Short Review: Timeline of the Electrochemical Lithium Recovery System Using the Spinel LiMn2O4 as a Positive Electrode
Abstract
1. Introduction
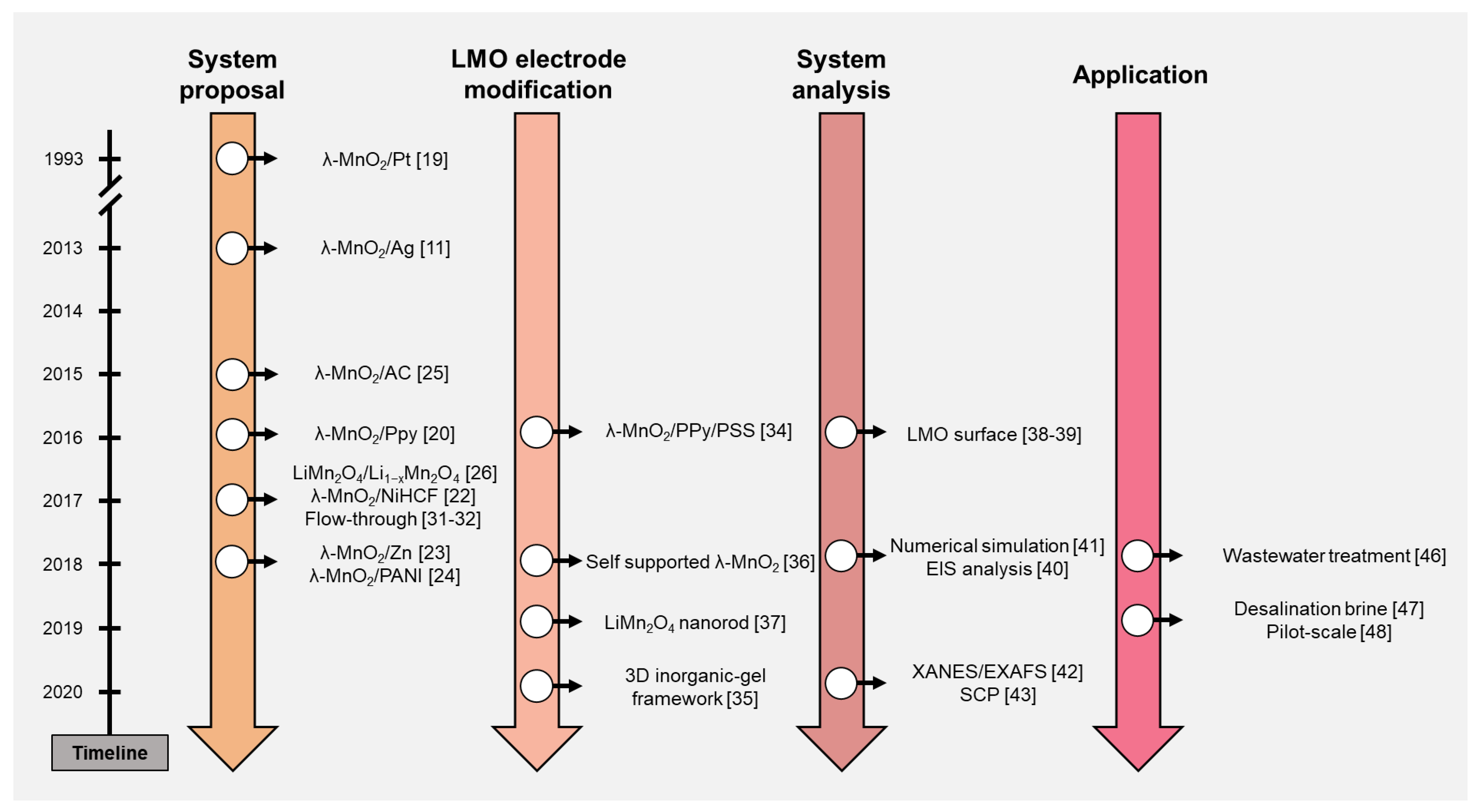
2. System Proposal
2.1. The System with the Hydrolysis (λ-MnO2/Pt System)
2.2. Asymmetric Battery System
2.3. Hybrid Supercapacitor System
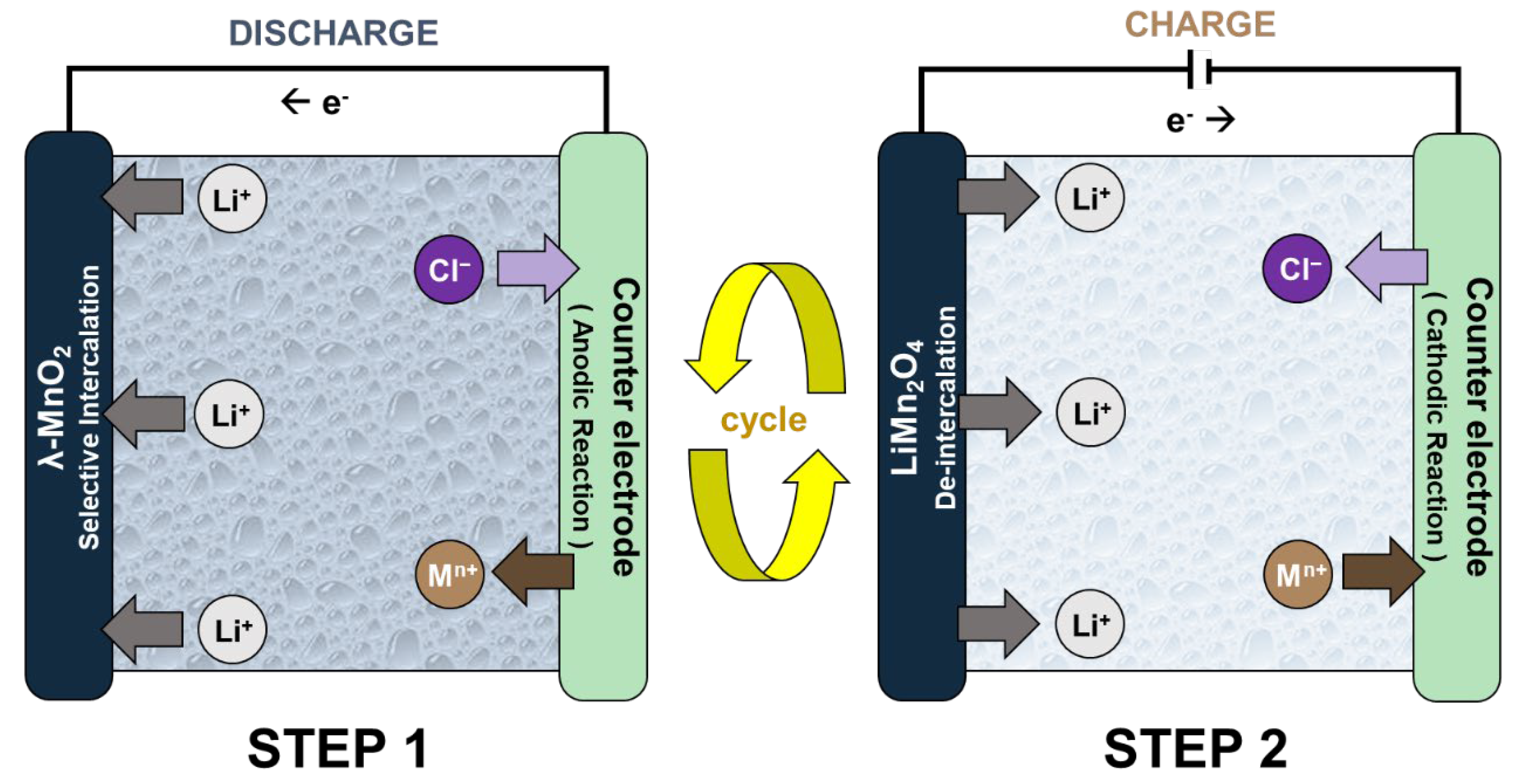
2.4. Symmetric Rocking Chair Battery-Liked System
2.5. Flow-Through Type Reactor
3. LMO Electrode Modification to Enhance the Li+ Recovery Performance
4. System Analysis
4.1. Electroanalysis
4.2. Numerical Simulation
4.3. X-ray Measurement
4.4. Selective Concentration Polarization
5. Application
5.1. Lithium Recovery with the Wastewater Treatment
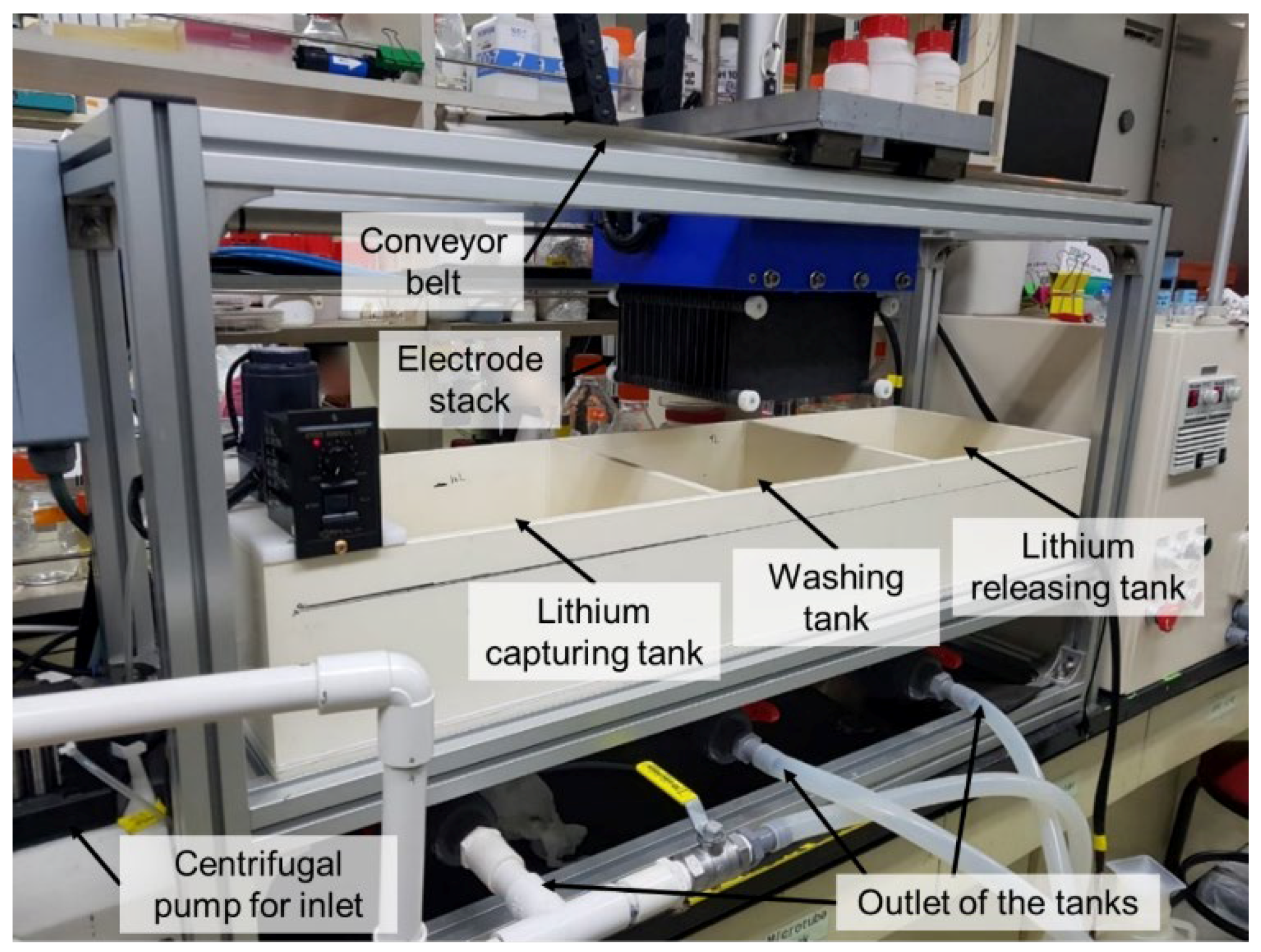
5.2. Lithium Recovery from the Desalination Concentrate
6. Summary of the System Performance and Perspectives
6.1. System Proposal and Operation
6.2. LMO Electrode Modification
6.3. System Analysis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| LMO | Mass Loading (mg cm−2) | Electrode Dimension (cm × cm or cm2) | Counter | Solution Composition | Operation Mode | Capacity (mAh gLMO−1) | Energy Consumption (Wh molLi−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| λ-MnO2 | - | 1.0 × 1.0 | Pt | Geothermal (Li+: 0.74 mM) | Forward sweep | 85 | - | [19] |
| λ-MnO2 | - | 3.0 × 3.0 | Ag | Simulated brine: Salar de Atacama (Li+: 210 mM) | CC mode (0.5 mA cm−2) | - | 1.0 | [11] |
| λ-MnO2 | - | - | AC | 30 mM LiCl, NaCl, KCl, MgCl2, and CaCl2 | CC mode (0.5 mA cm−2) | - | 4.2 | [25] |
| λ-MnO2 | 0.52 |
Plane: 3.0 × 2.0 Total: 56.0 cm2 | PPy | Brine (Salar de Olaroz) (Li+: 180 mM) | CC mode (2.5 mA cm−2) | - | 9.4 | [20] |
| λ-MnO2 /PPy/PSS | - |
5 × 5 (porous) | Pt | 30 mM LiCl and NaCl | CV mode | 135 | - | [34] |
| LMO | - | 2 cm2 | NiHCF | Simulated brine: Salar de Atacama (Li+: 42 mM) | CC mode (1 C) | 100 | 3.6 | [22] |
| LiMn2O4 | - | 3.5 × 3.0 | Li1−xMn2O4 | Simulated brine (Li+: 21 mM) and concentrated seawater (Li+: 32 mM) | CV mode (0.6 V) | 77–97 | 17.89–18.67 | [26] |
| λ-MnO2 | 28.6 | 8.75 cm2 | BDD | Wastewater: battery recycling plant (Li+: 276.5 mM) | CC mode (2.9 mA cm−2) | 88 | 8.71 | [46] |
| LiMn2O4 | - | 2.5 × 4.0 | Zn | Simulated brine: Salar de Atacama (Li+: 210 mM) | CC mode (0.5 mA cm−2) | - | 6.3 | [23] |
| Self-supported λ-MnO2 | - | 3.0 × 3.0 | Ag | 30 mM LiCl, NaCl, KCl, MgCl2, and CaCl2 | CC mode (50 mA g−1) | 100 | 4.14 | [36] |
| LiMn2O4 | 25–35 | 2.0 × 2.0 | PANI | Simulated brine: Taijinair (Li+: 64 mM) | CC mode (0.5 mA cm−2) | ~90 | 3.95 | [24] |
| LiMn2O4 nanorod | 5 | - | NiHCF | Simulated brine: Taijinair (Li+: 31.7 mM) | CC mode (1 C) | 105 | 1.76 | [37] |
| λ-MnO2 | - | Diameter: 5 cm | Ag | Desalination brine (Li+: 0.063 mM) | CC mode (0.01 mA cm−2) | - | 17.2 (1st step) | [47] |
| λ-MnO2 | 5 | 2.0 × 2.0 | LiMn2O4 | 30 mM LiCl | CC mode (0.0625 mA cm−2) | 128 | 0.56 | [28] |
| PPy/Al2O3/LMO | - | 1 cm2 | AC | Simulated diluted brine (Li+: 23.48 mM) | CC mode (0.75 mA) | ~50 | 1.41 | [35] |
| Li1−xMn2O4 | 0.76 | 15.5 × 4.5 (packed bed) | LiMn2O4 | Simulated brine: Hombre Muerto (Li+: 190 mM) | CC mode (1.43 mA cm−2) | ~100 | 2.76 | [29] |
References
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research—Global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Wanger, T.C. The Lithium future-resources, recycling, and the environment. Conserv. Lett. 2011, 4, 202–206. [Google Scholar] [CrossRef]
- Calvo, E.J. Electrochemical methods for sustainable recovery of lithium from natural brines and battery recycling. Curr. Opin. Electrochem. 2019, 15, 102–108. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Wan, P.; Gasem, K.; Wang, K.; He, T.; Adidharma, H.; Fan, M. Extraction of lithium with functionalized lithium ion-sieves. Prog. Mater. Sci. 2016, 84, 276–313. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.; Kim, S.; Yoon, J. Review of concepts and applications of electrochemical ion separation (EIONS) process. Sep. Purif. Technol. 2019, 215, 190–207. [Google Scholar] [CrossRef]
- Battistel, A.; Palagonia, M.S.; Brogioli, D.; La Mantia, F.; Trócoli, R. Electrochemical Methods for Lithium Recovery: A Comprehensive and Critical Review. Adv. Mater. 2020, 32. [Google Scholar] [CrossRef] [PubMed]
- Srimuk, P.; Su, X.; Yoon, J.; Aurbach, D.; Presser, V. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nat. Rev. Mater. 2020, 5, 517–538. [Google Scholar] [CrossRef]
- Pasta, M.; Battistel, A.; La Mantia, F. Batteries for lithium recovery from brines. Energy Environ. Sci. 2012, 5, 9487. [Google Scholar] [CrossRef]
- Xu, P.; Hong, J.; Qian, X.; Xu, Z.; Xia, H.; Tao, X.; Xu, Z.; Ni, Q.Q. Materials for lithium recovery from salt lake brine. J. Mater. Sci. 2020. [Google Scholar] [CrossRef]
- Lee, J.; Yu, S.-H.; Kim, C.; Sung, Y.-E.; Yoon, J. Highly selective lithium recovery from brine using a λ-MnO2–Ag battery. Phys. Chem. Chem. Phys. 2013, 15, 7690. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.; Miyai, Y.; Katoh, S.; Maeda, H.; Abe, M. Topotactic Li+ Insertion to λ-MnO2 in the Aqueous Phase. Langmuir 1989, 5, 150–157. [Google Scholar] [CrossRef]
- Ooi, K.; Miyai, Y.; Katoh, S.; Maeda, H.; Abe, M. Lithium-ion Insertion/Extraction Reaction with λ-MnO2 in the Aqueous Phase. Chem. Lett. 1988, 17, 989–992. [Google Scholar] [CrossRef]
- Greedan, J.E.; Raju, N.P.; Wills, A.S.; Morin, C.; Shaw, S.M.; Reimers, J.N. Structure and Magnetism in λ-MnO2. Geometric Frustration in a Defect Spinel. Chem. Mater. 1998, 10, 3058–3067. [Google Scholar] [CrossRef]
- Ooi, K.; Miyai, Y.; Sakakihara, J. Mechanism of Li Insertion in Spinel-Type Manganese Oxide. Redox and Ion-Exchange Reactions. Langmuir 1991, 7, 1167–1171. [Google Scholar] [CrossRef]
- Feng, Q.; Miyai, Y.; Kanoh, H.; Ooi, K. Li+ Extraction/Insertion with Spinel-Type Lithium Manganese Oxides. Characterization of Redox-Type and Ion-Exchange-Type Sites. Langmuir 1992, 8, 1861–1867. [Google Scholar] [CrossRef]
- Liu, W.; Kowal, K.; Farrington, G.C. Mechanism of the Electrochemical Insertion of Lithium into LiMn2O4 Spinels. J. Electrochem. Soc. 1998, 145, 459–465. [Google Scholar] [CrossRef]
- Kanoh, H.; Ooi, K.; Miyai, Y.; Katoh, S. Selective Electroinsertion of Lithium Ions into a Pt/λ-MnO2 Electrode in the Aqueous Phase. Langmuir 1991, 7, 1841–1842. [Google Scholar] [CrossRef]
- Kanoh, H.; Ooi, K.; Miyai, Y.; Katoh, S. Electrochemical recovery of lithium ions in the aqueous phase. Sep. Sci. Technol. 1993, 28, 643–651. [Google Scholar] [CrossRef]
- Missoni, L.L.; Marchini, F.; del Pozo, M.; Calvo, E.J. A LiMn2O4 -Polypyrrole System for the Extraction of LiCl from Natural Brine. J. Electrochem. Soc. 2016, 163, A1898–A1902. [Google Scholar] [CrossRef]
- Trócoli, R.; Battistel, A.; La Mantia, F. Nickel Hexacyanoferrate as Suitable Alternative to Ag for Electrochemical Lithium Recovery. ChemSusChem 2015, 8, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Trócoli, R.; Erinmwingbovo, C.; La Mantia, F. Optimized Lithium Recovery from Brines by using an Electrochemical Ion-Pumping Process Based on λ-MnO2 and Nickel Hexacyanoferrate. ChemElectroChem 2017, 4, 143–149. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Kim, S.; Kim, S.; Yoon, J. Electrochemical Lithium Recovery with a LiMn2O4–Zinc Battery System using Zinc as a Negative Electrode. Energy Technol. 2018, 6, 340–344. [Google Scholar] [CrossRef]
- Zhao, A.; Liu, J.; Ai, X.; Yang, H.; Cao, Y. Highly Selective and Pollution-Free Electrochemical Extraction of Lithium by a Polyaniline/LixMn2O4 Cell. ChemSusChem 2019, 12, 1361–1367. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Kang, J.S.; Jo, K.; Kim, S.; Sung, Y.E.; Yoon, J. Lithium recovery from brine using a λ-MnO2/activated carbon hybrid supercapacitor system. Chemosphere 2015, 125, 50–56. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Ji, Z.Y.; Zhang, Y.G.; Guo, Z.Y.; Zhao, Y.Y.; Liu, J.; Yuan, J.S. Study on lithium extraction from brines based on LiMn2O4/Li1−xMn2O4 by electrochemical method. Electrochim. Acta 2017, 252, 350–361. [Google Scholar] [CrossRef]
- Qu, Y.; Campbell, P.G.; Gu, L.; Knipe, J.M.; Dzenitis, E.; Santiago, J.G.; Stadermann, M. Energy consumption analysis of constant voltage and constant current operations in capacitive deionization. Desalination 2016, 400, 18–24. [Google Scholar] [CrossRef]
- Joo, H.; Jung, S.Y.; Kim, S.; Ahn, K.H.; Ryoo, W.S.; Yoon, J. Application of a Flow-Type Electrochemical Lithium Recovery System with λ-MnO2/LiMn2O4: Experiment and Simulation. ACS Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Romero, V.C.E.; Putrino, D.S.; Tagliazucchi, M.; Flexer, V.; Calvo, E.J. Electrochemical Flow Reactor for Selective Extraction of Lithium Chloride from Natural Brines. J. Electrochem. Soc. 2020, 167, 120522. [Google Scholar] [CrossRef]
- Marchini, F.; Williams, F.J.; Calvo, E.J. Sustainable Selective Extraction of Lithium Chloride from Natural Brine Using a Li1-xMn2O4 Ion Pump. J. Electrochem. Soc. 2018, 165, A3292–A3298. [Google Scholar] [CrossRef]
- Palagonia, M.S.; Brogioli, D.; Mantia, F.L. Influence of Hydrodynamics on the Lithium Recovery Efficiency in an Electrochemical Ion Pumping Separation Process. J. Electrochem. Soc. 2017, 164, E586–E595. [Google Scholar] [CrossRef]
- Palagonia, M.S.; Brogioli, D.; La Mantia, F. Effect of Current Density and Mass Loading on the Performance of a Flow-Through Electrodes Cell for Lithium Recovery. J. Electrochem. Soc. 2019, 166, E286–E292. [Google Scholar] [CrossRef]
- Xiao, J.L.; Sun, S.Y.; Wang, J.; Li, P.; Yu, J.G. Synthesis and adsorption properties of Li1.6Mn1.6O4 spinel. Ind. Eng. Chem. Res. 2013, 52, 11967–11973. [Google Scholar] [CrossRef]
- Du, X.; Guan, G.; Li, X.; Jagadale, A.D.; Ma, X.; Wang, Z.; Hao, X.; Abudula, A. A novel electroactive λ-MnO2/PPy/PSS core-shell nanorod coated electrode for selective recovery of lithium ions at low concentration. J. Mater. Chem. A 2016, 4, 13989–13996. [Google Scholar] [CrossRef]
- Zhao, X.; Jiao, Y.; Xue, P.; Feng, M.; Wang, Y.; Sha, Z. Efficient Lithium Extraction from Brine Using a Three-Dimensional Nanostructured Hybrid Inorganic-Gel Framework Electrode. ACS Sustain. Chem. Eng. 2020, 8, 4827–4837. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Feng, Z.; Kahn, N.U.; Haq Khan, Z.U.; Tang, Y.; Sun, Y.; Wan, P.; Chen, Y.; Fan, M. A Self-Supported λ-MnO2 Film Electrode used for Electrochemical Lithium Recovery from Brines. Chempluschem 2018, 83, 521–528. [Google Scholar] [CrossRef]
- Xie, N.; Li, Y.; Lu, Y.; Gong, J.; Hu, X. Electrochemically Controlled Reversible Lithium Capture and Release Enabled by LiMn2O4 Nanorods. ChemElectroChem 2019, 1–8. [Google Scholar] [CrossRef]
- Marchini, F.; Rubi, D.; Del Pozo, M.; Williams, F.J.; Calvo, E.J. Surface Chemistry and Lithium-Ion Exchange in LiMn2O4for the Electrochemical Selective Extraction of LiCl from Natural Salt Lake Brines. J. Phys. Chem. C 2016, 120, 15875–15883. [Google Scholar] [CrossRef]
- Marchini, F.; Calvo, E.J.; Williams, F.J. Effect of the electrode potential on the surface composition and crystal structure of LiMn2O4 in aqueous solutions. Electrochim. Acta 2018, 269, 706–713. [Google Scholar] [CrossRef]
- Marchini, F.; Williams, F.J.; Calvo, E.J. Electrochemical impedance spectroscopy study of the LixMn2O4 interface with natural brine. J. Electroanal. Chem. 2018, 819, 428–434. [Google Scholar] [CrossRef]
- Romero, V.C.E.; Tagliazucchi, M.; Flexer, V.; Calvo, E.J. Sustainable Electrochemical Extraction of Lithium from Natural Brine for Renewable Energy Storage. J. Electrochem. Soc. 2018, 165, A2294–A2302. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.S.; Joo, H.; Sung, Y.E.; Yoon, J. Understanding the Behaviors of λ-MnO2 in Electrochemical Lithium Recovery: Key Limiting Factors and a Route to the Enhanced Performance. Environ. Sci. Technol. 2020, 54, 9044–9051. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-Y.; Ji, Z.-Y.; Chen, H.-Y.; Liu, J.; Zhao, Y.-Y.; Li, F.; Yuan, J.-S. Effect of Impurity Ions in the Electrosorption Lithium Extraction Process: Generation and Restriction of “Selective Concentration Polarization”. ACS Sustain. Chem. Eng. 2020, 4. [Google Scholar] [CrossRef]
- Zhao, Z.; Si, X.; Liu, X.; He, L.; Liang, X. Li extraction from high Mg/Li ratio brine with LiFePO4/FePO4 as electrode materials. Hydrometallurgy 2013, 133, 75–83. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Asif, M.B.; Roychand, R.; Shu, L.; Jegatheesan, V.; Bhuiyan, M.; Hai, F.I. Lithium recovery from salt-lake brine: Impact of competing cations, pretreatment and preconcentration. Chemosphere 2020, 260, 127623. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Kim, S.; Lee, J.; Yoon, J. Electrochemical lithium recovery and organic pollutant removal from industrial wastewater of a battery recycling plant. Environ. Sci. Water Res. Technol. 2018, 4, 175–182. [Google Scholar] [CrossRef]
- Kim, S.; Joo, H.; Moon, T.; Kim, S.; Yoon, J. Environmental Science Processes & Impacts Rapid and selective lithium recovery from desalination brine using an electrochemical. Environ. Sci. Process. Impacts 2019, 667–676. [Google Scholar] [CrossRef]
- Joo, H.; Kim, S.; Kim, S.; Choi, M.; Kim, S.-H.; Yoon, J. Pilot-scale demonstration of an electrochemical system for lithium recovery from the desalination concentrate. Environ. Sci. Water Res. Technol. 2019. [Google Scholar] [CrossRef]
- Hawks, S.A.; Ramachandran, A.; Porada, S.; Campbell, P.G.; Suss, M.E.; Biesheuvel, P.M.; Santiago, J.G.; Stadermann, M. Performance metrics for the objective assessment of capacitive deionization systems. Water Res. 2019, 152, 126–137. [Google Scholar] [CrossRef]
- Ravikumar, B.; Mynam, M.; Rai, B. Effect of Salt Concentration on Properties of Lithium Ion Battery Electrolytes: A Molecular Dynamics Study. J. Phys. Chem. C 2018, 122, 8173–8181. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, H.; Lee, J.; Yoon, J. Short Review: Timeline of the Electrochemical Lithium Recovery System Using the Spinel LiMn2O4 as a Positive Electrode. Energies 2020, 13, 6235. https://doi.org/10.3390/en13236235
Joo H, Lee J, Yoon J. Short Review: Timeline of the Electrochemical Lithium Recovery System Using the Spinel LiMn2O4 as a Positive Electrode. Energies. 2020; 13(23):6235. https://doi.org/10.3390/en13236235
Chicago/Turabian StyleJoo, Hwajoo, Jaehan Lee, and Jeyong Yoon. 2020. "Short Review: Timeline of the Electrochemical Lithium Recovery System Using the Spinel LiMn2O4 as a Positive Electrode" Energies 13, no. 23: 6235. https://doi.org/10.3390/en13236235
APA StyleJoo, H., Lee, J., & Yoon, J. (2020). Short Review: Timeline of the Electrochemical Lithium Recovery System Using the Spinel LiMn2O4 as a Positive Electrode. Energies, 13(23), 6235. https://doi.org/10.3390/en13236235





