Effect of Biowastes on Soil Remediation, Plant Productivity and Soil Organic Carbon Sequestration: A Review
Abstract
1. Introduction
1.1. European, and World Standards and Regulations on the Use of Organic Waste in Soil
1.2. Biowastes
1.3. Remediation of Metal Contaminated Soils
1.4. Soil Conditioner and Risk of Contamination
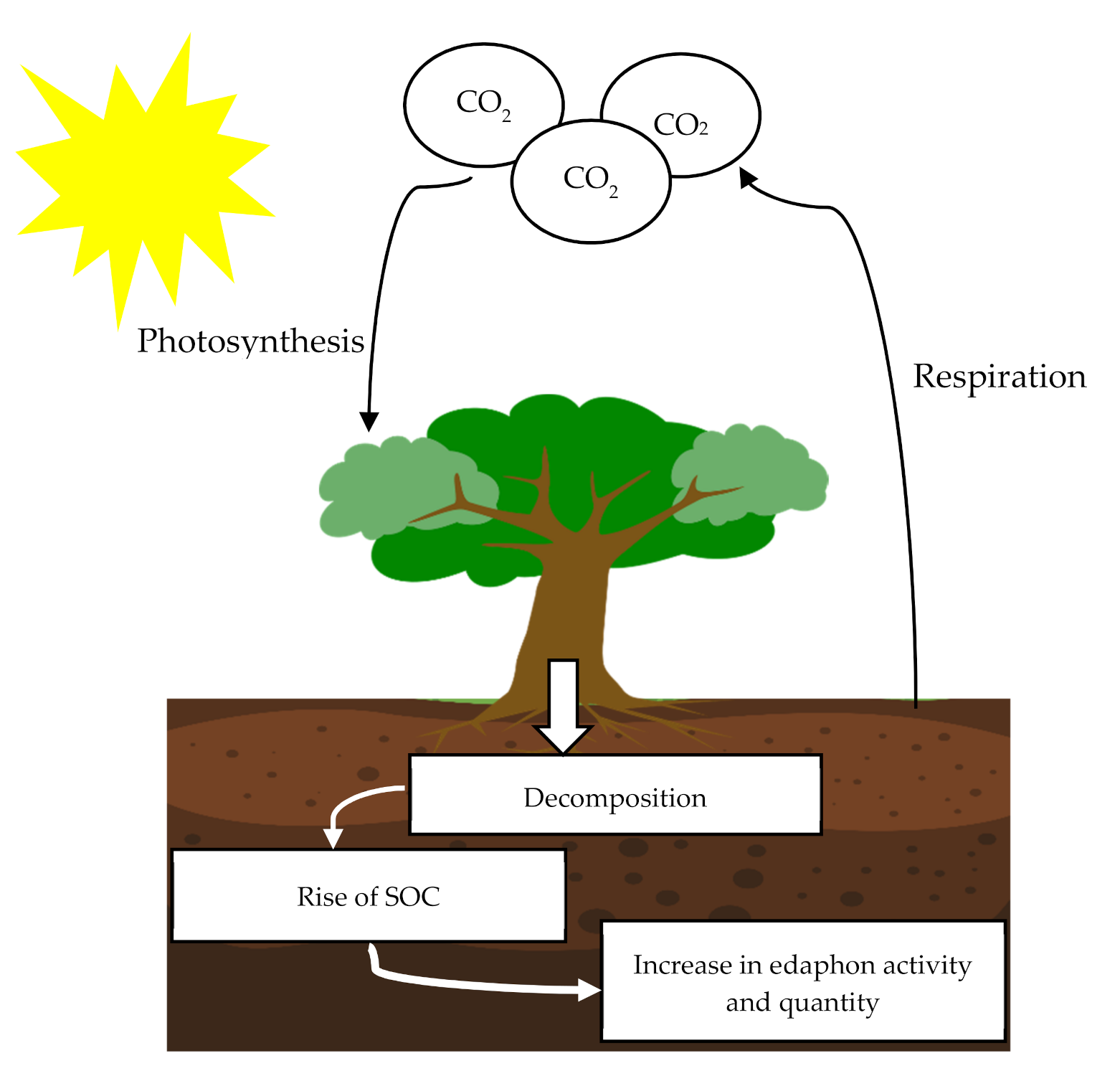
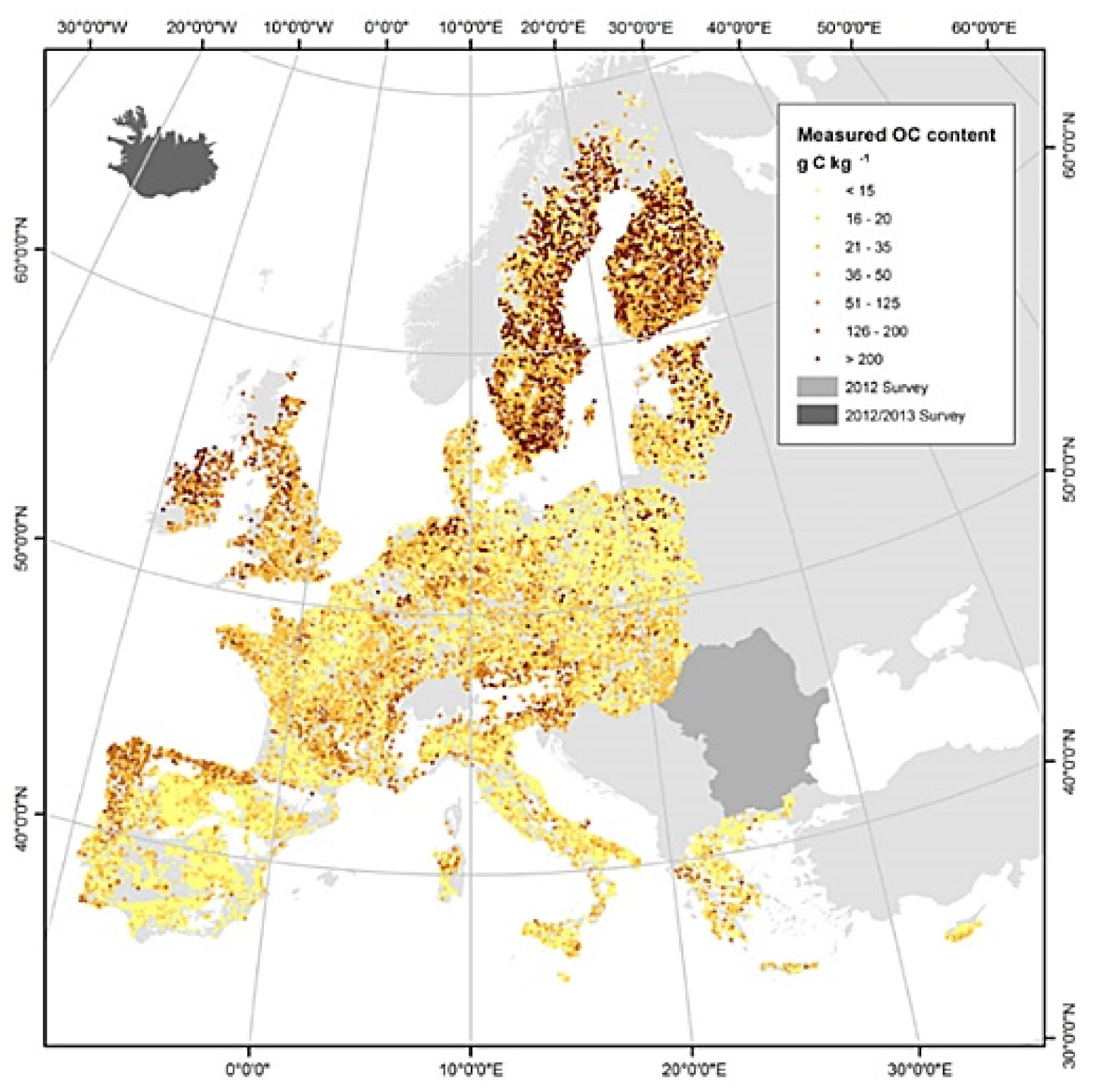
1.5. Carbon Sequestration
2. Soil Amendment with Biowaste
2.1. Sewage Sludge
2.2. Composts
2.3. Other Organic Wastes
3. Soil Property Changes after Biowastes Amendment
3.1. Physical and Chemical Soil Parameters
3.2. Impact on Biological and Biochemical Parameters
3.3. Remediation of Degraded Soil Using Biowaste
4. Plant Productivity in Biowaste Treated Soils—Benefits and Risks
| Plant | Plant Properties | Alternation | Reference | |
|---|---|---|---|---|
| SEWAGE SLUDGE | ||||
| Vigna radiata L. | Root length (cm plant−1) | Increase | [100] | |
| Shoot length (cm plant−1) | Increase | |||
| Leaf area (cm2 plant−1) | Increase | |||
| Number of leaves (plant−1) | Increase | |||
| Number of nodules (plant−1) | Increase | |||
| Total biomass (g plant−1) | Increase | |||
| Zea mays | Height (m) | Increase | [99] | |
| Stem diameter (cm) | Decrease | |||
| Number of leaves | Increase | |||
| Foliar area | Increase | |||
| Number of nodes | Increase | |||
| Number of corn cob | Increase | |||
| Productivity (t ha−1) | Increase | |||
| Scot Pine | Root biomass production (g) | Increase | [31] | |
| Giant Miscanthus | Root biomass production (g) | Increase | ||
| Lepidium sativum | Root growth (cm) | Increase | [78] | |
| Sinapis alba | increase | |||
| Sorghum saccharatum | Increase | |||
| Dactylis glomerate, Festuca arundinacea, F. rubra, Loliumperene | Biomass yield | Increase | [106] | |
| Eucalyptus, Poplar, Willow | Root biomass (g plant−1) | Increase | [107] | |
| Stem biomass (g plant−1) | Increase | |||
| Leaf biomass (g plant−1) | Increase | |||
| Aboveground biomass (g plant−1) | Increase | |||
| Total biomass(g plant−1) | Increase | |||
| Sunflower | Root biomass (g plant−1) | Decrease | ||
| Stem biomass (g plant−1) | Decrease | |||
| Leaf biomass (g plant−1) | Decrease | |||
| Aboveground biomass (g plant−1) | Decrease | |||
| Total biomass(g plant−1) | Decrease | |||
| Tomato | Fresh weight (kg) | Increase | [103] | |
| COMPOST | ||||
| Mustard | Grain yield (t ha−1) | Increase | [94] | |
| Straw yield (t ha−1) | Increase | |||
| Pearl millet | Yield (t ha−1) | Increase | ||
| Straw yield (t ha−1) | Increase | |||
| Tomato | Leaf length (cm plant−1) | Increase | [102] | |
| Leaf width (cm plant−1) | Increase | |||
| Chlorophyll | Increase | |||
| Chinese cabbage | Leaf length (cm plant−1) | Increase | ||
| Leaf width (cm plant−1) | Increase | |||
| Chlorophyll | Increase | |||
| Scot Pine | Root biomass production [g] | Increase | [31] | |
| Giant Miscanthus | Root biomass production [g] | Increase | ||
| Wheat | Grain yield | Increase | [108] | |
| Wheat (Triticum aestivum) | Yield (g/pot) CRI stage | Shoot | Increase | [104] |
| Root | Increase | |||
| Yield (g/pot) Maximum tillering stage | Shoot | Increase | ||
| Root | Increase | |||
| Yield (g/pot) Flowering stage | Shoot | Increase | ||
| Root | Increase | |||
| Yield (g/pot) Maturity stage | Grain | Increase | ||
| Shoot | Increase | |||
| Root | Increase | |||
| Winter wheat | Grain yield | Increase | [105] | |
| Lupin crops | Grain yield | Increase | ||
| Sorghum | Biomass yield | Increase | ||
5. Effect of Biowaste on Soil Organic Carbon Sequestration
5.1. Soil Organic Carbon Sequestration
5.2. Assessment Methods of Soil Organic Carbon Sequestration
Evaluation of the Effectiveness and Stability of Assessment Indicators and Modeling of the Degree of Organic Carbon Sequestration (SOC) of Soils
6. Conclusions and Research Perspective
Author Contributions
Funding
Conflicts of Interest
References
- He, Z.; Shentu, J.; Yang, X.; Baligar, V.; Zhang, T.; Stoffella, P. Heavy metal contamination of soils: Sources, indicators, and assessment. J. Environ. Indic. 2015, 9, 17–18. [Google Scholar]
- Różański, S.Ł.; Kwasowski, W.; Castejón, J.M.P.; Hardy, A. Heavy metal content and mobility in urban soils of public playgrounds and sport facility areas, Poland. Chemosphere 2018, 212, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 2016, 64, 56–74. [Google Scholar] [CrossRef]
- Kong, X. China must protect high-quality arable land. Nat. Cell Biol. 2014, 506, 7. [Google Scholar] [CrossRef] [PubMed]
- Radziemska, M. Study of applying naturally occurring mineral sorbents of Poland (dolomite halloysite, chalcedonite) for aided phytostabilization of soil polluted with heavy metals. Catena 2018, 163, 123–129. [Google Scholar] [CrossRef]
- European Parliament. Directive 1999/31/EC of 16/07/1999 on the Landfill of Waste. Off. J. 1991, 182, 1–19. [Google Scholar]
- Rozporządzenie Ministra Środowiska z dnia 11 maja 2015 r. w Sprawie Odzysku Odpadów Poza Instalacjami i Urządzeniami. (Regulation of the Minister of Environment dated 11 May 2015 in the Recovery of Waste Outside of Installations and Equipment). DzU 2015, poz. 796 z dnia 12 czerwca 2015 r. Available online: http://isap.sejm.gov.pl/DetailsServlet?id=WDU20150000796 (accessed on 28 May 2019).
- Grobelak, A.; Grosser, A.; Kacprzak, M.; Kamizela, T. Sewage sludge processing and management in small and medium-sized municipal wastewater treatment plant-new technical solution. J. Environ. Manag. 2019, 234, 90–96. [Google Scholar] [CrossRef]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Mininni, G.; Blanch, A.R.; Lucena, F.; Berselli, S. EU policy on sewage sludge utilization and perspectives on new approaches of sludge management. Environ. Sci. Pollut. Res. 2014, 22, 7361–7374. [Google Scholar] [CrossRef] [PubMed]
- NRMMC: National Resource Management Ministers Council. Guidelines for Sewage Sludge Systems—Biosolids Management. National Water Quality Management Strategy Paper 13; NRMMC: Canaberra, Australia, 2004.
- U.S. Environmental Protection Agency (USEPA). A plain English Guide to the EPA Part 503 Biosolids Rule. EPA832-R-93-003. 1994. Available online: https://www.epa.gov/sites/production/files/2018-12/documents/plain-english-guide-part503-biosolids-rule.pdf (accessed on 26 October 2020).
- Waste Management Rules. India. 2016. Available online: http://www.moef.gov.in/sites/default/files/SWM%202016.pdf (accessed on 26 October 2020).
- Cesaro, A.; Belgiorno, V.; Guida, M. Compost from organic solid waste: Quality assessment and European regulations for its sustainable use. Resour. Conserv. Recycl. 2015, 94, 72–79. [Google Scholar] [CrossRef]
- Eur-Lex. Commission Decision 2006/799/EC of 3 November 2006, Establishing Revised Ecological Criteria and the Related Assessment and Verification Requirements for the Award of the Community Eco-Label to Soil Improvers. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32006D0799 (accessed on 28 May 2019).
- Jędrczak, A. Composting and Fermentation of Biowaste—Advantages and Disadvantages of Processes. Civ. Environ. Eng. Rep. 2018, 28, 71–87. [Google Scholar] [CrossRef]
- ISWA. Circular Economy: Carbon, Nutrients and Soil. Report 4; ISWA. Available online: https://www.iswa.org/fileadmin/galleries/Task_Forces/Task_Force_Report_4.pdf (accessed on 26 October 2020).
- European Union. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC (Text with EEA Relevance). Off. J. Eur. Union 2009, 5, 39–85. [Google Scholar]
- Tóth, G.; Hermann, T.; Szatmári, G.; Pásztor, L. Maps of heavy metals in the soils of the European Union and proposed priority areas for detailed assessment. Sci. Total Environ. 2016, 565, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Nachtegaal, M.; Sparks, D.L. Speciation of Metals in Soils. In Chemical Processes in Soils; Tabatabai, M., Sparks, D., Eds.; Soil Science Society of America: Madison, WI, USA, 2005; Volume 8, pp. 619–654. [Google Scholar]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Almås, R.Å.; Singh, R.B. Trace Metal Contamination. In Encyclopedia of Soil Science, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 2364–2369. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 3, pp. 133–164. [Google Scholar] [CrossRef]
- Xu, Y.; Seshadri, B.; Sarkar, B.; Wang, H.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; Yang, X.; Bolan, N. Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci. Total Environ. 2018, 621, 148–159. [Google Scholar] [CrossRef]
- Hafeez, F.; Zafar, N.; Nazir, R.; Javeed, H.M.R.; Rizwan, M.; Faridullah; Asad, S.A.; Iqbal, A. Assessment of flood-induced changes in soil heavy metal and nutrient status in Rajanpur, Pakistan. Environ. Monit. Assess. 2019, 191, 234. [Google Scholar] [CrossRef]
- Lazo, P.; Steinnes, E.; Qarri, F.; Allajbeu, S.; Kane, S.; Stafilov, T.; Frontasyeva, M.V.; Harmens, H. Origin and spatial distribution of metals in moss samples in Albania: A hotspot of heavy metal contamination in Europe. Chemosphere 2018, 190, 337–349. [Google Scholar] [CrossRef]
- Song, B.; Niu, C.-G.; Gong, J.; Liang, J.; Xu, P.; Liu, Z.; Zhang, Y.; Zhang, C.; Cheng, M.; Liu, Y.; et al. Evaluation methods for assessing effectiveness of in situ remediation of soil and sediment contaminated with organic pollutants and heavy metals. Environ. Int. 2017, 105, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.; Ratering, S.; Felix-Henningsen, P.; Schnell, S. Stability of in situ immobilization of trace metals with different amendments revealed by microbial 13C-labelled wheat root decomposition and efflux-mediated metal resistance of soil bacteria. Sci. Total Environ. 2018, 659, 1082–1089. [Google Scholar] [CrossRef]
- Angin, I.; Aslantas, R.; Gunes, A.; Kose, M.; Ozkan, G. Effects of Sewage Sludge Amendment on Some Soil Properties, Growth, Yield and Nutrient Content of Raspberry (Rubus idaeus L.). Erwerbs-Obstbau 2016, 59, 93–99. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Placek, A.; Grobelak, A.; Hiller, J.; Stępień, W.; Jelonek, P.; Jaskulak, M.; Kacprzak, M. The Role of Organic and Inorganic Amendments in Carbon Sequestration and Immobilization of Heavy Metals in Degraded Soils. J. Sustain. Dev. Energy Water Environ. Syst. 2017, 5, 509–517. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, S.K.; Bahuguna, A.; Sharma, S.; Yadav, A. Assessment of effects of sewage-sludge, zinc, boron and sulphur application on concentration and uptake of nutrients by mustard. Int. J. Chem. Stud. 2020, 6, 363–367. [Google Scholar]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef]
- Singh, R.; Agrawal, M. Potential benefits and risks of land application of sewage sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Torri, S.; Corrêa, R.S.; Renella, G.; Corrê A, R.S. Soil Carbon Sequestration Resulting from Biosolids Application. Appl. Environ. Soil Sci. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Europe 2020. A Strategy for Smart, Sustainable and Inclusive Growth, 32Brussels: Communication from the Commission. European Commission, COM (2010 2020 Final 2). Available online: http://ec.europa.eu/growthandjobs/pdf/complet_en.pdf (accessed on 20 September 2020).
- Eurostats. GHG (Green House Gases) Emissions. Available online: https://ec.europa.eu/eurostat/web/products-datasets/-/med_en1 (accessed on 14 July 2019).
- Yang, Y.; Fang, J.; Tang, Y.; Ji, C.; Zheng, C.; He, J.; Zhu, B. Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Glob. Chang. Biol. 2008, 14, 1592–1599. [Google Scholar] [CrossRef]
- Kane, D. Carbon Sequestration Potential on Agricultural Lands: A Review of Current Science and Available Practices. Available online: https://sustainableagriculture.net/wp-content/uploads/2015/12/Soil_C_review_Kane_Dec_4-final-v4.pdf (accessed on 20 September 2020).
- Rumpel, C.; Chabbi, A.; Marschner, B. Carbon Storage and Sequestration in Subsoil Horizons: Knowledge, Gaps and Potentials. In Recarbonization of the Biospherel; Lal, R., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 445–464. [Google Scholar]
- Ghimire, R.; Lamichhane, S.; Acharya, B.S.; Bista, P.; Sainju, U.M. Tillage, crop residue, and nutrient management effects on soil organic carbon in rice-based cropping systems: A review. J. Integr. Agric. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Van Der Bliek, J.; McCornick, P.; Clarke, J. On Target for People and Planet: Setting and Achieving Water-Related Sustainable Development Goals. Water Intell. Online 2018, 17, 9781789060010. [Google Scholar] [CrossRef]
- Sewage Sludge Production and Disposal. Available online: https://ec.europa.eu/eurostat/web/products-datasets/product?code=env_ww_spd (accessed on 20 May 2019).
- Andriamananjara, A.; Rakotoson, T.; Razanakoto, O.; Razafimanantsoa, M.-P.; Rabeharisoa, L.; Smolders, E. Farmyard manure application in weathered upland soils of Madagascar sharply increase phosphate fertilizer use efficiency for upland rice. Field Crop. Res. 2018, 222, 94–100. [Google Scholar] [CrossRef]
- Vinodhini, V.; Das, N. Biowaste materials as sorbents to remove chromium (VI) from aqueous environment- a comparative study. ARPN J. Agric. Biol. Sci. 2009, 4, 19–23. [Google Scholar]
- Garau, G.; Porceddu, A.; Sanna, M.; Silvetti, M.; Castaldi, P. Municipal solid wastes as a resource for environmental recovery: Impact of water treatment residuals and compost on the microbial and biochemical features of As and trace metal-polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 445–454. [Google Scholar] [CrossRef]
- Soares, M.A.; Quina, M.J.; Quinta-Ferreira, R.M. Immobilisation of lead and zinc in contaminated soil using compost derived from industrial eggshell. J. Environ. Manag. 2015, 164, 137–145. [Google Scholar] [CrossRef]
- Fang, W.; Qi, G.; Wei, Y.; Kosson, D.S.; Van Der Sloot, H.A.; Liu, J. Leaching characteristic of toxic trace elements in soils amended by sewage sludge compost: A comparison of field and laboratory investigations. Environ. Pollut. 2018, 237, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Tsang, D.C.; Zhou, F.; Zhang, W.; Qiu, R. Stabilization of cationic and anionic metal species in contaminated soils using sludge-derived biochar. Chemosphere 2016, 149, 263–271. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Eurostats. Recycling of Biowaste. 2018. Available online: https://ec.europa.eu/eurostat/web/products-datasets/-/cei_wm030 (accessed on 25 June 2020).
- Mandal, S.; Kunhikrishnan, A.; Bolan, N.; Wijesekara, H.; Naidu, R. Application of Biochar Produced from Biowaste Materials for Environmental Protection and Sustainable Agriculture Production. In Environmental Materials and Waste: Resource Recovery and Pollution Prevention; Academic Press: Cambridge, MA, USA, 2016; pp. 73–89. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. J. Anal. Appl. Pyrolysis 2015, 112, 201–213. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.-Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Cieślik, B.M.; Namieśnik, J.; Konieczka, P. Review of sewage sludge management: Standards, regulations and analytical methods. J. Clean. Prod. 2015, 90, 1–15. [Google Scholar] [CrossRef]
- Bartkiewicz, B.; Pierścieniak, M. Management of biogas produce in the methane fermentation process in wastewater treatment plants. Ochr. Sr. Zasobów Nat. 2011, 47, 39. [Google Scholar]
- Šuňovská, A.; Horník, M.; Pipíška, M.; Lesný, J.; Augustín, J.; Hostin, S. Characterization of soil additive derived from sewage sludge. Nova Biotechnol. Chim. 2013, 12, 141–153. [Google Scholar] [CrossRef][Green Version]
- Kchaou, R.; Baccar, R.; Bouzid, J.; Rejeb, S. The impact of sewage sludge and compost on winter triticale. Environ. Sci. Pollut. Res. 2017, 25, 18314–18319. [Google Scholar] [CrossRef]
- Füleky, G.; Benedek, S. Composting to Recycle Biowaste. In Sociology, Organic Farming, Climate Change and Soil Science; Springer: Dordrecht, The Netherlands, 2010; Volume 3. [Google Scholar]
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef]
- Iqbal, M.K.; Shafiq, T.; Hussain, A.; Ahmed, K. Effect of enrichment on chemical properties of MSW compost. Bioresour. Technol. 2010, 101, 5969–5977. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.; Alburquerque, J.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Grosser, A.; Neczaj, E.; Singh, B.; Almås, Å.R.; Brattebø, H.; Kacprzak, M. Anaerobic digestion of sewage sludge with grease trap sludge and municipal solid waste as co-substrates. Environ. Res. 2017, 155, 249–260. [Google Scholar] [CrossRef]
- Pandey, A.K.; Gaind, S.; Ali, A.; Nain, L. Effect of bioaugmentation and nitrogen supplementation on composting of paddy straw. Biodegradation 2009, 20, 293–306. [Google Scholar] [CrossRef]
- Gaind, S. Effect of fungal consortium and animal manure amendments on phosphorus fractions of paddy-straw compost. Int. Biodeterior. Biodegrad. 2014, 94, 90–97. [Google Scholar] [CrossRef]
- Kalemelawa, F.; Nishihara, E.; Endo, T.; Ahmad, Z.; Yeasmin, R.; Tenywa, M.M.; Yamamoto, S. An evaluation of aerobic and anaerobic composting of banana peels treated with different inoculums for soil nutrient replenishment. Bioresour. Technol. 2012, 126, 375–382. [Google Scholar] [CrossRef]
- Domínguez, J. Relationships Between Composting and Vermicomposting. In Vermiculture Technology: Earthworms, Organic Wastes, and Environmental Management; Edwards, C.A., Arancon, N.Q., Sherman, R.L., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 11–26. [Google Scholar]
- Aksakal, E.L.; Sari, S.; Angin, I. Effects of Vermicompost Application on Soil Aggregation and Certain Physical Properties. Land Degrad. Dev. 2016, 27, 983–995. [Google Scholar] [CrossRef]
- Bang-Andreasen, T.; Nielsen, J.T.; Voriskova, J.; Heise, J.; Rønn, R.; Kjøller, R.; Hansen, H.C.B.; Jacobsen, C.S. Wood Ash Induced pH Changes Strongly Affect Soil Bacterial Numbers and Community Composition. Front. Microbiol. 2017, 8, 1400. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, A.; Nkana, J.V.; Verloo, M. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.; Patra, A.; Dwivedi, B. Changes in soil quality in response to short-term application of municipal sewage sludge in a typic haplustept under cowpea-wheat cropping system. Environ. Nanotechnol. Monit. Manag. 2015, 4, 37–41. [Google Scholar] [CrossRef]
- Navas, A.; Bermúdez, F.; Machín, J. Influence of sewage sludge application on physical and chemical properties of Gypsisols. Geoderma 1998, 87, 123–135. [Google Scholar] [CrossRef][Green Version]
- Roig, N.; Sierra, J.; Martí, E.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Long-term amendment of Spanish soils with sewage sludge: Effects on soil functioning. Agric. Ecosyst. Environ. 2012, 158, 41–48. [Google Scholar] [CrossRef]
- Méndez, A.; Gómez, A.; Paz-Ferreiro, J.; Gascó, G. Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 2012, 89, 1354–1359. [Google Scholar] [CrossRef]
- Grobelak, A.; Placek, A.; Grosser, A.; Singh, B.R.; Almås, Å.R.; Napora, A.; Kacprzak, M. Effects of single sewage sludge application on soil phytoremediation. J. Clean. Prod. 2017, 155, 189–197. [Google Scholar] [CrossRef]
- Mohamed, B.; Olivier, G.; François, G.; Laurence, A.-S.; Bourgeade, P.; Badr, A.-S.; Lotfi, A. Sewage sludge as a soil amendment in a Larix decidua plantation: Effects on tree growth and floristic diversity. Sci. Total Environ. 2018, 621, 291–301. [Google Scholar] [CrossRef]
- Urbaniak, M.; Wyrwicka, A.; Tołoczko, W.; Serwecińska, L.; Zieliński, M. The effect of sewage sludge application on soil properties and willow (Salix sp.) cultivation. Sci. Total Environ. 2017, 586, 66–75. [Google Scholar] [CrossRef]
- Aggelides, S.; Londra, P. Effects of compost produced from town wastes and sewage sludge on the physical properties of a loamy and a clay soil. Bioresour. Technol. 2000, 71, 253–259. [Google Scholar] [CrossRef]
- Oo, A.N.; Iwai, C.B.; Saenjan, P. Soil Properties and Maize Growth in Saline and Nonsaline Soils using Cassava-Industrial Waste Compost and Vermicompost with or Without Earthworms. Land Degrad. Dev. 2013, 26, 300–310. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Muirhead, B.; Wright, G.; Bird, M.I. Biochar and biochar-compost as soil amendments: Effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric. Ecosyst. Environ. 2015, 213, 72–85. [Google Scholar] [CrossRef]
- Fang, W.; Wei, Y.; Kosson, D.S. Comparative characterization of sewage sludge compost and soil: Heavy metal leaching characteristics. J. Hazard. Mater. 2016, 310, 1–10. [Google Scholar] [CrossRef]
- Brunetti, G.; Polo, A.; Plaza, C.; Senesi, N. Effects of sewage sludge amendment on humic acids and microbiological properties of a semiarid Mediterranean soil. Biol. Fertil. Soils 2004, 39, 320–328. [Google Scholar] [CrossRef]
- Yazdanpanah, N.; Mahmoodabadi, M.; Cerdà, A. The impact of organic amendments on soil hydrology, structure and microbial respiration in semiarid lands. Geoderma 2016, 266, 58–65. [Google Scholar] [CrossRef]
- Pérez-Piqueres, A.; Edel-Hermann, V.; Alabouvette, C.; Steinberg, C. Response of soil microbial communities to compost amendments. Soil Biol. Biochem. 2006, 38, 460–470. [Google Scholar] [CrossRef]
- Bailey, K.; Lazarovits, G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res. 2003, 72, 169–180. [Google Scholar] [CrossRef]
- Santos, E.S.; Magalhães, M.C.F.; Abreu, M.M.; Macías, F. Effects of organic/inorganic amendments on trace elements dispersion by leachates from sulfide-containing tailings of the São Domingos mine, Portugal. Time evaluation. Geoderma 2014, 226, 188–203. [Google Scholar] [CrossRef][Green Version]
- Hattab, N.; Motelica-Heino, M.; Faure, O.; Bouchardon, J.-L. Effect of fresh and mature organic amendments on the phytoremediation of technosols contaminated with high concentrations of trace elements. J. Environ. Manag. 2015, 159, 37–47. [Google Scholar] [CrossRef]
- Jaskulak, M.; Rorat, A.; Grobelak, A.; Kacprzak, M. Antioxidative enzymes and expression of rbcL gene as tools to monitor heavy metal-related stress in plants. J. Environ. Manag. 2018, 218, 71–78. [Google Scholar] [CrossRef]
- Lukić, B.; Panico, A.; Huguenot, D.; Fabbricino, M.; Van Hullebusch, E.D.; Esposito, G. A review on the efficiency of landfarming integrated with composting as a soil remediation treatment. Environ. Technol. Rev. 2017, 6, 94–116. [Google Scholar] [CrossRef]
- Adenuga, A.O.; Johnson, J.H.; Cannon, J.N.; Wan, L. Bioremediation of PAH-Contaminated Soil via In-Vessel Composting. Water Sci. Technol. 1992, 26, 2331–2334. [Google Scholar] [CrossRef]
- Lukić, B.; Huguenot, D.; Panico, A.; Fabbricino, M.; Van Hullebusch, E.D.; Esposito, G. Importance of organic amendment characteristics on bioremediation of PAH-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 15041–15052. [Google Scholar] [CrossRef]
- Moreno, J.L.; Hernández, T.; Garcia, C. Effects of a cadmium-contaminated sewage sludge compost on dynamics of organic matter and microbial activity in an arid soil. Biol. Fertil. Soils 1999, 28, 230–237. [Google Scholar] [CrossRef]
- Meena, M.D.; Joshi, P.K.; Narjary, B.; Sheoran, P.; Jat, H.S.; Chinchmalatpure, A.R.; Yadav, R.K.; Sharma, D.K. Effects of municipal solid waste compost, rice-straw compost and mineral fertilisers on biological and chemical properties of a saline soil and yields in a mustard–pearl millet cropping system. Soil Res. 2016, 54, 958–969. [Google Scholar] [CrossRef]
- Giusquiani, P.L.; Pagliai, M.; Gigliotti, G.; Businelli, D.; Benetti, A. Urban Waste Compost: Effects on Physical, Chemical, and Biochemical Soil Properties. J. Environ. Qual. 1995, 24, 175–182. [Google Scholar] [CrossRef]
- Foley, B.J.; Cooperband, L.R. Paper Mill Residuals and Compost Effects on Soil Carbon and Physical Properties. J. Environ. Qual. 2002, 31, 2086–2095. [Google Scholar] [CrossRef]
- Bitew, Y.; Alemayehu, M. Impact of Crop Production Inputs on Soil Health: A Review. Asian J. Plant Sci. 2017, 16, 109–131. [Google Scholar] [CrossRef]
- Hue, N.V.; Ranjith, S.A. Sewage sludges in Hawaii: Chemical composition and reactions with soils and plants. Water Air Soil Pollut. 1994, 72, 265–283. [Google Scholar] [CrossRef]
- Vaca, R.; Lugo, J.; Martinez, R.; Esteller, M.V.; Zavaleta, H. Effects of sewage sludge and sewage sludge compost amendment on soil properties and Zea mays L. plants (heavy metals, quality and productivity). Rev. Int. Contam. Ambient. 2011, 27, 303–311. [Google Scholar]
- Singh, R.; Agrawal, M. Effect of different sewage sludge applications on growth and yield of Vigna radiata L. field crop: Metal uptake by plant. Ecol. Eng. 2010, 36, 969–972. [Google Scholar] [CrossRef]
- Lakhdar, A.; Iannelli, M.A.; Debez, A.; Massacci, A.; Jedidi, N.; Abdelly, C. Effect of municipal solid waste compost and sewage sludge use on wheat (Triticum durum): Growth, heavy metal accumulation, and antioxidant activity. J. Sci. Food Agric. 2010, 90, 965–971. [Google Scholar] [CrossRef]
- Yoo, J.H.; Lee, Y.D.; Hussein, K.A.; Joo, J.H. The Effect of Food Waste Compost on Chinese Cabbage (Brassica rapa var. glabra) and Tomato (Solanum lycopersicum L.) Growth. Korean. J. Soil Sci. Fertil. 2018, 51, 596–607. [Google Scholar] [CrossRef]
- Waqas, M.; Li, G.; Khan, S.; Shamshad, I.; Reid, B.J.; Qamar, Z.; Chao, C. Application of sewage sludge and sewage sludge biochar to reduce polycyclic aromatic hydrocarbons (PAH) and potentially toxic elements (PTE) accumulation in tomato. Environ. Sci. Pollut. Res. 2015, 22, 12114–12123. [Google Scholar] [CrossRef] [PubMed]
- Nishanth, D.; Biswas, D. Kinetics of phosphorus and potassium release from rock phosphate and waste mica enriched compost and their effect on yield and nutrient uptake by wheat (Triticum aestivum). Bioresour. Technol. 2008, 99, 3342–3353. [Google Scholar] [CrossRef]
- Hall, D.J.; Bell, R.W. Biochar and Compost Increase Crop Yields but the Effect is Short Term on Sandplain Soils of Western Australia. Pedosphere 2015, 25, 720–728. [Google Scholar] [CrossRef]
- Kacprzak, M.; Grobelak, A.; Grosser, A.; Prasad, M.N.V. Efficacy of Biosolids in Assisted Phytostabilization of Metalliferous Acidic Sandy Soils with Five Grass Species. Int. J. Phytoremediation 2013, 16, 593–608. [Google Scholar] [CrossRef]
- Nissim, W.G.; Cincinelli, A.; Martellini, T.; Alvisi, L.; Palm, E.; Mancuso, S.; Azzarello, E. Phytoremediation of sewage sludge contaminated by trace elements and organic compounds. Environ. Res. 2018, 164, 356–366. [Google Scholar] [CrossRef]
- Elrahman, S.H.A.; Mostafa, M.; Taha, T.; ElSharawy, M.; Eid, M. Effect of different amendments on soil chemical characteristics, grain yield and elemental content of wheat plants grown on salt-affected soil irrigated with low quality water. Ann. Agric. Sci. 2012, 57, 175–182. [Google Scholar] [CrossRef]
- Bernal, M.P.; Clemente, R.; Walker, D.J. The Role of Organic Amendments in the Bio-Remediation of Heavy Metal-Polluted Soils. In Environmental Research at the Leading Edge; Gore, R.W., Ed.; Nova Science Pub Inc: New York, NY, USA, 2007; pp. 1–57. [Google Scholar]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, S.C.; Baidoo, S.K.; Chander, Y.; Rosen, C.J. Antibiotic Uptake by Plants from Soil Fertilized with Animal Manure. J. Environ. Qual. 2005, 34, 2082–2085. [Google Scholar] [CrossRef]
- Hoitink, H.A.J.; Kuter, G.A. Effects of Composts in Growth Media on Soilborne Pathogens. In The Role of Organic Matter in Modern Agriculture; Martinus Nijhoff Publishers: Leiden, The Netherlands, 2011; pp. 289–306. [Google Scholar] [CrossRef]
- Ontl, T.A.; Schulte, L.A. Soil Carbon Storage. Nat. Educ. Knowl. 2012, 3, 35. [Google Scholar]
- FAO. Soil Erosion: The Greatest Challenge to Sustainable Soil Management; FAO: Rome, Italy, 2019; p. 100. [Google Scholar]
- Schimel, D.; Braswell, B.H.; Holland, E.A.; McKeown, R.; Ojima, D.S.; Painter, T.H.; Parton, W.J.; Townsend, A.R. Climatic, edaphic, and biotic controls over storage and turnover of carbon in soils. Glob. Biogeochem. Cycles 1994, 8, 279–293. [Google Scholar] [CrossRef]
- De Brogniez, D.; Ballabio, C.; Stevens, A.; Jones, R.J.A.; Montanarella, L.; Van Wesemael, B. A map of the topsoil organic carbon content of Europe generated by a generalized additive model. Eur. J. Soil Sci. 2014, 66, 121–134. [Google Scholar] [CrossRef]
- FAO. Soil Organic Carbon; Yigini, Y., Olmedo, G.F., Reiter, S., Baritz, R., Viatkin, K., Vargas, R.R., Eds.; FAO: Rome, Italy, 2018. [Google Scholar]
- Ercoli, L.; Mariotti, M.; Masoni, A.; Bonari, E. Effect of irrigation and nitrogen fertilization on biomass yield and efficiency of energy use in crop production of Miscanthus. Field Crop. Res. 1999, 63, 3–11. [Google Scholar] [CrossRef]
- Angelini, L.G.; Ceccarini, L.; Bonari, E. Biomass yield and energy balance of giant reed (Arundo donax L.) cropped in central Italy as related to different management practices. Eur. J. Agron. 2005, 22, 375–389. [Google Scholar] [CrossRef]
- Hemmat, A.; Aghilinategh, N.; Rezainejad, Y.; Sadeghi, M. Long-term impacts of municipal solid waste compost, sewage sludge and farmyard manure application on organic carbon, bulk density and consistency limits of a calcareous soil in central Iran. Soil Tillage Res. 2010, 108, 43–50. [Google Scholar] [CrossRef]
- Kätterer, T.; Börjesson, G.; Kirchmann, H. Changes in organic carbon in topsoil and subsoil and microbial community composition caused by repeated additions of organic amendments and N fertilisation in a long-term field experiment in Sweden. Agric. Ecosyst. Environ. 2014, 189, 110–118. [Google Scholar] [CrossRef]
- Soriano-Disla, J.; Pedreno, J.N.; Gómez, I. Contribution of a sewage sludge application to the short-term carbon sequestration across a wide range of agricultural soils. Environ. Earth Sci. 2010, 61, 1613–1619. [Google Scholar] [CrossRef]
- Brian, B.; Richard, B.; David, C.; Dennis, D.; Stephen, F.; William, F.; Gleason, R.; Hawbaker, T.; Liu, J.; Shuguang, L.; et al. A Method for Assessing Carbon Stocks, Carbon Sequestration, and Greenhouse-Gas Fluxes in Ecosystems of the United States under Present Conditions and Future Scenarios; Zhiliang, Z., Ed.; U.S. Geological Survey Scientific Investigations Report 2010–5233; Supersedes Open-File Report2010–1144 2010; USGS: Reston, VA, USA, 2010; p. 188. Available online: http://pubs.usgs.gov/sir/2010/5233/ (accessed on 20 May 2020).
- Nayak, A.; Rahman, M.M.; Naidu, R.; Dhal, B.; Swain, C.; Tripathi, R.; Shahid, M.; Islam, M.R.; Pathak, H. Current and emerging methodologies for estimating carbon sequestration in agricultural soils: A review. Sci. Total Environ. 2019, 665, 890–912. [Google Scholar] [CrossRef]
- Goulden, M.L.; Munger, J.W.; Fan, S.-M.; Daube, B.C.; Wofsy, S.C. Measurements of carbon sequestration by long-term eddy covariance: Methods and a critical evaluation of accuracy. Glob. Chang. Biol. 1996, 2, 169–182. [Google Scholar] [CrossRef]
- Xu, M.G.; Wang, J.Z.; Lu, C.A. Soil Organic Carbon Sequestration Under Long-Term Manure and Straw Fertilization in North and Northeast China by Roth C Model Simulation. In Functions of Natural Organic Matter in Changing Environment; Xu, J., Ed.; Zhejiang University Press: Zhejiang, China; Springer Science & Business Media: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-5634-2. [Google Scholar]
- Barančíková, G.; Halas, J.; Gutteková, M.; Makovníková, J.; Novakova, M.; Skalský, R.; Tarasovičová, Z. Application of RothC model to predict soil organic carbon stock on agricultural soils of Slovakia. Soil Water Res. 2010, 5, 1–9. [Google Scholar] [CrossRef]
- Smith, J.; Smith, P.; Wattenbach, M.; Gottschalk, P.; Romanenkov, V.; Shevtsova, L.K.; Sirotenko, O.D.; Rukhovich, D.I.; Koroleva, P.V.; Romanenko, I.A.; et al. Projected changes in the organic carbon stocks of cropland mineral soils of European Russia and the Ukraine, 1990–2070. Glob. Chang. Biol. 2007, 13, 342–356. [Google Scholar] [CrossRef]
- Easter, M.; Paustian, K.; Killian, K.; Williams, S.; Feng, T.; Al-Adamat, R.; Batjes, N.H.; Bernoux, M.; Bhattacharyya, T.; Cerri, C.; et al. The GEFSOC soil carbon modelling system: A tool for conducting regional-scale soil carbon inventories and assessing the impacts of land use change on soil carbon. Agric. Ecosyst. Environ. 2007, 122, 13–25. [Google Scholar] [CrossRef]
- Shrestha, B.M.; Williams, S.; Easter, M.; Paustian, K.; Singh, B.R. Modeling soil organic carbon dynamics in a mountain watershed in Nepal watershed. Ecosyst. Environ. 2009, 132, 91–97. [Google Scholar] [CrossRef]
- Coleman, K.; Jenkinson, D.S. RothC-26.3—A Model for the turnover of carbon in soil. Eval. Soil Org. Matter Models 1996, I, 237–246. [Google Scholar] [CrossRef]
- FAO. Proceedings of the Global Symposium on Soil Organic Carbon 2017; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Farina, R.; Coleman, K.; Whitmore, A. Modification of the RothC model for simulations of soil organic C dynamics in dryland regions. Geoderma 2013, 200, 18–30. [Google Scholar] [CrossRef]
- Ludwig, B.; Hu, K.L.; Niu, L.A.; Liu, X.J. Modelling the dynamics of organic carbon in fertilization and tillage experiments in the North China Plain using the Rothamsted Carbon Model—Initialization and calculation of C inputs. Plant Soil 2010, 332, 193–206. [Google Scholar] [CrossRef]
- Kamoni, P.; Gicheru, P.; Wokabi, S.; Easter, M.; Milne, E.; Coleman, K.; Falloon, P.D.; Paustian, K.; Killian, K.; Kihanda, F. Evaluation of two soil carbon models using two Kenyan long term experimental datasets. Agric. Ecosyst. Environ. 2007, 122, 95–104. [Google Scholar] [CrossRef]
- Mondini, C.; Cayuela, M.L.; Sinicco, T.; Fornasier, F.; Galvez, A.; Sánchez-Monedero, M.A. Soil C Storage Potential of Exogenous Organic Matter at Regional Level (Italy) Under Climate Change Simulated by RothC Model Modified for Amended Soils. Front. Environ. Sci. 2018, 6. [Google Scholar] [CrossRef]
- Mondini, C.; Cayuela, M.L.; Sinicco, T.; Fornasier, F.; Galvez, A.; Sánchez-Monedero, M.A. Modification of the RothC model to simulate soil C mineralization of exogenous organic matter. Biogeosciences 2017, 14, 3253–3274. [Google Scholar] [CrossRef]
- Franko, U. Modellierung des umsatzes der organischen bodensubstanz. Arch. Agron. Soil Sci. 1997, 41, 527–547. [Google Scholar] [CrossRef]
- Bolinder, M.; Janzen, H.; Gregorich, E.; Angers, D.; Van den Bygaart, A. An approach for estimating net primary productivity and annual carbon inputs to soil for common agricultural crops in Canada. Agric. Ecosyst. Environ. 2007, 118, 29–42. [Google Scholar] [CrossRef]
- Grunwald, S. Current State of Digital Soil Mapping and What is Next. In Digital Soil Mapping. Progress in Soil Science; Boettinger, J.L., Howell, D.W., Moore, A.C., Hartemink, A.E., Kienast-Brown, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 2. [Google Scholar] [CrossRef]
- Dimassi, B.; Guenet, B.; Saby, N.; Muñoz, F.; Bardy, M.; Millet, F.; Martin, M.P. The impacts of CENTURY model initialization scenarios on soil organic carbon dynamics simulation in French long-term experiments. Geoderma 2018, 311, 25–36. [Google Scholar] [CrossRef]
- Qian, Y.L.; Bandaranayake, W.; Parton, W.J.; Mecham, B.; Harivandi, M.A.; Mosier, A.R. Long-Term Effects of Clipping and Nitrogen Management in Turfgrass on Soil Organic Carbon and Nitrogen Dynamics. J. Environ. Qual. 2003, 32, 1694–1700. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.E.; Minasny, B.; McBratney, A.B.; Courcelles, V.D.R.D.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
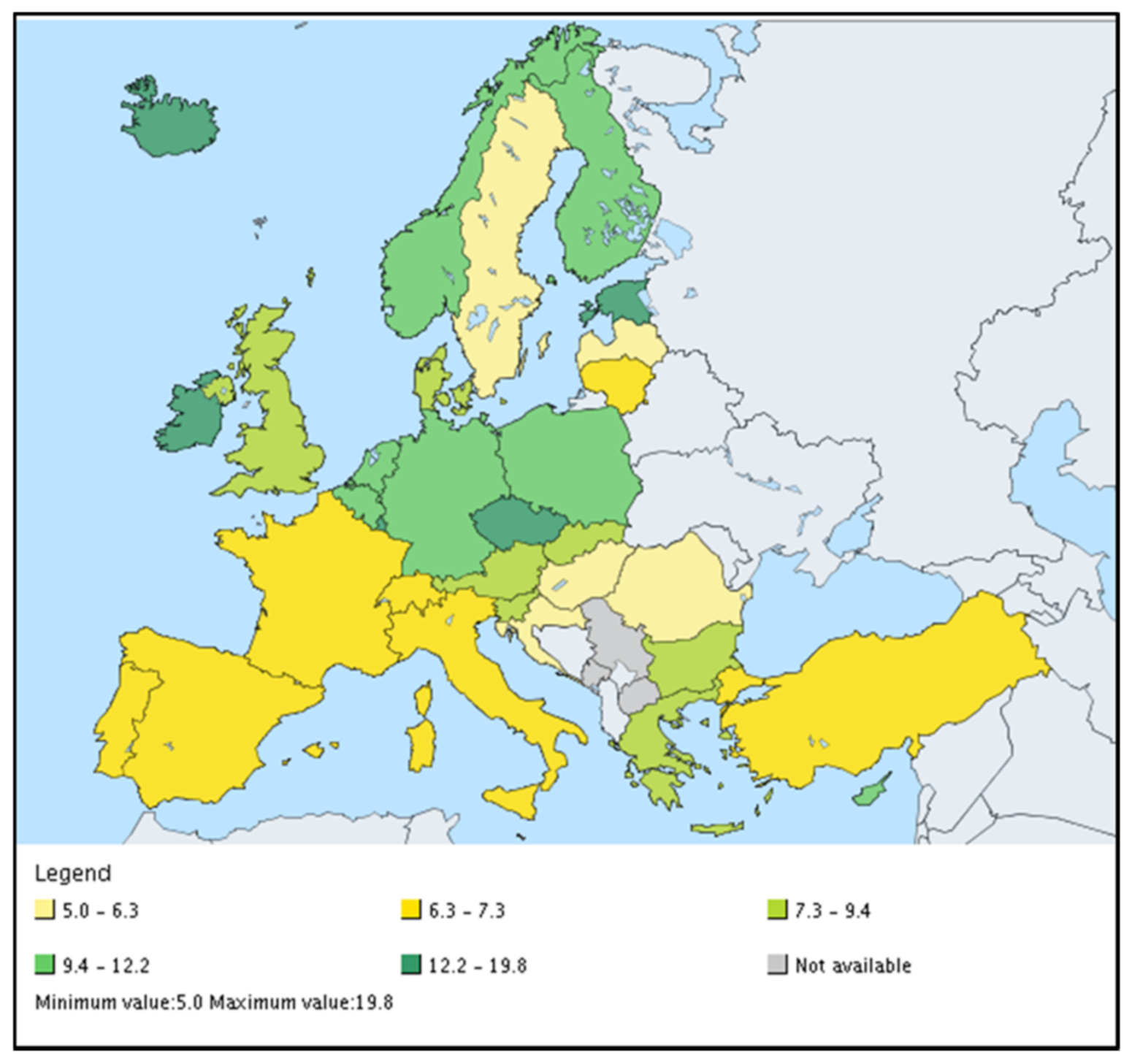
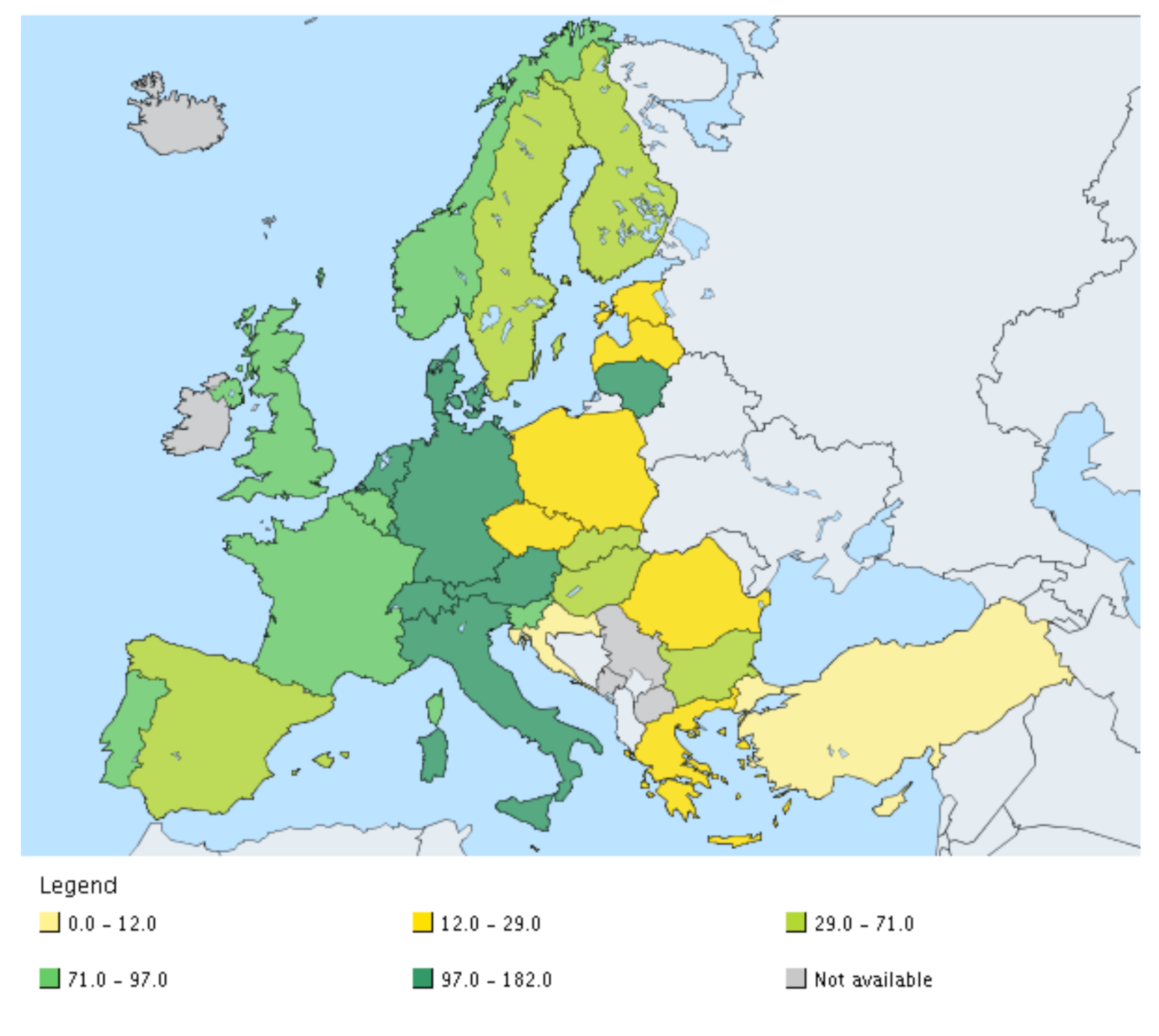
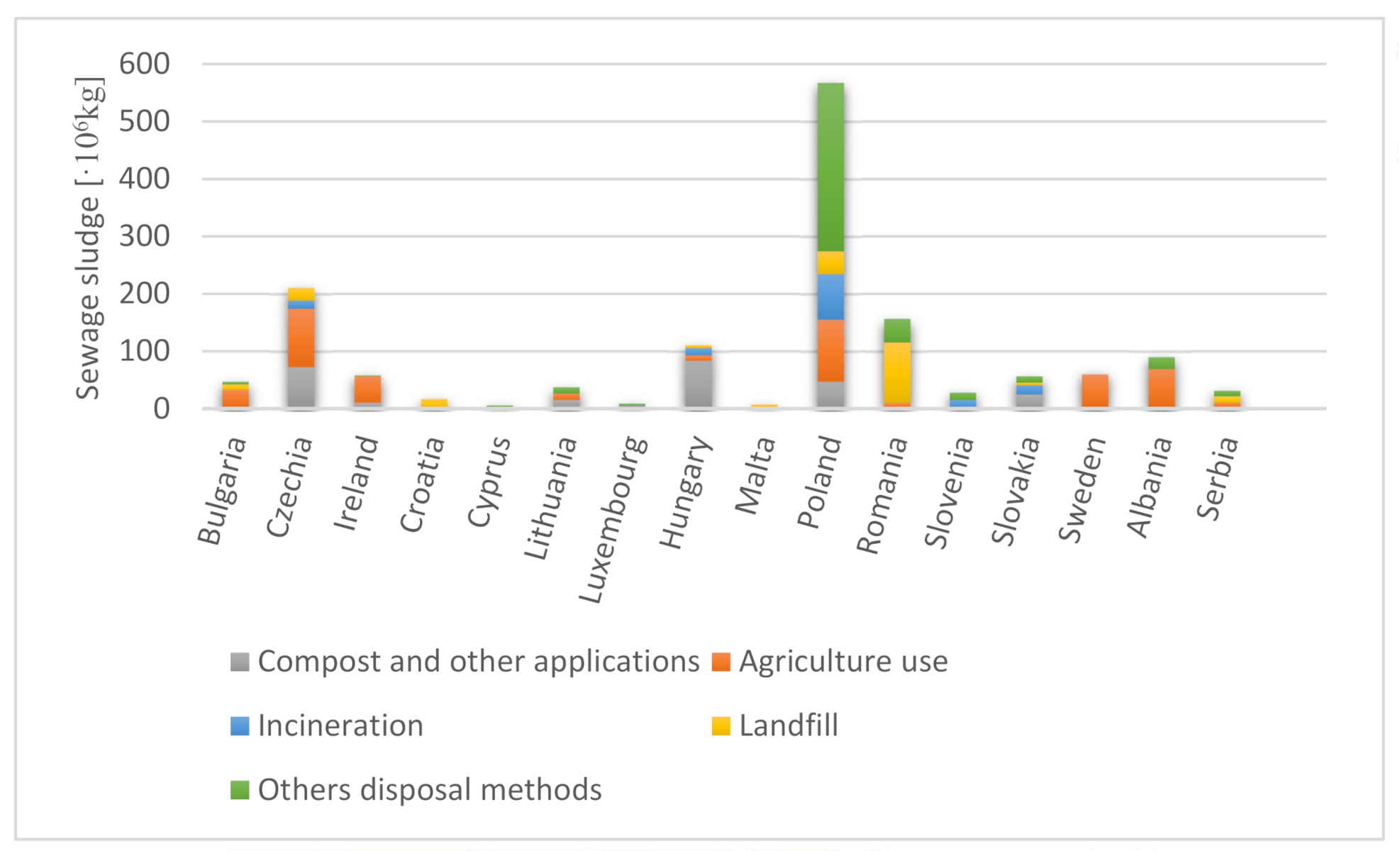
| Norm/Country | Cd | Cu | Hg | Ni | Pb | Zn | Cr | As | Co | Se |
|---|---|---|---|---|---|---|---|---|---|---|
| Directive 86/278/EEC | 20–40 | 1000–1750 | 16–25 | 300–400 | 750–1200 | 2500–4000 | ||||
| Czech republic | 5 | 500 | 4 | 100 | 200 | 2500 | 200 | 30 | ||
| Denmark | 0.8 | 1000 | 0.8 | 30 | 120 | 4000 | 100 | 25 | ||
| Finland | 3 | 600 | 2 | 100 | 150 | 1500 | 300 | |||
| France | 20 | 1000 | 10 | 200 | 800 | 3000 | 1000 | |||
| Germany (proposed new limits) | 2 | 600 | 1.4 | 60 | 100 | 1500 | 80 | |||
| Hungary | 10 | 1000 | 10 | 200 | 750 | 2500 | 1000-1 (Cr VI) | 75 | 50 | 100 |
| Luxemburg | 20–40 | 1000–1750 | 15–25 | 300–400 | 750–1200 | 2500–4000 | 1000–1750 | |||
| Netherlands | 1.25 | 75 | 0.75 | 30 | 100 | 300 | 75 | 15 | ||
| Poland | 10 | 800 | 5 | 100 | 500 | 2500 | 500 | |||
| Portugal | 20 | 1000 | 16 | 300 | 750 | 2500 | 1000 | |||
| Sweden | 2 | 300 | 2 | 70 | 100 | 1200 | 100 | |||
| Spain | 40 | 1750 | 25 | 400 | 1200 | 4000 | 1500 | |||
| Range in Europe | 0.5–40 | 75–1750 | 0.2–25 | 30–400 | 40–1200 | 100–4000 | ||||
| Australia | 1 | 100–200 | 1 | 60 | 150–300 | 200–250 | 100–400 | 20 | 3 | |
| United States | 85 | 4300 | 57 | 420 | 840 | 7500 | 3000 | 75 | 100 | |
| Mexico | 85 | 4300 | 57 | 420 | 840 | 7500 | 3000 | 75 | 100 | |
| China | 5–20 | 800–1500 | 5–15 | 100–200 | 300–1000 | 2000–3000 | 75 | |||
| Japan | 5 | 2 | 300 | 100 | 50 | |||||
| Russia | 15 | 750 | 7.5 | 200 | 250 | 1750 | 10 | |||
| India | 5 | 300 | 0.15 | 100 | 1000 | 10 |
| Organic Additive | Soil Properties | Effect | Reference |
|---|---|---|---|
| Sewage sludge | pH | In H2O Decrease | [76] |
| In KCl Increase | |||
| Decrease | [29,75] | ||
| Increase | [77] | ||
| Humic acids | Increase | [76,78] | |
| Organic matter | Increase | [30] | |
| Dissolved organic carbon | Increase | [74] | |
| Cation-exchange capacity | Increase | [30] | |
| Total organic carbon | Increase | [76,78,79] | |
| N Kjeldhal | Decrease | [76] | |
| Increase | [30,77] | ||
| Ntotal | Increase | [74] | |
| NO3-N | Increase | ||
| P, K, Fe | Increase | [30] | |
| Compost | Organic matter | Increase | [79] |
| CaCO3 | Increase | ||
| pH | Increase | ||
| Decrease | [80] | ||
| Cation-exchange capacity | Increase | [79,80] | |
| Soil bulk density | Increase | [81] | |
| Decrease | [79] | ||
| Soil water content | Increase | [81] | |
| Humic substances | Increase | [50] | |
| Electron conductivity | Increase | [50,80] | |
| Dissolved organic carbon | Increase | [50] | |
| Soil organic carbon | Increase | [80] | |
| Total organic carbon | Increase | [50] | |
| C:N ratio | Increase | [81] | |
| P | Decrease | ||
| NH4-N | Decrease | ||
| NO3-N | Increase |
| Metals | Heavy Metal Uptake (µg plant−1d−1) | Translocation Factor | ||||
|---|---|---|---|---|---|---|
| Unamended Soil | 20% Sewage Sludge Amendment | 40% Sewage Sludge Amendment | Unamended Soil | 20% Sewage Sludge Amendment | 40% Sewage Sludge Amendment | |
| Ni | 0.10 c | 0.16 b | 0.31 a | 0.14 c | 0.89 a | 0.73 b |
| Cd | 0.04 b | 1.34 a | 1.37 a | 0.96 a | 0.78 b | 0.91 a |
| Cu | 0.80 c | 1.17 b | 1.66 a | 1.23 b | 1.5 a | 0.46 c |
| Cr | 0.19 c | 0.28 b | 0.32 a | 0.32 a | 0.34 b | 0.29 b |
| Pb | 0.08 c | 0.16 a | 0.12 b | 0.92 a | 0.40 b | 0.60 b |
| Zn | 2.15 c | 5.88 b | 6.90 a | 0.83 a | 0.58 b | 0.35 c |
| Mn | 3.18 a | 2.00 b | 1.41 c | 0.99 a | 0.90 b | 0.67 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, A.; Grobelak, A.; Almås, Å.R.; Singh, B.R. Effect of Biowastes on Soil Remediation, Plant Productivity and Soil Organic Carbon Sequestration: A Review. Energies 2020, 13, 5813. https://doi.org/10.3390/en13215813
Kowalska A, Grobelak A, Almås ÅR, Singh BR. Effect of Biowastes on Soil Remediation, Plant Productivity and Soil Organic Carbon Sequestration: A Review. Energies. 2020; 13(21):5813. https://doi.org/10.3390/en13215813
Chicago/Turabian StyleKowalska, Aneta, Anna Grobelak, Åsgeir R. Almås, and Bal Ram Singh. 2020. "Effect of Biowastes on Soil Remediation, Plant Productivity and Soil Organic Carbon Sequestration: A Review" Energies 13, no. 21: 5813. https://doi.org/10.3390/en13215813
APA StyleKowalska, A., Grobelak, A., Almås, Å. R., & Singh, B. R. (2020). Effect of Biowastes on Soil Remediation, Plant Productivity and Soil Organic Carbon Sequestration: A Review. Energies, 13(21), 5813. https://doi.org/10.3390/en13215813






