A Review on Synthetic Ester Liquids for Transformer Applications
Abstract
1. Introduction
- -
- Chemical properties of synthetic esters;
- -
- AC and DC breakdown voltage of synthetic esters;
- -
- Lightning impulse breakdown voltage and pre-breakdown phenomena of synthetic esters;
- -
- Synthetic ester-based nanofluids;
- -
- Combined paper-synthetic ester-based insulating systems;
- -
- Application of synthetic esters for retro-filling and drying of mineral oil-immersed transformers;
- -
- DGA-based diagnosis of synthetic ester-filled transformers;
- -
- Static electrification of synthetic esters.
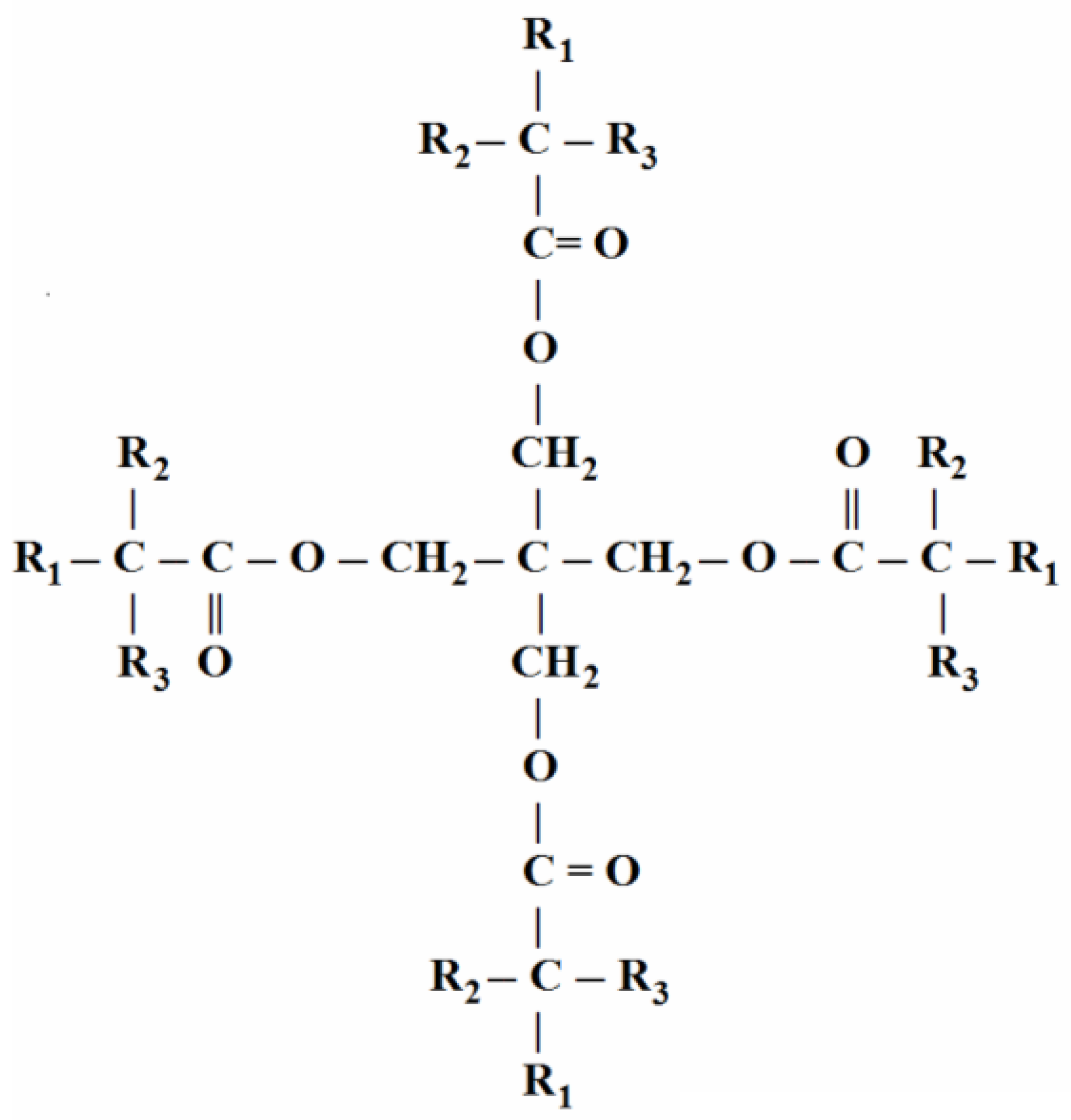
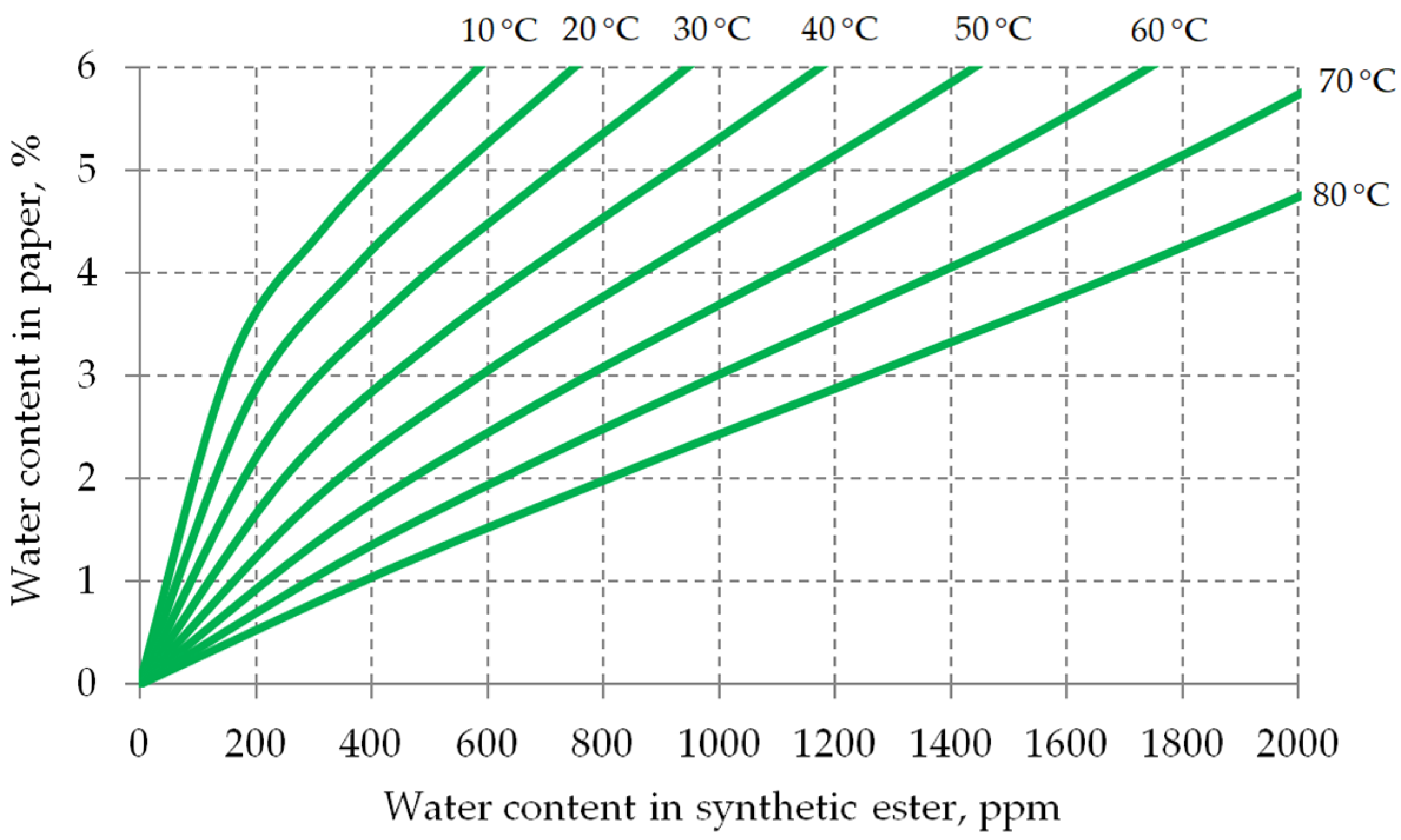
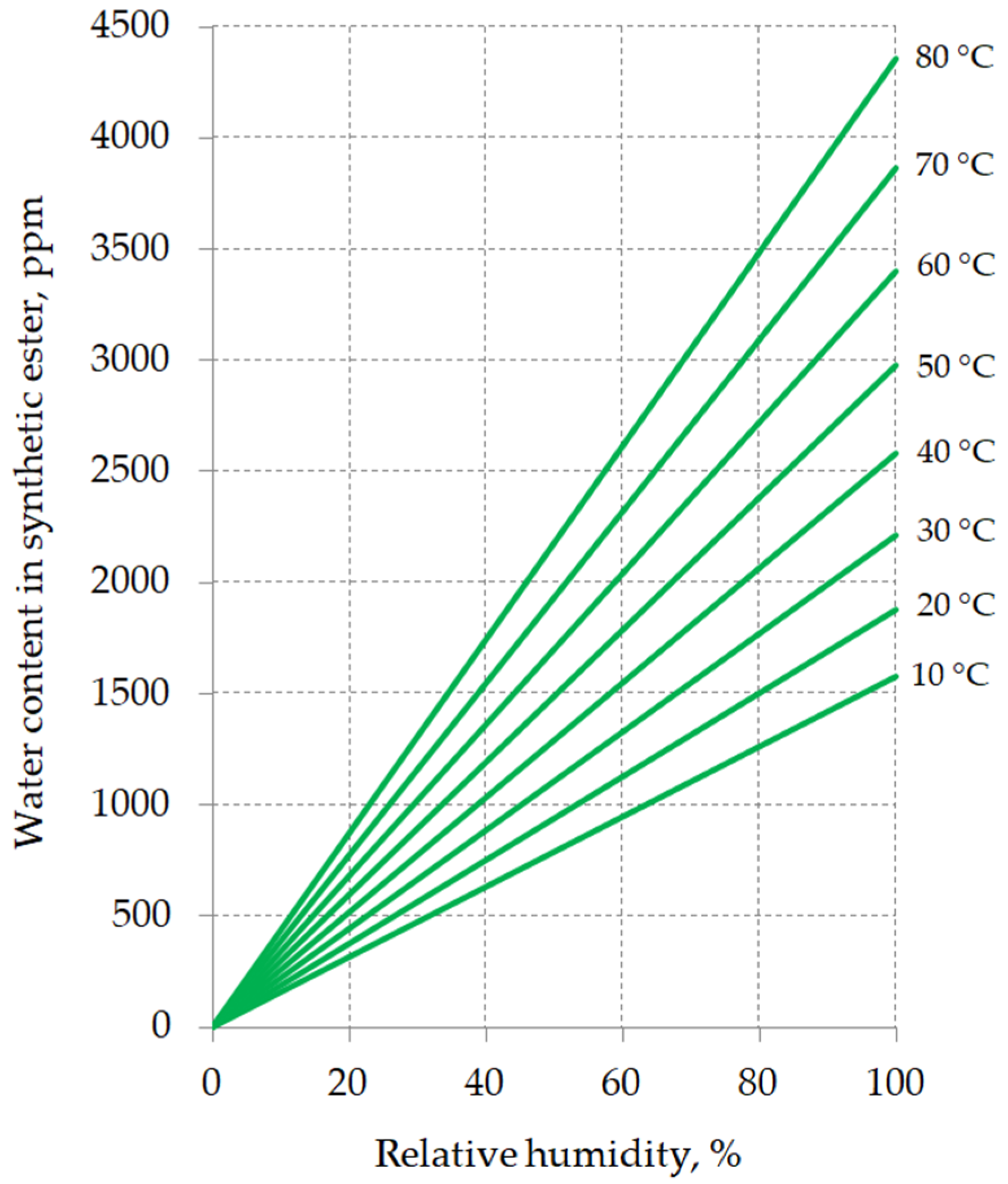
2. Chemical Properties of Synthetic Esters
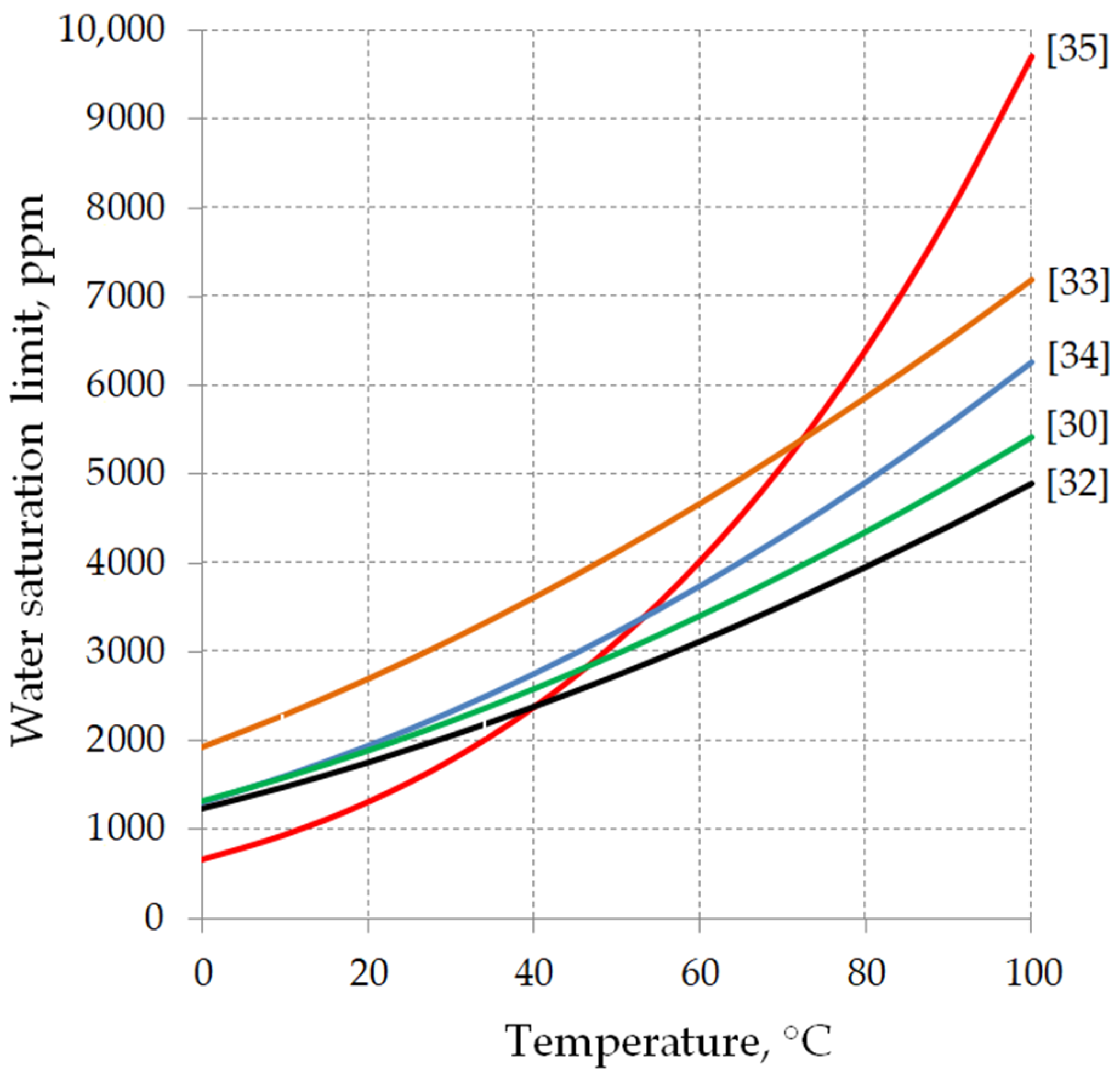
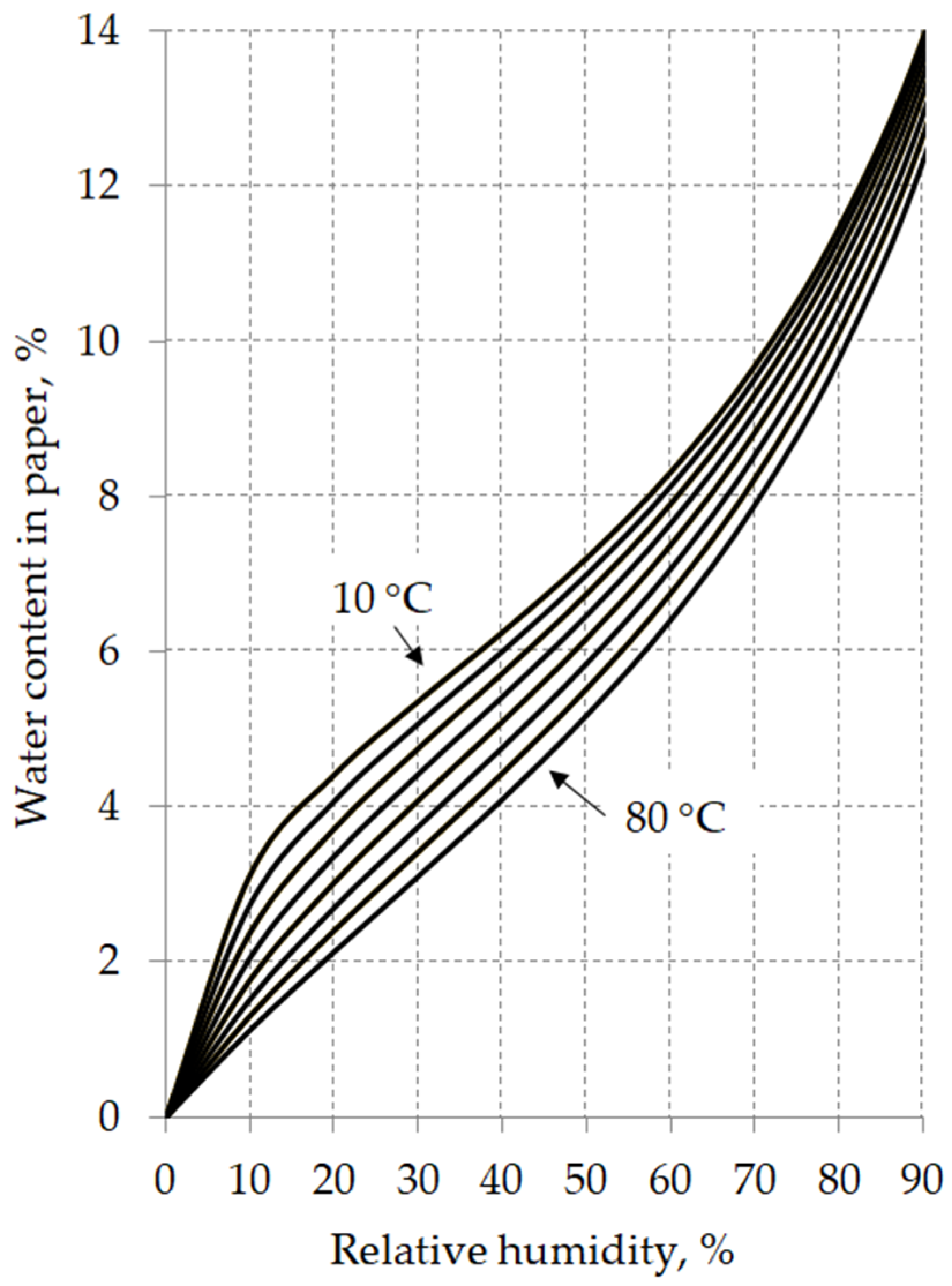
- A and B—water saturation coefficients characteristic for the given insulating liquid,
- T—liquid temperature in Kelvin.
3. Breakdown Characteristics of Synthetic Esters
3.1. AC and DC Breakdown Voltage
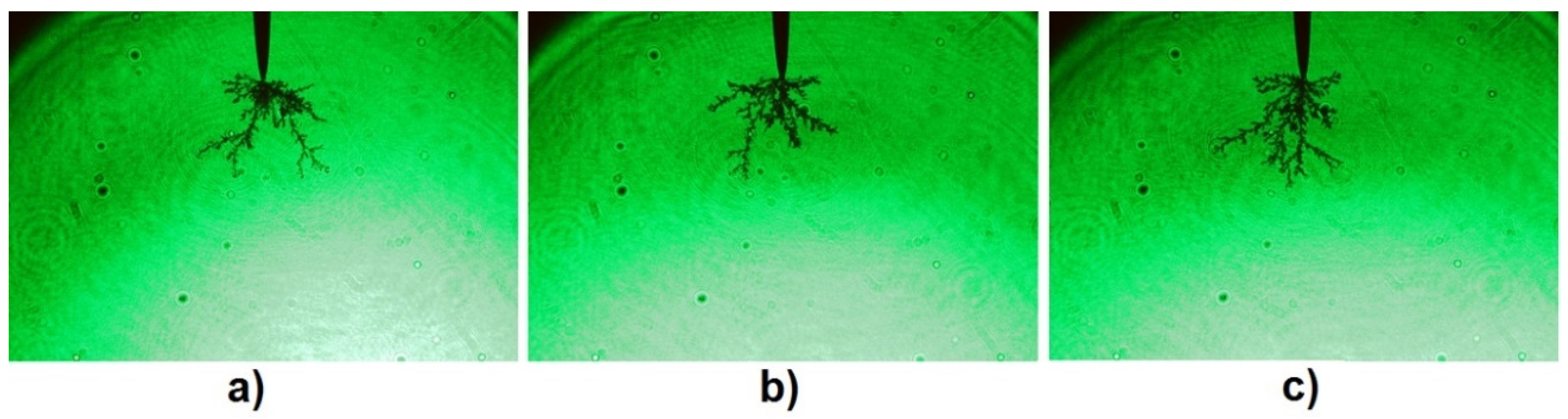
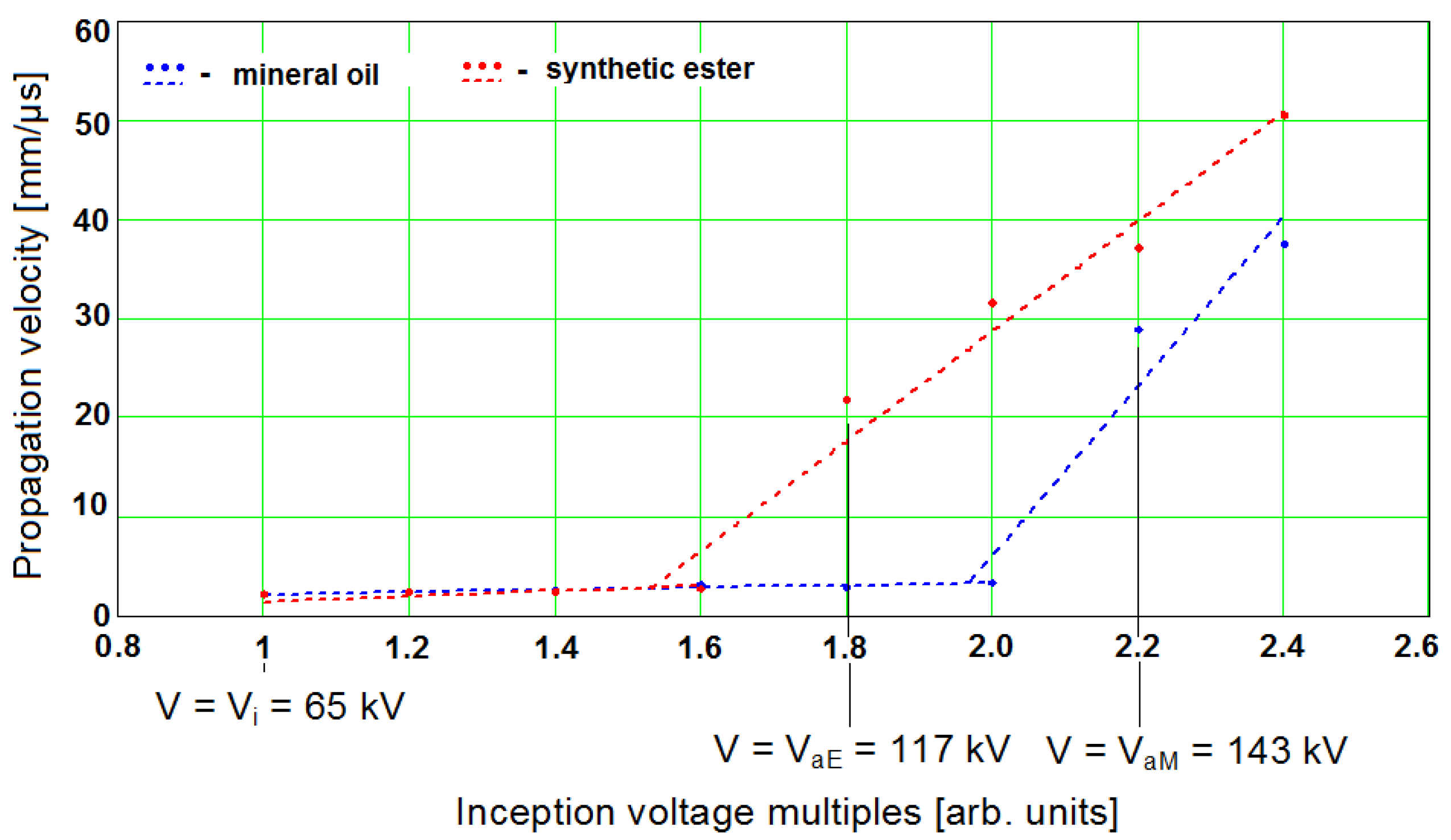
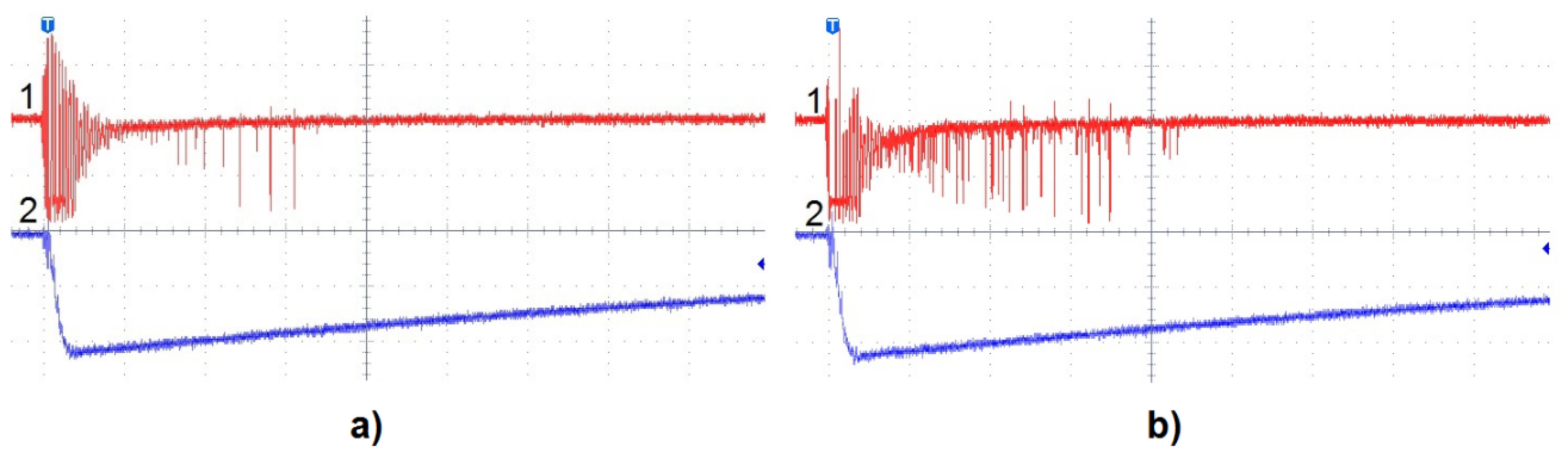
3.2. LI Breakdown Voltage and Associated Phenomena
4. Synthetic Ester-Based Nanofluids
4.1. Influence of Nanoparticles on the Breakdown Voltage of Synthetic Estes-Based Nanofluids
4.2. Processes Involved in Breakdown Voltage Mechanisms of Synthetic Esters-Based Nanofluids
5. Combined Paper-Synthetic Ester-Based Insulating Systems
5.1. Moisture Equilibrium Curves
5.2. Impregnation of Solid Insulation with Synthetic Esters
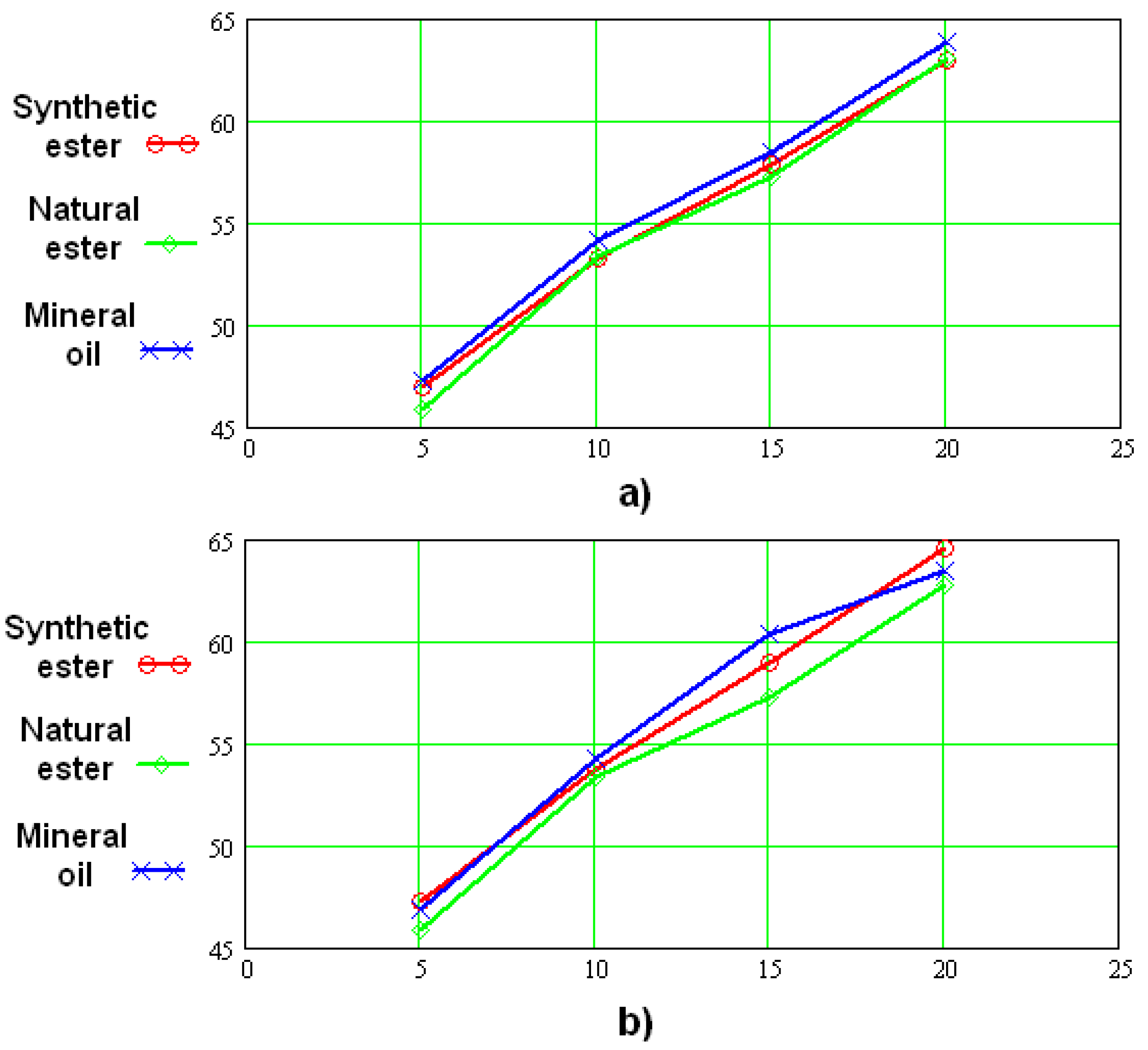
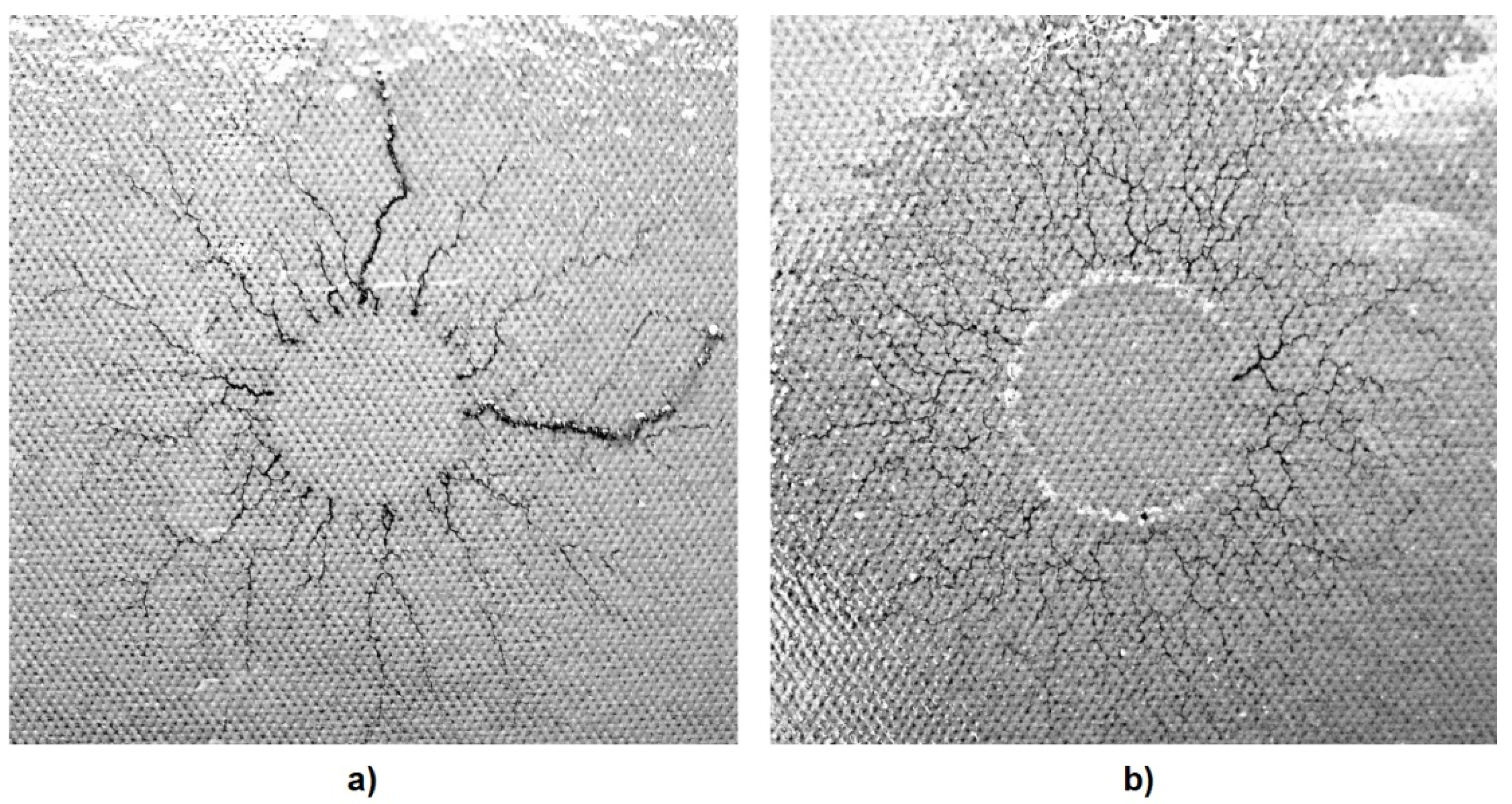
5.3. Breakdown Characteristics of Solid Insulation Components Impregnated with Synthetic Esters
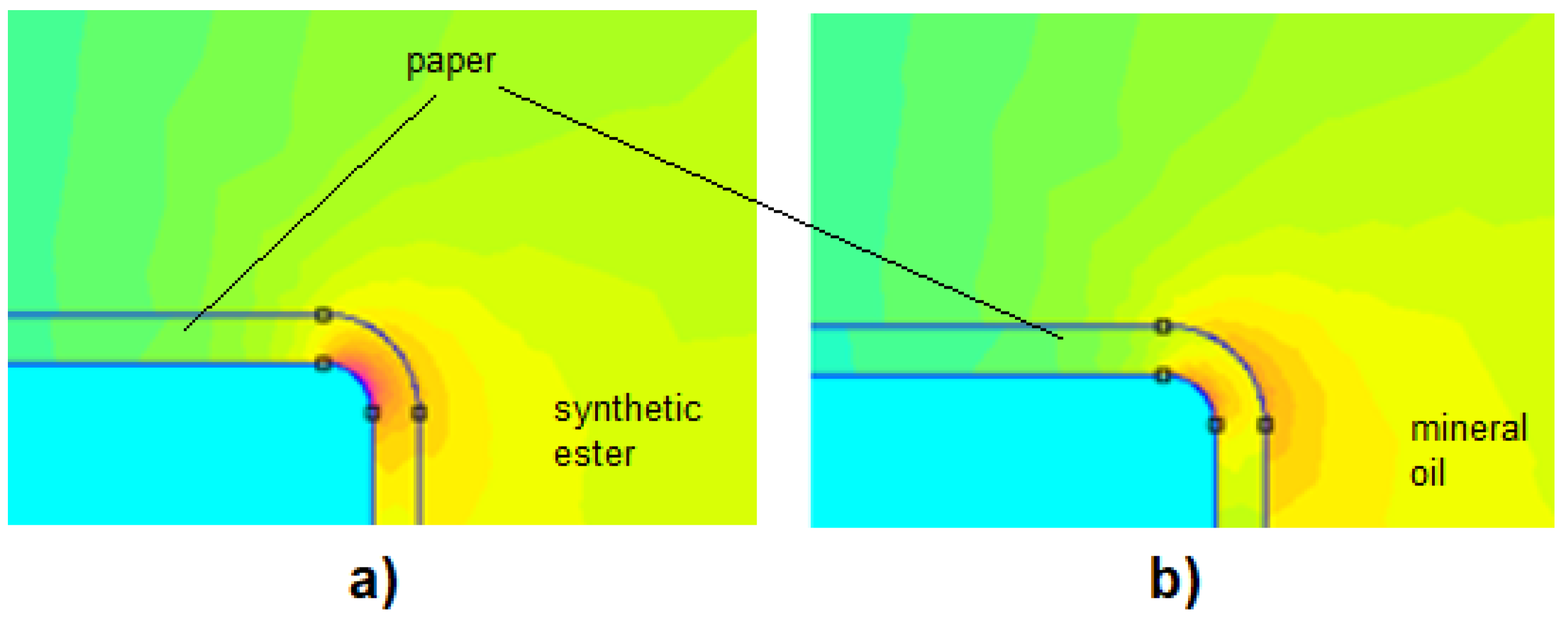
5.4. Electric Field Distribution in Insulating Systems with Synthetic Esters
6. Application of Synthetic Esters for Retro-Filling and Drying of Mineral Oil-Immersed Transformers
6.1. Miscibility
6.2. Retro-Filling
- increasing the transformer life; which is related to the superior water tolerance properties of synthetic esters, which increase the life of solid insulation’
- lowering the insurance costs by reducing the likelihood of a serious fire;
- and demonstrating the social responsibility of the transformer owner.
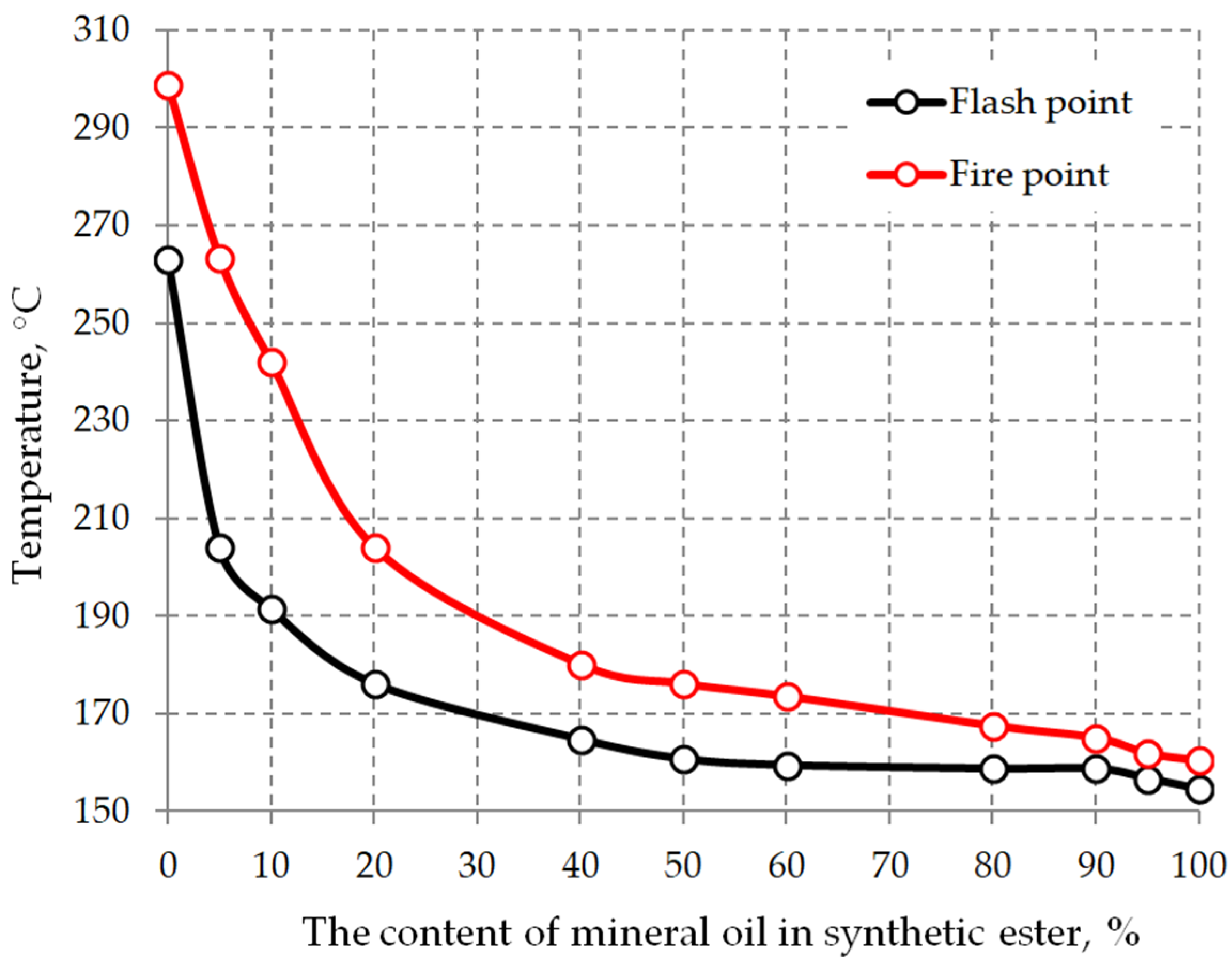
6.3. Drying
- Drying temperature;
- Thickness of cellulose materials;
- Moisture content in the synthetic ester;
- Moisture content in cellulose;
- Weight ratio of ester to cellulose.
- The moisture content in cellulose materials before retro-filling is equal to 3.0%;
- The average operation temperature of the insulation is equal to 60 °C;
- The initial water content in synthetic ester is equal to 60 ppm;
- The weight ratio of the cellulose insulation to the electro-insulating liquid equals 0.0715 for the chosen transformer with a capacity of 10 MVA, a mean of 522 kg of cellulose and 7300 kg of mineral oil.
- Replacement of mineral oil with a synthetic ester
- Drying the cellulose insulation by means of the ester, which circulates between the transformer and the equipment for ester drying and heating
- Filling back the transformer tank with mineral oil.
7. DGA-Based Diagnosis of Synthetic Ester-Filled Transformers
8. Static Electrification of Synthetic Esters
9. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Oommen, T.; Claiborne, C.; Mullen, J. Biodegradable electrical insulation fluids. In Proceedings of the Electrical Insulation Conference and Electrical Manufacturing and Coil Winding Conference, Rosemont, IL, USA, 25 September 1997; pp. 465–468. [Google Scholar] [CrossRef]
- Gockenbach, E.; Borsi, H. Natural and Synthetic Ester Liquids as alternative to mineral oil for power transformers. In Proceedings of the 2008 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Quebec City, QC, Canada, 26–29 October 2008; pp. 521–524. [Google Scholar] [CrossRef]
- Perrier, C.; Beroual, A. Experimental investigations on insulating liquids for power transformers: Mineral, ester, and silicone oils. IEEE Electr. Insul. Mag. 2009, 25, 6–13. [Google Scholar] [CrossRef]
- Martin, R.; Athanassatou, H.; Duart, J.C.; Perrier, C.; Sitar, I.; Walker, J.; Claiborne, C.; Boche, T.; Cherry, D.; Darwin, A.; et al. Experiences in Service with New Insulating Liquids; Cigré Technical Brochure 436; International Council on Large Electric Systems (CIGRE): Paris, France, 2010. [Google Scholar]
- Fofana, I. 50 years in the development of insulating liquids. IEEE Electr. Insul. Mag. 2013, 29, 13–25. [Google Scholar] [CrossRef]
- Fernández, I.; Ortiz, A.; Delgado, F.; Renedo, C.; Pérez, S. Comparative evaluation of alternative fluids for power transformers. Electr. Power Syst. Res. 2013, 98, 58–69. [Google Scholar] [CrossRef]
- Bathina, V.; Sood, Y.R.; Jarial, R.K. Ester Dielectrics: Current Perspectives and Future Challenges. IETE Tech. Rev. 2016, 34, 448–459. [Google Scholar] [CrossRef]
- Rao, U.M.; Fofana, I.; Jaya, T.; Rodriguez-Celis, E.M.; Jalbert, J.; Picher, P. Alternative Dielectric Fluids for Transformer Insulation System: Progress, Challenges, and Future Prospects. IEEE Access 2019, 7, 184552–184571. [Google Scholar] [CrossRef]
- Oommen, T. Vegetable oils for liquid-filled transformers. IEEE Electr. Insul. Mag. 2002, 18, 6–11. [Google Scholar] [CrossRef]
- McShane, C.; Corkran, J.; Rapp, K.; Luksich, J. Natural Ester Dielectric Fluid Development. In Proceedings of the 2005/2006 IEEE/PES Transmission and Distribution Conference and Exhibition, Dallas, TX, USA, 21–24 May 2006; pp. 18–22. [Google Scholar] [CrossRef]
- Liao, R.; Hao, J.; Chen, G.; Ma, Z.; Yang, L. A comparative study of physicochemical, dielectric and thermal properties of pressboard insulation impregnated with natural ester and mineral oil. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1626–1637. [Google Scholar] [CrossRef]
- Rapp, K.J.; Luksich, J.; Sbravati, A. Application of natural ester insulating liquids in power transformers. In Proceedings of the My Transfo 2014, Turin, Italy, 18–19 November 2014; pp. 1–7. [Google Scholar]
- Sitorus, H.B.; Setiabudy, R.; Bismo, S.; Beroual, A. Jatropha curcas methyl ester oil obtaining as vegetable insulating oil. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2021–2028. [Google Scholar] [CrossRef]
- Haegele, S.; Vahidi, F.; Tenbohlen, S.; Rapp, K.J.; Sbravati, A. Lightning Impulse Withstand of Natural Ester Liquid. Energies 2018, 11, 1964. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, X.; Li, J.; Wang, F.; Zhang, R.; Mehmood, M.A.; Liang, S.; Jiang, T. Streamer characteristics of dielectric natural ester-based liquids under long gap distances. AIP Adv. 2018, 8, 105129. [Google Scholar] [CrossRef]
- Tokunaga, J.; Nikaido, M.; Koide, H.; Hikosaka, T. Palm fatty acid ester as biodegradable dielectric fluid in transformers: A review. IEEE Electr. Insul. Mag. 2019, 35, 34–46. [Google Scholar] [CrossRef]
- Szewczyk, R.; Vercesi, G. Innovative insulation materials for liquid-immersed transformers. Presented at the DuPont Webinar, 13 March 2015. [Google Scholar]
- Rozga, P.; Stanek, M. Comparative analysis of lightning breakdown voltage of natural ester liquids of different viscosities supported by light emission measurement. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 991–999. [Google Scholar] [CrossRef]
- Rozga, P. Comparative assessment of impregnation efficiency of insulating pressboard by selected dielectric esters and mineral oil using capillary action test. Przegląd Elektrotech. 2018, 10, 69–72. (In Polish) [Google Scholar] [CrossRef]
- Available online: https://www.cargill.com/bioindustrial/dielectric-fluids (accessed on 12 October 2020).
- Available online: https://www.midel.com/ (accessed on 12 October 2020).
- Russel, M.; Lashbrook, M.; Satija, N. Synthetic esters for Power transformers AT > 100 kV. ITMA J. 2013. [Google Scholar]
- Dohnal, D.; Frotscher, F. The use of natural and synthetic esters in tap changers for power transformers. CEPSI 2014, E9, 1–9. [Google Scholar]
- IEC 61099. Insulating Liquids—Specifications for Unused Synthetic Organic Esters for Electrical Purposes; International Electrotechnical Commission (IEC): Geneva, Switzerland, 2010. [Google Scholar]
- Stanek, M. The Use of Measuring the Intensity of Light Emitted by Electrical Discharges Developing in Biodegradable Insulating Esters to Assess Their Lightning Strength. Ph.D. Thesis, Lodz University of Technology, Lodz, Poland, 2019. [Google Scholar]
- Liu, Q.; Wang, Z.D. Breakdown and withstand strengths of ester transformer liquids in a quasi-uniform field under impulse voltages. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 571–579. [Google Scholar] [CrossRef]
- Pukel, G.J.; Schwarz, R.; Baumann, F.; Muhr, H.M.; Eberhardt, R.; Wieser, B.; Chu, D. Power transformers with environmentally friendly and low flammability ester liquids. Cigre Sess. 2013, 1–6. [Google Scholar] [CrossRef]
- Asano, R.; Page, S.A. Reducing Environmental Impact and Improving Safety and Performance of Power Transformers with Natural Ester Dielectric Insulating Fluids. IEEE Trans. Ind. Appl. 2013, 50, 134–141. [Google Scholar] [CrossRef]
- IEC 60814. IEC Insulating Liquids—Oil-Impregnated Paper and Pressboard—Determination of Water by Automatic Coulometric Karl Fischer Titration; International Electrotechnical Commission (IEC): Geneva, Switzerland, 1997. [Google Scholar]
- Atanasova-Höhlein, I.; Končan-Gradnik, M.; Gradnik, T.; Čuček, B.; Przybylek, P.; Siodla, K.; Liland, K.B.; Leivo, S.; Liu, Q. Moisture Measurement and Assessment in Transformer Insulation—Evaluation of Chemical Methods and Moisture Capacitive Sensors; Cigré Technical Brochure 741; International Council on Large Electric Systems (CIGRE): Paris, France, 2018. [Google Scholar]
- Przybyłek, P. Water solubility in synthetic ester and mixture of ester with mineral oil in aspect of cellulose insulation drying. Prz. Elektrotech. 2016, 10, 92–95. [Google Scholar] [CrossRef]
- Przybylek, P. Water saturation limit of insulating liquids and hygroscopicity of cellulose in aspect of moisture determination in oil-paper insulation. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1886–1893. [Google Scholar] [CrossRef]
- Aralkellian, V.G.; Fofana, I. Water in Oil-Filled, High-Voltage Equipment, Part I: States, Solubility, and Equilibrium in Insulating Materials. IEEE Electr. Insul. Mag. 2007, 23, 15–27. [Google Scholar] [CrossRef]
- Jovalekic, M.; Kolb, D.; Tenbohlen, S.; Bates, L.; Szewczyk, R. A methodology for determining water saturation limits and moisture equilibrium diagrams of alternative insulation systems. In Proceedings of the 2011 IEEE International Conference on Dielectric Liquids, Trondheim, Norway, 26–30 June 2011. [Google Scholar] [CrossRef]
- Water solubility algorithms for dielectric liquids. In DOMINOTM Application Bulletin; Serial number: MKT-AB 11 Rev B; Doble: Marlborough, MA, USA, 1999.
- Dombek, G.; Nadolny, Z.; Przybylek, P.; Lopatkiewicz, R.; Marcinkowska, A.; Druzynski, L.; Boczar, T.; Tomczewski, A. Effect of Moisture on the Thermal Conductivity of Cellulose and Aramid Paper Impregnated with Various Dielectric Liquids. Energies 2020, 13, 4433. [Google Scholar] [CrossRef]
- IEC 61203. Synthetic Organic Esters for Electrical Purposes—Guide for Maintenance of Transformer Esters in Equipment; International Electrotechnical Commission (IEC): Geneva, Switzerland, 1992. [Google Scholar]
- Martins, M.A.G. Vegetable oils, an alternative to mineral oil for power transformers- experimental study of paper aging in vegetable oil versus mineral oil. IEEE Electr. Insul. Mag. 2010, 26, 7–13. [Google Scholar] [CrossRef]
- Martins, M.A.G.; Gomes, A.R. Comparative study of the thermal degradation of synthetic and natural esters and mineral oil: Effect of oil type in the thermal degradation of insulating kraft paper. IEEE Electr. Insul. Mag. 2012, 28, 22–28. [Google Scholar] [CrossRef]
- IEC 60156. Insulating Liquids—Determination of the Breakdown Voltage at Power Frequency—Test Method; International Electrotechnical Commission (IEC): Geneva, Switzerland, 2018. [Google Scholar]
- Scigala, K. Analysis of the Influence of the Used Dielectric Liquid on the Distribution of the Electric Field in the Insulation System-Solid Insulation-Dielectric Liquid. Master’s Thesis, Lodz University of Technology, Lodz, Poland, 2018. (In Polish). [Google Scholar]
- Dombek, G.; Gielniak, J. Fire safety and electrical properties of mixtures of synthetic ester/mineral oil and synthetic ester/natural ester. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1846–1852. [Google Scholar] [CrossRef]
- Khaled, U.; Beroual, A. AC dielectric strength of synthetic ester-based Fe3O4, Al2O3 and SiO2 nanofluids—Conformity with normal and weibull distributions. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 625–633. [Google Scholar] [CrossRef]
- Mohamad, M.S.; Zainuddin, H.; Ghani, S.A.; Chairul, I.S. AC breakdown voltage and viscosity of palm fatty acid ester. J. Electr. Eng. Technol. 2017, 12, 2333–2341. [Google Scholar] [CrossRef]
- Mentlik, V.; Trnka, P.; Hornak, J.; Totzauer, P. Development of a Biodegradable Electro-Insulating Liquid and Its Subsequent Modification by Nanoparticles. Energies 2018, 11, 508. [Google Scholar] [CrossRef]
- Beroual, A.; Khaled, U.; Alghamdi, A.M. DC Breakdown Voltage of Synthetic Ester Liquid-Based Nanofluids. IEEE Access 2020, 8, 125797–125805. [Google Scholar] [CrossRef]
- Khaled, U.; Beroual, A. DC breakdown voltage of natural ester oil-based Fe3O4, Al2O3, and SiO2 nanofluids. Alex. Eng. J. 2020. [Google Scholar] [CrossRef]
- Mahidhar, G.D.P.; Sarathi, R.; Taylor, N.; Edin, H. Study on performance of silica nanoparticle dispersed synthetic ester oil under AC and DC voltages. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1958–1966. [Google Scholar] [CrossRef]
- Fafana, I.; Wasserberg, V.; Borsil, H.; Gockenbach, E. Retrofilling conditions of high voltage transformers. IEEE Electr. Insul. Mag. 2001, 17, 17–30. [Google Scholar] [CrossRef]
- Fofana, I.; Wasserberg, V.; Borsi, H.; Gockenbach, E. Preliminary investigations for the retrofilling of perchlorethylene based fluid filled transformer. IEEE Trans. Dielectr. Electr. Insul. 2002, 9, 97–103. [Google Scholar] [CrossRef]
- Fatyga, P.; Morańda, H. Evaluation of the Mineral Oil and Synthetic Ester Percentage Composition after Replacing Oil with Ester Fluid in Power Transformer. In Proceedings of the International Conference Transformation, Toruń, Poland, 9–11 May 2017. (In Polish). [Google Scholar]
- Retrofiling–Upgrading Your Transformer. Available online: https://www.midel.com/midel-in-use/retrofilling/ (accessed on 25 August 2020).
- Insulect-Energy Blog. Available online: https://insulect.com/energy-blog/can-i-use-midel-fluids-to-retrofill-transformers-at-high-voltages (accessed on 25 August 2020).
- Wasserberg, V.; Borsi, H.; Gockenbach, E. A new method for drying the paper insulation of power transformers during service. In Proceedings of the Conference Record of the 2000 IEEE International Symposium on Electrical Insulation (Cat. No.00CH37075), Anaheim, CA, USA, 5 April 2002; pp. 251–254. [Google Scholar] [CrossRef]
- Przybylek, P. Drying transformer cellulose insulation by means of synthetic ester. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2643–2648. [Google Scholar] [CrossRef]
- Przybylek, P.; Moranda, H.; Moscicka-Grzesiak, H.; Szczesniak, D. Application of Synthetic Ester for Drying Distribution Transformer Insulation—The Influence of Cellulose Thickness on Drying Efficiency. Energies 2019, 12, 3874. [Google Scholar] [CrossRef]
- Przybylek, P.; Moranda, H.; Moscicka-Grzesiak, H.; Cybulski, M. Laboratory Model Studies on the Drying Efficiency of Transformer Cellulose Insulation Using Synthetic Ester. Energies 2020, 13, 3467. [Google Scholar] [CrossRef]
- Przybylek, P.; Moranda, H.; Moscicka-Grzesiak, H.; Cybulski, M. Analysis of factors affecting the effectiveness of drying cellulose materials with synthetic ester. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1538–1545. [Google Scholar] [CrossRef]
- Dang, V.-H.; Beroual, A.; Perrier, C. Comparative study of streamer phenomena in mineral, synthetic and natural ester oils under lightning impulse voltage. In Proceedings of the 2010 International Conference on High Voltage Engineering and Application, New Orleans, LA, USA, 11–14 October 2010; pp. 560–563. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.D. Streamer characteristic and breakdown in synthetic and natural ester transformer liquids under standard lightning impulse voltage. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 285–294. [Google Scholar] [CrossRef]
- Rozga, P. Streamer propagation in small gaps of synthetic ester and mineral oil under lightning impulse. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2754–2762. [Google Scholar] [CrossRef]
- Rozga, P.; Stanek, M.; Rapp, K. Lightning properties of selected insulating synthetic esters and mineral oil in point-to-sphere electrode system. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1699–1705. [Google Scholar] [CrossRef]
- Dang, V.-H.; Beroual, A.; Perrier, C. Investigations on streamers phenomena in mineral, synthetic and natural ester oils under lightning impulse voltage. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1521–1527. [Google Scholar] [CrossRef]
- Rozga, P. Studies on behavior of dielectric synthetic ester under the influence of concentrated heat flux. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 908–914. [Google Scholar] [CrossRef]
- Livesey, P.; Lashbrook, M.; Martin, R. Investigation of the factors affecting the dielectric dissipation factor of synthetic and natural esters. In Proceedings of the 2019 IEEE 20th International Conference on Dielectric Liquids (ICDL), Roma, Italy, 23–27 June 2019. [Google Scholar] [CrossRef]
- Graczkowski, A.; Gielniak, J. Influence of impregnating liquids on dielectric response of impregnated cellulose insulation. In Proceedings of the 2010 10th IEEE International Conference on Solid Dielectrics, Potsdam, Germany, 4–9 July 2010. [Google Scholar] [CrossRef]
- Beroual, A.; Sadaoui, F.; Coulibaly, M.-L.; Perrier, C. Investigation on static electrification phenomenon of ester liquids and mineral oil. In Proceedings of the 2015 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Ann Arbor, MI, USA, 18–21 October 2015; pp. 391–394. [Google Scholar] [CrossRef]
- Zdanowski, M. Streaming electrification of mineral insulating oil and synthetic ester MIDEL. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1127–1132. [Google Scholar] [CrossRef]
- Duval, M.; Hoehlein, I.; Scatiggio, F.; Cyr, M.; Grisaru, M.; Frotscher, R.; Martins, M.; Bates, L.; Boman, P.; Hall, A.C.; et al. DGA in Non-Mineral Oils and Load Tap Changers and Improved DGA Diagnosis Criteria; CIGRE brochure 443; International Council on Large Electric Systems (CIGRE): Paris, France, 2010. [Google Scholar]
- Perrier, C.; Marugan, M.; Beroual, A. DGA comparison between ester and mineral oils. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1609–1614. [Google Scholar] [CrossRef]
- Martin, D.; Wang, Z.D. Statistical analysis of the AC breakdown voltages of ester based transformer oils. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1044–1050. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Noakhes, J. Motion of conductive particles and the effect on AC breakdown strengths of esters. In Proceedings of the 2011 IEEE International Conference on Dielectric Liquids, Trondheim, Norway, 26–30 June 2011. [Google Scholar] [CrossRef]
- Dang, V.-H.; Beroual, A.; Perrier, C. Comparative study of statistical breakdown in mineral, synthetic and natural ester oils under AC voltage. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1508–1513. [Google Scholar] [CrossRef]
- Reffas, A.; Moulai, H.; Béroual, A. Comparison of dielectric properties of olive oil, mineral oil, and other natural and synthetic ester liquids under AC and lightning impulse stresses. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1822–1830. [Google Scholar] [CrossRef]
- Beroual, A.; Khaled, U.; Noah, P.S.M.; Sitorus, H. Comparative Study of Breakdown Voltage of Mineral, Synthetic and Natural Oils and Based Mineral Oil Mixtures under AC and DC Voltages. Energies 2017, 10, 511. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, Q.; Wang, Z.D. Streamer characteristic and breakdown in a mineral oil and a synthetic ester liquid under DC voltage. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1636–1643. [Google Scholar] [CrossRef]
- Reffas, A.; Beroual, A.; Moulai, H. Comparison of breakdown voltage of vegetable olive with mineral oil, natural and synthetic ester liquids under DC voltage. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1691–1697. [Google Scholar] [CrossRef]
- Hestad, Ø.L.; Ingebrigsten, S.; Lundgaard, L. Streamer initiation in cyclohexane, midel 7131 and nytro 10X. In Proceedings of the IEEE International Conference on Dielectric Liquids, Coimbra, Portugal, 26 June–1 July 2005; pp. 123–126. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Perrot, F. Impulse breakdown voltages of ester-based transformer oils determined by using different test methods. In Proceedings of the 2009 IEEE Conference on Electrical Insulation and Dielectric Phenomena, Virginia Beach, VA, USA, 18–21 October 2009; pp. 608–612. [Google Scholar] [CrossRef]
- Ngoc, M.N.; Lesaint, O.; Bonifaci, N.; Denat, A.; Hassanzadeh, M. A comparison of breakdown properties of natural and synthetic esters at high voltage. In Proceedings of the 2010 Annual Report Conference on Electrical Insulation and Dielectic Phenomena, West Lafayette, IN, USA, 17–20 October 2010. [Google Scholar] [CrossRef]
- Rozga, P.; Stanek, M.; Cieslinski, D. Comparison of properties of electrical discharges developing in natural and synthetic ester at inception voltage. In Proceedings of the 2013 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Shenzhen, China, 20–23 October 2013; pp. 891–894. [Google Scholar] [CrossRef]
- Denat, A.; Lesaint, O.; Mc Cluskey, F. Breakdown of liquids in long gaps: Influence of distance, impulse shape, liquid nature, and interpretation of measurements. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2581–2591. [Google Scholar] [CrossRef]
- Rozga, P. Using the light emission measurement in assessment of electrical discharge development in different liquid dielectrics under lightning impulse voltage. Electr. Power Syst. Res. 2016, 140, 321–328. [Google Scholar] [CrossRef]
- Rozga, P. Streamer Propagation and Breakdown in a Very Small Point-Insulating Plate Gap in Mineral Oil and Ester Liquids at Positive Lightning Impulse Voltage. Energies 2016, 9, 467. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.D. Streamer characteristic and breakdown in synthetic and natural ester transformer liquids with pressboard interface under lightning impulse voltage. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1908–1917. [Google Scholar] [CrossRef]
- Wang, K.; Wang, F.; Shen, Z.; Lou, Z.; Han, Q.; Li, J.; Trnka, P.; Rozga, P. Breakdown and streamer behavior in homogeneous synthetic trimethylolpropane triesters insulation oil. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1501–1507. [Google Scholar] [CrossRef]
- IEC 60897. Methods for the Determination of the Lightning Impulse Breakdown Voltage of Insulating Liquids; International Electrotechnical Commission (IEC): Geneva, Switzerland, 1987. [Google Scholar]
- Tobazéon, R. Prebreakdown phenomena in dielectric liquids. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 1132–1147. [Google Scholar] [CrossRef]
- Lesaint, O.; Massala, G. Positive streamer propagation in large oil gaps: Experimental characterization of propagation modes. IEEE Trans. Dielectr. Electr. Insul. 1998, 5, 360–370. [Google Scholar] [CrossRef]
- Yamashita, H.; Amano, H. Prebreakdown phenomena in hydrocarbon liquids. IEEE Trans. Electr. Insul. 1988, 23, 739–750. [Google Scholar] [CrossRef]
- Lesaint, O. Prebreakdown phenomena in liquids: Propagation ‘modes’ and basic physical properties. J. Phys. D Appl. Phys. 2016, 49, 144001. [Google Scholar] [CrossRef]
- Rao, U.M.; Fofana, I.; Beroual, A.; Rozga, P.; Pompili, M.; Calcara, L.; Rapp, K.J. A review on pre-breakdown phenomena in ester fluids: Prepared by the international study group of IEEE DEIS liquid dielectrics technical committee. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1546–1560. [Google Scholar] [CrossRef]
- Choi, S. Enhancing thermal conductivity of fluids with nanoparticles, Developments and Applications of Non-Newtonian flows. ASME J. Fluids Eng. 1995, 231, 99–105. [Google Scholar]
- Xuan, Y.; Li, Q. Heat transfer enhancement of nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, S.U.-S.; Li, S.; Yu, W.; Thompson, L.J. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Choi, S.-S.; Zhang, Z.G.; Yu, W.; Lockwood, F.E.; Grulke, E.A. Anomalous thermal conductivity enhancement in nanotube suspensions. Appl. Phys. Lett. 2001, 79, 2252–2254. [Google Scholar] [CrossRef]
- Godson, L.; Raja, B.; Lal, D.M.; Wongwises, S. Enhancement of heat transfer using nanofluids—An overview. Renew. Sustain. Energy Rev. 2010, 14, 629–641. [Google Scholar] [CrossRef]
- Puspitasari, P.; Permanasari, A.A.; Shaharun, M.S.; Tsamroh, D.I. Heat transfer characteristics of NiO nanofluid in heat exchanger. AIP Conf. Proc. 2020, 2228, 030023. [Google Scholar] [CrossRef]
- Rafiq, M.; Lv, Y.; Li, C. A Review on Properties, Opportunities, and Challenges of Transformer Oil-Based Nanofluids. J. Nanomater. 2016, 1–23. [Google Scholar] [CrossRef]
- Segal, V.; Hjortsberg, A.; Rabinovich, A.; Nattrass, D.; Raj, K. AC (60 Hz) and impulse breakdown strength of a colloidal fluid based on transformer oil and magnetite nanoparticles. In Proceedings of the Conference Record of the 1998 IEEE International Symposium on Electrical Insulation (Cat. No.98CH36239), Arlington, VA, USA, 7–10 June 1998; pp. 619–622. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Wang, Y.S.; Tian, J.H.; Sha, Y.C.; Jiang, X.X.; Gao, S.Y.; Sun, Q.H.; Nie, Q. Breakdown characteristics in transformer oil modified by nanoparticles. High Volt. Eng. 2010, 36, 1155–1159. [Google Scholar]
- Saidur, R.; Leong, K.; Mohammed, H. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Mergos, J.A.; Athanassopoulou, M.D.; Argyropoulos, T.G.; Dervos, C.T. Dielectric properties of nanopowder dispersions in paraffin oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1502–1507. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhong, Y.; Zhou, J.; Li, X.; Zhou, Y. Effect of semiconductive nanoparticles on insulating performances of transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 770–776. [Google Scholar] [CrossRef]
- Taha-Tijerina, J.; Narayanan, T.N.; Gao, G.; Rohde, M.; Tsentalovich, D.A.; Pasquali, M.; Ajayan, P.M. Electrically Insulating Thermal Nano-Oils Using 2D Fillers. ACS Nano 2012, 6, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Kharthik, R.; Raja, T.; Madavan, R. Enhancement of critical characteristics of transformer oil using nanomaterials. Arab. J. Sci. Eng. 2013, 38, 2725–2733. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, W.; Ma, K.; Zhang, S.; Zhou, Y.; Li, C.; Wang, Q. Nanoparticle Effect on Dielectric Breakdown Strength of Transformer Oil-Based Nanofluids. In Proceedings of the 2013 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Shenzhen, China, 20–23 October 2013; pp. 680–682. [Google Scholar] [CrossRef]
- Lv, Y.Z.; Zhou, Y.; Li, C.R.; Wang, Q.; Qi, B. Recent progress in nanofluids based on transformer oil: Preparation and electrical insulation properties. IEEE Electr. Insul. Mag. 2014, 30, 23–32. [Google Scholar] [CrossRef]
- Mdavan, R.; Balaraman, S. Investigation on effects of different types of nanoparticles on critical parameters of nano-liquid insulation systems. J. Mol. Liq. 2017, 230, 437–444. [Google Scholar] [CrossRef]
- Huang, Z.; Li, J.; Yao, W.; Wang, F.; Wan, F.; Tan, Y.; Mehmood, M.A. Electrical and thermal properties of insulating oil-based nanofluids: A comprehensive overview. IET Nanodielectr. 2019, 2, 27–40. [Google Scholar] [CrossRef]
- Khaled, U.; Beroual, A. AC Dielectric Strength of Mineral Oil-Based Fe3O4 and Al2O3 Nanofluids. Energies 2018, 11, 3505. [Google Scholar] [CrossRef]
- Khaled, U.; Beroual, A. Statistical Investigation of AC Dielectric Strength of Natural Ester Oil-Based Fe3O4, Al2O3, and SiO2 Nano-Fluids. IEEE Access 2019, 7, 60594–60601. [Google Scholar] [CrossRef]
- Peppas, G.D.; Danikas, M.G.; Bakandritsos, A.; Charalampakos, V.P.; Pyrgioti, E.C.; Gonos, I.F. Statistical investigation of AC breakdown voltage of nanofluids compared with mineral and natural ester oil. IET Sci. Meas. Technol. 2016, 10, 644–652. [Google Scholar] [CrossRef]
- Peppas, G.D.; Charalampakos, V.P.; Pyrgioti, E.C.; Bakandritsos, A.; Polykrati, A.D.; Gonos, I.F. A study on the Breakdown Characteristics of Natural Ester Based Nanofluids with Magnetic Iron Oxide and SiO2 Nanoparticles. In Proceedings of the 2018 IEEE International Conference on High Voltage Engineering and Application (ICHVE), Athens, Greece, 10–13 September 2018. [Google Scholar] [CrossRef]
- Makmud, M.Z.H.; Illias, H.A.; Ching, Y.C.; Sarjadi, M.S. Influence of Conductive and Semi-Conductive Nanoparticles on the Dielectric Response of Natural Ester-Based Nanofluid Insulation. Energies 2018, 11, 333. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Zou, P.; Grzybowski, S.; Zahn, M. Preparation of a vegetable oil-based nanofluid and investigation of its breakdown and dielectric properties. IEEE Electr. Insul. Mag. 2012, 28, 43–50. [Google Scholar] [CrossRef]
- Beroual, A.; Khaled, U. Statistical Investigation of Lightning Impulse Breakdown Voltage of Natural and Synthetic Ester Oils-Based Fe3O4, Al2O3 and SiO2 Nanofluids. IEEE Access 2020, 8, 112615–112623. [Google Scholar] [CrossRef]
- Khaled, U.; Beroual, A. Lightning impulse breakdown voltage of synthetic and natural ester liquids-based Fe3O4, Al2O3 and SiO2 nanofluids. Alex. Eng. J. 2020, 59, 3709–3713. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Yin, H.; Huang, Z.; Wang, Q.; Liu, L.; Sun, J.; He, J. Analysis of Dielectric Properties and Breakdown Characteristics of Vegetable Insulating Oil with Modified by ZnO Nanoparticles. In Proceedings of the 2018 IEEE International Conference on High Voltage Engineering and Application (ICHVE), Athens, Greece, 10–13 September 2018. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhou, J.; Li, X.; Zhou, Y.; Tu, Y. Effect of electron shallow trap on breakdown performance of transformer oil-based nanofluids. J. Appl. Phys. 2011, 110, 104104. [Google Scholar] [CrossRef]
- Hwang, J.G.; Zahn, M.; O’Sullivan, F.M.; Pettersson, L.A.A.; Hjortstam, O.; Liu, R. Effects of nanoparticle charging on streamer development in transformer oil-based nanofluids. J. Appl. Phys. 2010, 107, 014310. [Google Scholar] [CrossRef]
- Miao, J.; Dong, M.; Ren, M.; Wu, X.; Shen, L.; Wang, H. Effect of nanoparticle polarization on relative permittivity of transformer oil-based nanofluids. J. Appl. Phys. 2013, 113, 204103. [Google Scholar] [CrossRef]
- Sima, W.; Shi, J.; Yang, Q.; Huang, S.; Cao, X. Effects of conductivity and permittivity of nanoparticle on transformer oil insulation performance: Experiment and theory. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 380–390. [Google Scholar] [CrossRef]
- Ibrahim, M.E.; Abd-Elhady, A.M.; Izzularab, M.A. Effect of nanoparticles on transformer oil breakdown strength: Experiment and theory. IET Sci. Meas. Technol. 2016, 10, 839–845. [Google Scholar] [CrossRef]
- Madawan, R.; Kumar, S.S.; Iruthyarajan, M.W. A comparative investigation on effects of nanoparticles on characteristics of natural esters-based nanofluids. Colloids Surf. A 2018, 556, 30–36. [Google Scholar] [CrossRef]
- Beroual, A. Electronic and gaseous processes in the prebreakdown phenomena of dielectric liquids. J. Appl. Phys. 1993, 73, 4528–4533. [Google Scholar] [CrossRef]
- Beroual, A. Pre-breakdown mechanisms in dielectric liquids and predicting models. In Proceedings of the 2016 IEEE Electrical Insulation Conference (EIC), Montreal, QC, Canada, 19–22 June 2016. [Google Scholar] [CrossRef]
- Beroual, A.; Zahn, M.; Badent, A.; Kist, K.; Schwabe, A.J.; Yamashita, H.; Yamazawa, K.; Danikas, M.; Chadband, W.D.; Torshin, Y. Propagation and structure of streamers in liquid dielectrics. IEEE Electr. Insul. Mag. 1998, 14, 6–17. [Google Scholar] [CrossRef]
- Oommen, T.V. Moisture equilibrium in paper-oil insulation systems. In Proceedings of the 1983 EIC 6th Electrical/Electronical Insulation Conference, Chicago, IL, USA, 3–6 October 1983; pp. 162–166. [Google Scholar]
- Liao, R.; Lin, Y.; Guo, P.; Liu, H.; Xia, H. Thermal aging effects on the moisture equilibrium curves of mineral and mixed oil-paper insulation systems. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 842–850. [Google Scholar] [CrossRef]
- Koch, M.; Tenbohlen, S.; Stirl, T. Diagnostic Application of Moisture Equilibrium for Power Transformers. IEEE Trans. Power Deliv. 2010, 25, 2574–2581. [Google Scholar] [CrossRef]
- IEC 60422. Mineral Insulating Oils in Electrical Equipment—Supervision and Maintenance Quidance; International Electrotechnical Commission (IEC): Geneva, Switzerland, 2013. [Google Scholar]
- Dai, J.; Wang, Z.D. A Comparison of the Impregnation of Cellulose Insulation by Ester and Mineral oil. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 374–381. [Google Scholar] [CrossRef]
- IEC 60243-1. Electric Strength of Insulating Materials-Test Methods-Part 1: Tests at Power Frequencies; International Electrotechnical Commission (IEC): Geneva, Switzerland, 2013. [Google Scholar]
- Rozga, P. Using the three-parameter Weibull distribution in assessment of threshold strength of pressboard impregnated by different liquid dielectrics. IET Sci. Meas. Technol. 2016, 10, 665–670. [Google Scholar] [CrossRef]
- Sbravati, A.; Rapp, K.; Schmitt, P.; Krause, C. Transformer insulation structure for dielectric liquids with higher permittivity. In Proceedings of the 2017 IEEE 19th International Conference on Dielectric Liquids (ICDL), Manchester, UK, 25–29 June 2017. [Google Scholar] [CrossRef]
- Dombek, G.; Nadolny, Z.; Marcinkowska, A. Effects of Nanoparticles Materials on Heat Transfer in Electro-Insulating Liquids. Appl. Sci. 2018, 8, 2538. [Google Scholar] [CrossRef]
- Villarroel, R.; García, B.; García, D.F.; Burgos, J.C. Assessing the Use of Natural Esters for Transformer Field Drying. IEEE Trans. Power Deliv. 2014, 29, 1894–1900. [Google Scholar] [CrossRef]
- Moore, S.; Rapp, K.; Baldyga, R. Transformer insulation dry out as a result of retrofilling with natural ester fluid. In Proceedings of the IEEE PES Transmission & Distribution Conference & Exposition, Orlando, FL, USA, 7–10 May 2012. [Google Scholar] [CrossRef]
- IEC 60599. Mineral Oil-Impregnated Electical Equipment in Service–Guide to the Interpretation of Dissolved and Free Gases Analysis, 2nd ed.; International Electrotechnical Commission (IEC): Geneva, Switzerland, 1999. [Google Scholar]
- IEEE C57.104. IEEE Guide for the Interpretation of Gases Generated in Oil-Immersed Transformers; IEEE: New York, NY, USA, 2008. [Google Scholar]
- Duval, M. The duval triangle for load tap changers, non-mineral oils and low temperature faults in transformers. IEEE Electr. Insul. Mag. 2008, 24, 22–29. [Google Scholar] [CrossRef]
- Duval, M.; Baldyga, R. Stray gassing of FR3 oils in transformers in service. In Proceedings of the 76th International Conference on Doble Clients, Boston, USA, 29 March 2009. [Google Scholar]
- Mnisi, H.; Nyamupangedengu, C. Dissolved Gases Analysis of canola-based ester oil under creepage discharge. In Proceedings of the 2020 International SAUPEC/RobMech/PRASA Conference, Cape Town, South Africa, 29–31 January 2020. [Google Scholar] [CrossRef]
- Gómez, N.A.; Wilhelm, H.M.; Santos, C.C.; Stocco, G.B. Dissolved gas analysis (DGA) of natural ester insulating fluids with different chemical compositions. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1071–1078. [Google Scholar] [CrossRef]
- Hanson, D.; Li, K.; Plascencia, J.; Beauchemin, C.; Claiborne, C.; Cherry, D.; Frimpong, G.; Luksich, J.; Lemm, A.; Martin, R. Understanding dissolved gas analysis of ester liquids: An updated review of gas generated in ester liquid by stray gassing, thermal decomposition and electrical discharge. In Proceedings of the 2016 IEEE Electrical Insulation Conference (EIC), Montreal, QC, Canada, 19–22 June 2016; pp. 138–144. [Google Scholar] [CrossRef]
- Przybyłek, P.; Gielniak, J. Concentration analysis of gases formed in mineral oil, natural ester, and synthetic ester by discharges of high energy. Ekspolatacja Niezawodn. Maint. Reliab. 2018, 20, 435–442. [Google Scholar] [CrossRef]
- Przybylek, P.; Gielniak, J. Analysis of Gas Generated in Mineral Oil, Synthetic Ester, and Natural Ester as a Consequence of Thermal Faults. IEEE Access 2019, 7, 65040–65051. [Google Scholar] [CrossRef]
- Lashbrook, M.; Al-Amin, H.; Martin, R. Natural ester and synthetic ester fluids, applications and maintenance. In Proceedings of the 2017 10th Jordanian International Electrical and Electronics Engineering Conference (JIEEEC), Amman, Jordan, 16 May 2017. [Google Scholar]
- Crofts, D. The electrification phenomena in power transformers. IEEE Trans. Electr. Insul. 1988, 23, 137–146. [Google Scholar] [CrossRef]
- Yamada, N.; Kishi, A.; Nitta, T.; Tanaka, T.; Ima, Y. Model Approach to the Static Electrification Phenomena Induced by the Flow of Oil in Large Power Transformers. IEEE Trans. Power Appar. Syst. 1980, 99, 1097–1106. [Google Scholar] [CrossRef]
- Shimizu, S.; Murata, H.; Honda, M. Electrostatics in Power Transformers. IEEE Trans. Power Appar. Syst. 1979, 98, 1244–1250. [Google Scholar] [CrossRef]
- Radwan, R.; El-Dewieny, R.; Metwally, I.A. Investigation of static electrification phenomenon due to transformer oil flow in electric power apparatus. IEEE Trans. Electr. Insul. 1992, 27, 278–286. [Google Scholar] [CrossRef]
- Peyraque, L.; Beroual, A.; Buret, F. Static electrification induced by oil flow in power transformers. Intern. Symp. Electr. Insul. 1996, 2, 745–749. [Google Scholar] [CrossRef]
- Kolcunová, I.; Kurimský, J.; Cimbala, R.; Petráš, J.; Dolnik, B.; Džmura, J.; Balogh, J. Contribution to static electrification of mineral oils and natural esters. J. Electrost. 2017, 88, 60–64. [Google Scholar] [CrossRef]
- Paillat, T.; Zelu, Y.; Morin, G.; Perrier, C. Ester oils and flow electrification hazards in power transformers. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1537–1543. [Google Scholar] [CrossRef]
- Vihacencu, M.; Dumitran, L.M.; Nothinger, P.V. Transformer Mineral Oil Electrification. In Proceedings of the 7th International Symposium on Advanced Topics in Electrical Engineering (ATEE), Bucharest, Romania, 12–14 May 2011. [Google Scholar]
- Kedzia, J.; Willner, B. Electrification current in the spinning disk system. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 58–62. [Google Scholar] [CrossRef]
- Jaroszewski, M. Electrification currents measurements of natural ester obtained by rotating disc method. Przegląd Elektrotech. 2016, 1, 90–93. [Google Scholar] [CrossRef]
- Zdanowski, M.; Wolny, S.; Zmarzly, D.; Kedzia, J. The analysis and selection of the spinning disk system parameters for the measurement of static electrification of insulation oils. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 480–486. [Google Scholar] [CrossRef]
- N’Cho, J.S.; Fofana, I.; Beroual, A. Parameters affecting the static electrification of aged transformer oils. In Proceedings of the 2011 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 16–19 October 2011; pp. 571–574. [Google Scholar] [CrossRef]
- Zelu, Y.; Paillat, T.; Morin, G.; Perrier, C.; Saravolac, M. Study on flow electrification hazards with ester oils. In Proceedings of the 2011 IEEE International Conference on Dielectric Liquids, Trondheim, Norway, 26–30 June 2011. [Google Scholar] [CrossRef]


| Properties | Unit | Standard for Evaluation | Required Value |
|---|---|---|---|
| Density at 20 °C | kg/dm3 | ISO 3675 | Max. 1 |
| Viscosity at 40 °C | mm2/s | ISO 3104 | Max. 35 |
| Flash Point | °C | ISO 2719 | 250 |
| Fire Point | °C | ISO 2592 | 300 |
| Pour Point | °C | ISO 3016 | Max. −45 |
| AC Breakdown Voltage | kV | IEC 60156 | Min. 45 |
| Dissipation Factor at 90 °C | - | IEC 60247 | Max. 0.03 |
| Resistivity at 90 °C | GΩm | IEC 60247 | Min. 2 |
| Water Saturation Coefficients | Water Saturation Limit, ppm | Reference | ||
|---|---|---|---|---|
| A | B | 20 °C | 50 °C | |
| 5.42 | 629 | 1881 | 2975 | [30] |
| 5.320 | 608.28 | 1758 | 2739 | [32] |
| 5.4166 | 581.95 | 2700 | 4128 | [33] |
| 5.6614 | 695.74 | 1941 | 3224 | [34] |
| 7.1790 | 1191 | 1307 | 3115 | [35] |
| Method | Synthetic Ester (kV) | Natural Ester (kV) | Mineral Oil (kV) |
|---|---|---|---|
| Rising-voltage 1 shot/step | 258.0 | 239.3 | 276.4 |
| Rising-voltage 3 shots/step | 205.0 | 200.4 | 251.9 |
| Up-and-down | 223.2 | 212.9 | 232.8 |
| Multiple-level | 248.9 | 230.8 | 270.0 |
| Natural Ester | Fe3O4 (50 nm) | Al2O3 (50 nm) | Al2O3 (13 nm) | SiO2 (50 nm) | |
|---|---|---|---|---|---|
| Natural ester/0.05 (g/L) Nanofluids | |||||
| Breakdown voltage (kV) | 68.77 | 57.53 | 61.53 | 73.93 | 58.20 |
| Natural ester/0.2 (g/L) Nanofluids | |||||
| Breakdown voltage (kV) | 68.77 | 66.90 | 66.10 | 69.87 | 72.23 |
| Natural ester/0.3 (g/L) Nanofluids | |||||
| Breakdown voltage (kV) | 68.77 | 69.53 | 73.00 | 67.33 | 72.73 |
| Natural ester/0.4 (g/L) Nanofluids | |||||
| Breakdown voltage (kV) | 68.77 | 73.63 | 69.07 | 67.30 | 67.13 |
| Synthetic Ester | Fe3O4 (50 nm) | Al2O3 (50 nm) | Al2O3 (13 nm) | SiO2 (50 nm) | |
|---|---|---|---|---|---|
| Synthetic ester/0.05 (g/L) Nanofluids | |||||
| Breakdown voltage (kV) | 60 | 56.57 | 66.3 | 80.83 | 71.5 |
| Synthetic ester/0.2 (g/L) Nanofluids | |||||
| Breakdown voltage (kV) | 60 | 59.97 | 74.3 | 79.87 | 72.5 |
| Synthetic ester/0.3 (g/L) Nanofluids | |||||
| Breakdown voltage (kV) | 60 | 70.07 | 75.27 | 72.3 | 74.6 |
| Synthetic ester/0.4 (g/L) Nanofluids | |||||
| Breakdown voltage (kV) | 60 | 88.67 | 74.03 | 69.9 | 78.9 |
| Relative Saturation, % | Limit Values of Water Content, ppm @ 50 °C | Condition of Cellulosic Insulation [132] |
|---|---|---|
| <5 | <149 | Dry insulation |
| 5 to 20 | 149 to 595 | Moderately wet, low values indicate fairly dry to moderate levels of moisture content in the insulation. The values toward the upper limit show moderately wet cellulose insulation |
| 20 to 30 | 595 to 893 | Wet insulation |
| >30 | >893 | Extremely wet insulation |
| Impregnation Cycle | Mineral Oil | Natural Ester | Synthetic Ester |
|---|---|---|---|
| 8 h, 60 °C | 38.85 | 38.10 | 37.55 |
| 8 h, 100 °C | 45.50 | 41.80 | 44.00 |
| Full, 24 h, 100 °C | 81.95 | 83.40 | 88.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozga, P.; Beroual, A.; Przybylek, P.; Jaroszewski, M.; Strzelecki, K. A Review on Synthetic Ester Liquids for Transformer Applications. Energies 2020, 13, 6429. https://doi.org/10.3390/en13236429
Rozga P, Beroual A, Przybylek P, Jaroszewski M, Strzelecki K. A Review on Synthetic Ester Liquids for Transformer Applications. Energies. 2020; 13(23):6429. https://doi.org/10.3390/en13236429
Chicago/Turabian StyleRozga, Pawel, Abderrahmane Beroual, Piotr Przybylek, Maciej Jaroszewski, and Konrad Strzelecki. 2020. "A Review on Synthetic Ester Liquids for Transformer Applications" Energies 13, no. 23: 6429. https://doi.org/10.3390/en13236429
APA StyleRozga, P., Beroual, A., Przybylek, P., Jaroszewski, M., & Strzelecki, K. (2020). A Review on Synthetic Ester Liquids for Transformer Applications. Energies, 13(23), 6429. https://doi.org/10.3390/en13236429








