Processing of Water Treatment Sludge by Bioleaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Inoculum Preparation
2.3. Bioleaching Experiments
2.4. Analysis
2.5. Statistical Analysis
3. Results and Discussion
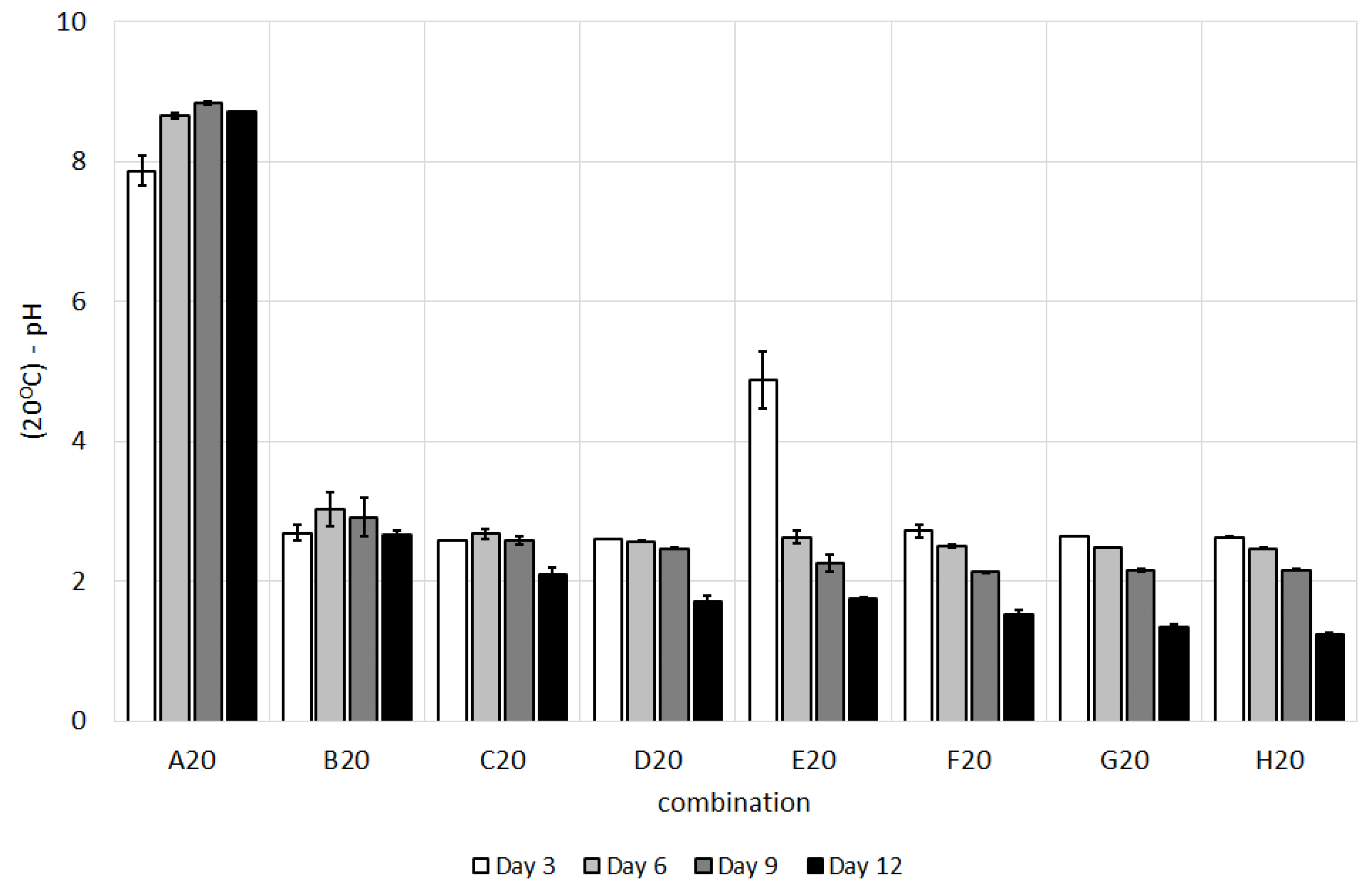
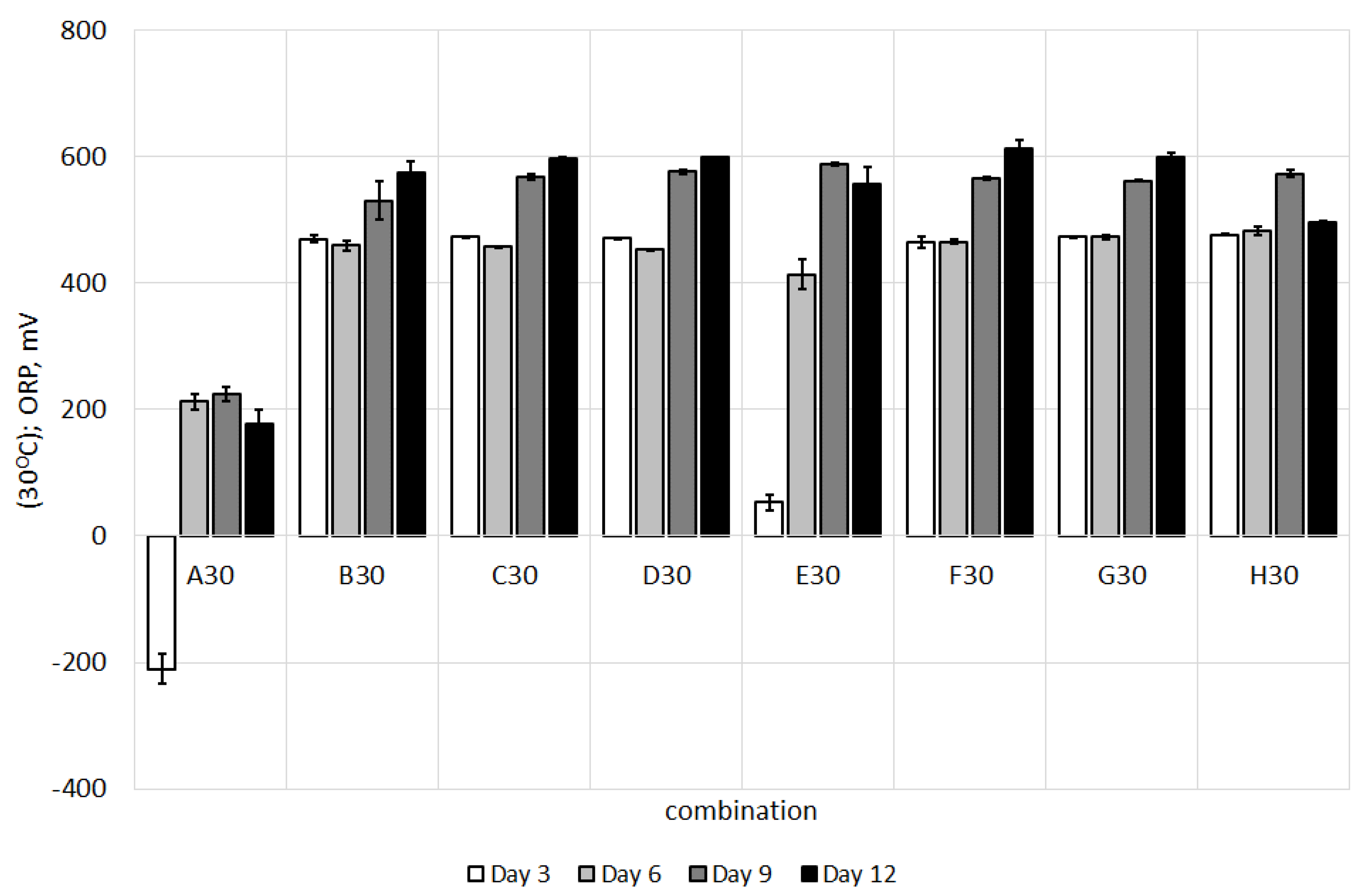
3.1. Changes in pH and Oxidation-Reduction Potential during Study
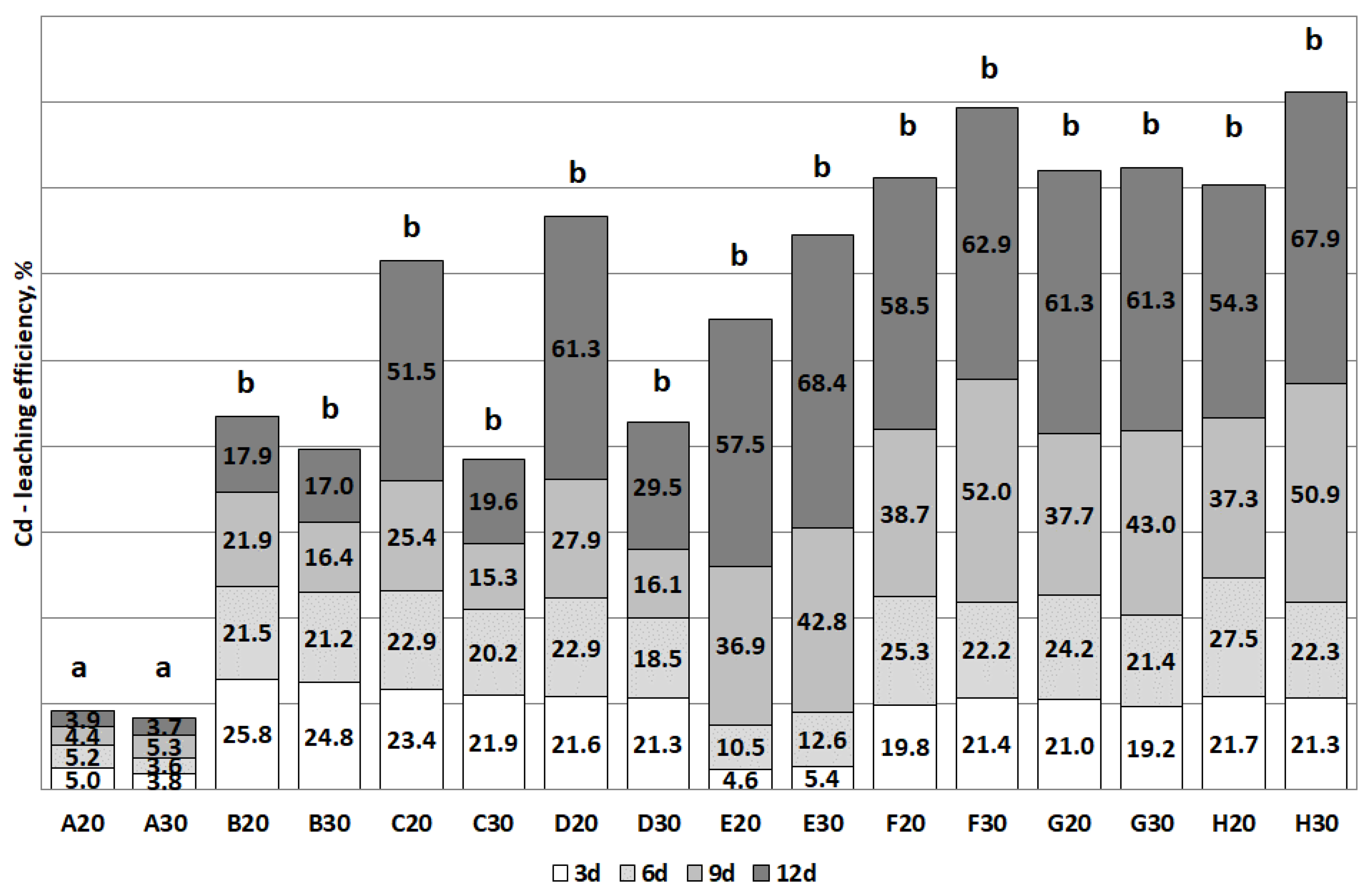
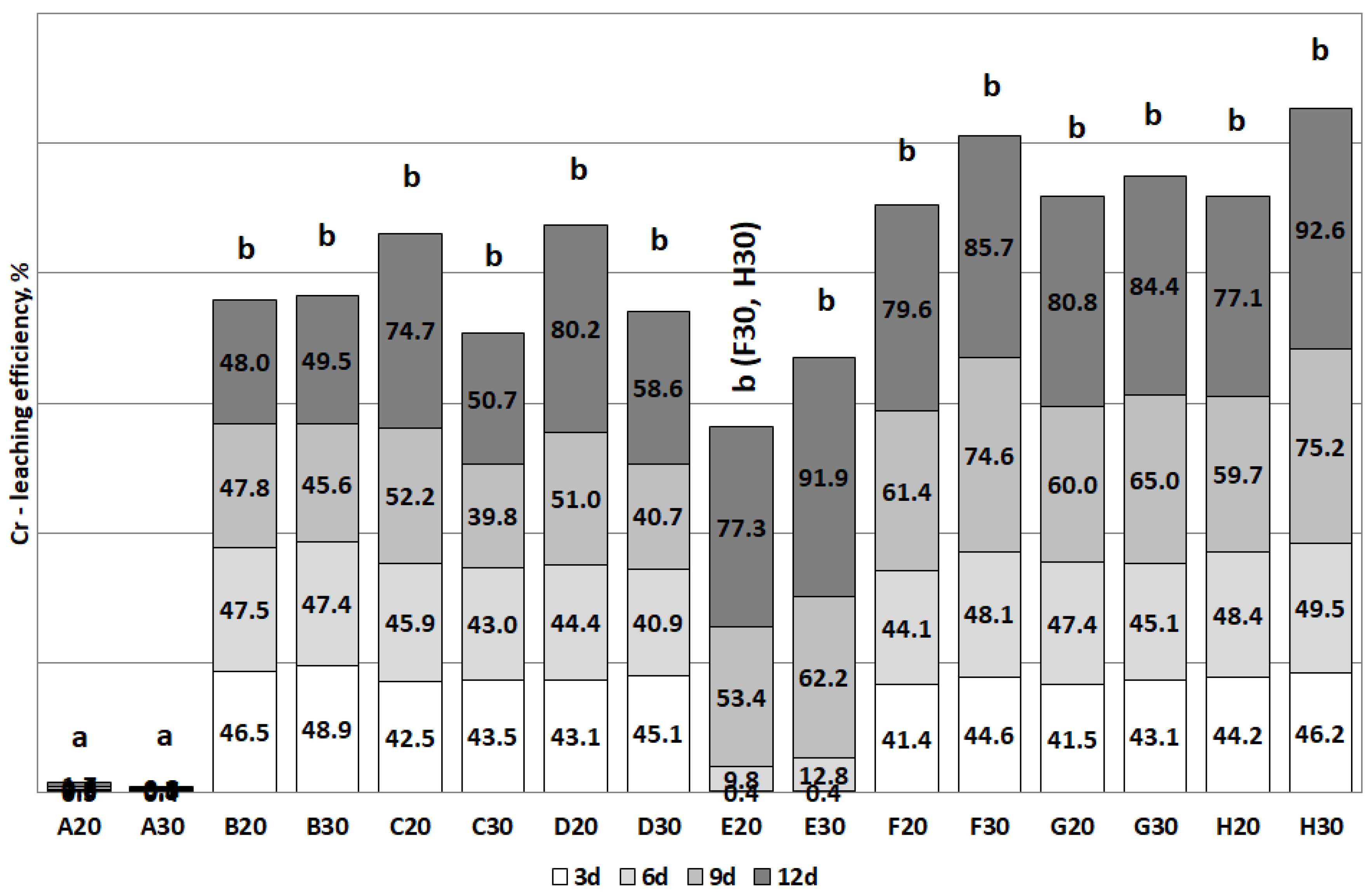
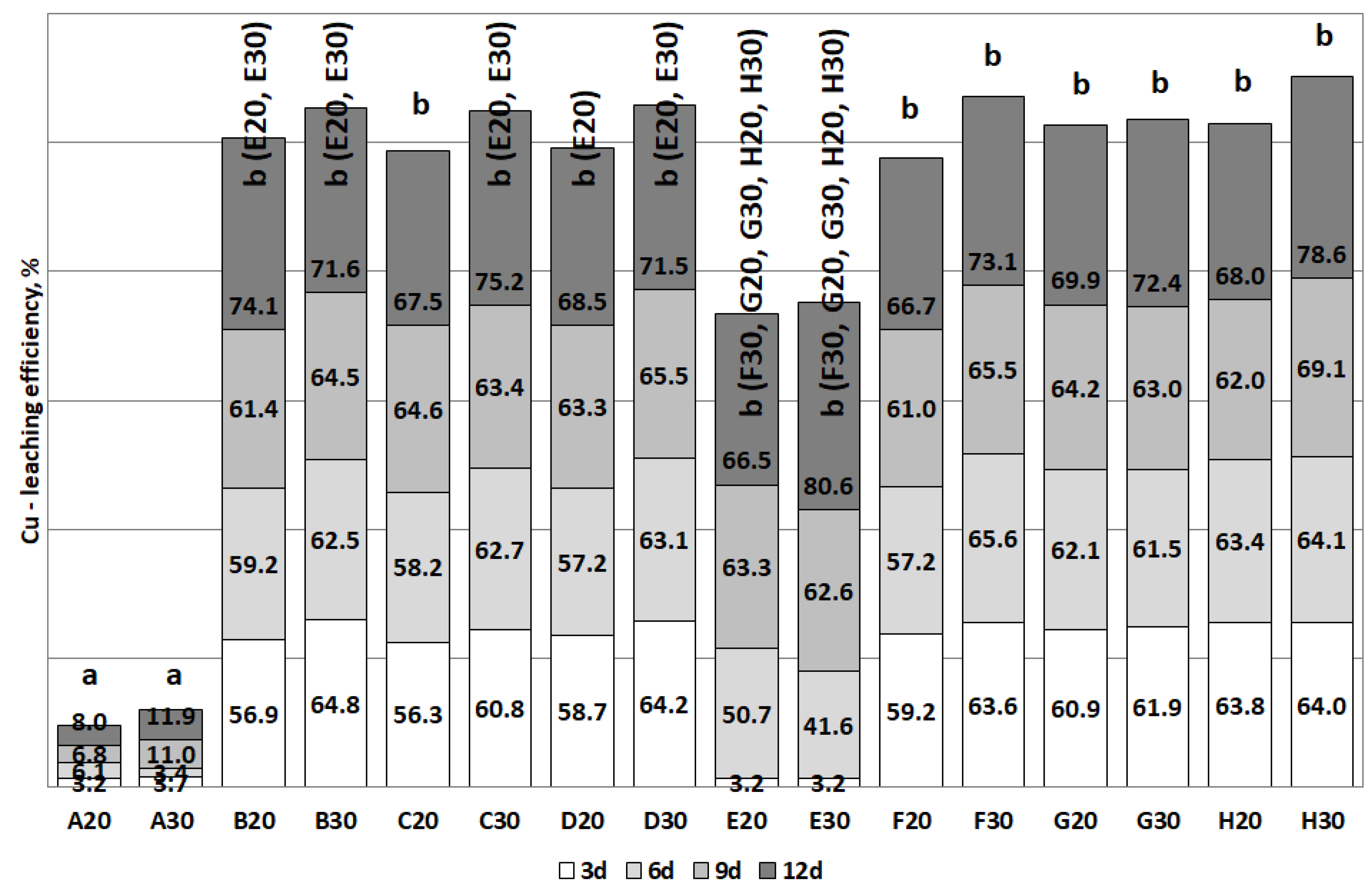
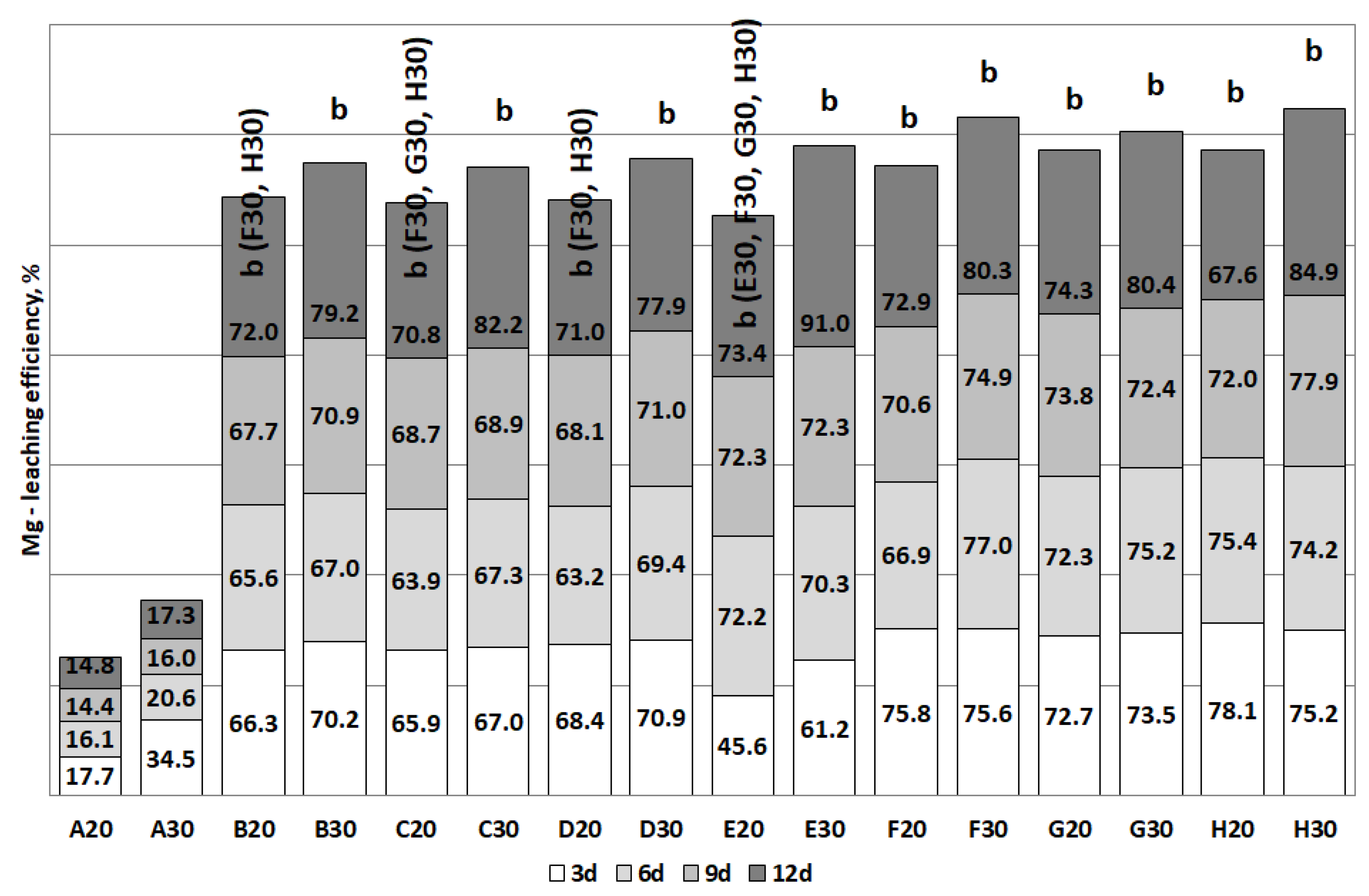
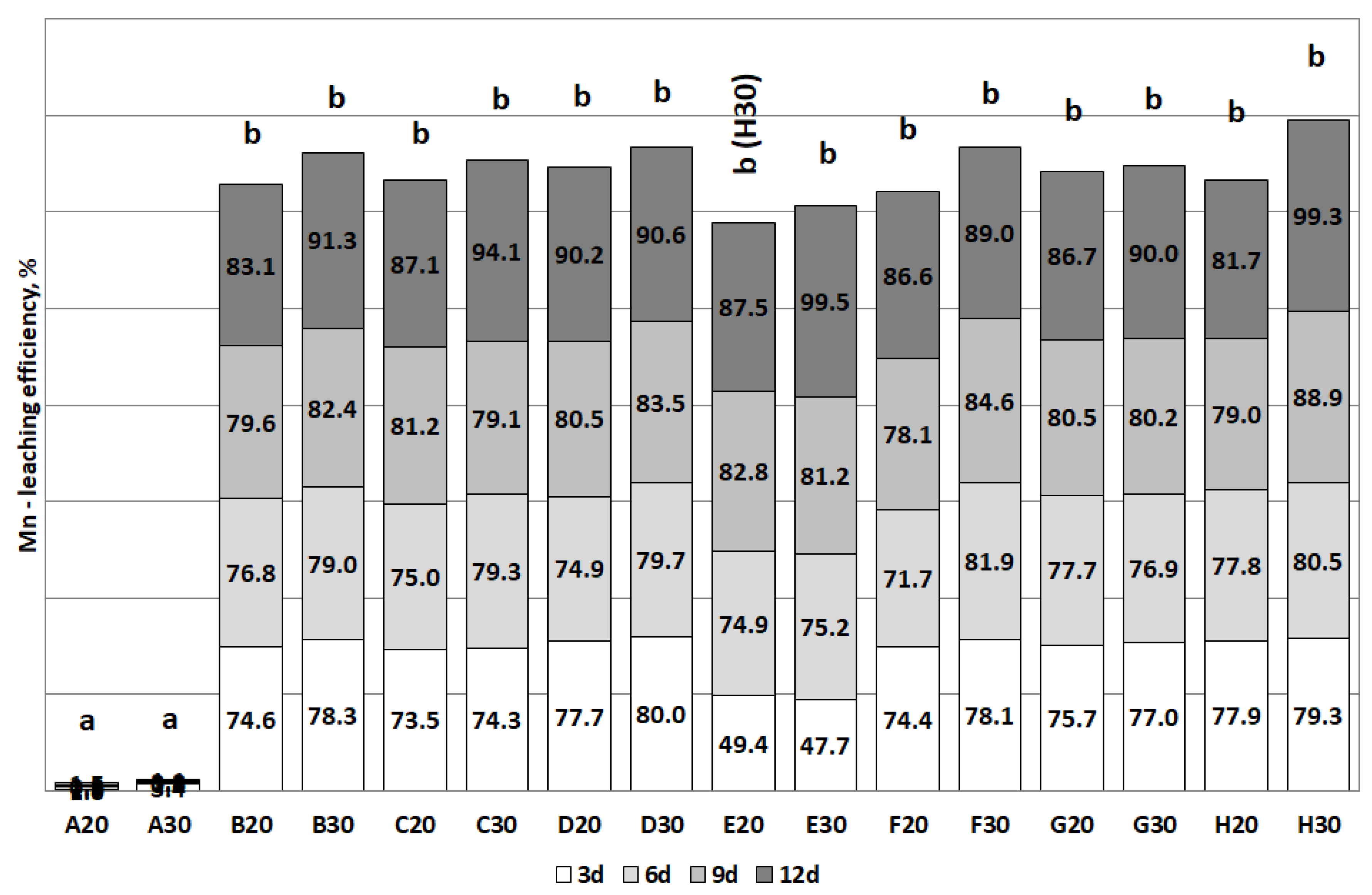
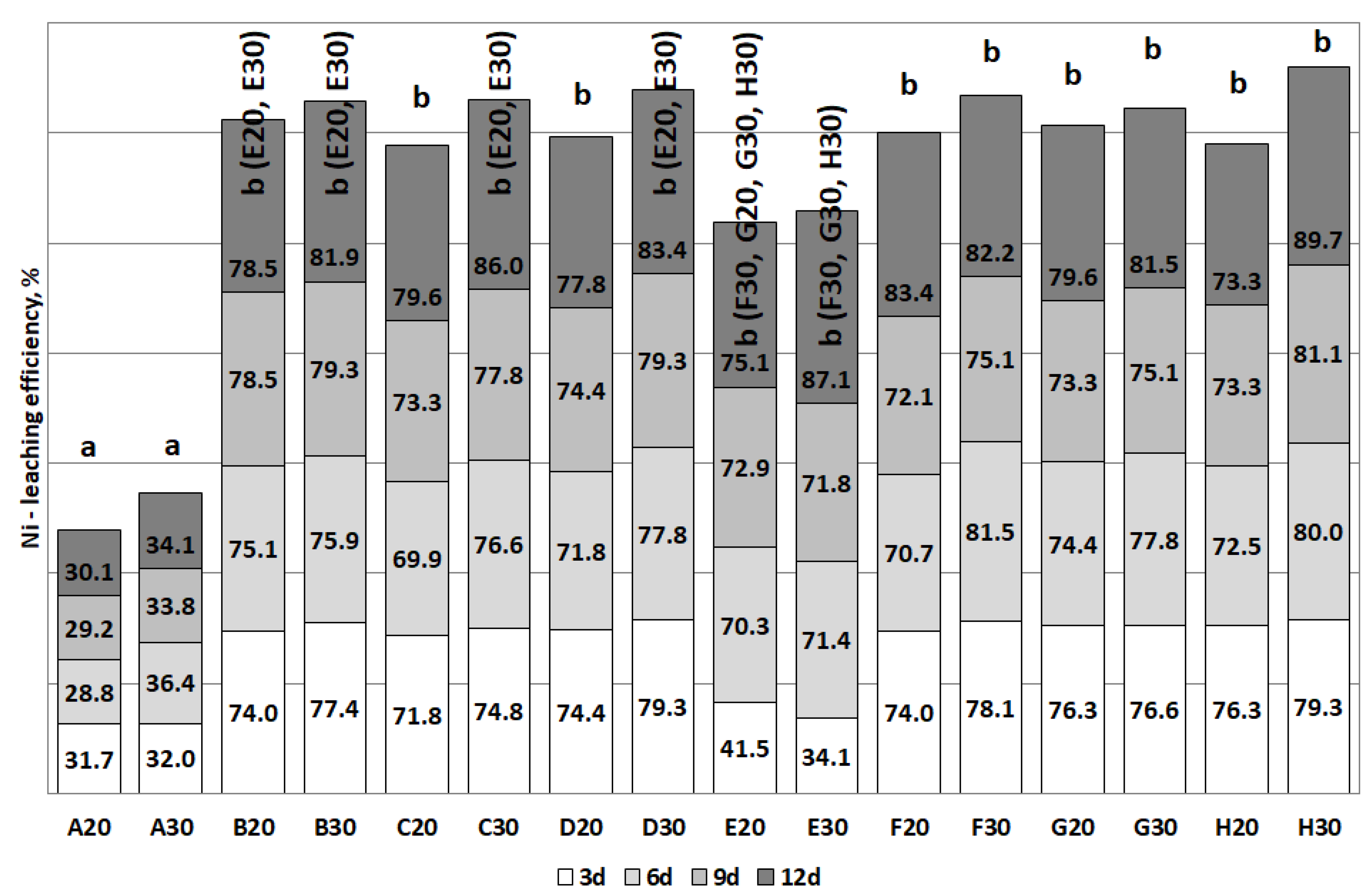
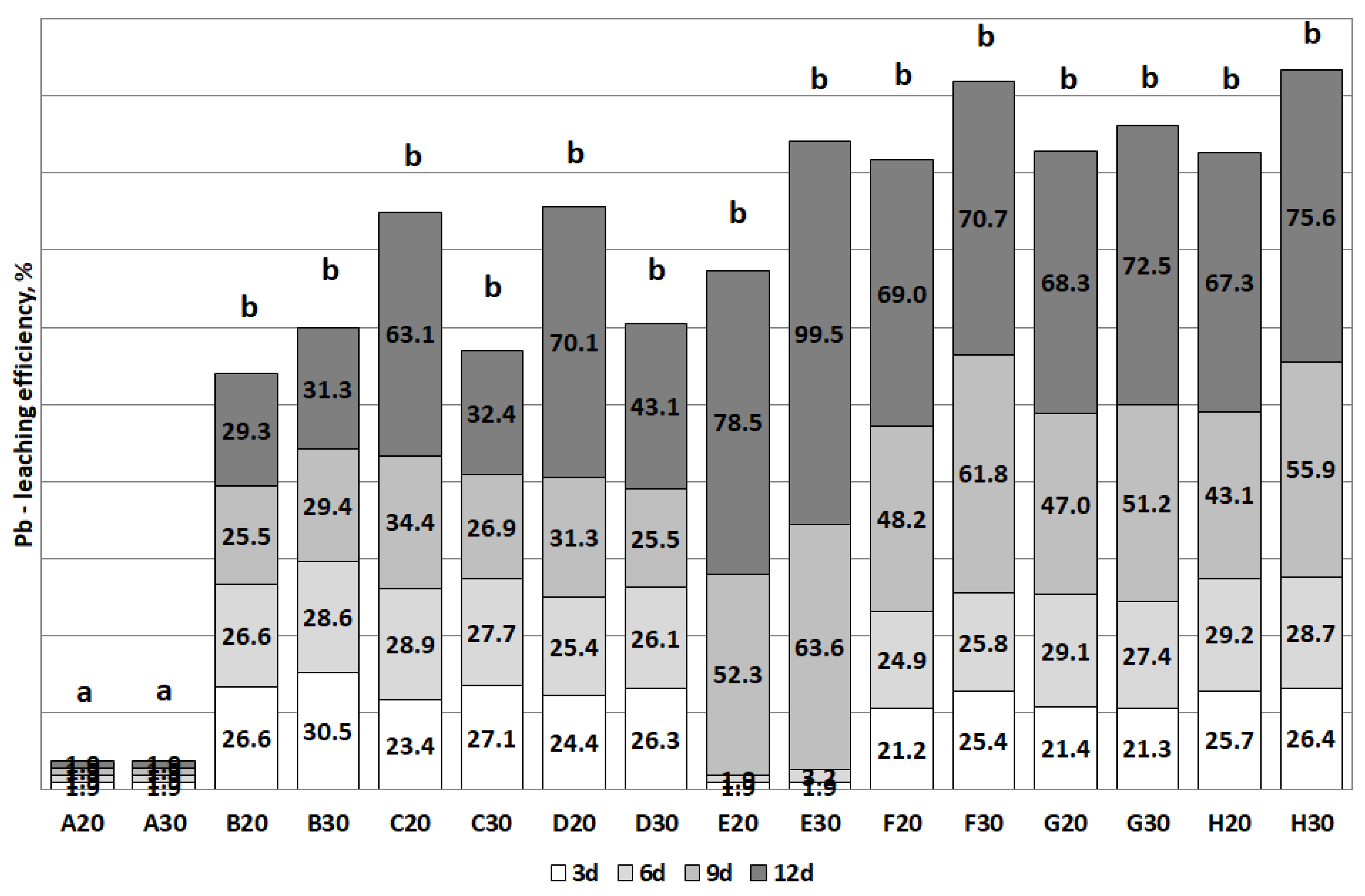
3.2. The Removal of Heavy Metals during the Bioleaching
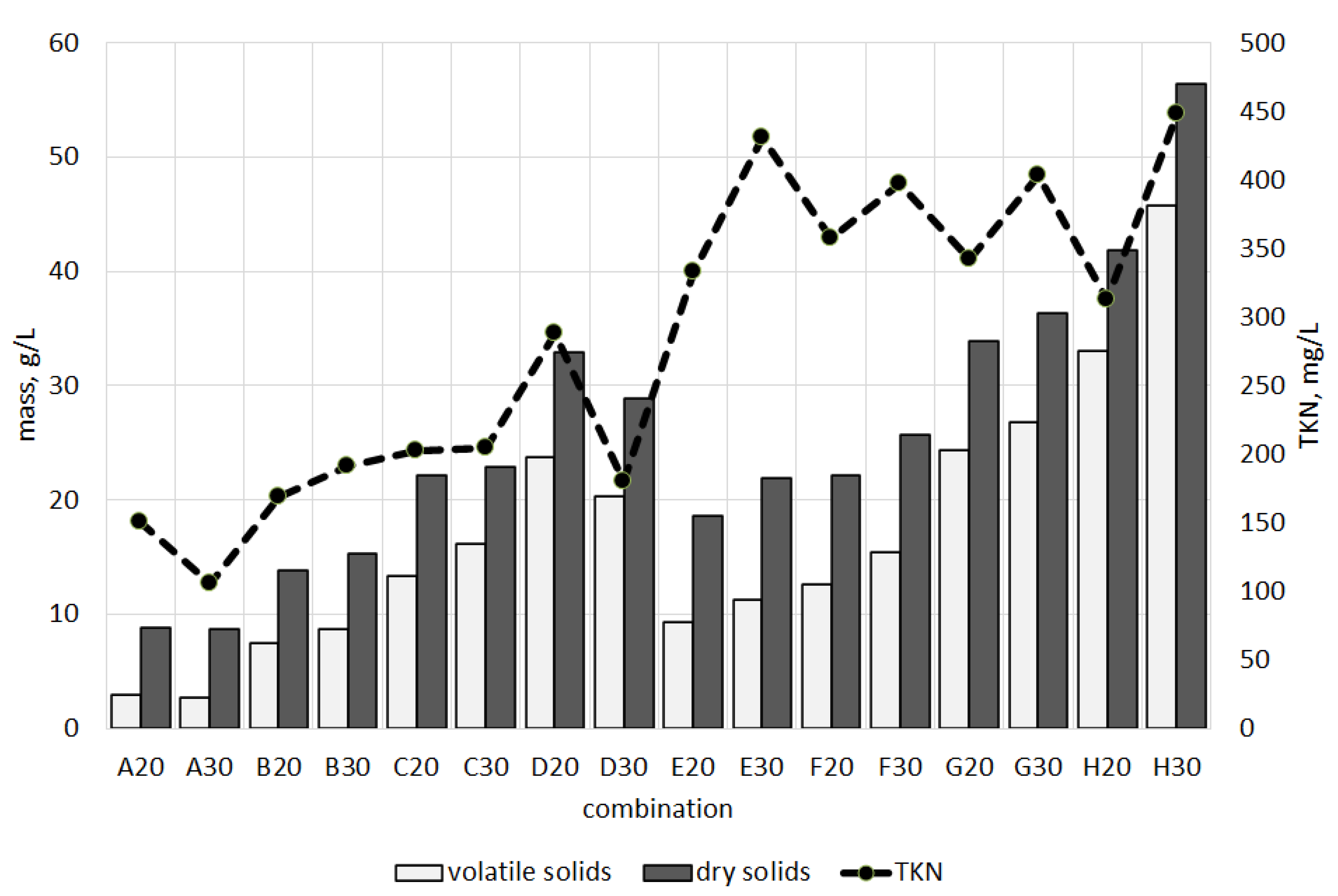
3.3. Mass Balance in Bioleaching
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, T.; Ahmad, K.; Alam, M. Characterization of water treatment plant’s sludge and its safe disposal options. Procedia Environ. Sci. 2016, 35, 950–955. [Google Scholar] [CrossRef]
- Keeley, J.; Smith, A.D.; Judd, S.J.; Jarvis, P. Reuse of recovered coagulants in water treatment: An investigation on the effect coagulant purity has on treatment performance. Sep. Purif. Technol. 2014, 131, 69–78. [Google Scholar] [CrossRef]
- Marousek, J.; Stehel, V.; Vochozka, M.; Kolar, L.; Marouskova, A.; Strunecký, O.; Peterka, J.; Kopecký, M.; Shreedhar, S. Ferrous sludge from water clarification: Changes in waste management practices advisable. J. Clean. Prod. 2019, 218, 459–464. [Google Scholar] [CrossRef]
- Cremades, L.; Cusido, J.A.; Arteaga, F. Recycling of sludge from drinking water treatment as ceramic material for the manufacture of tiles. J. Clean. Prod. 2018, 201, 1071–1080. [Google Scholar] [CrossRef]
- Mymrin, V.; Hackbart, F.M.; Alekseev, K.; Avancim, M.A.; Winter, E., Jr.; Marinho, G.P.; Iarozinski, A.N.; Catai, R.E. Construction materials wastes use to neutralize hazardous municipal water treatment sludge. Construct. Build. Mater. 2019, 204, 800–808. [Google Scholar] [CrossRef]
- Ling, Y.P.; Tham, R.-H.; Lim, S.-M.; Fahim, M.; Ooi, C.-H.; Krishnan, P.; Matsumoto, A.; Yeoh, F.-Y. Evaluation and reutilization of water sludge from fresh water processing plant as a green clay substituent. Appl. Clay Sci. 2017, 143, 300–306. [Google Scholar] [CrossRef]
- Gastaldini, A.L.G.; Hengen, M.F.; Gastaldini, M.C.C.; Amaralm, F.D.; Antolini, M.B.; Coletto, T. The use of water treatment plant sludge ash as a mineral addition. Construct. Build. Mater. 2015, 94, 513–520. [Google Scholar] [CrossRef]
- Carvalho Gomes, S.; Zhou, J.L.; Li, W.; Long, G. Progress in manufacture and properties of construction materials incorporating water treatment sludge: A review. Resour. Conserv. Recycl. 2019, 145, 148–159. [Google Scholar] [CrossRef]
- Ferone, C.; Capasso, I.; Bonati, A.; Roviello, G.; Montagnaro, F.; Santoro, L.; Turco, R.; Cioffi, R. Sustainable management of water potabilization sludge by means of geopolymers production. J. Clean. Prod. 2019, 229, 1–9. [Google Scholar] [CrossRef]
- Guan, X.-H.; Chen, G.-H.; Shang, C. Re-use of water treatment works sludge to enhance particulate pollutant removal from sewage. Water Res. 2005, 39, 3433–3440. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Ahad, A.; Alam, M. Characterization of water treatment sludge and its reuse as coagulant. J. Environ. Manag. 2016, 182, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Bal Krishna, K.C.; Aryal, A.; Jansen, T. Comparative study of ground water treatment plants sludges to remove phosphorous from wastewater. J. Environ. Manag. 2016, 180, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Al-Tahmazi, T.; Babatundea, A.O. Mechanistic study of P retention by dewatered waterworks sludges. Environ. Technol. Innov. 2016, 6, 38–48. [Google Scholar] [CrossRef]
- Keeley, J.; Smith, A.D.; Judd, S.J.; Jarvis, P. Acidified and ultrafiltered recovered coagulants from water treatment works sludge for removal of phosphorus from wastewater. Water Res. 2016, 88, 380–388. [Google Scholar] [CrossRef]
- Keeley, J.; Jarvis, P.; Smith, A.D.; Judd, S.J. Coagulant recovery and reuse for drinking water treatment. Water Res. 2016, 88, 502–509. [Google Scholar] [CrossRef]
- Asada, L.N.; Sundefeld, G.C.; Alvarez, C.R.; Ferreira Filho, S.S.; Pivelli, R.P. Water treatment plant sludge discharge to wastewater treatment plant works: Effects on the operation of upflow anareobic sludge blanket reactor and activated sludge systems. Water Environ. Res. 2010, 82, 392–400. [Google Scholar] [CrossRef]
- Nair, A.T.; Ahammed, M.M. The reuse of water treatment sludge as a coagulant for post-treatment of UASB reactor treating urban wastewater. J. Clean. Prod. 2015, 96, 272–281. [Google Scholar] [CrossRef]
- Luiz, M.A.; Sidney Seckler, F.F.; Passos, P.R. Full-scale effects of addition of sludge from water treatment stations into processes of sewage treatment by conventional activated sludge. J. Environ. Manag. 2018, 215, 283–293. [Google Scholar]
- Li, J.; Liu, L.; Liu, J.; Ma, T.; Yan, A.; Ni, Y. Effect of adding alum sludge from water treatment plant on sewage sludge dewatering. J. Environ. Chem. Eng. 2016, 4, 746–752. [Google Scholar] [CrossRef]
- Ebrahimi-Nik, M.; Heidari, A.; Azghandi, S.R.; Mohammadi, F.A.; Younesi, H. Drinking water treatment sludge as an effective additive for biogas production from food waste; kinetic evaluation and biomethane potential test. Bioresour. Technol. 2018, 260, 421–426. [Google Scholar] [CrossRef]
- Shahin, S.A.; Mossad, M.; Fouad, M. Evaluation of copper removal efficiency using water treatment sludge. Water Sci. Eng. 2019, 12, 37–44. [Google Scholar] [CrossRef]
- Sanchis, R.; Dejoz, A.; Vazquez, I.; Vilarrasa-García, E.; Jimenez-Jimenez, J.; Rodríguez-Castellon, E.; Lopez Nieto, J.M.; Solsona, B. Ferric sludge derived from the process of water purification as an efficient catalyst and/or support for the removal of volatile organic compounds. Chemosphere 2019, 219, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Jerez, C.A. Metal Extraction and Biomining. In Encyclopedia of Microbiology, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 407–420. [Google Scholar]
- Babel, S.; del Mundo, D.D. Heavy metal removal from contaminated sludge for land application: A review. Waste Manag. 2006, 26, 988–1004. [Google Scholar] [CrossRef]
- Johnson, D.B. Biomining-biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30C, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Tian, G.; Liu, J.; Bao, Q.; Zang, L. Removal of heavy metals from sewage sludge with a combination of bioleaching and electrokinetic remediation technology. Desalination 2011, 271, 100–104. [Google Scholar] [CrossRef]
- Pathak, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioleaching of heavy metals from sewage sludge: A review. J. Environ. Manag. 2009, 90, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Erust, C.; Ozdemiroglu, S.; Fonti, V.; Beolchini, F. A review of approaches and techniques used in aquatic contaminated sediments: Metal removal and stabilization by chemical and biotechnological processes. J. Clean. Prod. 2014, 86, 24–36. [Google Scholar] [CrossRef]
- Fonti, V.; Dell’Anno, A.; Beolchini, F. Does bioleaching represent a biotechnological strategy for remediation of contaminated sediments? Sci. Total Environ. 2016, 563–564, 302–319. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Hong, Y.; Valix, M. Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria. J. Clean. Prod. 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Funari, V.; Mäkinen, J.; Salminen, J.; Braga, R.; Dinelli, E.; Revitzer, H. Metal removal from Municipal Solid Waste Incineration fly ash: A comparison between chemical leaching and bioleaching. Waste Manag. 2017, 60, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Rastegar, S.O.; Mousavi, S.M.; Li, M.; Zhou, M. Advances in bioleaching for recovery of metals and bioremediation of fuel ash and sewage sludge. Bioresour. Technol. 2018, 261, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Kim, D.-J.; Ralph, D.E.; Ahn, J.-H.; Rhee, Y.-H. Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus Ferrooxidans. Waste Manag. 2008, 28, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Zhang, D.; Zhang, X.; Xia, Y.; Wu, F.; Chen, S.; Li, L. Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulphur-oxidizing and iron-oxidizing bacteria. Bioresour. Technol. 2009, 24, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Polish Committee for Standardization. Determination of Selected Elements by Means of Optical Emission Spectrometry with Inductively Excited Plasma (ICP-OES); PN-EN ISO 11885:2009; Polish Committee for Standardization: Warsaw, Poland, 2009. [Google Scholar]
- Zhang, P.; Zhu, Y.; Zhang, G.M.; Wu, Z. Sewage sludge bioleaching by indigenous sulfur-oxidizing bacteria: Effects of ratio of substrate dosage to solid content. Bioresour. Technol. 2009, 100, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Li, B.; Sun, C.; Deng, F.; Song, C.; Wang, S. Isolation of Thiobacillus spp. and its application in the removal of heavy metals from activated sludge. Afr. J. Biotechnol. 2012, 11, 16336–16341. [Google Scholar] [CrossRef]
- Asghari, I.; Mousavi, S.M. Effects of key parameters in recycling of metals from petroleum refinery waste catalysts in bioleaching process. Rev. Environ. Sci. Biol. 2014, 13, 139–161. [Google Scholar] [CrossRef]
- Yu, R.; Shi, L.; Gu, G.; Zhou, D.; You, L.; Chen, M.; Qiu, G.; Zeng, W. The shift of microbial community under the adjustment of initial and processing pH during bioleaching of chalcopyrite concentrate by moderate thermophiles. Bioresour. Technol. 2014, 162, 300–307. [Google Scholar] [CrossRef]
- Christensen, T.H.; Kjeldsen, P.; Bjerg, P.L.; Jensen, D.L.; Christensen, B.J.; Baun, A.; Albrechtsen, H.; Heron, G. Biogeochemistry of landfill leachate plumes. Appl. Geochem. 2001, 16, 659–718. [Google Scholar] [CrossRef]
- Liu, H.H.; Sang, S.H. Study on the law of heavy metal leaching in municpal solid waste landfill. Environ. Monit. Assesss. 2010, 165, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and Long-Term Composition of MSW Landfill Leachate: A review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Nareshkumar, R.; Nagendran, R.; Parvathi, K. Bioleaching of heavy metals from contaminated soil using Acidithiobacillus thiooxidans: Effect of sulfur/soil ratio. World J. Microbiol. Biotechnol. 2008, 24, 1539–1546. [Google Scholar] [CrossRef]
- Xiang, L.; Chan, L.C.; Wong, J.W.C. Removal of heavy metals from anaerobically digested sewage sludge by isolated indigenous iron-oxidizing bacteria. Chemosphere 2000, 41, 283–287. [Google Scholar] [CrossRef]
- Seidel, A.; Zimmels, Y.; Armon, R. Mechanism of bioleaching of coal fly ash by Thiobacillus thiooxidans. Chem. Eng. J. 2001, 83, 123–130. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Kappler, U.; McEwan, A.G.; Lindsay, I.S. Probing the archaeal diversity of a mixed thermophilic bioleaching culture by TGGE and FISH. Syst. Appl. Microbiol. 2009, 32, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, D.; Silver, S. Mining with microbes. Biotechnology 1995, 13, 773–778. [Google Scholar] [CrossRef]
- Krebs, W.; Brombacher, C.; Bosshard, P.P.; Bachofen, R.; Brandl, H. Microbial recovery of metals from solids. FEMS Microbiol. Rev. 1997, 20, 605–617. [Google Scholar] [CrossRef]
- Rawlings, D.E. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Fact. 2005, 4, 13. [Google Scholar] [CrossRef]
- Tsai, L.J.; Yu, K.C.; Chen, S.F.; Kung, P.Y.; Chang, C.Y.; Lin, C.H. Partitioning variation of heavy metals in contaminated river sediment via bioleaching: Effect of sulfur added to total solids ratio. Water Res. 2003, 37, 4623–4630. [Google Scholar] [CrossRef] [PubMed]
- Löser, C.; Zehnsdorf, A.; Görsch, K.; Seidel, H. Bioleaching of heavy metal polluted sediment: Kinetics of leaching and microbial sulfur oxidation. Eng. Life Sci. 2005, 5, 535–549. [Google Scholar] [CrossRef]
- Löser, C.; Zehnsdorf, A.; Hoffmann, P.; Seidel, H. Bioleaching of heavy metal polluted sediment: Influence of sediment properties (part 2). Eng. Life Sci. 2006, 6, 364–371. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—Part A. App. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Rezza, I.; Salinasa, E.; Elorzaa, M.; Sanz de Tosettia, M.; Donatib, E. Mechanisms involved in bioleaching of an aluminosilicate by heterotrophic microorganisms. Process Biochem. 2001, 36, 495–500. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.P. Microbial leaching of heavy metals using Escherichia coli and evaluation of bioleaching mechanism. Bioresour. Technol. Rep. 2020, 9, 100368. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.P. Sequential biological process for molybdenum extraction from hydrodesulphurization spent catalyst. Chemosphere 2016, 160, 7–12. [Google Scholar] [CrossRef]






| Index | Units | Value |
|---|---|---|
| Dry solids (DS) | (g/L) | 8.9 ± 0.1 |
| Volatile solids (VS) | (g/L) | 5.0 ± 0.1 |
| pH | - | 6.9 ± 0.3 |
| Alkalinity | (mg CaCO3/L) | 571.7 ± 8.5 |
| Total Kjeldahl nitrogen | (mg N/L) | 721 |
| Carbon content | (%DS) | 27.80 |
| Hydrogen content | (%DS) | 4.41 |
| Nitrogen content | (%DS) | 4.84 |
| Sulfur content | (%DS) | 0.79 |
| Dissolved organic carbon | (mg C/L) | 73.9 ± 0.03% |
| Dissolved total Kjeldahl nitrogen | (mg N/L) | 25.2 |
| Dissolved ammonium nitrogen | (mg N-NH4+/L) | 2.2 |
| Metal | mg/kg | % DS |
|---|---|---|
| Al | 1110.4 | 0.1110 |
| Ca | 26,703.7 | 2.6704 |
| Cd | 20.5 | 0.0021 |
| Cr | 446.8 | 0.0447 |
| Cu | 135.4 | 0.0135 |
| Fe | 242,474.7 | 24.2475 |
| Mg | 2978.1 | 0.2978 |
| Mn | 738.7 | 0.0739 |
| Ni | 30.1 | 0.0030 |
| P | 15,796.9 | 1.5797 |
| Pb | 152.7 | 0.0153 |
| Zn | 5042.0 | 0.5042 |
| Treatment | pH Adjustment | Sulfur Addition, g | Incubation Temperature, °C | |
|---|---|---|---|---|
| A20 | WPS (135 mL) + water (15 mL) | - | - | 20 |
| A30 | 30 | |||
| B20 | WPS (135 mL) + water (15 mL) | 2.0 | - | 20 |
| B30 | 30 | |||
| C20 | WPS (135 mL) + water (15 mL) | 2.0 | 0.75 | 20 |
| C30 | 30 | |||
| D20 | WPS (135 mL) + water (15 mL) | 2.0 | 1.5 | 20 |
| D30 | 30 | |||
| E20 | WPS (135 mL) + A. thiooxidans (15 mL) | - | - | 20 |
| E30 | 30 | |||
| F20 | WPS (135 mL) + A. thiooxidans (15 mL) | 2.0 | - | 20 |
| F30 | 30 | |||
| G20 | WPS (135 mL) + A. thiooxidans (15 mL) | 2.0 | 0.75 | 20 |
| G30 | 30 | |||
| H20 | WPS (135 mL) + A. thiooxidans (15 mL) | 2.0 | 1.5 | 20 |
| H30 | 30 | |||
| Element | Incubation Temperature | |||
|---|---|---|---|---|
| 20 °C | 30 °C | 20 °C | 30 °C | |
| Combination (Efficiency, %) | ||||
| Combinations A–D | Combinations E–H | |||
| Al | C, D (>40) | B, C, D (>30) | H (>50) | E, H (>50) |
| Ca | B, C, D (>80) | C (>90) | E, G (>80) | E, H (>90) |
| Cd | D (>60) | D>20 | G (>60) | E, F, G, H (>60) |
| Cr | D (>80) | C, D (>50) | G (>80) | E, H (>90) |
| Cu | B (>70) | B, C, D (>70) | E, F, G, H (>60) | E (>80) |
| Fe | D (>80) | D (>30) | F, G (>80) | E, H (>90) |
| Mg | B, C, D (>70) | C (>80) | E, F, G (>70) | E (>90) |
| Mn | D (>90) | B, C, D (>90) | E, F, G, H (>80) | E, G, H (>90) |
| Ni | B, C, D (>70) | B, C, D (>80) | F (>80) | E, F, G, H (>80) |
| Pb | D (>70) | D (>40) | E (>70) | E (>90) |
| Zn | D (>80) | B, C, D (>80) | E, G (>80) | E, H (>90) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamizela, T.; Worwag, M. Processing of Water Treatment Sludge by Bioleaching. Energies 2020, 13, 6539. https://doi.org/10.3390/en13246539
Kamizela T, Worwag M. Processing of Water Treatment Sludge by Bioleaching. Energies. 2020; 13(24):6539. https://doi.org/10.3390/en13246539
Chicago/Turabian StyleKamizela, Tomasz, and Malgorzata Worwag. 2020. "Processing of Water Treatment Sludge by Bioleaching" Energies 13, no. 24: 6539. https://doi.org/10.3390/en13246539
APA StyleKamizela, T., & Worwag, M. (2020). Processing of Water Treatment Sludge by Bioleaching. Energies, 13(24), 6539. https://doi.org/10.3390/en13246539






