Mercury Migration Behavior from Flue Gas to Fly Ashes in a Commercial Coal-Fired CFB Power Plant
Abstract
1. Introduction
2. Experimental Section
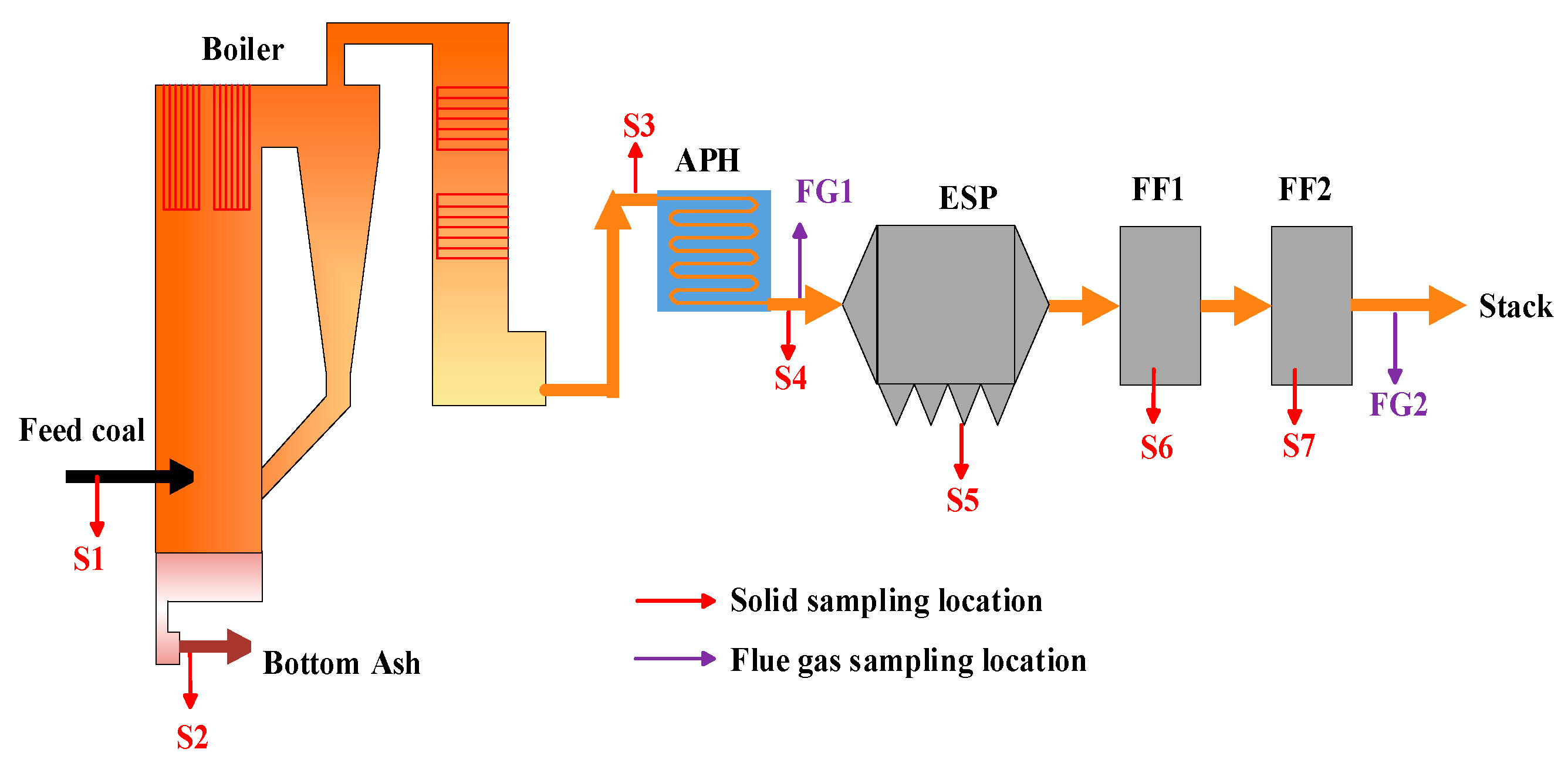
2.1. CFB Boiler Unit and Measurement Method
2.2. Characterization of Fly Ash Samples
3. Results and Discussions
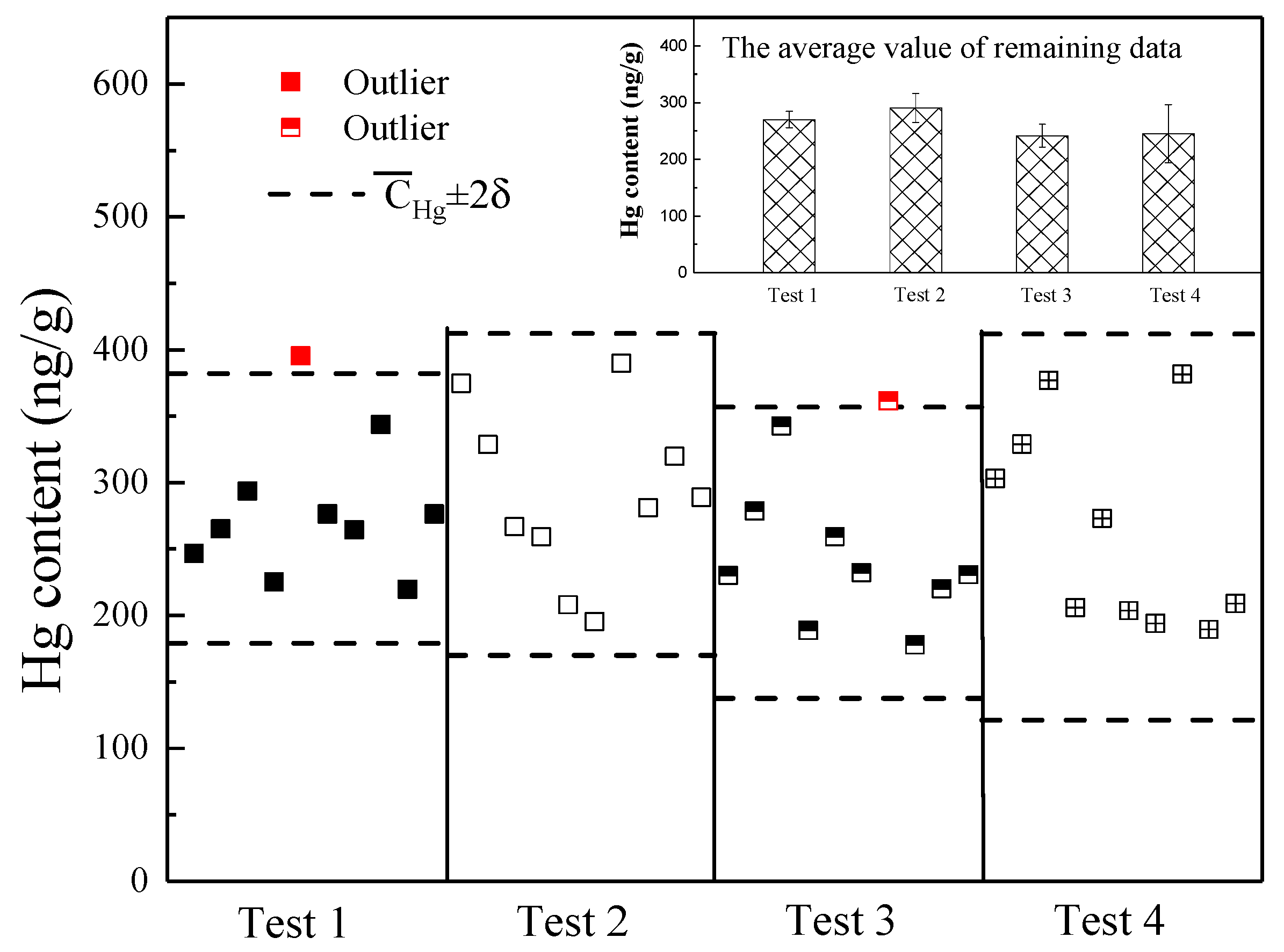
3.1. Hg Content in Feed Fuel
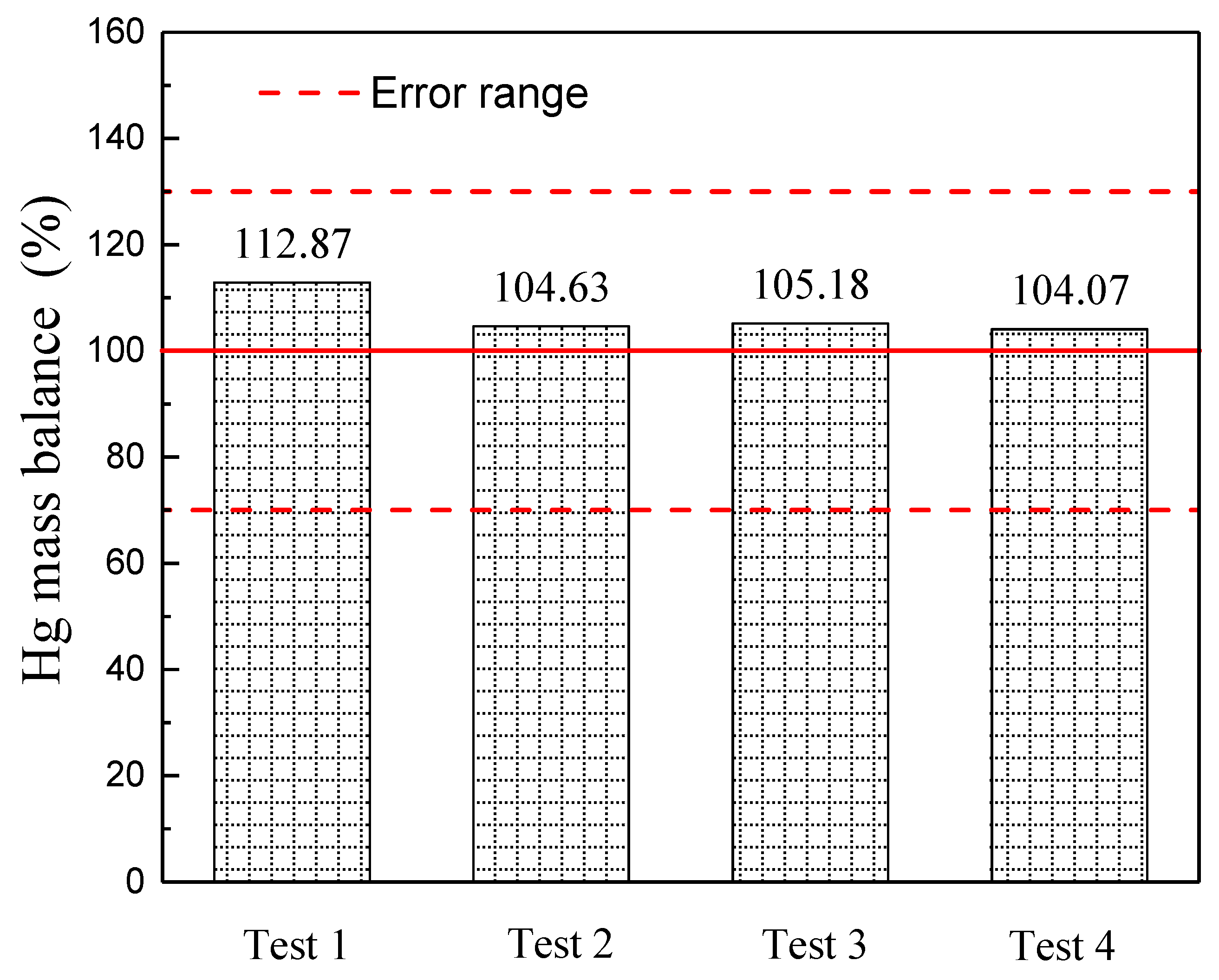
3.2. Hg Mass Balance and Distribution in the CFB Unit
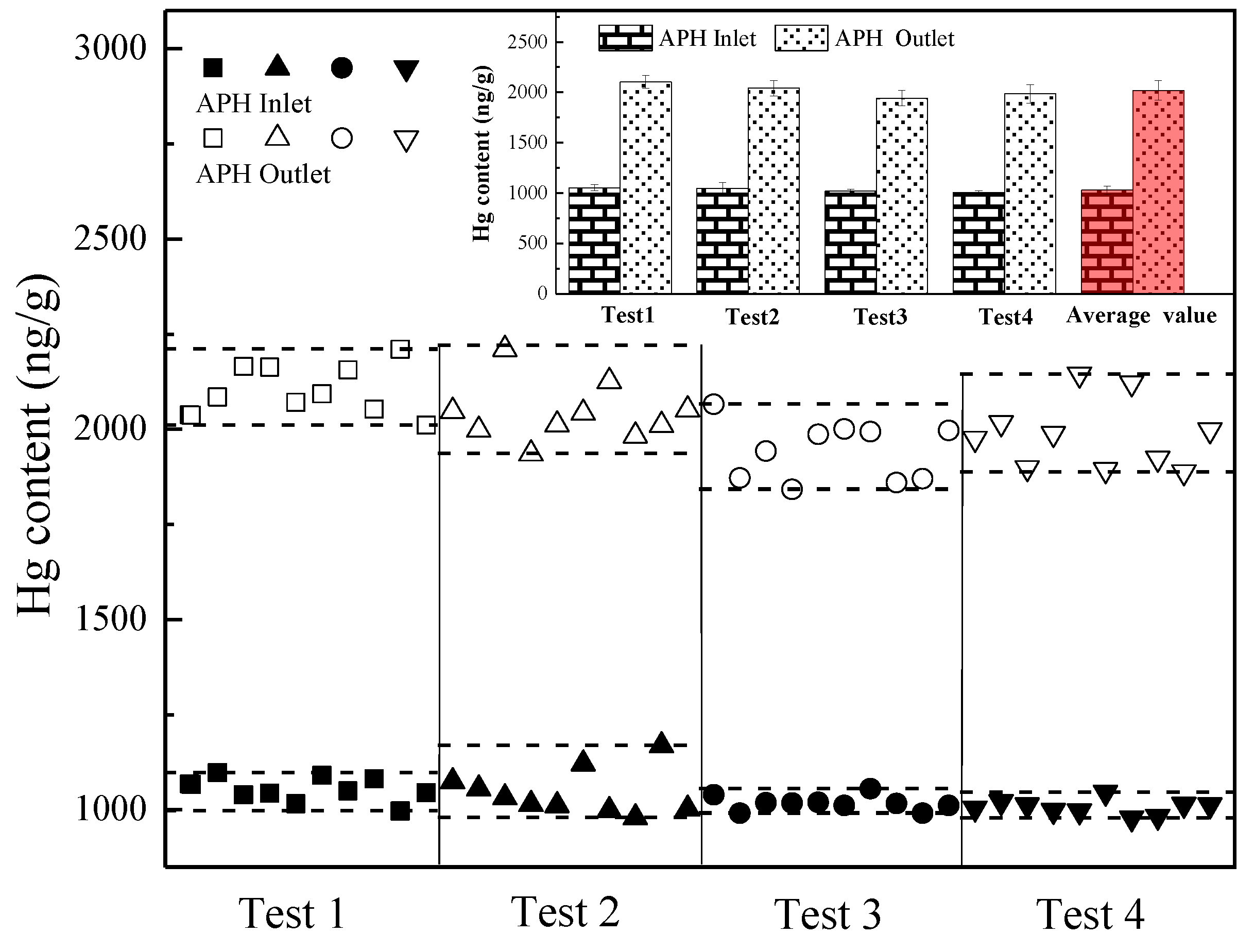
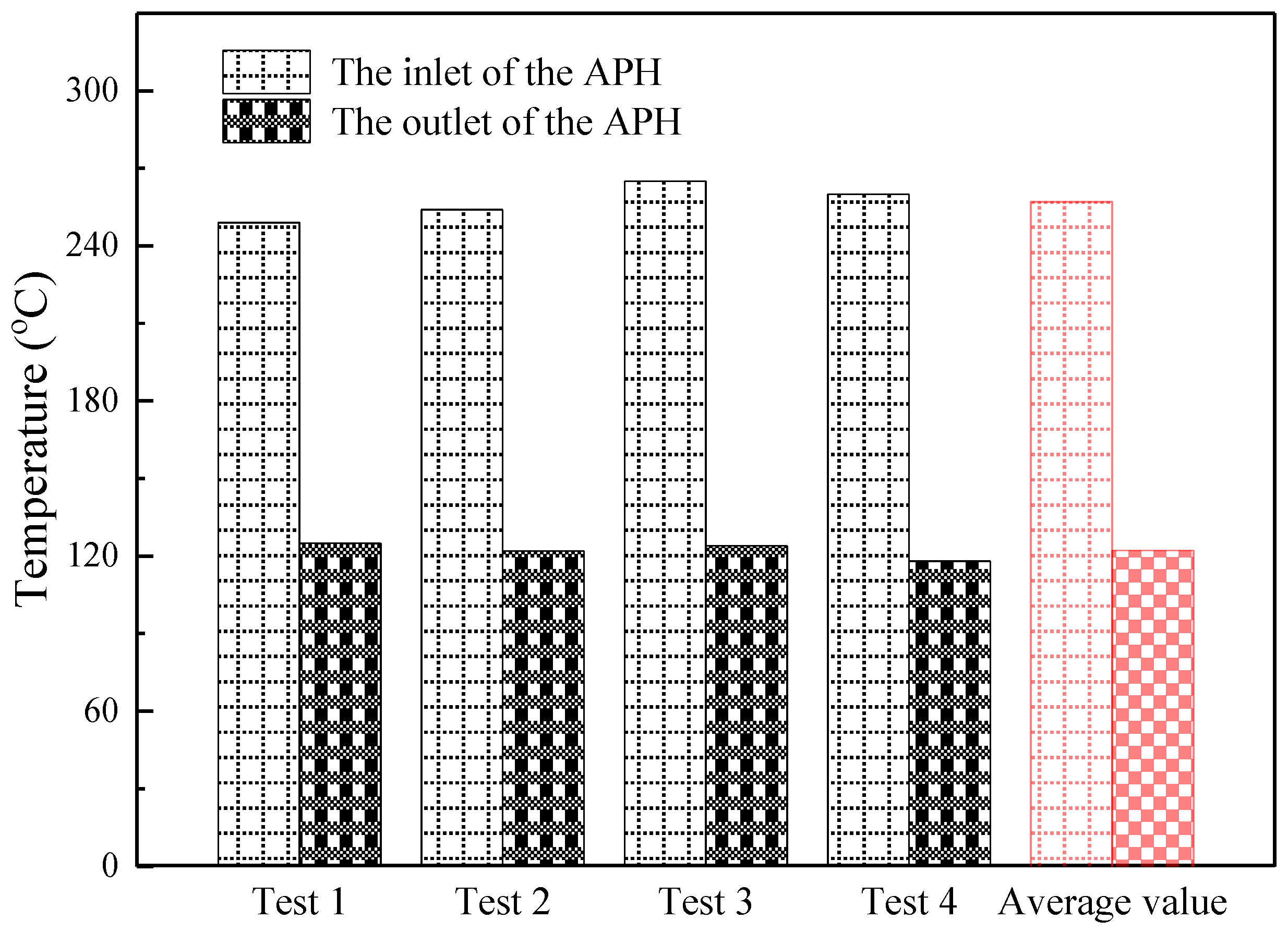
3.3. Gaseous Hg migration across APH
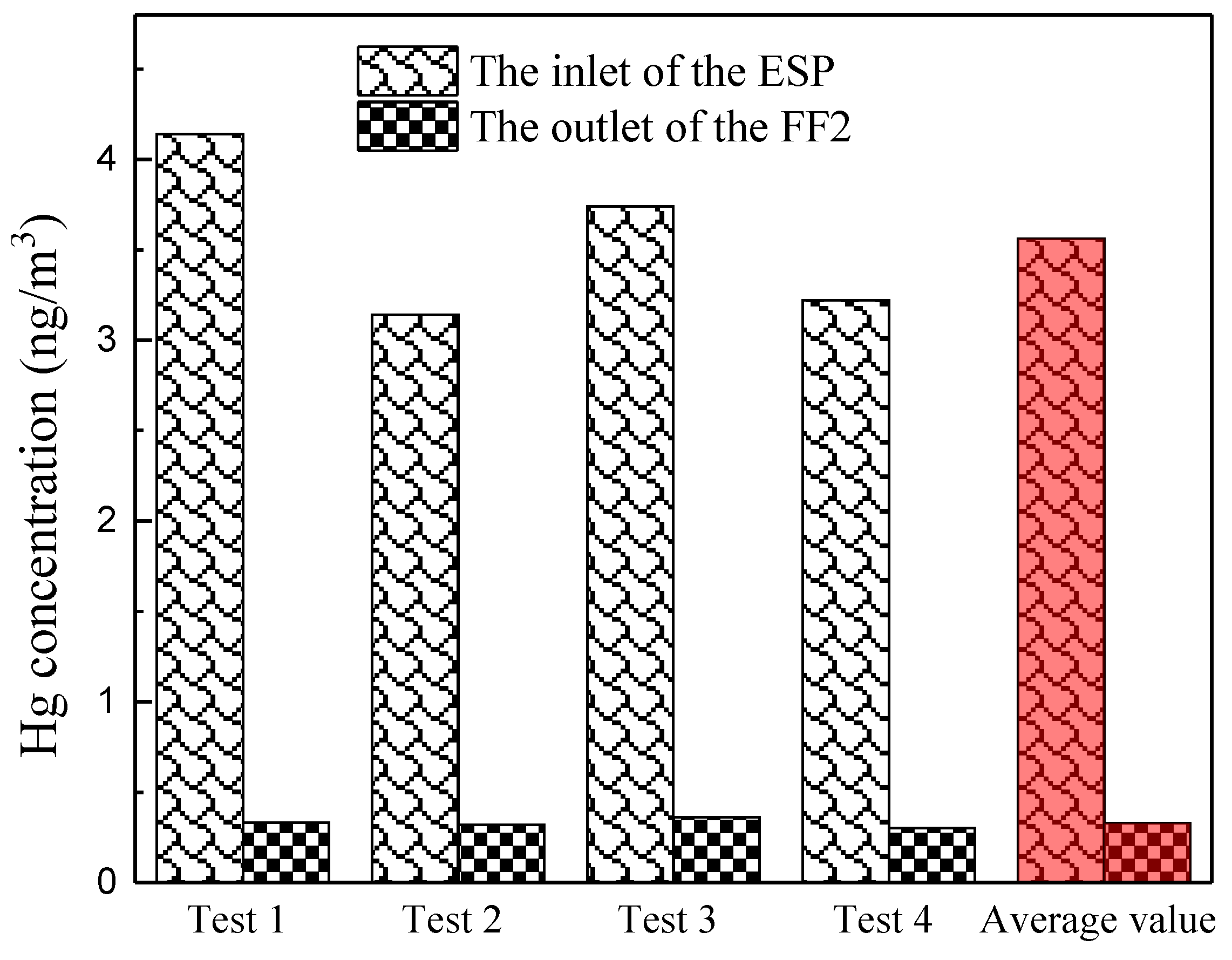
3.4. Gaseous Hg Migration Across PCD
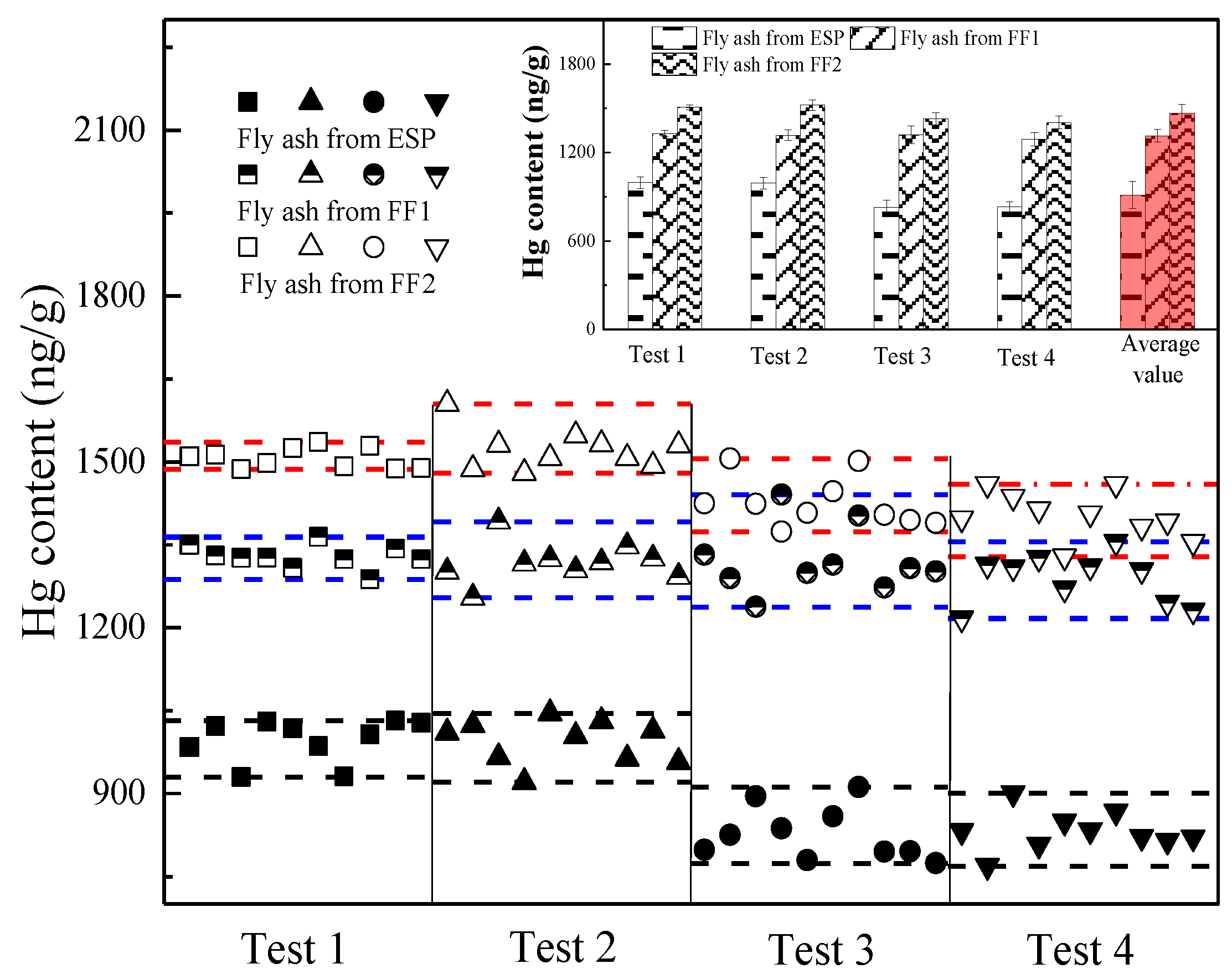
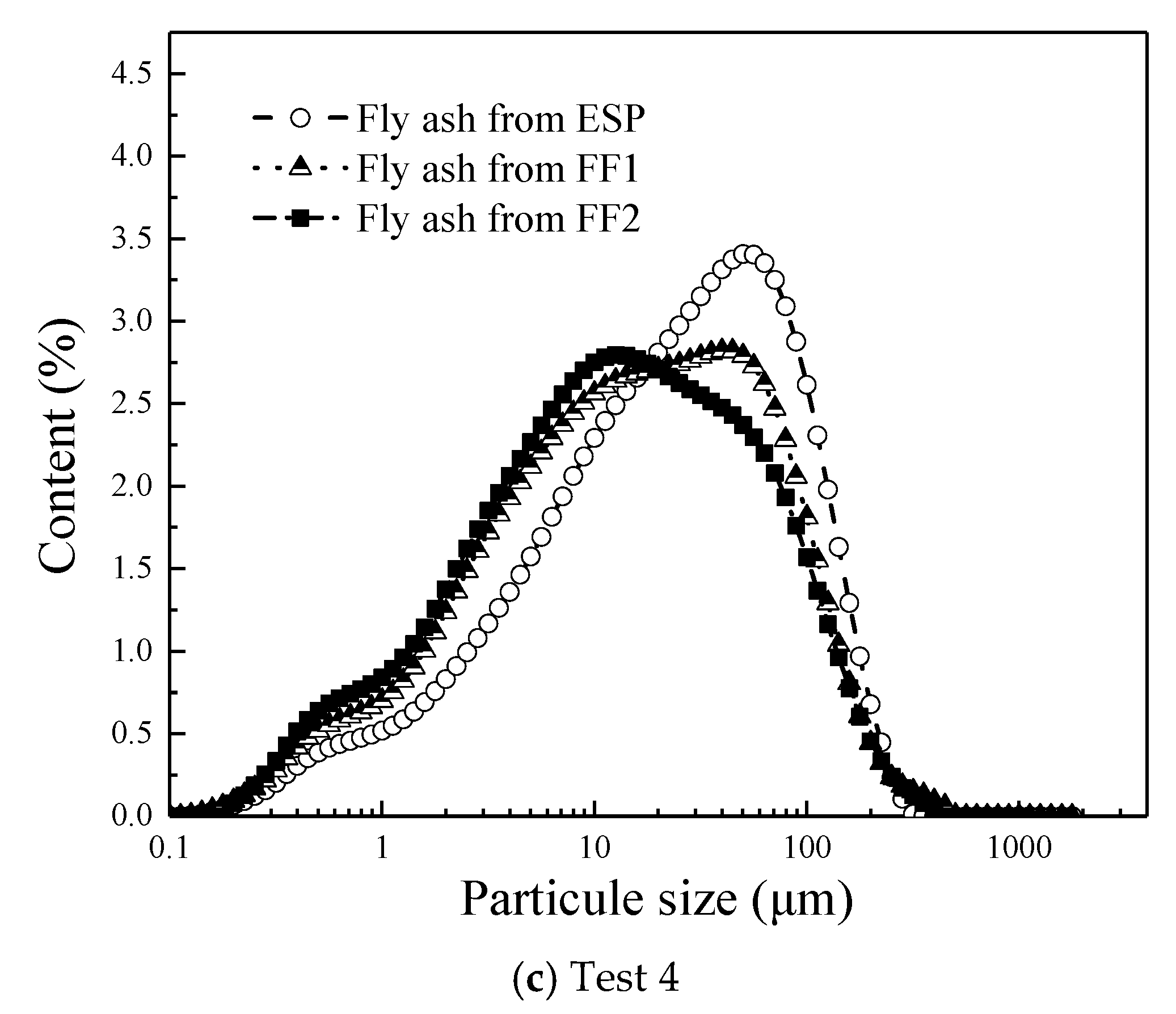
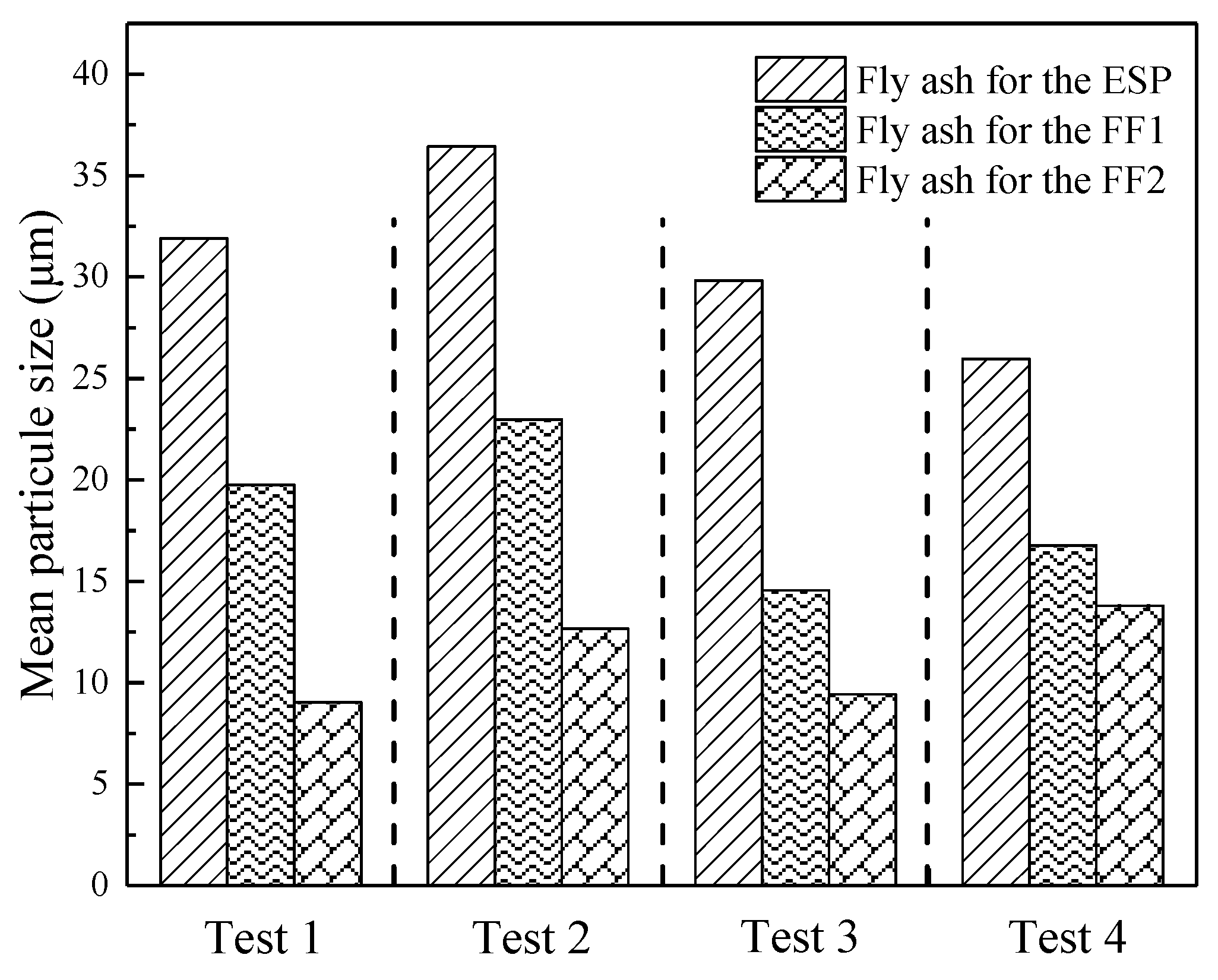
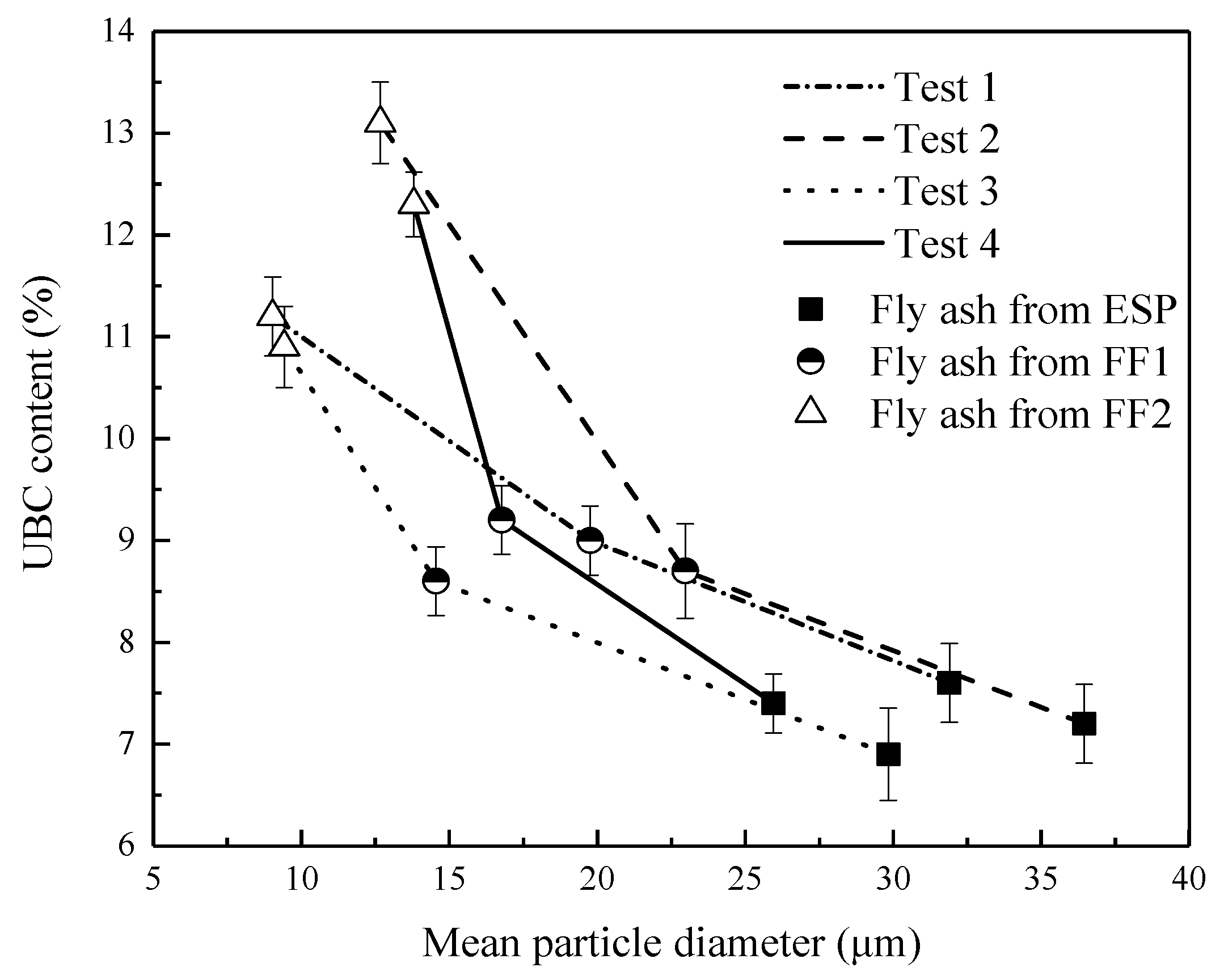
3.5. The Effect of Fly Ash Properties on Hg Migration
3.6. Comparison of Hg Behavior between PC Boiler Unit and CFB Boiler Unit
4. Conclusions
- (1)
- The Hg mass balance across the CFB boiler unit for four tests are between 100.38% and 112.88%. More than 99% of Hg contained in the feed fuel is captured by the fly ash, whilst less than 1% of Hg remained in the bottom ash or the flue gas at the outlet of the PCD system.
- (2)
- The migration of the gaseous Hg from the flue gas to the fly ash is enhanced in the APH, where the flue gas temperature decreases from 250 to 120 °C, and the average Hg content in the fly ash samples increases from 1030.9 to 2018.6 ng/g.
- (3)
- Continuous migration of Hg from the flue gas to the fly ash is observed across the PCD system. The gaseous Hg could further migrate to the fly ash in the subsequent FFs due to a long gas–solid contact time.
- (4)
- The gaseous Hg easily migrates onto the small-sized fly ash particles in the CFB boiler unit, due to higher UBC content and specific surface area of the small fly ash particle.
- (5)
- The migration of the gaseous Hg from the flue gas to the fly ash downstream of the CFB boiler unit is easier than that downstream of the PC boiler unit due to high UBC content and specific surface area of the fly ash.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heidel, B.; Rogge, T.; Scheffknecht, G. Controlled desorption of mercury in wet FGD waste water treatment. Appl. Energy 2016, 162, 1211–1217. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.X.; Meng, Y.; Hao, J.M. Influence of mercury and chlorine content of coal on mercury emissions from coal-fired power plants in china. Environ. Sci. Technol. 2012, 46, 6385–6392. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.; Rupp, E.; Ying, S.C.; Lim, D.H.; Negreira, A.S.; Kirchofer, A.; Feng, F.; Lee, K. Mercury adsorption and oxidation in coal combustion and gasification processes. Int. J. Coal Geol. 2012, 90–91, 4–20. [Google Scholar] [CrossRef]
- Masoomi, I.; Kamata, H.; Yukimura, A.; Ohtsubo, K.; Schmid, M.C.; Scheffknecht, G. Investigation on the behavior of mercury across the flue gas treatment of coal combustion power plants using a lab-scale firing system. Fuel Process. Technol. 2020, 201, 106340. [Google Scholar] [CrossRef]
- Arctic Monitoring and Assessment Programme (AMAP) and United Nations Environment Programme (UNEP). Technical Background Report to the Global Atmospheric Mercury Assessment; AMAP and UNEP: Geneva, Switzerland, 2008. [Google Scholar]
- Zhao, S.L.; Pudasainee, D.; Duan, Y.F.; Gupta, R.; Liu, M.; Lu, J.H. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Prog. Energy Combust. Sci. 2019, 73, 26–64. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. National Emission Standards for Hazardous Air Pollutants from Coal- and Oil-Fired Electric Utility Steam Generating Units and Standards of Performance for Fossil-Fuel-Fired Electric Utility, Industrial-Commercial-Institutional, and Small Industrial-Commercial-Institutional Steam Generating Units; Federal Register; Proposed Rule, United States Environmental Protection Agency: Washington, DC, USA, 2011; Volume 76.
- MEP, Ministry of Environmental Protection of China. Emission Standard of Air Pollutants for Thermal Power Plants, GB 13223–2011; Ministry of Environmental Protection of China: Beiging, China, 2011. (In Chinese)
- Galbreath, K.C.; Zygarlicke, C.J. Mercury speciation in coal combustion and gasification flue gases. Environ. Sci. Technol. 1996, 30, 2421–2426. [Google Scholar] [CrossRef]
- Xu, M.H.; Qiao, Y.; Zheng, C.G.; Li, L.C.; Liu, J. Modeling of homogeneous mercury speciation using detailed chemical kinetics. Combust. Flame 2003, 132, 208–218. [Google Scholar] [CrossRef]
- Zeng, M.; Du, L.; Liao, D.; Chu, W.X.; Wang, Q.W.; Luo, Y.; Sun, Y. Investigation on pressure drop and heat transfer performances of plate-fin iron air preheater unit with experimental and Genetic Algorithm methods. Appl. Energy 2012, 92, 725–732. [Google Scholar] [CrossRef]
- Schofield, K. Mercury emission control from coal combustion systems: A modified air preheater solution. Combust. Flame 2012, 159, 1741–1747. [Google Scholar] [CrossRef]
- Schofield, K. Mercury emission control: Phase II. Let’s now go passive. Energy 2017, 122, 311–318. [Google Scholar] [CrossRef]
- Romero, C.E.; Li, Y.; Bilirgen Sarunac, N.; Levy, E.K. Modification of boiler operating conditions for mercury emissions reductions in coal-fired utility boilers. Fuel 2006, 85, 204–212. [Google Scholar] [CrossRef]
- Senior, C. Mercury behavior in coal combustion systems. In Mercury Control: For Coal-Derived Gas Streams; Granite, E.J., Pennline, H.W., Eds.; US Department of Energy: Washington, DC, USA, 2014. [Google Scholar]
- Pudasainee, D.; Kim, J.H.; Yoon, Y.S.; Seo, Y.C. Oxidation, reemission and mass distribution of mercury in bituminous coal-fired power plants with SCR, CS-ESP and wet FGD. Fuel 2012, 93, 312–318. [Google Scholar] [CrossRef]
- Pudasainee, D.; Seo, Y.C.; Sung, J.H.; Jang, N.N.; Gupta, R. Mercury co-beneficial capture in air pollution control devices of coal-fired power plants. Int. J. Coal Geol. 2017, 170, 48–53. [Google Scholar] [CrossRef]
- Senior, C.L.; Johnson, S.A. Impact of carbon-in-ash on mercury removal across particulate control devices in coal-fired power plants. Energy Fuels 2005, 19, 859–863. [Google Scholar] [CrossRef]
- EPRI. An Assessment of Mercury Emissions from U.S. Coal-Fired Power Plants; EPRI: Palo Alto, CA, USA, 2000; p. 1000608. [Google Scholar]
- Wang, S.X.; Zhang, L.; Li, G.H.; Wu, Y.; Hao, J.M.; Pirrone, N.; Sproviveri, F.; Ancora, M.P. Mercury emission and speciation of coal-fired power plants in China. Atmos. Chem. Phys. Discuss. 2009, 10, 1183–1192. [Google Scholar] [CrossRef]
- Pavlish, J.H.; Sondreal, E.A.; Mann, M.D.; Olson, E.S.; Galbreath, K.C.; Laudal, D.L.; Benson, S.A. Status review of mercury control options for coal-fired power plants. Fuel Process. Technol. 2003, 82, 89–165. [Google Scholar] [CrossRef]
- Cao, Y.; Cheng, C.M.; Chen, C.W.; Liu, M.C.; Wang, C.W.; Pan, W.P. Abatement of mercury emissions in the coal combustion process equipped with a Fabric Filter Baghouse. Fuel 2008, 87, 3322–3330. [Google Scholar] [CrossRef]
- Zhao, S.L.; Duan, Y.F.; Chen, L.; Li, Y.N.; Yao, T.; Liu, S.; Liu, M.; Lu, J.H. Study on emission of hazardous trace elements in a 350MW coal-fired power plant. Part 1. Mercury. Environ. Pollut. 2017, 229, 863–870. [Google Scholar] [CrossRef]
- Gao, M.M.; Hong, F.; Liu, J.Z. Investigation on energy storage and quick load change control of subcritical circulating fluidized bed boiler units. Appl. Energy 2017, 185, 463–471. [Google Scholar] [CrossRef]
- Wichlinski, M.; Wielgosz, G.; Kobyłecki, R. The effect of circulating fluidized bed boiler load on the emission of mercury. J. Energy Inst. 2019, 92, 1800–1806. [Google Scholar] [CrossRef]
- Wang, Y.J.; Duan, Y.F.; Zhao, C.S. Comparison of mercury emissions between circulating fluidized bed boiler and pulverized coal boiler. In Proceedings of the 20th International Conference on Fluidized Bed Combustion; Yue, G., Zhang, H., Chao, C., Luo, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 256–261. [Google Scholar]
- Zhang, L.; Zhuo, Y.Q.; Chen, L.; Xu, X.C.; Chen, C.H. Mercury emissions from six coal-fired power plants in China. Fuel Process. Technol. 2008, 89, 1033–1040. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, Y.; Su, Y.J.; Teng, Y.; Guan, Y.J.; Zhang, K. Difference of fly ash characteristics from PC and CFB boilers and its effect on mercury adsorption capability. CIESC J. 2019, 70, 1075–1082. (In Chinese) [Google Scholar]
- Fan, M.H.; Brown, R.C. Comparison of the loss-on-ignition and thermos-gravimetric analysis techniques in measuring unburned carbon in coal fly ash. Energy Fuels 2002, 43, 252. [Google Scholar]
- Yokoyama, T.; Asakura, K.; Matsuda, H.; Ito, S.; Noda, N. Mercury emissions from a coal-fired power plant in Japan. Environ. Sci. Technol. 2000, 259, 97–103. [Google Scholar] [CrossRef]
- Hall, B.; Schager, P.; Weesmaa, J. The homogeneous gas phase reaction of mercury with oxygen, and the corresponding heterogeneous reactions in the presence of activated carbon and fly ash. Chemosphere 1995, 30, 611–627. [Google Scholar] [CrossRef]
- Senior, C.L.; Sarofim, A.F.; Zeng, T.F.; Helble, J.J.; Mamani-Paco, R. Gas-phase transformations of mercury in coal-fired power plants. Fuel Process. Technol. 2000, 63, 197–213. [Google Scholar] [CrossRef]
- Niksa, S.; Helble, J.J.; Fujiwara, N. Kinetic modeling of homogeneous mercury oxidation: The importance of NO and H2O in predicting oxidation in coal-derived systems. Environ. Sci. Technol. 2001, 35, 3701–3706. [Google Scholar] [CrossRef]
- Zhong, L.C.; Zhang, Y.S.; Liu, Z.; Sui, Z.F.; Cao, Y.; Pan, W.P. Study of mercury adsorption by selected Chinese coal fly ashes. J. Therm. Anal. Calorim. 2014, 116, 1197–1203. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, H.G.; Lu, J.Z.; Teng, Y.; Zhang, K. Kinetics and mechanism of mercury adsorption on fly ashes from pulverized coal boiler and circulating fluidized bed boiler. CIESC J. 2019, 70, 4397–4409. (In Chinese) [Google Scholar]
- Hower, J.C.; Senior, C.L.; Suuberg, E.M.; Hurtet, R.H.; Wilcox, J.L.; Olson, E.S. Mercury capture by native fly ash carbons in coal-fired power plants. Prog. Energy Combust. Sci. 2010, 36, 510–529. [Google Scholar] [CrossRef]
- Hower, J.C.; Clack, H.L.; Hood, M.M.; Hopps, S.G.; Thomas, G.H. Impact of coal source changes on mercury content in fly ash: Examples from a Kentucky power plant. Int. J. Coal Geol. 2017, 170, 2–6. [Google Scholar] [CrossRef]
- Kostova, I.J.; Hower, J.C.; Mastalerz, M.; Vassilev, S.V. Mercury capture by selected Bulgarian fly ashes: Influence of coal rank and fly ash carbon pore structure on capture efficiency. Appl. Geochem. 2011, 26, 18–27. [Google Scholar] [CrossRef]
- Wu, J.; Wang, P.; He, L.; Pan, W.; Ren, J.; He, P.; Wu, Q.; Zhang, J. Experimental study of the effect of fly ash particle size on its mercury adsorption capability in the flue gas. Adv. Mater. Res. 2012, 356–360, 1664–1667. [Google Scholar] [CrossRef]
- Chen, C.; Li, Q.; Tao, D.J.; Zhai, J.P.; Tao, C.L. Physical and chemical prosperities difference between pulverized coal boiler fly ash and circulating fluidized bed combustion ash. Asia J. Chem. 2012, 24, 4538–4540. [Google Scholar]
- Xiao, X.B.; Yang, H.R.; Zhang, H.; Lu, J.F.; Yue, G.X. Research on carbon content in fly ash from circulating fluidized bed boilers. Energy Fuels 2005, 19, 1520–1525. [Google Scholar] [CrossRef]



| Proximate Analysis (Dry Base Weight %) | Ultimate Analysis (Dry Base Weight %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Moisture | Ash | Volatile | Fixed carbon | C | H | O | N | S |
| 2.74 | 55.17 | 17.33 | 24.76 | 31.85 | 2.53 | 5.00 | 0.59 | 2.12 |
| Test | Hg Distribution (%) | ||
|---|---|---|---|
| Fly Ash from the PCD System | Bottom Ash from the Residue Extraction | Flue Gas Leaving from the PCD System | |
| Test1 | 99.43 | 0.08 | 0.49 |
| Test2 | 99.45 | 0.07 | 0.48 |
| Test3 | 99.29 | 0.07 | 0.64 |
| Test4 | 99.40 | 0.07 | 0.53 |
| Test | The Inlet of the APH | The Outlet of the APH | ||
|---|---|---|---|---|
| UBC Content (%) | Specific Surface Area (m2/g) | UBC Content (%) | Specific Surface Area (m2/g) | |
| Test1 | 7.0 | 6.30 | 7.4 | 6.75 |
| Test2 | 5.9 | 6.34 | 5.8 | 6.69 |
| Test3 | 6.3 | 6.58 | 6.6 | 6.18 |
| Test4 | 6.9 | 6.64 | 7.2 | 6.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Teng, Y.; Zhang, K.; Peng, H.; Cheng, F.; Yoshikawa, K. Mercury Migration Behavior from Flue Gas to Fly Ashes in a Commercial Coal-Fired CFB Power Plant. Energies 2020, 13, 1040. https://doi.org/10.3390/en13051040
Li X, Teng Y, Zhang K, Peng H, Cheng F, Yoshikawa K. Mercury Migration Behavior from Flue Gas to Fly Ashes in a Commercial Coal-Fired CFB Power Plant. Energies. 2020; 13(5):1040. https://doi.org/10.3390/en13051040
Chicago/Turabian StyleLi, Xiaohang, Yang Teng, Kai Zhang, Hao Peng, Fangqin Cheng, and Kunio Yoshikawa. 2020. "Mercury Migration Behavior from Flue Gas to Fly Ashes in a Commercial Coal-Fired CFB Power Plant" Energies 13, no. 5: 1040. https://doi.org/10.3390/en13051040
APA StyleLi, X., Teng, Y., Zhang, K., Peng, H., Cheng, F., & Yoshikawa, K. (2020). Mercury Migration Behavior from Flue Gas to Fly Ashes in a Commercial Coal-Fired CFB Power Plant. Energies, 13(5), 1040. https://doi.org/10.3390/en13051040





