.; Hong, S.P.; Jang, H.W. Triple planar heterojunction of SnO2/WO3/BiVO4 with enhanced photoelectrochemical performance under front illumination. Appl. Sci. 2018, 8, 1765. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Suh, J.M.; Choi, S.; Hong, S.P.; Lee, S.A.; Kim, C.; Moon, C.W.; Lee, M.G.; Jang, H.W. Substantially enhanced front illumination photocurrent in porous SnO2 nanorods/networked BiVO4 heterojunction photoanodes. J. Mater. Chem. A 2018, 6, 14633–14643. [Google Scholar] [CrossRef]
- Lee, B.R.; Lee, M.G.; Park, H.; Lee, T.H.; Lee, S.A.; Bhat, S.S.M.; Kim, C.; Lee, S.; Jang, H.W. All-Solution-Processed WO3/BiVO4 Core-Shell Nanorod Arrays for Highly Stable Photoanodes. ACS Appl. Mater. Interfaces 2019, 11, 20004–20012. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Jang, H.W. Recent Advances in Bismuth-Based Nanomaterials for Photoelectrochemical Water Splitting. ChemSusChem 2017, 10, 3001–3018. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Sundaram, N.G. Photocatalysis of Bi4NbO8Cl hierarchical nanostructure for degradation of dye under solar/UV irradiation. New J. Chem. 2015, 39, 3956–3963. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Swain, D.; Feygenson, M.; Neuefeind, J.C.; Mishra, A.K.; Hodala, J.L.; Narayana, C.; Shanbhag, G.V.; Sundaram, N.G. Bi4TaO8Cl Nano-Photocatalyst: Influence of Local, Average, and Band Structure. Inorg. Chem. 2017, 56, 5525–5536. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.S.M.; Sundaram, N.G. Efficient visible light photocatalysis of Bi4TaO8Cl nanoparticles synthesized by solution combustion technique. RSC Adv. 2013, 3, 14371–14378. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Maeda, K.; Domen, K.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Sulfur-mediated synthesis of carbon nitride: Band-gap engineering and improved functions for photocatalysis. Energy Environ. Sci. 2011, 4, 675–678. [Google Scholar] [CrossRef]
- Qin, D.D.; Quan, J.J.; Duan, S.F.; San Martin, J.; Lin, Y.; Zhu, X.; Yao, X.Q.; Su, J.Z.; Rodríguez-Gutiérrez, I.; Tao, C.L.; et al. High-Performance Photoelectrochemical Water Oxidation with Phosphorus-Doped and Metal Phosphide Cocatalyst-Modified g-C3N4 Formation Through Gas Treatment. ChemSusChem 2019, 12, 898–907. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Xu, J.; Tang, H.; Chen, K.; Jiang, Y. Tuning the Morphology of g-C3N4 for Improvement of Z-Scheme Photocatalytic Water Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 15285–15293. [Google Scholar] [CrossRef]
- Ho, W.; Zhang, Z.; Lin, W.; Huang, S.; Zhang, X.; Wang, X.; Huang, Y. Copolymerization with 2,4,6-triaminopyrimidine for the rolling-up the layer structure, tunable electronic properties, and photocatalysis of g-C3N4. ACS Appl. Mater. Interfaces 2015, 7, 5497–5505. [Google Scholar] [CrossRef]
- Iqbal, W.; Yang, B.; Zhao, X.; Rauf, M.; Waqas, M.; Gong, Y.; Zhang, J.; Mao, Y. Controllable synthesis of graphitic carbon nitride nanomaterials for solar energy conversion and environmental remediation: The road travelled and the way forward. Catal. Sci. Technol. 2018, 8, 4576–4599. [Google Scholar] [CrossRef]
- Liao, Y.; Zhu, S.; Ma, J.; Sun, Z.; Yin, C.; Zhu, C.; Lou, X.; Zhang, D. Tailoring the Morphology of g-C3N4 by Self-Assembly towards High Photocatalytic Performance. ChemCatChem 2014, 6, 3419–3425. [Google Scholar] [CrossRef]
- Jung, H.; Pham, T.T.; Shin, E.W. Effect of g-C3N4 precursors on the morphological structures of g-C3N4/ZnO composite photocatalysts. J. Alloys Compd. 2019, 788, 1084–1092. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Li, C. A comparison of graphitic carbon nitrides synthesized from different precursors through pyrolysis. J. Photochem. Photobiol. A Chem. 2017, 332, 32–44. [Google Scholar] [CrossRef]
- Pham, T.T.; Shin, E.W. Influence of g-C3N4 Precursors in g-C3N4/NiTiO3 Composites on Photocatalytic Behavior and the Interconnection between g-C3N4 and NiTiO3. Langmuir 2018, 34, 13144–13154. [Google Scholar] [CrossRef]
- Shalom, M.; Inal, S.; Fettkenhauer, C.; Neher, D.; Antonietti, M. Improving carbon nitride photocatalysis by supramolecular preorganization of monomers. J. Am. Chem. Soc. 2013, 135, 7118–7121. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.K. In situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Kainth, S.; Basu, S. A comparative study on the effect of different precursors for synthesis and efficient photocatalytic activity of g-C3N4/TiO2/bentonite nanocomposites. J. Mater. Sci. 2018, 53, 13126–13142. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Wang, S.; Chen, P.; Yun, J.H.; Hu, Y.; Wang, L. An Electrochemically Treated BiVO4 Photoanode for Efficient Photoelectrochemical Water Splitting. Angew. Chem. Int. Ed. 2017, 56, 8500–8504. [Google Scholar] [CrossRef]
- Yang, M.; Liu, J.; Zhang, X.; Qiao, S.; Huang, H.; Liu, Y.; Kang, Z. C3N4-sensitized TiO2 nanotube arrays with enhanced visible-light photoelectrochemical performance. Phys. Chem. Chem. Phys. 2015, 17, 17887–17893. [Google Scholar] [CrossRef]
- Kang, S.; Jang, J.; Pawar, R.C.; Ahn, S.; Lee, C.S. Direct coating of a g-C3N4 layer onto one-dimensional TiO2 nanocluster/nanorod films for photoactive applications. Dalt. Trans. 2018, 47, 7237–7244. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, T.; Zhang, M.; Li, Q.; Yang, J. Interfacial Construction of Zero-Dimensional/One-Dimensional g-C3N4 Nanoparticles/TiO2 Nanotube Arrays with Z-Scheme Heterostructure for Improved Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 2483–2491. [Google Scholar] [CrossRef]
- Fan, X.; Wang, T.; Gao, B.; Gong, H.; Xue, H.; Guo, H.; Song, L.; Xia, W.; Huang, X.; He, J. Preparation of the TiO2/Graphic Carbon Nitride Core-Shell Array as a Photoanode for Efficient Photoelectrochemical Water Splitting. Langmuir 2016, 32, 13322–13332. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Rathi, A.K.; Kmentová, H.; Naldoni, A.; Goswami, A.; Gawande, M.B.; Varma, R.S.; Kment, Š.; Zbořil, R. Significant Enhancement of Photoactivity in Hybrid TiO2/g-C3N4 Nanorod Catalysts Modified with Cu–Ni-Based Nanostructures. ACS Appl. Nano Mater. 2018, 1, 2526–2535. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, P.; Guo, L.; Chen, Z.; Wu, Q.; Ding, Y.; Zheng, W.; Cao, Y. The design of TiO2 nanostructures (nanoparticle, nanotube, and nanosheet) and their photocatalytic activity. J. Phys. Chem. C 2014, 118, 12727–12733. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, F.; Li, X.; Li, C.; Xu, J.; Wang, B. Fabrication of novel cyanuric acid modified g-C3N4/kaolinite composite with enhanced visible light-driven photocatalytic activity. Minerals 2018, 8, 437. [Google Scholar] [CrossRef]
- Martin, D.J.; Qiu, K.; Shevlin, S.A.; Handoko, A.D.; Chen, X.; Guo, Z.; Tang, J. Highly efficient photocatalytic H2 evolution from water using visible light and structure-controlled graphitic carbon nitride. Angew. Chem. Int. Ed. 2014, 53, 9240–9245. [Google Scholar] [CrossRef]
- Ye, L.; Chen, S. Fabrication and high visible-light-driven photocurrent response of g-C3N4 film: The role of thiourea. Appl. Surf. Sci. 2016, 389, 1076–1083. [Google Scholar] [CrossRef]
- Wang, R.; Liu, H.; Fan, Z.; Li, L.; Cai, Y.; Xu, G.; Luo, W.; Yang, B.; Zhou, Y.; Zou, Z. Unconventional gas-based bottom-up, meter-area-scale fabrication of hydrogen-bond free g-CN nanorod arrays and coupling layers with TiO2 toward high-efficiency photoelectrochemical performance. Nanoscale 2018, 10, 3342–3349. [Google Scholar] [CrossRef]
- Wei, Q.; Yan, X.; Kang, Z.; Zhang, Z.; Cao, S.; Liu, Y.; Zhang, Y. Carbon Quantum Dots Decorated C3N4/TiO2 Heterostructure Nanorod Arrays for Enhanced Photoelectrochemical Performance. J. Electrochem. Soc. 2017, 164, H515–H520. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Jiao, Y.; Wang, Z.; Xiao, M.; Du, A.; Li, Y.; Wang, J.; Wang, L. An Unusual Red Carbon Nitride to Boost the Photoelectrochemical Performance of Wide Bandgap Photoanodes. Adv. Funct. Mater. 2018, 28, 1–10. [Google Scholar] [CrossRef]
| Photoanode | Photocurrent Density (mA/cm2) | Reference |
|---|---|---|
| TiO2/g-C3N4 core shell array | 0.045 | [31] |
| CuNi@g-C3N4/TiO2 nanorods | 0.89 | [33] |
| TiO2/P-g-C3N4 | 0.20 | [15] |
| 0D 1D g-C3N4/TiO2 nanotube arrays | 0.12 | [30] |
| g-C3N4/TiO2 nanorod | 0.29 | [29] |
| C3N4/TiO2 nanotube | 1.5 | [28] |
| C3N4/TiO2 nanorod | 2.74 | This Work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, S.S.M.; Jun, S.E.; Lee, S.A.; Lee, T.H.; Jang, H.W. Influence of C3N4 Precursors on Photoelectrochemical Behavior of TiO2/C3N4 Photoanode for Solar Water Oxidation. Energies 2020, 13, 974. https://doi.org/10.3390/en13040974
Bhat SSM, Jun SE, Lee SA, Lee TH, Jang HW. Influence of C3N4 Precursors on Photoelectrochemical Behavior of TiO2/C3N4 Photoanode for Solar Water Oxidation. Energies. 2020; 13(4):974. https://doi.org/10.3390/en13040974
Chicago/Turabian StyleBhat, Swetha S. M., Sang Eon Jun, Sol A Lee, Tae Hyung Lee, and Ho Won Jang. 2020. "Influence of C3N4 Precursors on Photoelectrochemical Behavior of TiO2/C3N4 Photoanode for Solar Water Oxidation" Energies 13, no. 4: 974. https://doi.org/10.3390/en13040974
APA StyleBhat, S. S. M., Jun, S. E., Lee, S. A., Lee, T. H., & Jang, H. W. (2020). Influence of C3N4 Precursors on Photoelectrochemical Behavior of TiO2/C3N4 Photoanode for Solar Water Oxidation. Energies, 13(4), 974. https://doi.org/10.3390/en13040974