The Prospects of Agricultural and Food Residue Hydrolysates for Sustainable Production of Algal Products
Abstract
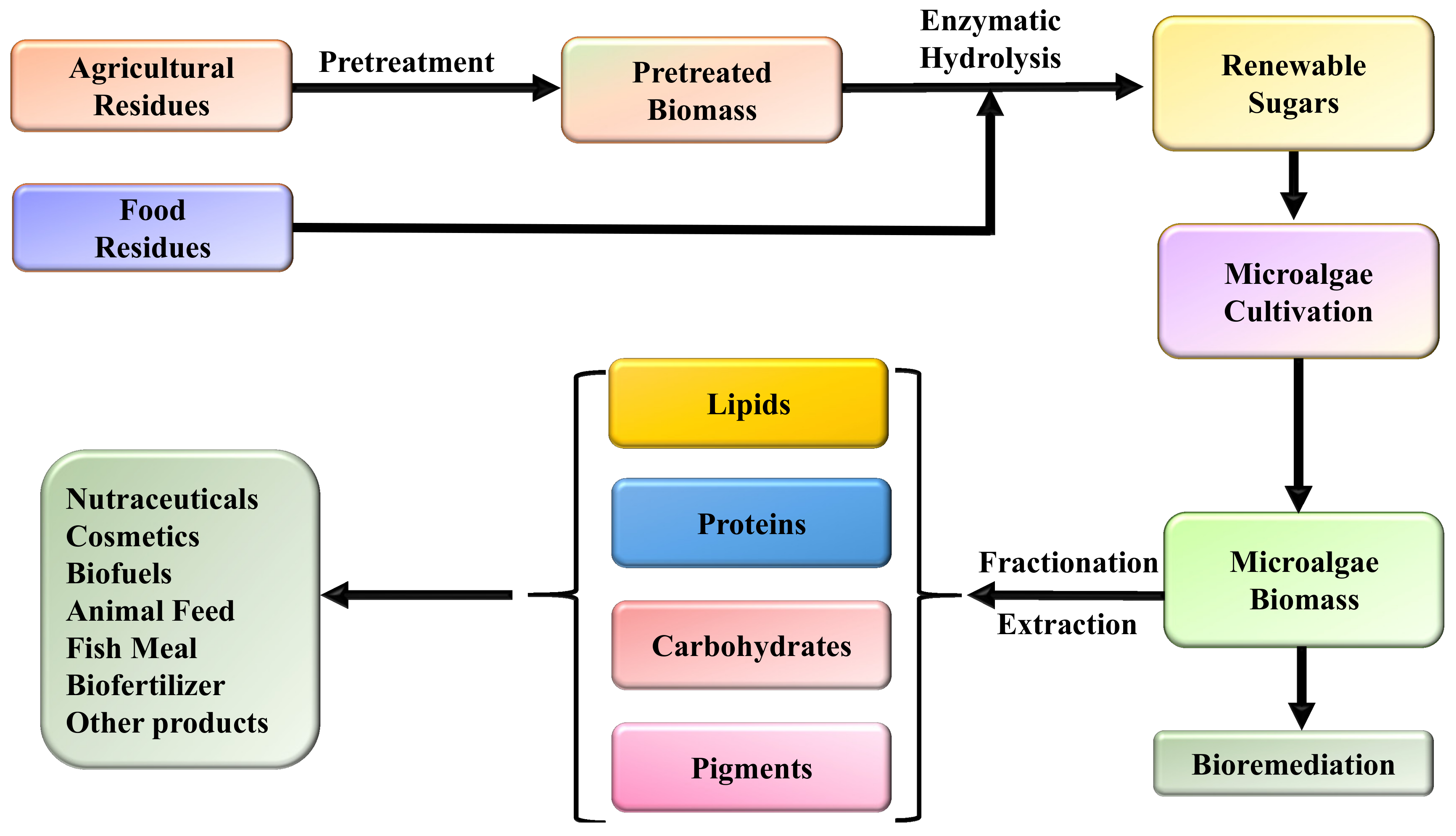
1. Introduction
2. Microalgal Products
3. The Role of Agricultural and Food Residues
3.1. Agricultural Residues as Feedstock for Microalgae
3.2. Food Residues as Feedstock for Microalgae
4. Conversion of Low-Cost Residues to Microalgal Biomass
4.1. Agricultural Residues
4.1.1. Sucrose-Rich Agricultural Residues
4.1.2. Fructose-Rich Agricultural Residues
4.1.3. Starch-Rich Agricultural Residues
4.1.4. Sugar Industry Vinasse
4.1.5. Lignocellulosic Residues
4.1.6. Other Agricultural Residues
4.2. Food Residues
4.2.1. Food Waste
4.2.2. Fruit Waste
4.2.3. Dairy Waste
4.2.4. Wine Industry and Other Waste
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Harada, R.; Nomura, T.; Yamada, K.; Mochida, K.; Suzuki, K. Genetic Engineering Strategies for Euglena gracilis and Its Industrial Contribution to Sustainable Development Goals: A Review. Front. Bioeng. Biotechnol. 2020, 8, 790. [Google Scholar] [CrossRef]
- D’Imporzano, G.; Veronesi, D.; Salati, S.; Adani, F. Carbon and nutrient recovery in the cultivation of Chlorella vulgaris: A life cycle assessment approach to comparing environmental performance. J. Clean. Prod. 2018, 194, 685–694. [Google Scholar] [CrossRef]
- Arora, N.; Patel, A.; Pruthi, P.A.; Pruthi, V. Synergistic dynamics of nitrogen and phosphorous influences lipid productivity in Chlorella minutissima for biodiesel production. Bioresour. Technol. 2016, 213, 79–87. [Google Scholar] [CrossRef]
- Vigani, M.; Parisi, C.; Rodríguez-Cerezo, E.; Barbosa, M.J.; Sijtsma, L.; Ploeg, M.; Enzing, C. Food and feed products from micro-algae: Market opportunities and challenges for the EU. Trends Food Sci. Technol. 2015, 42, 81–92. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential microalgae derived pharmaceutical and biological reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Cultivation and downstream processing of microalgae and cyanobacteria to generate protein-based technofunctional food ingredients. Crit. Rev. Food Sci. Nutr. 2019, 1–29. [Google Scholar] [CrossRef]
- Koutra, E.; Tsafrakidou, P.; Sakarika, M.; Kornaros, M. Microalgal Biorefinery. In Microalgae Cultivation for Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–185. ISBN 978-0-12-817536-1. [Google Scholar]
- Hossain, N.; Zaini, J.; Indra Mahlia, T.M. Life cycle assessment, energy balance and sensitivity analysis of bioethanol production from microalgae in a tropical country. Renew. Sustain. Energy Rev. 2019, 115, 109371. [Google Scholar] [CrossRef]
- Zhang, Y.; Kendall, A. Effects of system design and Co-product treatment strategies on the life cycle performance of biofuels from microalgae. J. Clean. Prod. 2019, 230, 536–546. [Google Scholar] [CrossRef]
- Bussa, M.; Eisen, A.; Zollfrank, C.; Röder, H. Life cycle assessment of microalgae products: State of the art and their potential for the production of polylactid acid. J. Clean. Prod. 2019, 213, 1299–1312. [Google Scholar] [CrossRef]
- Zanette, C.M.; Mariano, A.B.; Yukawa, Y.S.; Mendes, I.; Rigon Spier, M. Microalgae mixotrophic cultivation for β-galactosidase production. J. Appl. Phycol. 2019, 31, 1597–1606. [Google Scholar] [CrossRef]
- Arora, N.; Laurens, L.M.L.; Sweeney, N.; Pruthi, V.; Poluri, K.M.; Pienkos, P.T. Elucidating the unique physiological responses of halotolerant Scenedesmus sp. cultivated in sea water for biofuel production. Algal Res. 2019, 37, 260–268. [Google Scholar] [CrossRef]
- Xin, X.; Huang, G.; Liu, X.; An, C.; Yao, Y.; Weger, H.; Zhang, P.; Chen, X. Molecular toxicity of triclosan and carbamazepine to green algae Chlorococcum sp.: A single cell view using synchrotron-based Fourier transform infrared spectromicroscopy. Environ. Pollut. 2017, 226, 12–20. [Google Scholar] [CrossRef]
- Alonso, D.L.; Maroto, F.G. Plants as ‘chemical factories’ for the production of polyunsaturated fatty acids. Biotechnol. Adv. 2000, 18, 481–497. [Google Scholar] [CrossRef]
- Manisali, A.Y.; Sunol, A.K.; Philippidis, G.P. Effect of macronutrients on phospholipid production by the microalga Nannochloropsis oculata in a photobioreactor. Algal Res. 2019, 41, 101514. [Google Scholar] [CrossRef]
- Sniffen, K.D.; Price, J.R.; Sales, C.M.; Olson, M.S. Influence of Scale on Biomass Growth and Nutrient Removal in an Algal–Bacterial Leachate Treatment System. Environ. Sci. Technol. 2017, 51, 13344–13352. [Google Scholar] [CrossRef]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A Review of Algae-Based Produced Water Treatment for Biomass and Biofuel Production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Oswald, W.J.; Gotaas, H.B.; Ludwig, H.F.; Lynch, V. Algae symbiosis in oxidation ponds: III. Photosynthetic oxygenenation. JSTOR 1953, 25, 692–705. [Google Scholar]
- Gupta, P.L.; Choi, H.-J.; Pawar, R.R.; Jung, S.P.; Lee, S.-M. Enhanced biomass production through optimization of carbon source and utilization of wastewater as a nutrient source. J. Environ. Manag. 2016, 184, 585–595. [Google Scholar] [CrossRef]
- Dogaris, I.; Loya, B.; Cox, J.; Philippidis, G. Study of landfill leachate as a sustainable source of water and nutrients for algal biofuels and bioproducts using the microalga Picochlorum oculatum in a novel scalable bioreactor. Bioresour. Technol. 2019, 282, 18–27. [Google Scholar] [CrossRef]
- Arora, N.; Jaiswal, K.K.; Kumar, V.; Vlaskin, M.S.; Nanda, M.; Pruthi, V.; Chauhan, P. Small-scale phyco-mitigation of raw urban wastewater integrated with biodiesel production and its utilization for aquaculture. Bioresour. Technol. 2020, 297, 122489. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef]
- Sim, S.J.; Joun, J.; Hong, M.E.; Patel, A.K. Split mixotrophy: A novel cultivation strategy to enhance the mixotrophic biomass and lipid yields of Chlorella protothecoides. Bioresour. Technol. 2019, 291, 121820. [Google Scholar] [CrossRef]
- Salati, S.; D’Imporzano, G.; Menin, B.; Veronesi, D.; Scaglia, B.; Abbruscato, P.; Mariani, P.; Adani, F. Mixotrophic cultivation of Chlorella for local protein production using agro-food by-products. Bioresour. Technol. 2017, 230, 82–89. [Google Scholar] [CrossRef]
- Bentsen, N.S.; Felby, C.; Thorsen, B.J. Agricultural residue production and potentials for energy and materials services. Prog. Energy Combust. Sci. 2014, 40, 59–73. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Kyazze, G.; Keshavarz, T. Advances in the valorization of lignocellulosic materials by biotechnology: An overview. BioResources 2013, 8, 3157–3176. [Google Scholar]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech 2013, 3, 415–431. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Mtui, G.Y. Recent advances in pretreatment of lignocellulosic wastes and production of value added products. Afr. J. Biotechnol. 2009, 8, 1398–1415. [Google Scholar]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste—Extent, Causes and Prevention. Food and Agriculture Organization (FAO). 2011. Available online: http://www.fao.org/3/a-i2697e.pdf (accessed on 4 October 2020).
- Lipinski, B.; Hanson, C.; Lomax, J.; Kitinoja, L.; Waite, R.; Searchinger, T. Reducing food loss and waste. World Resour. Inst. Work. Paper 2013, 1, 1–40. [Google Scholar]
- Buzby, J.C.; Farah-Wells, H.; Hyman, J. The estimated amount, value, and calories of postharvest food losses at the retail and consumer levels in the United States. USDA-ERS Econ. Inf. Bull. 2014, 121, 1–39. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Sustainable bioconversion of food waste into high-value products by immobilized enzymes to meet bio-economy challenges and opportunities–A review. Food Res. Int. 2019, 123, 226–240. [Google Scholar] [PubMed]
- Liang, Y.; Sarkany, N.; Cui, Y.; Yesuf, J.; Trushenski, J.; Blackburn, J.W. Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresour. Technol. 2010, 101, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhai, Y.; Ding, Y.; Wu, Q. Application of sweet sorghum for biodiesel production by heterotrophic microalga Chlorella protothecoides. Appl. Energy 2010, 87, 756–761. [Google Scholar]
- Cheng, Y.; Zhou, W.; Gao, C.; Lan, K.; Gao, Y.; Wu, Q. Biodiesel production from Jerusalem artichoke (Helianthus Tuberosus L.) tuber by heterotrophic microalgae Chlorella protothecoides. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 777–781. [Google Scholar]
- Kim, M.; Cho, J.M.; Kim, H.S.; Lee, H.; Oh, H.-M.; Chang, Y.K. Heterotrophic cultivation of Ettlia sp. based on sequential hydrolysis of Helianthus tuberosus and algal residue. Energy Convers. Manag. 2020, 211, 112769. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.; Wu, Q. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 2006, 126, 499–507. [Google Scholar]
- Wei, A.; Zhang, X.; Wei, D.; Chen, G.; Wu, Q.; Yang, S.-T. Effects of cassava starch hydrolysate on cell growth and lipid accumulation of the heterotrophic microalgae Chlorella protothecoides. J. Ind. Microbiol. Biotechnol. 2009, 36, 1383. [Google Scholar]
- Lu, Y.; Zhai, Y.; Liu, M.; Wu, Q. Biodiesel production from algal oil using cassava (Manihot esculenta Crantz) as feedstock. J. Appl. Phycol. 2010, 22, 573–578. [Google Scholar]
- Lu, Y.; Ding, Y.; Wu, Q. Simultaneous saccharification of cassava starch and fermentation of algae for biodiesel production. J. Appl. Phycol. 2011, 23, 115–121. [Google Scholar]
- Salim, M.A. Biomass and lipid content of heterotrophic Spirogyra sp by using cassava starch hydrolysate. Int. J. Eng. Res. Dev. 2012, 6, 21–26. [Google Scholar]
- Salim, M.A. Heterotrophic growth of Ankistrodesmus sp. for lipid production using cassava starch hydrolysate as a carbon source. Int. J. Biotechnol. 2013, 2, 42–51. [Google Scholar]
- Barrocal, V.M.; García-Cubero, M.T.; González-Benito, G.; Coca, M. Production of biomass by Spirulina maxima using sugar beet vinasse in growth media. New Biotechnol. 2010, 27, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Coca, M.; Barrocal, V.M.; Lucas, S.; González-Benito, G.; García-Cubero, M.T. Protein production in Spirulina platensis biomass using beet vinasse-supplemented culture media. Food Bioprod. Process. 2015, 94, 306–312. [Google Scholar] [CrossRef]
- Marques, S.S.I.; Nascimento, I.A.; De Almeida, P.F.; Chinalia, F.A. Growth of Chlorella vulgaris on sugarcane vinasse: The effect of anaerobic digestion pretreatment. Appl. Biochem. Biotechnol. 2013, 171, 1933–1943. [Google Scholar] [CrossRef]
- Ramirez, N.N.V.; Farenzena, M.; Trierweiler, J.O. Growth of microalgae Scenedesmus sp in ethanol vinasse. Braz. Arch. Biol. Technol. 2014, 57, 630–635. [Google Scholar] [CrossRef]
- Dos Santos, R.R.; Araújo, O.; De Medeiros, J.L.; Chaloub, R.M. Cultivation of Spirulina maxima in medium supplemented with sugarcane vinasse. Bioresour. Technol. 2016, 204, 38–48. [Google Scholar] [CrossRef]
- Santana, H.; Cereijo, C.R.; Teles, V.C.; Nascimento, R.C.; Fernandes, M.S.; Brunale, P.; Campanha, R.C.; Soares, I.P.; Silva, F.C.; Sabaini, P.S. Microalgae cultivation in sugarcane vinasse: Selection, growth and biochemical characterization. Bioresour. Technol. 2017, 228, 133–140. [Google Scholar] [CrossRef]
- El-sheekh, M.M.; Bedaiwy, M.Y.; Osman, M.E.; Ismail, M.M. Mixotrophic and heterotrophic growth of some microalgae using extract of fungal-treated wheat bran. Int. J. Recycl. Org. Waste Agric. 2012, 1, 12. [Google Scholar] [CrossRef]
- Gélinas, M.; Pham, T.T.H.; Boëns, B.; Adjallé, K.; Barnabé, S. Residual corn crop hydrolysate and silage juice as alternative carbon sources in microalgae production. Algal Res. 2015, 12, 33–42. [Google Scholar] [CrossRef]
- Li, P.; Miao, X.; Li, R.; Zhong, J. In situ biodiesel production from fast-growing and high oil content Chlorella pyrenoidosa in rice straw hydrolysate. BioMed Res. Int. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Bindra, S.; Kulshrestha, S. Converting waste to energy: Production and characterization of biodiesel from Chlorella pyrenoidosa grown in a medium designed from waste. Renew. Energy 2019, 142, 415–425. [Google Scholar] [CrossRef]
- Joe, M.-H.; Kim, J.-Y.; Lim, S.; Kim, D.-H.; Bai, S.; Park, H.; Lee, S.G.; Han, S.J.; Choi, J. Microalgal lipid production using the hydrolysates of rice straw pretreated with gamma irradiation and alkali solution. Biotechnol. Biofuels 2015, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Nolasco, N.A.; Vasavada, A.; Johnson, M.; Kuehnle, A. Algae-mediated valorization of industrial waste streams. Ind. Biotechnol. 2015, 11, 229–234. [Google Scholar] [CrossRef]
- Miazek, K.; Remacle, C.; Richel, A.; Goffin, D. Beech wood Fagus sylvatica dilute-acid hydrolysate as a feedstock to support Chlorella sorokiniana biomass, fatty acid and pigment production. Bioresour. Technol. 2017, 230, 122–131. [Google Scholar] [CrossRef]
- Lage, S.; Kudahettige, N.P.; Ferro, L.; Matsakas, L.; Funk, C.; Rova, U.; Gentili, F.G. Microalgae Cultivation for the Biotransformation of Birch Wood Hydrolysate and Dairy Effluent. Catalysts 2019, 9, 150. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Simultaneous production of DHA and squalene from Aurantiochytrium sp. grown on forest biomass hydrolysates. Biotechnol. Biofuels 2019, 12, 255. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Wei, D.; Chen, G. High yields of fatty acid and neutral lipid production from cassava bagasse hydrolysate (CBH) by heterotrophic Chlorella protothecoides. Bioresour. Technol. 2015, 191, 281–290. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Lim, P.-E.; Wei, D. Enhanced single cell oil production by mixed culture of Chlorella pyrenoidosa and Rhodotorula glutinis using cassava bagasse hydrolysate as carbon source. Bioresour. Technol. 2018, 255, 140–148. [Google Scholar] [CrossRef]
- Mu, J.; Li, S.; Chen, D.; Xu, H.; Han, F.; Feng, B.; Li, Y. Enhanced biomass and oil production from sugarcane bagasse hydrolysate (SBH) by heterotrophic oleaginous microalga Chlorella protothecoides. Bioresour. Technol. 2015, 185, 99–105. [Google Scholar] [CrossRef]
- Pelizer, L.H.; De Carvalho, J.C.M.; De Oliveira Moraes, I. Protein production by Arthrospira (Spirulina) platensis in solid state cultivation using sugarcane bagasse as support. Biotechnol. Rep. 2015, 5, 70–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arora, N.; Patel, A.; Pruthi, P.A.; Pruthi, V. Boosting TAG accumulation with improved biodiesel production from novel oleaginous microalgae Scenedesmus sp. IITRIND2 utilizing waste sugarcane bagasse aqueous extract (SBAE). Appl. Biochem. Biotechnol. 2016, 180, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Ahmad, Q.; Aslam, A.; Jabeen, F.; Rasul, A.; Schenk, P.M.; Qazi, J.I. Mixotrophic cultivation of Scenedesmus dimorphus in sugarcane bagasse hydrolysate. Environ. Prog. Sustain. Energy 2020, 39, e13334. [Google Scholar]
- Nguyen, H.C.; Su, C.-H.; Yu, Y.-K.; Huong, D.T.M. Sugarcane bagasse as a novel carbon source for heterotrophic cultivation of oleaginous microalga Schizochytrium sp. Ind. Crops Prod. 2018, 121, 99–105. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.; Jiang, M.; Liang, Z.; Jin, H.; Hu, X.; Wan, X.; Hu, C. Improvement of omega-3 docosahexaenoic acid production by marine dinoflagellate Crypthecodinium cohnii using rapeseed meal hydrolysate and waste molasses as feedstock. PLoS ONE 2015, 10, e0125368. [Google Scholar]
- Ali, M.; Rajewski, J.; Baenziger, P.; Gill, K.; Eskridge, K.; Dweikat, I. Assessment of genetic diversity and relationship among a collection of US sweet sorghum germplasm by SSR markers. Mol. Breed. 2008, 21, 497–509. [Google Scholar] [CrossRef]
- Matsakas, L.; Sterioti, A.-A.; Rova, U.; Christakopoulos, P. Use of dried sweet sorghum for the efficient production of lipids by the yeast Lipomyces starkeyi CBS 1807. Ind. Crop. Prod. 2014, 62, 367–372. [Google Scholar] [CrossRef]
- Lo, E.; Brabo-Catala, L.; Dogaris, I.; Ammar, E.M.; Philippidis, G.P. Biochemical conversion of sweet sorghum bagasse to succinic acid. J. Biosci. Bioeng. 2020, 129, 104–109. [Google Scholar] [CrossRef]
- Ammar, E.M.; Martin, J.; Brabo-Catala, L.; Philippidis, G.P. Propionic acid production by Propionibacterium freudenreichii using sweet sorghum bagasse hydrolysate. Appl. Microbiol. Biotechnol. 2020, 104, 9619–9629. [Google Scholar] [CrossRef]
- Rodrigues Reis, C.E.; Hu, B. Vinasse from Sugarcane Ethanol Production: Better Treatment or Better Utilization? Front. Energy Res. 2017, 5, 7. [Google Scholar] [CrossRef]
- Kwan, T.H.; Pleissner, D.; Lau, K.Y.; Venus, J.; Pommeret, A.; Lin, C.S.K. Techno-economic analysis of a food waste valorization process via microalgae cultivation and co-production of plasticizer, lactic acid and animal feed from algal biomass and food waste. Bioresour. Technol. 2015, 198, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.-M.; Sun, Z.; Liu, S.-F.; Qin, Z.-H.; Mou, J.-H.; Zhou, Z.-G.; Lin, C.S.K. Sustainable lipid and lutein production from Chlorella mixotrophic fermentation by food waste hydrolysate. J. Hazard. Mater. 2020, 400, 123258. [Google Scholar] [CrossRef] [PubMed]
- Sloth, J.K.; Jensen, H.C.; Pleissner, D.; Eriksen, N.T. Growth and phycocyanin synthesis in the heterotrophic microalga Galdieria sulphuraria on substrates made of food waste from restaurants and bakeries. Bioresour. Technol. 2017, 238, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Ling, T.C.; Arya, S.S.; Chang, J.-S. Food waste compost as an organic nutrient source for the cultivation of Chlorella vulgaris. Bioresour. Technol. 2018, 267, 356–362. [Google Scholar] [CrossRef]
- Hou, Q.; Pei, H.; Hu, W.; Jiang, L.; Yu, Z. Mutual facilitations of food waste treatment, microbial fuel cell bioelectricity generation and Chlorella vulgaris lipid production. Bioresour. Technol. 2016, 203, 50–55. [Google Scholar] [CrossRef]
- Pleissner, D.; Lin, C.S.K. Valorisation of food waste in biotechnological processes. Sustain. Chem. Process. 2013, 1, 21. [Google Scholar] [CrossRef]
- Pleissner, D.; Lau, K.Y.; Schneider, R.; Venus, J.; Lin, C.S.K. Fatty acid feedstock preparation and lactic acid production as integrated processes in mixed restaurant food and bakery wastes treatment. Food Res. Int. 2015, 73, 52–61. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L.; Hrůzová, K.; Rova, U.; Christakopoulos, P. Biosynthesis of Nutraceutical Fatty Acids by the Oleaginous Marine Microalgae Phaeodactylum tricornutum Utilizing Hydrolysates from Organosolv-Pretreated Birch and Spruce Biomass. Mar. Drugs 2019, 17, 119. [Google Scholar] [CrossRef]
- Haske-Cornelius, O.; Vu, T.; Schmiedhofer, C.; Vielnascher, R.; Dielacher, M.; Sachs, V.; Grasmug, M.; Kromus, S.; Guebitz, G.M. Cultivation of heterotrophic algae on enzymatically hydrolyzed municipal food waste. Algal Res. 2020, 50, 101993. [Google Scholar] [CrossRef]
- Wang, X.; Balamurugan, S.; Liu, S.-F.; Zhang, M.-M.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y.; Lin, C.S.K. Enhanced polyunsaturated fatty acid production using food wastes and biofuels byproducts by an evolved strain of Phaeodactylum tricornutum. Bioresour. Technol. 2020, 296, 122351. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Mining of squalene as a value-added byproduct from DHA producing marine thraustochytrid cultivated on food waste hydrolysate. Sci. Total Environ. 2020, 736, 139691. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Yang, Z.; Chen, S.; Pei, H. Using an anaerobic digestion tank as the anodic chamber of an algae-assisted microbial fuel cell to improve energy production from food waste. Water Res. 2020, 170, 115305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, J.; Pei, H.; Pan, J.; Jiang, L.; Hou, Q.; Han, F. Cultivation of microalgae using anaerobically digested effluent from kitchen waste as a nutrient source for biodiesel production. Renew. Energy 2018, 115, 276–287. [Google Scholar] [CrossRef]
- Yu, Z.; Song, M.; Pei, H.; Han, F.; Jiang, L.; Hou, Q. The growth characteristics and biodiesel production of ten algae strains cultivated in anaerobically digested effluent from kitchen waste. Algal Res. 2017, 24, 265–275. [Google Scholar] [CrossRef]
- Kamal, S.; El-Sayed, A.; Hassan, A.; El-Shazly, H.; Ibrahim, M. Use Of Okara Waste For Algae Nutrition. Arab Univ. J. Agric. Sci. 2017, 25, 271–279. [Google Scholar] [CrossRef]
- Limbu, B.; Sibi, G. Fruit Wastes Hydrolysates as Feedstock: Pre-Treatment Strategies for Cost-Saving and Sustainable Microalgae Cultivation. Int. J. Res. Environ. Sci. 2017, 3. [Google Scholar] [CrossRef]
- Nazir, Y.; Halim, H.; Al-Shorgani, N.K.N.; Manikan, V.; Hamid, A.A.; Song, Y. Efficient conversion of extracts from low-cost, rejected fruits for high-valued Docosahexaenoic acid production by Aurantiochytrium sp. SW1. Algal Res. 2020, 50, 101977. [Google Scholar] [CrossRef]
- Park, W.-K.; Moon, M.; Shin, S.-E.; Cho, J.M.; Suh, W.I.; Chang, Y.K.; Lee, B. Economical DHA (Docosahexaenoic acid) production from Aurantiochytrium sp. KRS101 using orange peel extract and low cost nitrogen sources. Algal Res. 2018, 29, 71–79. [Google Scholar] [CrossRef]
- Park, W.-K.; Moon, M.; Kwak, M.-S.; Jeon, S.; Choi, G.-G.; Yang, J.-W.; Lee, B. Use of orange peel extract for mixotrophic cultivation of Chlorella vulgaris: Increased production of biomass and FAMEs. Bioresour. Technol. 2014, 171, 343–349. [Google Scholar] [CrossRef]
- Heller, W.P.; Kissinger, K.R.; Matsumoto, T.K.; Keith, L.M. Utilization of papaya waste and oil production by Chlorella protothecoides. Algal Res. 2015, 12, 156–160. [Google Scholar] [CrossRef]
- Ghosh, U.K. An approach for phycoremediation of different wastewaters and biodiesel production using microalgae. Environ. Sci. Pollut. Res. 2018, 25, 18673–18681. [Google Scholar] [CrossRef]
- Rumiani, L.A.; Jalili, H.; Amrane, A. Enhanced docosahexaenoic acid production by Crypthecodinium cohnii under combined stress in two-stage cultivation with date syrup based medium. Algal Res. 2018, 34, 75–81. [Google Scholar] [CrossRef]
- Abreu, A.P.; Fernandes, B.; Vicente, A.A.; Teixeira, J.; Dragone, G. Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012, 118, 61–66. [Google Scholar] [CrossRef] [PubMed]
- De Melo, R.G.; De Andrade, A.F.; Bezerra, R.P.; Correia, D.S.; De Souza, V.C.; Brasileiro-Vidal, A.C.; De Araújo Viana Marques, D.; Porto, A.L.F. Chlorella vulgaris mixotrophic growth enhanced biomass productivity and reduced toxicity from agro-industrial by-products. Chemosphere 2018, 204, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Gonzalez, I.; Parashar, A.; Bressler, D.C. Heterotrophic growth and lipid accumulation of Chlorella protothecoides in whey permeate, a dairy by-product stream, for biofuel production. Bioresour. Technol. 2014, 155, 170–176. [Google Scholar] [CrossRef]
- Patel, A.K.; Choi, Y.Y.; Sim, S.J. Emerging prospects of mixotrophic microalgae: Way forward to sustainable bioprocess for environmental remediation and cost-effective biofuels. Bioresour. Technol. 2020, 300, 122741. [Google Scholar] [CrossRef]
- Ribeiro, J.E.S.; Martini, M.; Altomonte, I.; Salari, F.; Nardoni, S.; Sorce, C.; Da Silva, F.L.H.; Andreucci, A. Production of Chlorella protothecoides biomass, chlorophyll and carotenoids using the dairy industry by-product scotta as a substrate. Biocatal. Agric. Biotechnol. 2017, 11, 207–213. [Google Scholar] [CrossRef]
- Mondal, M.; Ghosh, A.; Sharma, A.S.; Tiwari, O.N.; Gayen, K.; Mandal, M.K.; Halder, G.N. Mixotrophic cultivation of Chlorella sp. BTA 9031 and Chlamydomonas sp. BTA 9032 isolated from coal field using various carbon sources for biodiesel production. Energy Convers. Manag. 2016, 124, 297–304. [Google Scholar] [CrossRef]
- Girard, J.-M.; Roy, M.-L.; Hafsa, M.B.; Gagnon, J.; Faucheux, N.; Heitz, M.; Tremblay, R.; Deschênes, J.-S. Mixotrophic cultivation of green microalgae Scenedesmus obliquus on cheese whey permeate for biodiesel production. Algal Res. 2014, 5, 241–248. [Google Scholar] [CrossRef]
- Girard, J.-M.; Tremblay, R.; Faucheux, N.; Heitz, M.; Deschênes, J.-S. Phycoremediation of cheese whey permeate using directed commensalism between Scenedesmus obliquus and Chlorella protothecoides. Algal Res. 2017, 22, 122–126. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Chen, P.; Ruan, R. Semi-continuous Cultivation of Chlorella vulgaris for Treating Undigested and Digested Dairy Manures. Appl. Biochem. Biotechnol. 2010, 162, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- León-Vaz, A.; León, R.; Díaz-Santos, E.; Vigara, J.; Raposo, S. Using agro-industrial wastes for mixotrophic growth and lipids production by the green microalga Chlorella sorokiniana. New Biotechnol. 2019, 51, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-D.; Li, Z.-H.; Guo, D.-B.; Huang, F.; Nugroho, Y.; Xia, K. Cultivation of Chlorella sp. with livestock waste compost for lipid production. Bioresour. Technol. 2017, 223, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.-F.; Shen, C.; Xu, X.-R.; Kuang, R.-D.; Guo, Y.-J.; Zeng, L.-S.; Gao, L.-L.; Lin, X.; Xie, J.-F.; Xia, E.-Q.; et al. Potential of Fruit Wastes as Natural Resources of Bioactive Compounds. IJMS 2012, 13, 8308–8323. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Ur Rahman, U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and utilization of dairy industrial waste: A review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Giakoundis, A. Current Strategies for Dairy Waste Management: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 379–390. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
| Microalga | Hydrolysate | Sugar Composition | Mode of Cultivation | Dry Cell Weight (g/L) | Microalgal Bioproduct | Reference |
|---|---|---|---|---|---|---|
| Sucrose-rich agricultural residues | ||||||
| Schizochytrium limacinum | Sweet sorghum juice | Total sugars (242.6 g/L), sucrose (142.3 g/L), fructose (61 g/L), glucose (39.3 g/L) | Heterotrophic, batch | 9.4 | Lipids + DHA | [45] |
| Chlorella protothecoides | Sweet sorghum juice | Sucrose (101.7 g/L), fructose (33.1 g/L), glucose (25 g/L) | Heterotrophic, batch | 5.1 | Lipids | [46] |
| Fructose-rich agricultural residues | ||||||
| Chlorella protothecoides | Jerusalem artichoke (Helianthus tuberosus) | Reducing sugars (45 g/L) | Heterotrophic, batch | 16–18 | Lipids | [47] |
| Ettlia sp. | Jerusalem artichoke (Helianthus tuberosus) | Fructose (56 g/L), glucose (12.2 g/L), amino acids (7.9 g/L) | Heterotrophic, batch | 23.6 | Lipids | [48] |
| Starch-rich agricultural residues | ||||||
| Chlorella protothecoides | Corn powder | Glucose (21.9 g/L) | Heterotrophic, batch + fed-batch | 15.5 | Lipids | [49] |
| Chlorella protothecoides | Cassava starch | Glucose (80.7%), maltose (16.1%), maltotriose (3.2%) | Heterotrophic, batch | 15.8 | Lipids | [50] |
| Chlorella protothecoides | Cassava (Manihot esculenta) starch | Reducing sugars (≥30 g/L) | Heterotrophic, batch + fed-batch | 49.34–53.6 | Lipids | [51,52] |
| Spirogyra sp. | Corn starch | NA | Heterotrophic, batch | 12 | Lipids | [53] |
| Ankistrodesmus sp. | Corn starch | NA | Heterotrophic, batch | 1.3 | Lipids | [54] |
| Sugar industry vinasse | ||||||
| Spirulina maxima | Sugar beet vinasse | NA | Mixotrophic, batch + continuous | 8 | Proteins | [55] |
| Spirulina platensis | Sugar beet vinasse | NA | Mixotrophic, batch and continuous | 6.5 | Proteins | [56] |
| Chlorella vulgaris | Sugarcane vinasse | NA | Mixotrophic, batch | NA | Lipids | [57] |
| Scenedesmus sp. | Sugarcane vinasse | NA | Mixotrophic, batch | 0.3–0.7 | Biomass | [58] |
| Spirulina maxima | Sugarcane vinasse | NA | Autotrophic + Heterotrophic, batch + fed-batch | 0.5–0.6 | Proteins | [59] |
| Micractinium sp. and Chlamydomonas biconvexa | Sugarcane vinasse. | NA | Mixotrophic, batch | 2 | Proteins + Carbohydrates | [60] |
| Lignocellulosic residues | ||||||
| Chlorella vulgaris and Scenedesmus obliquus | Wheat bran extract | Reducing sugars (54 mg/g wheat bran) | Mixotrophic + Heterotrophic, batch | 3.4–3.7 and 3.3–5, respectively | Proteins + Carbohydrates + Lipids + Pigments | [61] |
| Bacteria-microalgae consortium dominated by Chlorella sp. | Corn biomass | Reducing sugars (55.6 g/L), glucose (49.9%), xylose (49.9%) | Mixotrophic + Heterotrophic, batch | 0.6–0.7 | Biomass | [62] |
| Chlorella pyrenoidosa | Rice straw | Reducing sugars (13.7 g/L) | Mixotrophic, batch | 2.83 | Lipids | [63] |
| Chlorella pyrenoidosa | Rice straw | NA | Mixotrophic, batch | 2.15 | Lipids | [64] |
| Chlorella protothecoides | Rice straw | Glucose (~110 g/L) | Mixotrophic + Heterotrophic, batch | 6.5 | Lipids | [65] |
| Hawaiian species of Chlorella and Scenedesmus sp. | Hardwoods | Total sugars (18.7, 87.9, 21.5, 44.3, 27.5 g/L) | Heterotrophic, batch | 7–8 | Biomass | [66] |
| Chlorella sorokiniana | Beech wood (Fagus sylvatica) | Glucose (1.2 g/L), xylose (4.7 g/L) | Mixotrophic + Heterotrophic, batch | > 0.5 | Biomass + Lipids + Pigments | [67] |
| Chlorella sorokiniana, Chlorella saccharophila, Chlorella vulgaris, Coelastrella sp. | Silver Birch wood (Betula pendula) | Glucose (61.7 g/L), xylose (42.4 g/L) | Mixotrophic + Heterotrophic, batch | 2 | Biomass + Lipids | [68] |
| Aurantiochytrium sp. | Birch wood | Glucose (77 g/L) | Heterotrophic, batch | 11.2 | DHA + Squalene | [69] |
| Chlorella protothecoides | Cassava bagasse | Reducing sugars (46.2 g/L), glucose (34.9 g/L) | Heterotrophic, batch + fed-batch | 9.7 | Lipids | [70] |
| Chlorella pyrenoidosa co-cultured with yeast (Rhodotorula glutinis) | Cassava bagasse | Reducing sugars (> 60 g/L) | Mixotrophic, batch + fed-batch | 20.4 | Lipids | [71] |
| Chlorella protothecoides | Sugarcane bagasse | Glucose (13.9 g/L), xylose (5.3 g/L), arabinose (3 g/L) | Heterotrophic, batch + fed-batch | 24 | Biomass + Lipids | [72] |
| Arthrospira platensis (spirulina) | Sugarcane bagasse (untreated) | NA | Mixotrophic, batch | NA | Proteins | [73] |
| Scenedesmus sp. | Sugarcane bagasse aqueous extract | Total carbohydrates (50 g/L), sucrose (18.2 g/L), glucose (9.2 g/L), fructose (8.8 g/L), xylose (4.3 g/L) | Mixotrophic, batch | 2.2 | Lipids | [74] |
| Scenedesmus dimorphus | Sugarcane bagasse | Total sugars (>10 g/L) | Mixotrophic, batch | NA | Lipids | [75] |
| Schizochytrium sp. | Sugarcane bagasse | Glucose (56.1 g/L), xylose (12.5 g/L) | Heterotrophic, batch | 10.5 | Biomass + Lipids + DHA | [76] |
| Other agricultural residues | ||||||
| Crypthecodinium cohnii | Rapeseed meal | Soluble sugars (0.235 g/L) | Heterotrophic, batch | 3.43 | DHA | [77] |
| Microalga | Hydrolysate | Sugar Composition | Mode of Cultivation | Microalgal Bioproduct | Dry Cell Weight (g/L) | Reference |
|---|---|---|---|---|---|---|
| Food waste | ||||||
| Chlorella vulgaris | Food waste | Total carbon (30.84%) | Mixotrophic, batch | Lipids | 1.72 | [86] |
| NA | Bioelectricity + lipids | 1.2 | [87] | |||
| Chlorella pyrenoidosa | Glucose (5 g/L) | Lactic acid + plasticizer | - | [83] | ||
| Total carbon (20 g/L) | Heterotrophic, batch | Lipids + proteins + carbohydrates | 10 | [88] | ||
| Glucose (17 g/L), fructose (0.6 g/L) | Heterotrophic, continuous | 25 | [89] | |||
| Chlorella sp. | Total carbon (495 mg/g) | Mixotrophic, semi-continuous | Lipids + Lutein | 6.9 | [84] | |
| Auxenochlorella protothecoides | Glucose (20 g/L), fructose (6.10 g/L) | Heterotrophic, batch | Lipids | 9.02 | [90] | |
| Chlorella sorokinana | Glucose (42 g/L), fructose (10 g/L) | Proteins | 9.5 | [91] | ||
| Phaeodactylum tricornutum | NA | Mixotrophic, batch | PUFA | - | [92] | |
| Glucose (245.5 g/L) | 0.50 | |||||
| Aurantiochytrium sp. T66 | Glucose (78.34 g/L), fructose (24.96 g/L) | Heterotrophic, batch | DHA + squalene | 14.7 | [93] | |
| Golenkinia sp. | Anaerobically digested food waste | NA | Mixotrophic, batch | Bioelectricity + Biogas + Lipids | 0.8 | [94] |
| Galdieria sulphuraria | Restaurant waste | Glucose (103 g/L), sucrose (6 g/L), fructose (13 g/L), xylose (14 g/L) | Heterotrophic, batch | Lipids | 0.65 g/g glucose | [85] |
| Bakery waste | Glucose (128 g/L), sucrose (72 g/L), fructose (6 g/L) | 0.72 g/g glucose | ||||
| Chlorella sorokinana | Anaerobically digested kitchen waste | Total organic carbon (3.761 g/L) | Mixotrophic, batch | 0.42 | [95] | |
| Scenedesmus sp. | 0.55 | |||||
| Chlorella sp. | NA | 0.53 | [96] | |||
| Scenedesmus sp. | 0.52 | |||||
| Chlorella vulgaris | Okara waste | Lipids + carbohydrates | 1.92 | [97] | ||
| Nannochloropsis oculata | 1 | |||||
| Fruit waste | ||||||
| Chlorella vulgaris | Fruit waste | Total reducing sugar (43.6 g/L) | Mixotrophic, batch | Lipids | - | [98] |
| Aurantiochytrium sp. | Musa acuminate colla (Banana) | Total sugar content (0.72 g/g) | Mixotrophic, fed-batch | DHA | 13 | [99] |
| Ananas comosus MD2 (Pineapples) | Total sugar content (0.70 g/g) | 12 | ||||
| Orange peel extract | Glucose (5.1–5.9 g/L), fructose (5.1–5.6 g/L) | Heterotrophic, batch | 4 | [100] | ||
| Chlorella vulgaris | Glucose (4.2 g/L), sucrose (2.7 g/L | Mixotrophic, batch | Lipids | 2.20 | [101] | |
| Chlorella protothecoides | Papaya waste | Total sugar (101 g/Kg) | Heterotrophic, batch | 34.76 g/Kg | [102] | |
| Tetraselmis indica | Kinnow peel waste | NA | Mixotrophic, batch | 0.98 | [103] | |
| Crypthecodinium cohnii | Date syrup | Glucose (37%), fructose (43%), sucrose (3%) | DHA | 42 | [104] | |
| Dairy waste | ||||||
| Chlorella vulgaris | Cheese whey | - | Mixotrophic, batch | Proteins | 2.59 | [31] |
| Glucose (5 g/L), galactose (5 g/L) | Proteins + Lipids | 3.58 | [105] | |||
| Lactose (10 g/L) | Proteins + lipids | 2.10 | [106] | |||
| Chlorella protothecoides | Glucose (10 g/L), galactose (10 g/L) | Heterotrophic, Fed-batch | Lipids | 2.8 | [107] | |
| Lactose (9.52 g/L), glucose (4.53 g/L), galactose (0.24 g/L) | Mixotrophic, batch | 4.54 | [108] | |||
| Lactose (4.69 g/L) | Pigments | 3.60 | [109] | |||
| Chlorella sp. | Lactose (3.37 g/L) | Lipids | 1.62 | [110] | ||
| Chlamydomonas sp. | 1.15 | |||||
| Scenedesmus obliqqus | Lactose (40 g/L) | 4.9 | [111] | |||
| Scenedesmus obliqqus + Chlorella protothecoides | 10.6 | [112] | ||||
| Chlorella sp. | Dairy manure | NA | - | [113] | ||
| Wine industry and other waste | ||||||
| Chlorella vulgaris | White wine lees | NA | Mixotrophic, batch | Proteins | 1.75 | [31] |
| Chlorella sorokinana | Mixotrophic, Fed-batch | Lipids | 4 | [114] | ||
| Chlorella sp. | Livestock compost | NA | Mixotrophic, batch | 2.88 | [115] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, E.M.; Arora, N.; Philippidis, G.P. The Prospects of Agricultural and Food Residue Hydrolysates for Sustainable Production of Algal Products. Energies 2020, 13, 6427. https://doi.org/10.3390/en13236427
Ammar EM, Arora N, Philippidis GP. The Prospects of Agricultural and Food Residue Hydrolysates for Sustainable Production of Algal Products. Energies. 2020; 13(23):6427. https://doi.org/10.3390/en13236427
Chicago/Turabian StyleAmmar, Ehab M., Neha Arora, and George P. Philippidis. 2020. "The Prospects of Agricultural and Food Residue Hydrolysates for Sustainable Production of Algal Products" Energies 13, no. 23: 6427. https://doi.org/10.3390/en13236427
APA StyleAmmar, E. M., Arora, N., & Philippidis, G. P. (2020). The Prospects of Agricultural and Food Residue Hydrolysates for Sustainable Production of Algal Products. Energies, 13(23), 6427. https://doi.org/10.3390/en13236427







