A Study on the Prediction of the Temperature and Mass of Hydrogen Gas inside a Tank during Fast Filling Process
Abstract
1. Introduction
2. Test Methods and Procedure
3. Hydrogen Charging Modeling inside the High Pressure Vessel
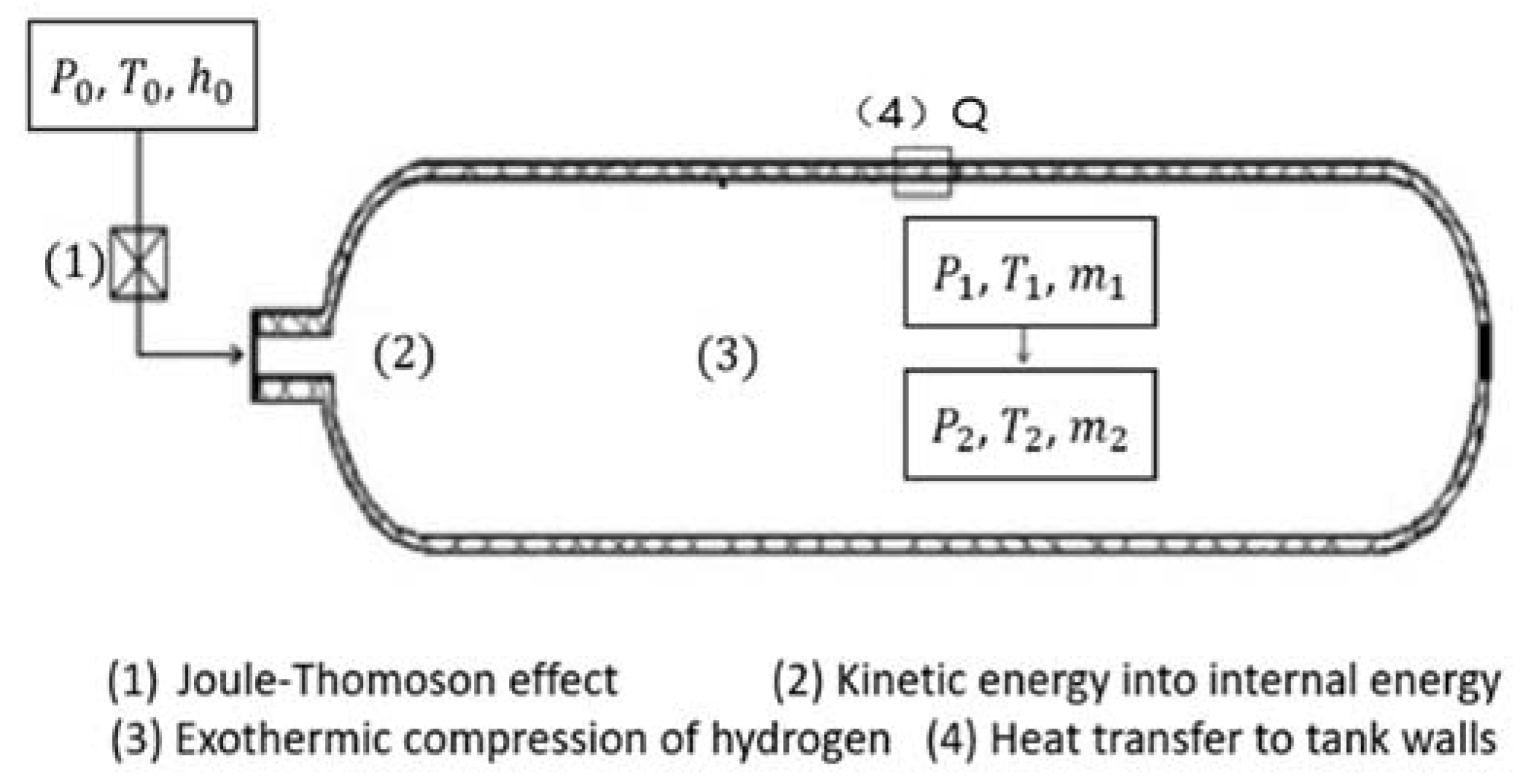
3.1. System Analysis
3.2. Thermodynamic Analysis of the Filling Process
3.2.1. Model Assumptions
- −
- During the filling process, the natural convection heat exchange coefficient of the outer wall of the hydrogen tank is constant, and the ambient temperature is constant, = Measured value.
- −
- The material of the hydrogen cylinder is stainless steel, and the thermal conductivity of stainless steel is regarded as isotropic, and its thermal conductivity is considered constant;
- −
- The temperature of the hydrogen that flows into the hydrogen tank is constant.
- −
- The gas in the compressed hydrogen storage tank is evenly distributed in pressure and temperature, and the temperature of the tank wall is also evenly distributed.
- −
- Consider the buoyancy effect caused by gravity.
- −
- The inlet boundary of the high-pressure hydrogen tank is a pressure inlet boundary constrained by variable conditions.
- −
- Solid components in the hydrogen gas cycling are rigid bodies without mechanical deformation.
- −
- Since the temperature and pressure changes drastically during filling, the corresponding physical parameters vary greatly. In order to get the simulation analysis closer to the actual working conditions, the gas in the filling process is regarded as a real gas. Functions of temperature and pressure used real gas parameters.
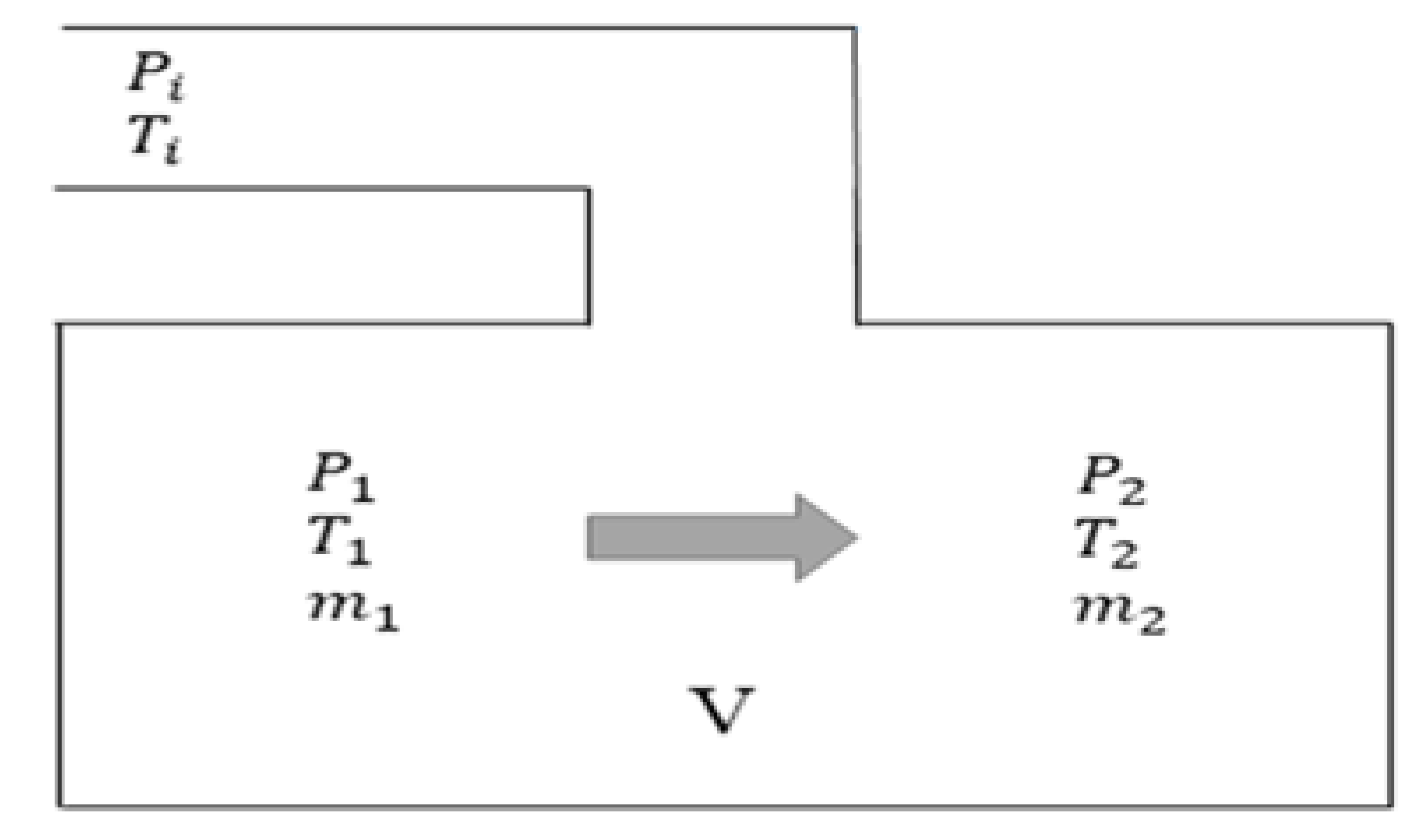
3.2.2. Model Equations
3.3. CFD Simulation Method
3.4. Thermodynamic Method
- −
- The temperature and pressure in the initial hydrogen tank are constant.
- −
- The volume of the hydrogen tank is constant, ignoring the kinetic energy and potential energy of the hydrogen.
- −
- The filling process of the compressed hydrogen in a high-pressure container through a compressor is an adiabatic process, and no heat exchange is made with the outside.
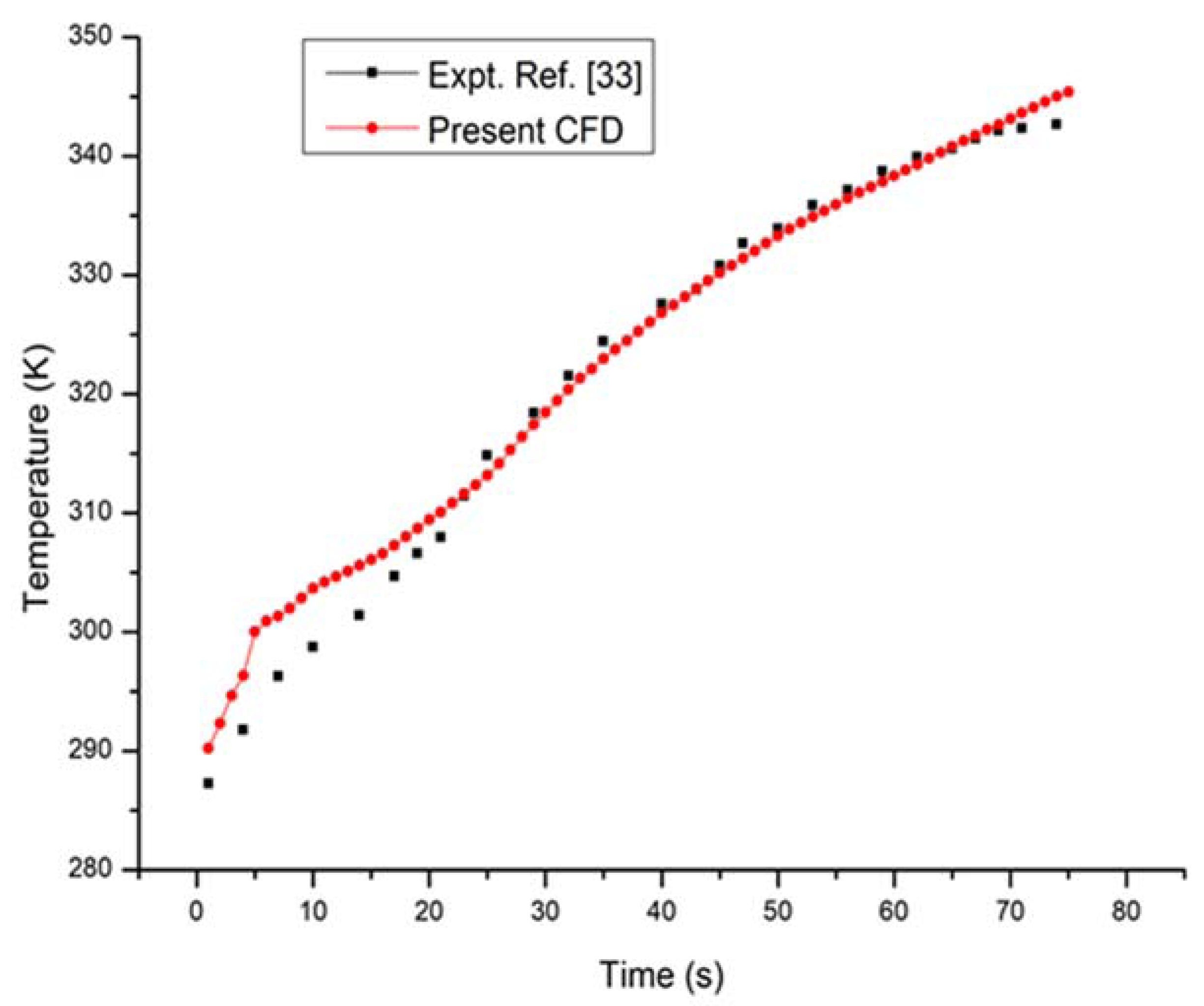
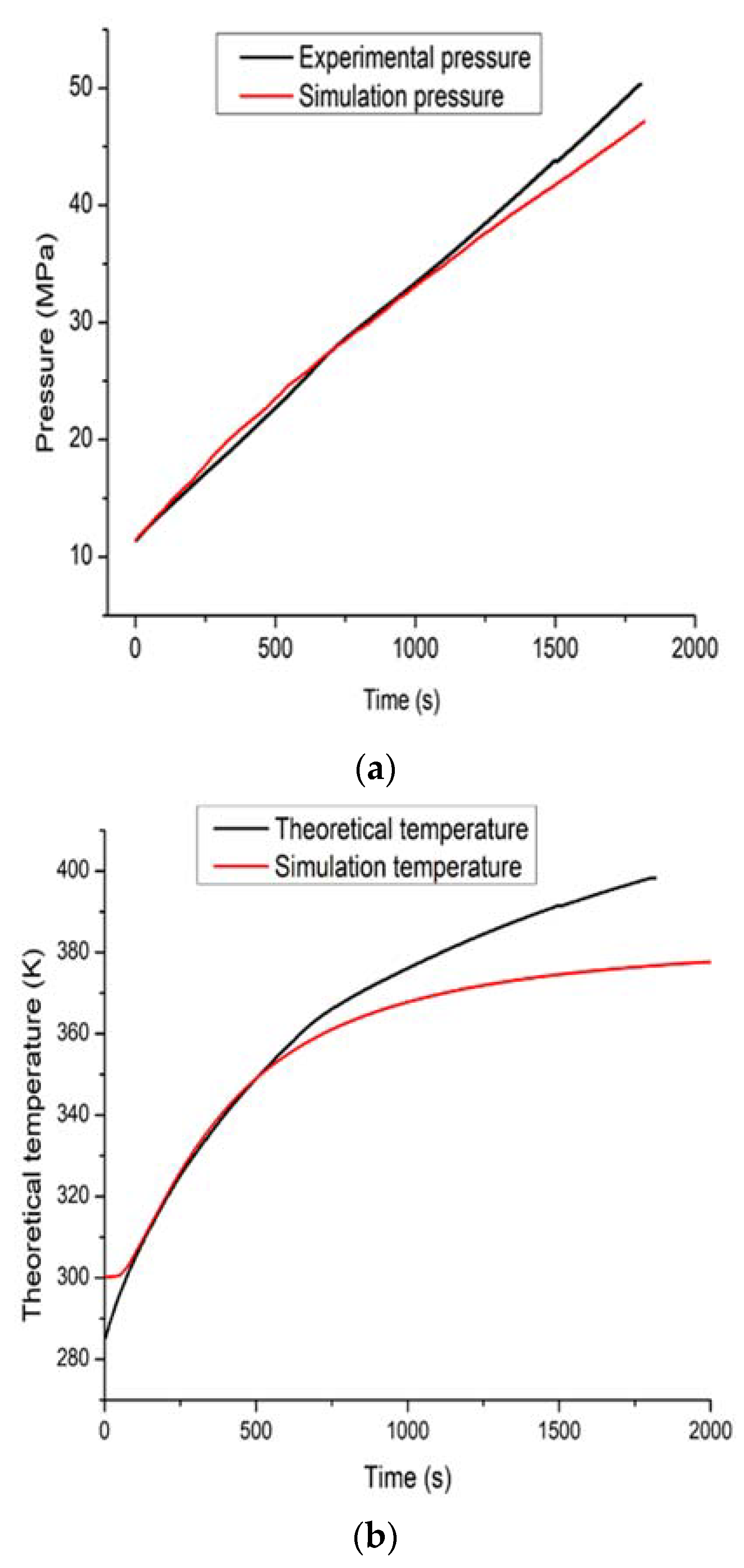
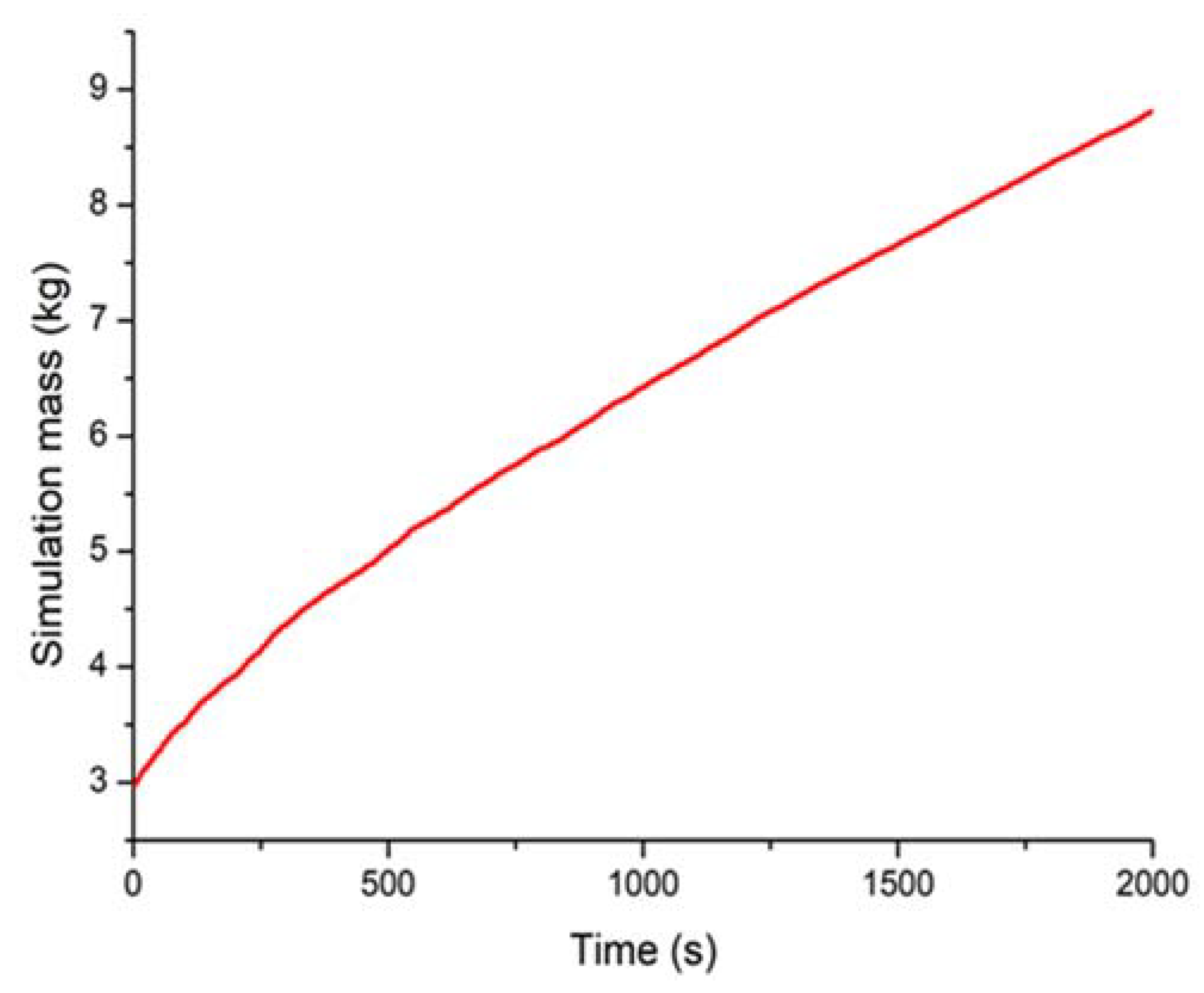
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Internal surface area of tank, (m2) | |
| External surface area of tank, (m2) | |
| Heat transfer coefficient between hydrogen and tank wall, (W/m2·K) | |
| Heat transfer coefficient between tank wall and ambient fluid, (W/m2·K) | |
| Specific enthalpy of inflow hydrogen, (J/kg) | |
| Specific enthalpy of outflow hydrogen, (J/kg) | |
| Specific heat at constant pressure, (J/kg·K) | |
| Specific heat at constant volume, (J/kg·K) | |
| Diameter of the injector of the tank, (m) | |
| Internal diameter, (m) | |
| External diameter, (m) | |
| L | Length, (m) |
| m | mass of hydrogen mass in the tank, (kg) |
| Initial hydrogen mass, (kg) | |
| Hydrogen mass inflow rate for charging, (kg/s) | |
| Hydrogen mass outflow rate for discharging, (kg/s) | |
| Nusselt number of the gas, dimensionless. | |
| Reynolds number of the flow, | |
| Rayleigh number of the gas. | |
| P | Pressure of the gas, (MPa) |
| Settled pressure in the tank, (MPa) | |
| Initial pressure, (MPa) | |
| Final pressure in the tank, (MPa) | |
| T | Temperature of the gas, (K) |
| Initial temperature, (K) | |
| The temperature of inlet gas, (K) | |
| Final temperature in the tank, (K) | |
| Temperature of the ambient, = measured value., (K) | |
| u | Specific internal energy, (J/kg). |
| k | Thermal conductivity of the gas (W/m·K.) |
| Density of the gas, (kg/m3) | |
| B | Abel-Nobel constant, B = 0.007691 m3 for hydrogen gas |
References
- Kim, Y.M.; Shin, D.G.; Kim, C.G. On-Board Cold Thermal Energy Storage System for Hydrogen Fueling Process. Energies 2019, 12, 561. [Google Scholar] [CrossRef]
- Li, M.; Bai, Y.; Zhang, C.; Song, Y.; Jiang, S.; Grouset, D.; Zhang, M. Review on the research of hydrogen storage system fast refueling in fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 10677–10693. [Google Scholar] [CrossRef]
- Xiao, J.; Bénard, P.; Chahine, R. Charge-discharge cycle thermodynamics for compression hydrogen storage system. Int. J. Hydrogen Energy 2016, 12, 5531–5539. [Google Scholar] [CrossRef]
- Kim, K.; Shin, D.; Kim, Y.; Karng, S.W. Adiabatic Performance of Layered Insulating Materials for Bulk LH2 Storage Tanks. Trans. Korean Hydrogen New Energy Soc. 2016, 6, 642–650. [Google Scholar] [CrossRef]
- Wellnitz, J. Hydrogen storage systems for automotive applications: Project Storhy. Int. J. Sustain. Des. 2008, 1, 93–109. [Google Scholar] [CrossRef]
- Wang, L.; Ye, F.; Xiao, J.; Bénard, P.; Chahine, R. Heat transfer analysis for fast filling of on board hydrogen tank. Energy Procedia 2019, 158, 1910–1916. [Google Scholar] [CrossRef]
- An, G. Numerical analysis of fast-filling process of hydrogen storage cylinder for vehicles. Missiles Space Veh. 2009, 3, 50–55. [Google Scholar]
- Newhouse, N.L.; Liss, W.E. Fast filling of NGV fuel containers. SAE Trans. 1999, 108, 568–574. [Google Scholar]
- GTR Number 13. Global Technical Regulation on Hydrogen and Fuel Cell Vehicles; United Nations Economic Commission for Europe: Geneva, Switzerland, 2013. [Google Scholar]
- Society of Automotive Engineers. SAE J2579: Standard for Fuel Systems in Fuel Cell and Other Hydrogen Vehicles; Society of Automotive Engineers: Warrendale, PA, USA, 2013. [Google Scholar]
- International Organization for Standardization. ISO/TS 15869: Gaseous Hydrogen and Hydrogen Blends—Land Vehicle Fuel Tanks; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- American national Standards Institude. ANSI HGV 2: Compressed Hydrogen Gas Vehicle Fuel Containers; American National Standards Institude: Washington, DC, USA, 2014. [Google Scholar]
- Tomioka, J.I.; Kiguchi, K.; Tamura, Y.; Mitsuishi, H. Influence of temperature on the fatigue strength of compressed-hydrogen tanks for vehicles. Int. J. Hydrogen Energy 2011, 36, 2513–2519. [Google Scholar] [CrossRef]
- Koshimizu, T.; Kasao, D.; Takata, Y.; Monde, M. Thermal Analysis on Filling Process in Hydrogen Tank. ASME Int. Mech. Eng. Congr. Expo. 2005, 42215, 141–148. [Google Scholar]
- Hirotani, R.; Terada, T.; Tamura, Y.; Mitsuishi, H.; Watanabe, S. Thermal Behavior in Hydrogen Storage Tank for Fuel Cell Vehicle on Fast Filling; SAE Technical Paper No. 2007-01-0688; SAE: Lyon, France, 2007. [Google Scholar]
- Khan, M.T.I.; Monde, M.; Setoguchi, T. Hydrogen gas filling into an actual tank at high pressure and optimization of its thermal characteristics. J. Therm. Sci. 2009, 18, 235–240. [Google Scholar] [CrossRef]
- Woodfield, P.L.; Monde, M.; Takano, T. Heat transfer characteristics for practical hydrogen pressure vessels being filled at high pressure. J. Therm. Sci. Technol. 2008, 3, 241–253. [Google Scholar] [CrossRef]
- Woodfield, P.L.; Monde, M.; Mitsutake, Y. Measurement of averaged heat transfer coefficients in high-pressure vessel during charging with hydrogen, nitrogen or argon gas. J. Therm. Sci. Technol. 2007, 2, 180–191. [Google Scholar] [CrossRef]
- Monde, M.; Woodfield, P.; Takano, T.; Kosaka, M. Estimation of temperature change in practical hydrogen pressure tanks being filled at high pressures of 35 and 70 MPa. Int. J. Hydrogen Energy 2012, 7, 5723–5734. [Google Scholar] [CrossRef]
- Galassi, M.C.; Papanikolaou, E.; Heitsch, M.; Baraldi, D.; Acosta Iborra, B.; Moretto, P. Validation of CFD models for hydrogen fast filling simulations. In Proceedings of the Fourth International Conference on Hydrogen Safety, San Francisco, CA, USA, 12–14 September 2011. [Google Scholar]
- Galassi, M.C.; Acosta-Iborra, B.; Baraldi, D.; Bonato, C.; Harskamp, F.; Frischauf, N.; Moretto, P. Onboard compressed hydrogen storage: Fast filling experiments and simulations. Energy Procedia 2012, 29, 192–200. [Google Scholar] [CrossRef]
- Galassi, M.C.; Baraldi, D.; Iborra, B.A.; Moretto, P. CFD analysis of fast filling scenarios for 70 MPa hydrogen type IV tanks. Int. J. Hydrogen Energy 2012, 37, 6886–6892. [Google Scholar] [CrossRef]
- Galassi, M.C.; Papanikolaou, E.; Heitsch, M.; Baraldi, D.; Iborra, B.A.; Moretto, P. Assessment of CFD models for hydrogen fast filling simulations. Int. J. Hydrogen Energy 2015, 39, 6252–6260. [Google Scholar] [CrossRef]
- Heitsch, M.; Baraldi, D.; Moretto, P. Numerical investigations on the fast filling of hydrogen tanks. Int. J. Hydrogen Energy 2011, 36, 2606–2612. [Google Scholar] [CrossRef]
- Acosta, B.; Moretto, P.; de Miguel, N.; Ortiz, R.; Harskamp, F.; Bonato, C. JRC reference data from experiments of on-board hydrogen tanks fast filling. Int. J. Hydrogen Energy 2014, 39, 20531–20537. [Google Scholar] [CrossRef]
- Melideo, D.; Baraldi, D.; Galassi, M.C.; Cebolla, R.O.; Iborra, B.A.; Moretto, P. CFD model performance benchmark of fast filling simulations of hydrogen tanks with pre-cooling. Int. J. Hydrogen Energy 2014, 39, 4389–4395. [Google Scholar] [CrossRef]
- Dicken, C.J.B.; Mérida, W. Temperature distribution within a compressed gas cylinder during fast filling. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2007; Volume 15, pp. 281–286. [Google Scholar]
- Dicken, C.J.B.; Merida, W. Measured effects of filling time and initial mass on the temperature distribution within a hydrogen cylinder during refuelling. J. Power Sources 2007, 165, 324–336. [Google Scholar] [CrossRef]
- Hosseini, M.; Dincer, I.; Naterer, G.F.; Rosen, M.A. Thermodynamic analysis of filling compressed gaseous hydrogen storage tanks. Int. J. Hydrogen Energy 2012, 37, 5063–5071. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhao, Y.Z.; Zhao, L.; Li, X.; Chen, H.G.; Zhang, L.F.; Zheng, J.Y. Experimental studies on temperature rise within a hydrogen cylinder during refueling. Int. J. Hydrogen Energy 2010, 35, 2627–2632. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Yang, J.; Zhao, Y.; Zheng, J.; Bie, H.; Liu, X. Numerical simulation of temperature rise within hydrogen vehicle cylinder during refueling. Int. J. Hydrogen Energy 2010, 35, 8092–8100. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, G.; Liu, Y.; Zheng, J.; Chen, Y.; Zhao, L.; Guo, J.; He, Y. Numerical study on fast filling of 70 MPa type III cylinder for hydrogen vehicle. Int. J. Hydrogen Energy 2012, 22, 17517–17522. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, J.; Yang, J.; Zhao, Y.; Zhao, L.; Pan, X.; Zhang, L. Experimental and numerical study on temperature rise within a 70 MPa type III cylinder during fast refueling. Int. J. Hydrogen Energy 2013, 38, 10956–10962. [Google Scholar] [CrossRef]
- Kim, S.C.; Lee, S.H.; Yoon, K.B. Thermal characteristics during hydrogen fueling process of type IV cylinder. Int. J. Hydrogen Energy 2010, 35, 6830–6835. [Google Scholar] [CrossRef]
- Suryan, A.; Kim, H.D.; Setoguchi, T. Three dimensional numerical computations on the fast filling of a hydrogen tank under different conditions. Int. J. Hydrogen Energy 2012, 37, 7600–7611. [Google Scholar] [CrossRef]
- Striednig, M.; Brandstätter, S.; Sartory, M.; Klell, M. Thermodynamic real gas analysis of a tank filling process. Int. J. Hydrogen Energy 2014, 39, 8495–8509. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, X.; You, H.; Gao, M. Research on the design of hydrogen supply system of 70 MPa hydrogen storage cylinder for vehicles. Int. J. Hydrogen Energy 2018, 43, 19189–19195. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, S.; Zhang, Z.; Zheng, J.; Zhao, Y. Numerical study on the fast filling of on-bus gaseous hydrogen storage cylinder. Int. J. Hydrogen Energy 2020, 45, 9241–9251. [Google Scholar] [CrossRef]
- Song, B.H.; Myoung, N.S.; Jang, S.J.; Kwon, J.T. Hydrogen Compressor Cycle Analysis for the Operating Pressure of 50 MPa and High Charging Capacity. J. Korea Acad. Ind. Coop. Soc. 2020, 21, 66–73. [Google Scholar]
- Zirkel, A.; Laurien, E. Turbulence Modelling for CFD-Methods for Containment Flows. In High Performance Computing in Science and Engineering’11; Springer: Berlin/Heidelberg, Germany, 2012; pp. 451–467. [Google Scholar]
- Bourgeois, T.; Ammouri, F.; Weber, M.; Knapik, C. Evaluating the temperature inside a tank during a filling with highly-pressurized gas. Int. J. Hydrogen Energy 2015, 40, 11748–11755. [Google Scholar] [CrossRef]
- NIST Reference Fluid Thermodynamic and Transport Properties Database; Version 9.5; NIST: Gaithersburg, MD, USA, 2019.
- Younglove, B.A.; McLinden, M.O. An International Standard Equation of State for the Thermodynamic Properties of Refrigerant 123 (2, 2-Dichloro-1,1,1-Trifluoroethane). J. Phys. Chem. Ref. Data 1994, 23, 731–779. [Google Scholar] [CrossRef]
- Leachman, J.W.; Jacobsen, R.T.; Penoncello, S.G.; Lemmon, E.W. Fundamental equations of state for parahydrogen, normal hydrogen, and orthohydrogen. J. Phys. Chem. Ref. Data 2009, 38, 721–748. [Google Scholar] [CrossRef]
- Menter, F.R.; Kuntz, M.; Langtry, R. Ten years of industrial experience with the SST turbulence model. Turbulence. Heat Mass Transf. 2003, 4, 625–632. [Google Scholar]
- Winters, B. 70MPa Fast-Fill Modeling &Validation; Sandia National Laboratories: Albuquerque, NM, USA, 2010.
- Wang, L.; Zheng, C.; Wei, S.; Wang, B.; Wei, Z. Thermo-mechanical investigation of composite high-pressure hydrogen storage cylinder during fast filling. Int. J. Hydrogen Energy 2015, 40, 6853–6859. [Google Scholar] [CrossRef]
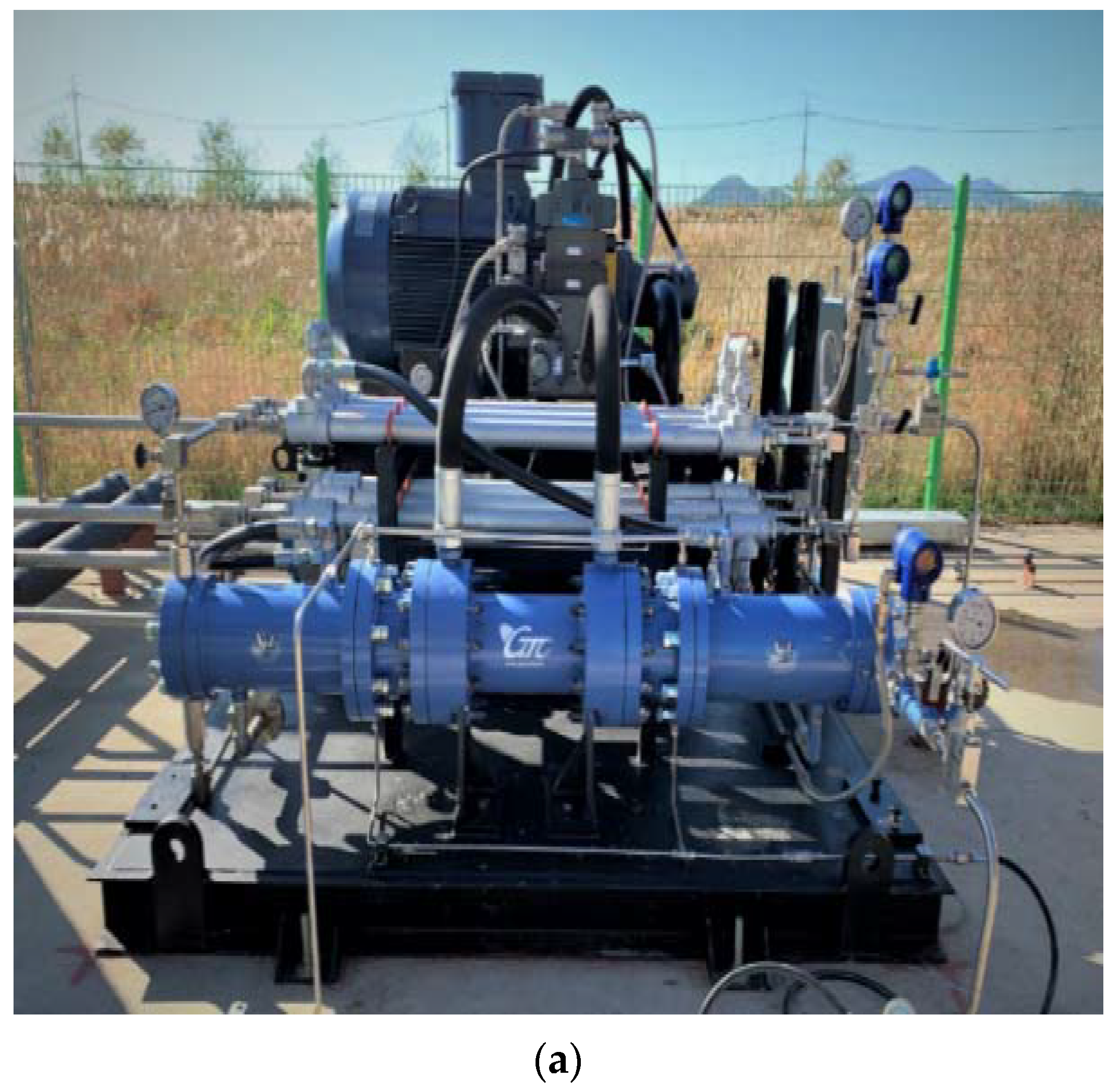
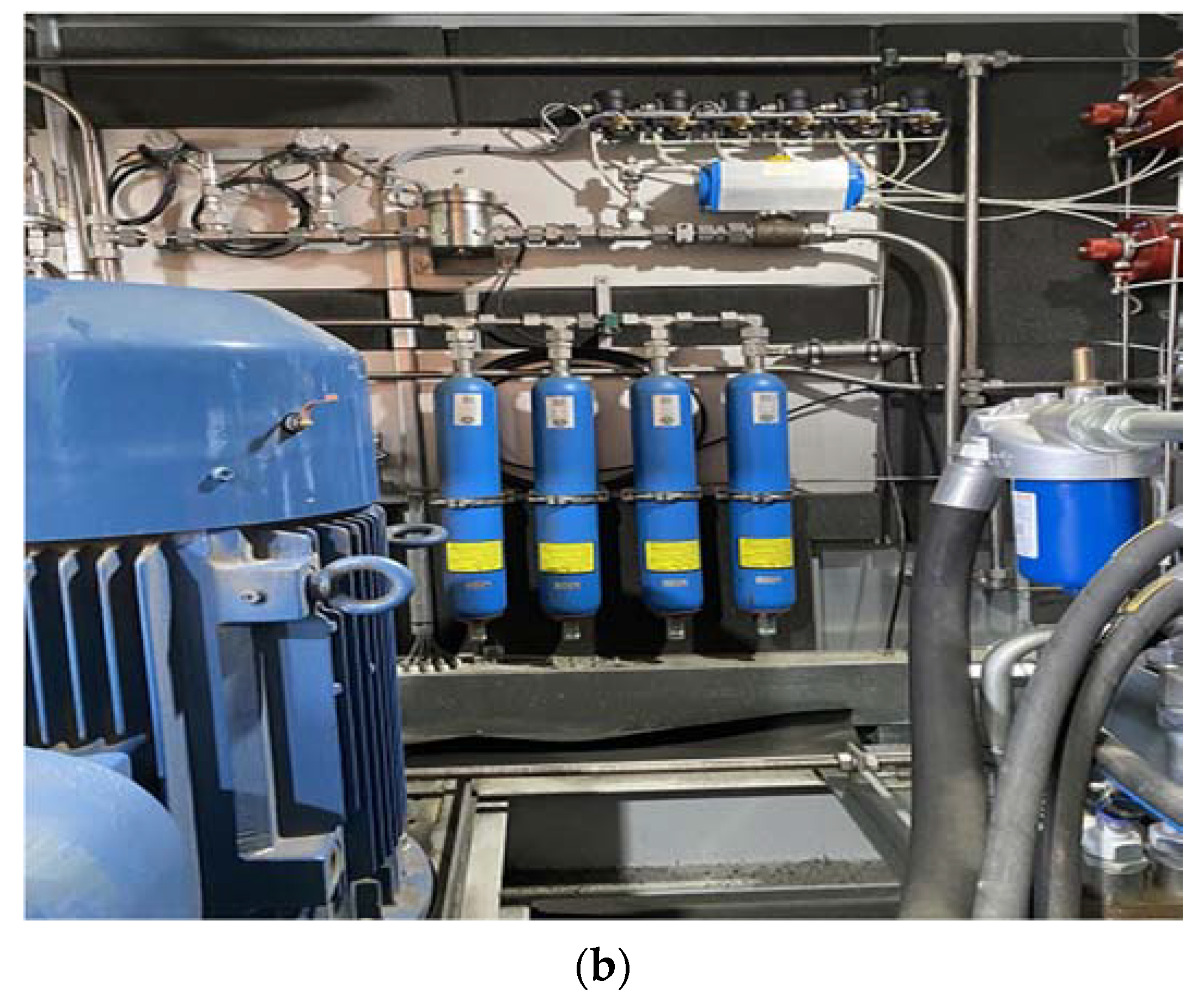

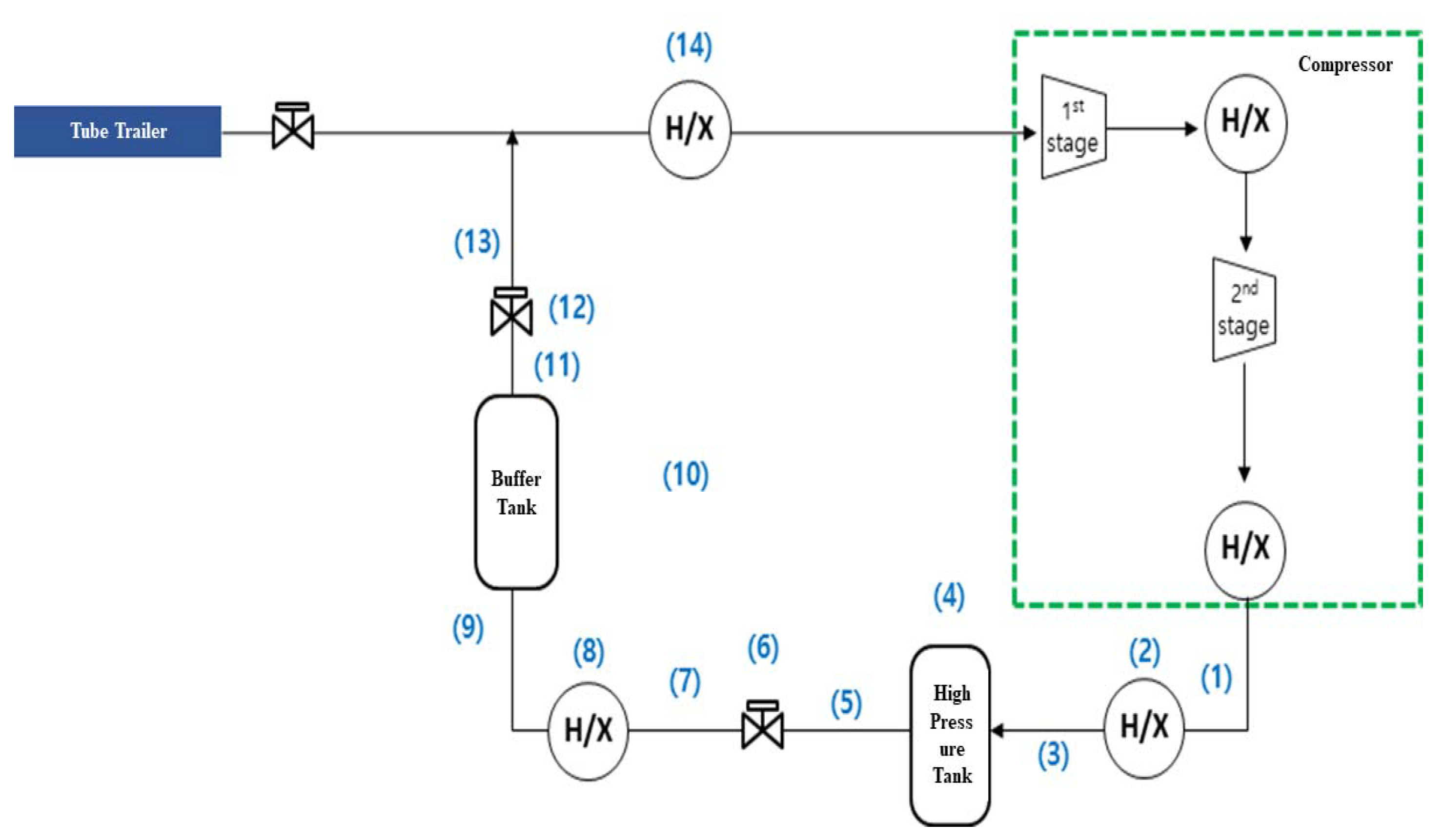


(MPa) | (K) | (MPa) | (K) | (kg) | (MPa) | (K) | (kg) |
|---|---|---|---|---|---|---|---|
| 50 | 313.13 | 11.3 | 300.15 | Initial mass | 50 | Final temperature | Final mass |
| Specification | |
| L (m) | 4.4197 |
| (m) | 0.3143 |
| (m) | 0.3985 |
| (m) | 0.0143 |
| T (m) | 0.0842 |
| Properties | |
| K (W/m·K) | 16.3 |
| (W/m2·K) | 113.49 |
| (W/m2·K) | 40 |
| Density | Specific Heat | Thermal Conductivity | |
|---|---|---|---|
(50 MPa, 313.15 K) | 29.651 | 14,957 | 0.233 |
| Cylinder (Stainless steel) | 7830 | 500 | 16.3 |
| Condition | Theoretical Method (Adiabatic Filling) | Simulation Method (Non-Adiabatic Filling) |
|---|---|---|
| Final temperature (K) | 397.12 | 378 |
| Final mass (kg) | 8.41 | 8.85 |
| Charged mass (kg) | 5.48 | 5.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-Q.; Myoung, N.-S.; Kwon, J.-T.; Jang, S.-J.; Lee, T. A Study on the Prediction of the Temperature and Mass of Hydrogen Gas inside a Tank during Fast Filling Process. Energies 2020, 13, 6428. https://doi.org/10.3390/en13236428
Li J-Q, Myoung N-S, Kwon J-T, Jang S-J, Lee T. A Study on the Prediction of the Temperature and Mass of Hydrogen Gas inside a Tank during Fast Filling Process. Energies. 2020; 13(23):6428. https://doi.org/10.3390/en13236428
Chicago/Turabian StyleLi, Ji-Qiang, No-Seuk Myoung, Jeong-Tae Kwon, Seon-Jun Jang, and Taeckhong Lee. 2020. "A Study on the Prediction of the Temperature and Mass of Hydrogen Gas inside a Tank during Fast Filling Process" Energies 13, no. 23: 6428. https://doi.org/10.3390/en13236428
APA StyleLi, J.-Q., Myoung, N.-S., Kwon, J.-T., Jang, S.-J., & Lee, T. (2020). A Study on the Prediction of the Temperature and Mass of Hydrogen Gas inside a Tank during Fast Filling Process. Energies, 13(23), 6428. https://doi.org/10.3390/en13236428





