Gel Pills for Downhole Pressure Control during Oil and Gas Well Drilling
Abstract
1. Introduction
2. Experiments with Viscous Pills and Gel Pills
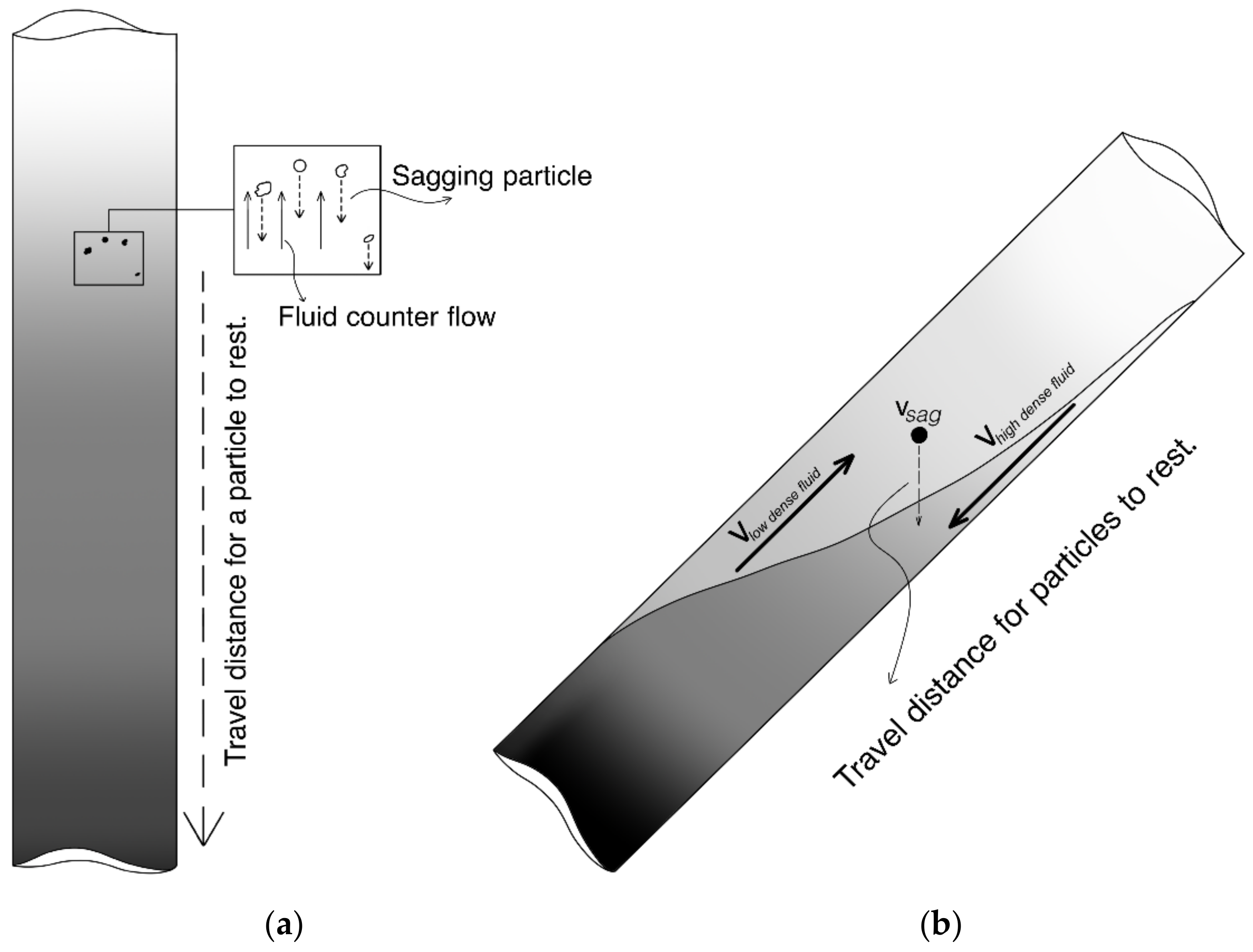
2.1. Benchtop Viscous Pill Test Overview
2.2. Model Drilling Fluid and Viscous Pills
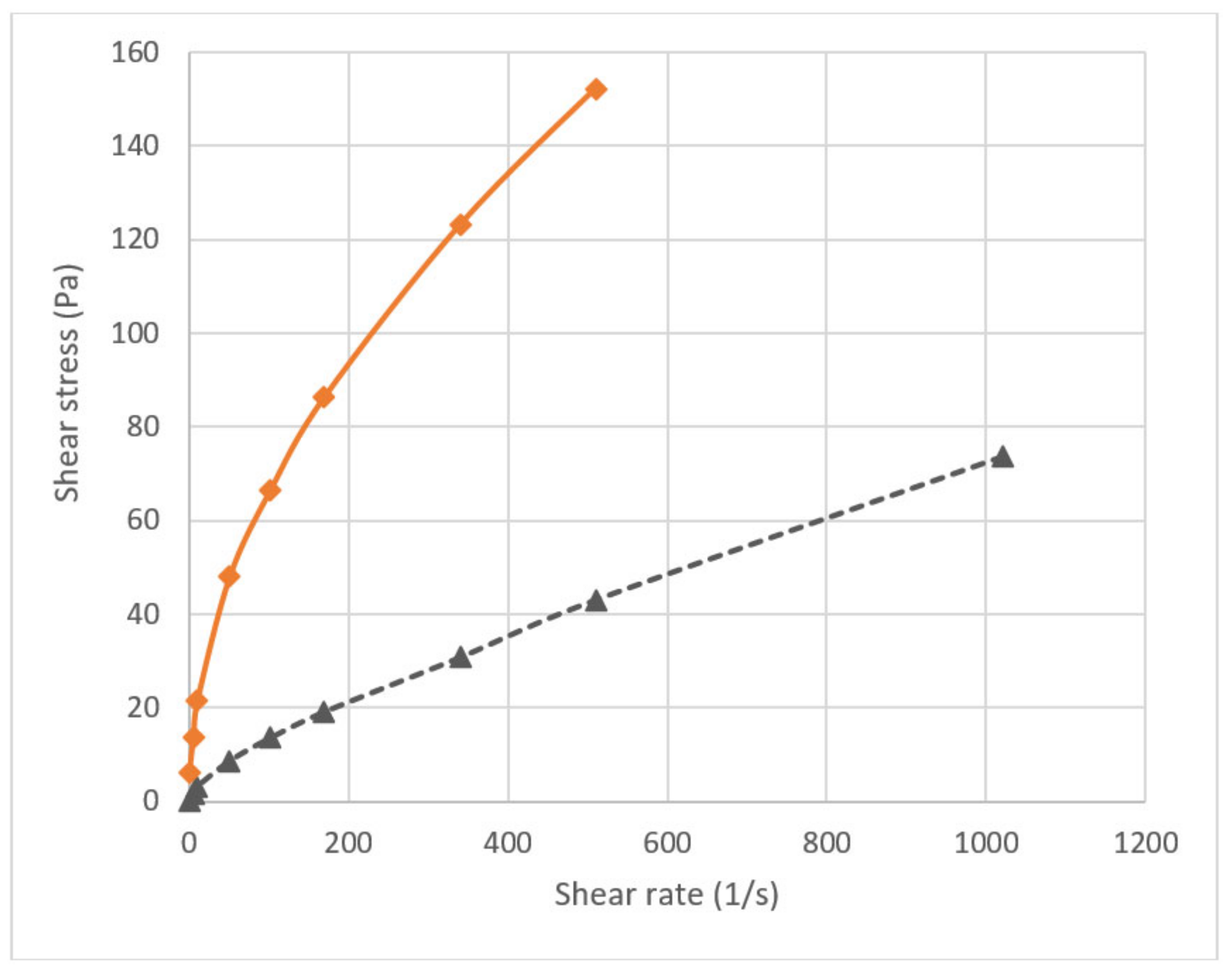
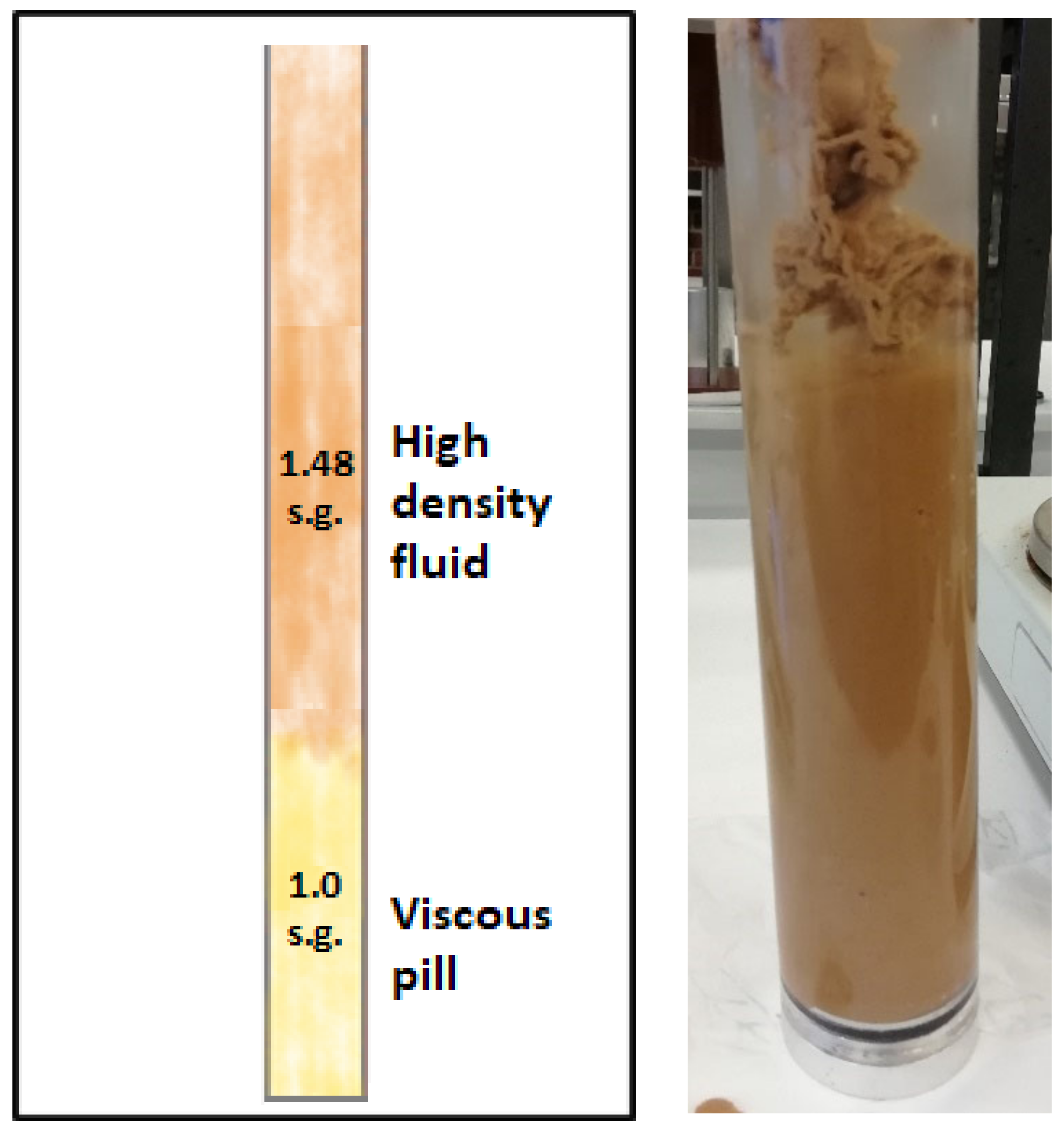
2.3. Performance of the Viscous Pills in the Benchtop Tests
2.4. Design and Development of Bentonite-Based Gel Pill
2.5. Equipment and Test Setup
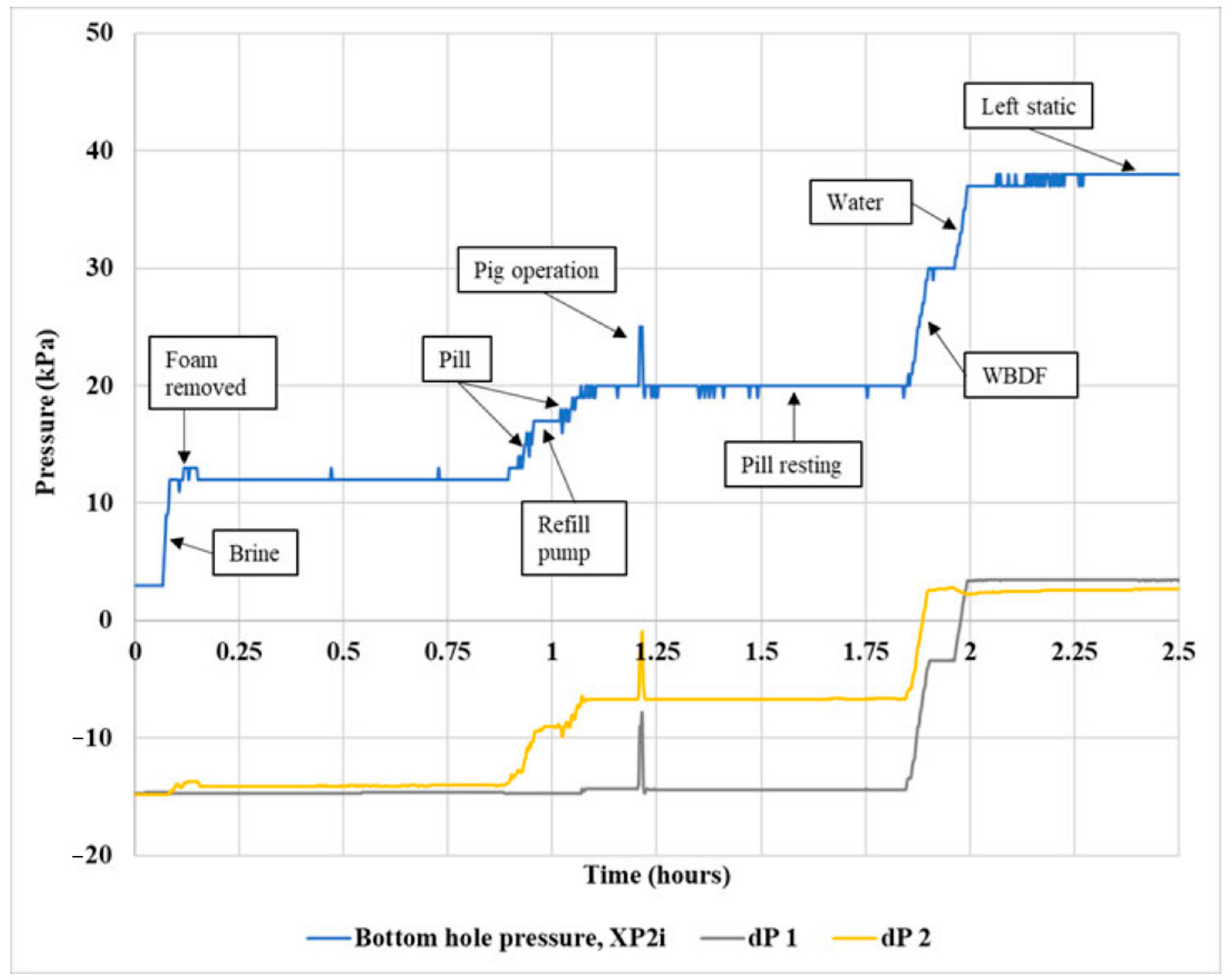
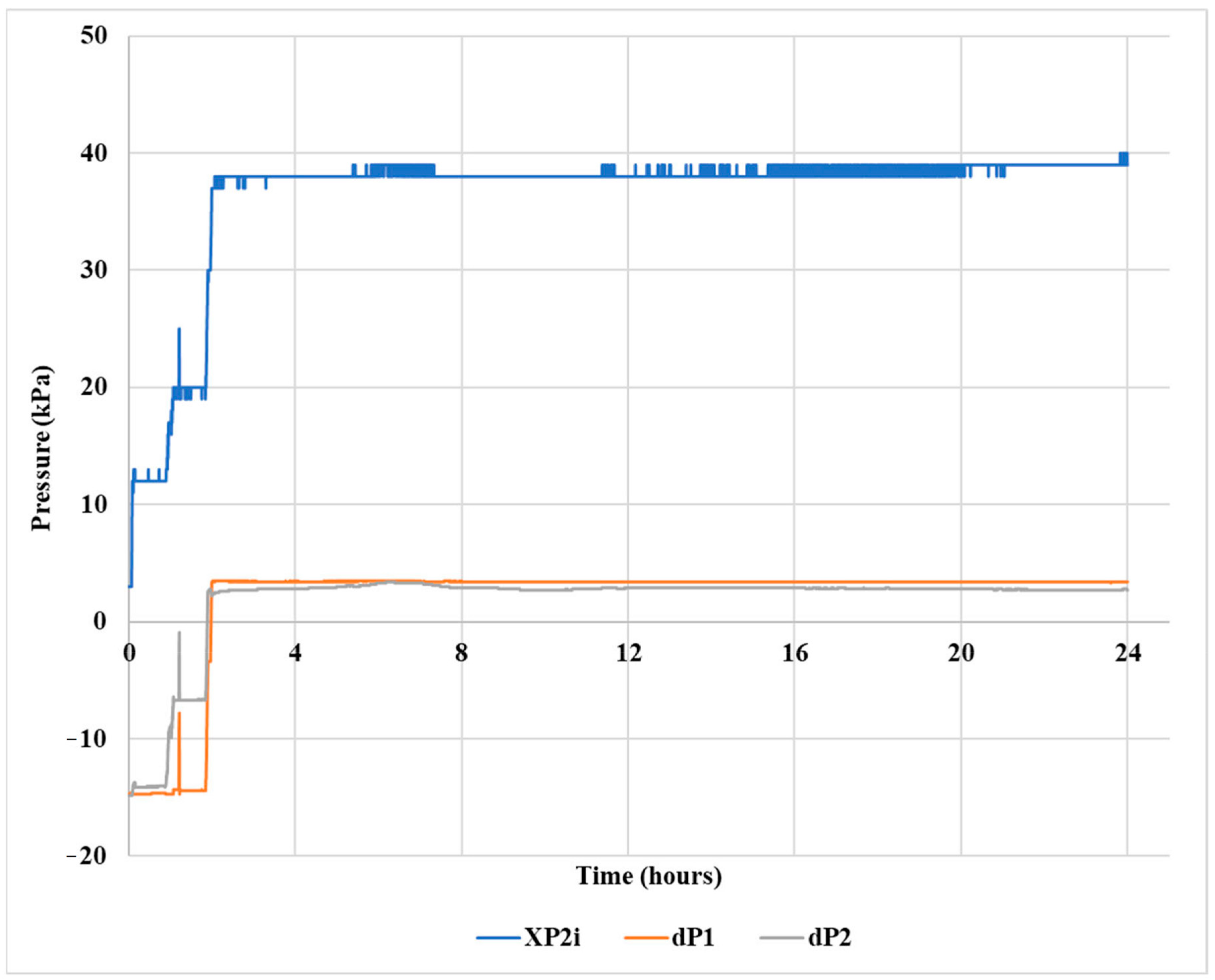
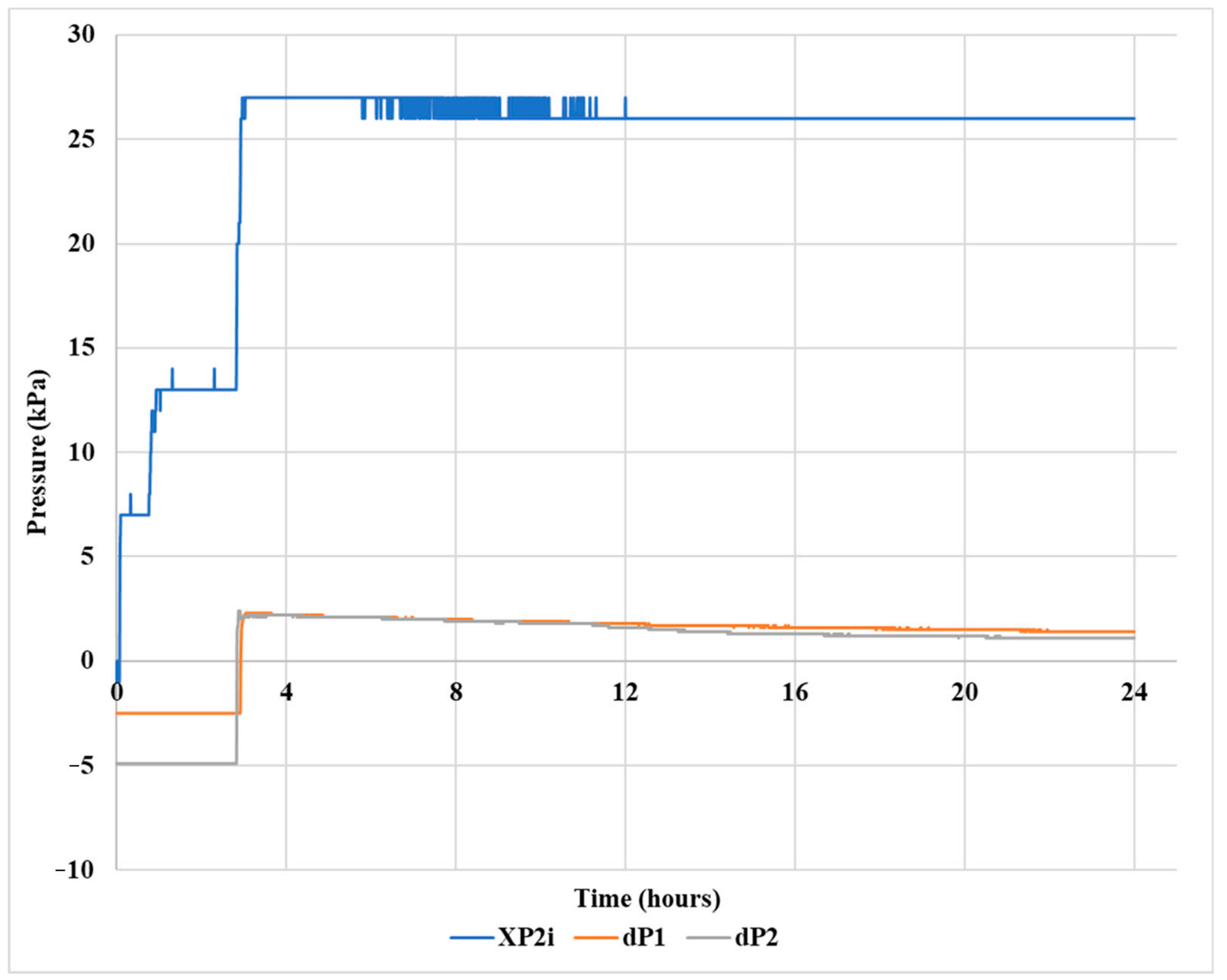
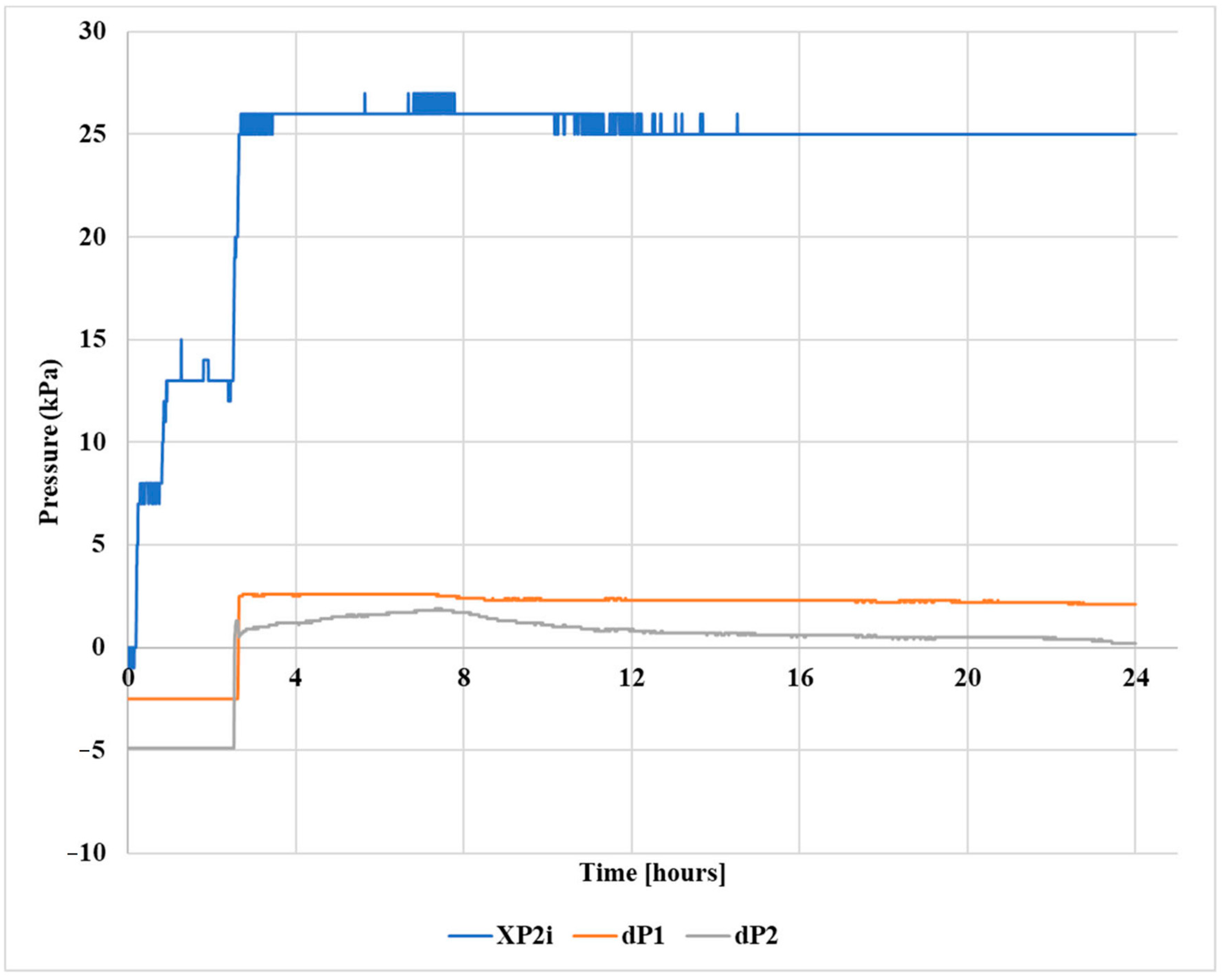
2.6. Gel Pill Performance in Vertical Hole
2.7. Gel Pill Performance in a 40° Deviated Hole
3. Discussion and Remarks
4. Conclusions
- A purely viscous pill should not be used for separating a high density fluid from a lighter fluid underneath.
- A bentonite or laponite gel, can be placed in a well for temporary prevention of intermixing of the fluids above and underneath the gel pill.
- Both the bentonite and laponite gel pills function as a good collection bed for settled barite.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ofei, T.N.; Kalaga, D.V.; Lund, B.; Saasen, A.; Linga, H.; Sangesland, S.; Gyland, K.R.; Kawaji, M. Laboratory Evaluation of Static and Dynamic Sag in Oil-Based Drilling Fluids. SPE J. 1995. SPE-199567-PA. [Google Scholar] [CrossRef]
- Omland, T.H.; Albertsen, T.; Taugbol, K.; Saasen, A.; Svanes, K.; Amundsen, P.A. The Effect of the Synthetic- and Oil-Based Drilling Fluid’s Internal Water-Phase Composition on Barite Sag. SPE Drill. Complet. 2006, 21, 91–98. [Google Scholar] [CrossRef]
- Omland, T.H.; Saasen, A.; Van Der Zwaag, C.; Amundsen, P.A. The Effect of Weighting Material Sag on Drilling Operation Efficiency. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 30 October–1 November 2007. [Google Scholar]
- Saasen, A.; Liu, D.; Marken, C. Prediction of Barite Sag Potential of Drilling Fluids From Rheological Measurements. In Proceedings of the SPE/IADC Drilling Conference, Amsterdam, The Netherlands, 28 February–2 March 1995. [Google Scholar]
- Boycott, A.E. Sedimentation of Blood Corpuscles. Nat. Cell Biol. 1920, 104, 532. [Google Scholar] [CrossRef]
- Palma, S.; Ihle, C.F.; Tamburrino, A. Characterization of a sediment layer of concentrated fluid-solid mixtures in tilted ducts at low Reynolds numbers. Powder Technol. 2018, 325, 192–201. [Google Scholar] [CrossRef]
- Movahedi, H.; Shad, S.; Mokhtari-Hosseini, Z.B. Modeling and simulation of barite deposition in an annulus space of a well using CFD. J. Pet. Sci. Eng. 2018, 161, 476–496. [Google Scholar] [CrossRef]
- Nguyen, T.; Miska, S.; Yu, M.; Takach, N.; Ahmed, R.; Saasen, A.; Omland, T.H.; Maxey, J. Experimental study of dynamic barite sag in oil-based drilling fluids using a modified rotational viscometer and a flow loop. J. Pet. Sci. Eng. 2011, 78, 160–165. [Google Scholar] [CrossRef]
- Kynch, G.J. A theory of sedimentation. Trans. Faraday Soc. 1952, 48, 166–176. [Google Scholar] [CrossRef]
- Taylor, G.I. The instability of liquid surfaces when accelerated in a direction perpendicular to their planes. Proc. Lond. Math. Soc. A 1950, 201, 192–196. [Google Scholar]
- Saasen, A.; Tyvand, P.A. Rayleigh-Taylor instability and Rayleigh-type waves on a Maxwell-fluid. J. Appl. Math. Phys. ZAMP 1990, 41, 284–292. [Google Scholar] [CrossRef]
- Sharma, R.C. Rayleigh-taylor instability of two viscoelastic superposed fluids. Acta Phys. Acad. Sci. Hung. 1978, 45, 213–220. [Google Scholar] [CrossRef]
- Harestad, K.; Herigstad, T.P.; Torsvoll, A.; Nodland, N.E.; Saasen, A. Optimization of Balanced-Plug Cementing. SPE Drill. Complet. 1997, 12, 168–173. [Google Scholar] [CrossRef]
- Dickinson, E. An Introduction to Food Colloids; Oxford University Press: Oxford, UK, 1992; ISBN 0198552238. [Google Scholar]
- Fosso, S.W.; Tina, M.; Frigaard, I.; Crawshaw, J. Viscous-Pill Design Methodology Leads to Increased Cement Plug Success Rates; Application and Case Studies from Southern Algeria. IADC SPE Asia Pac. Drill. Technol. 2000. [Google Scholar] [CrossRef]
- Saasen, A.; Ytrehus, J.D. Viscosity Models for Drilling Fluids—Herschel-Bulkley Parameters and Their Use. Energies 2020, 13, 5271. [Google Scholar] [CrossRef]
- Christidis, G.E.; Aldana, C.; Chryssikos, G.D.; Gionis, V.; Kalo, H.; Stöter, M.; Breu, J.; Robert, J.-L. The Nature of Laponite: Pure Hectorite or a Mixture of Different Trioctahedral Phases? Minerals 2018, 8, 314. [Google Scholar] [CrossRef]
- Huang, W.; Leong, Y.-K.; Chen, T.; Au, P.-I.; Liu, X.; Qiu, Z. Surface chemistry and rheological properties of API bentonite drilling fluid: pH effect, yield stress, zeta potential and ageing behaviour. J. Pet. Sci. Eng. 2016, 146, 561–569. [Google Scholar] [CrossRef]
- American Petroleum Institute. Recommended Practice for Testing Oil-based Drilling Fluids; API: Washington, DC, USA, 2014; API Recommended Practice 13B2. [Google Scholar]
- Pek-Ing, A.; Yee-Kwong, L. Surface chemistry and rheology of Laponite dispersions—Zeta potential, yield stress, ageing, fractal dimension and pyrophosphate. Appl. Clay Sci. 2015, 107, 36–45. [Google Scholar] [CrossRef]
- Zamora, M.; Power, D. Making a case for AADE hydraulics and the unified rheological model. In Proceedings of the AADE 2002 Technology Conference, Houston, TX, USA, 2–3 April 2002. [Google Scholar]
- Power, D.; Zamora, M. Drilling Fluid Yield Stress: Measurement Techniques for Improved Understanding of Critical Drilling Fluid Parameters. In Proceedings of the AADE 2003 National Technology Conference, Houston, TX, USA, 1–3 April 2003. [Google Scholar]
- Petroleum and Natural Gas Industries. Field Testing of Drilling Fluids. Part 1: Water-Based Fluids; International Organization for Standardization: Geneva, Switzerland, 2008; Report ISO 10414-1. [Google Scholar]
- Petroleum and Natural Gas Industries. Field Testing of Drilling Fluids. Part 2: Oil-Based Fluids; International Organization for Standardization: Geneva, Switzerland, 2001; Report ISO 10414-2. [Google Scholar]
- Ronaes, E.; Prebensen, O.I.; Mikalsen, R.; Taugbol, K.; Syltoy, S.; Torvund, S. An Innovative Fluid Pressure Transmission Pill Successfully Used During Managed-Pressure Drilling Operations in an HTHP Environment. In Proceedings of the IADC/SPE Drilling Conference, Orlando, FL, USA, 4–6 March 2008. [Google Scholar]








| Ingredients | Quantity (g) | Mixing Time (min) |
|---|---|---|
| Tap water | 249 | NA |
| Xanthan biopolymer | 0.3 | 20 |
| Xanthan concentration (g/L) | 1.2 | |
| Barite | 185 | 30 |
| Ingredients | Pill 1 (g) | Pill 2 (g) |
|---|---|---|
| Tap water | 350 | 350 |
| Xanthan biopolymer | 3.0 | 4.0 |
| Xanthan concentration (g/L) | 8.6 | 11.4 |
| Ingredients | Quantity (g) | Mixing Time (min) |
|---|---|---|
| Tap water | 343 | NA |
| NaCO3 | 0.02 | 7 |
| NaOH | 1.0 | 3 |
| Bentonite | 20 | 65 * |
| Ingredients | Quantity (g) | Mixing Time (min) |
|---|---|---|
| Tap water | 343 | NA |
| NaCO3 | 0.02 | 7 |
| NaOH | 1.0 | 3 |
| Laponite RD | 12 | 65 * |
| Shear Stress (Pa) | Bentonite | Laponite |
|---|---|---|
| Yield stress | 33.1 | 25.4 |
| 10 s gel strength | 25.7 | 20.2 |
| 10 min gel strength | 35.1 | 59.9 |
| Ingredients | Quantity (g) | Mixing Time (min) |
|---|---|---|
| Tap water | 249 | NA |
| Xanthan Gum | 0.181 | 15 |
| CaCl2*2H2O | 113.6 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifeh, M.; Penkala, L.; Saasen, A.; Aase, B.; Omland, T.H.; Taugbøl, K.; Reinås, L. Gel Pills for Downhole Pressure Control during Oil and Gas Well Drilling. Energies 2020, 13, 6318. https://doi.org/10.3390/en13236318
Khalifeh M, Penkala L, Saasen A, Aase B, Omland TH, Taugbøl K, Reinås L. Gel Pills for Downhole Pressure Control during Oil and Gas Well Drilling. Energies. 2020; 13(23):6318. https://doi.org/10.3390/en13236318
Chicago/Turabian StyleKhalifeh, Mahmoud, Larisa Penkala, Arild Saasen, Bodil Aase, Tor Henry Omland, Knut Taugbøl, and Lorents Reinås. 2020. "Gel Pills for Downhole Pressure Control during Oil and Gas Well Drilling" Energies 13, no. 23: 6318. https://doi.org/10.3390/en13236318
APA StyleKhalifeh, M., Penkala, L., Saasen, A., Aase, B., Omland, T. H., Taugbøl, K., & Reinås, L. (2020). Gel Pills for Downhole Pressure Control during Oil and Gas Well Drilling. Energies, 13(23), 6318. https://doi.org/10.3390/en13236318





