Systems Analysis of SO2-CO2 Co-Capture from a Post-Combustion Coal-Fired Power Plant in Deep Eutectic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Flue Gas and Baseline Process
2.2. Modeling of Deep Eutectic Solvents
2.3. Process Model for CO2 Absorption
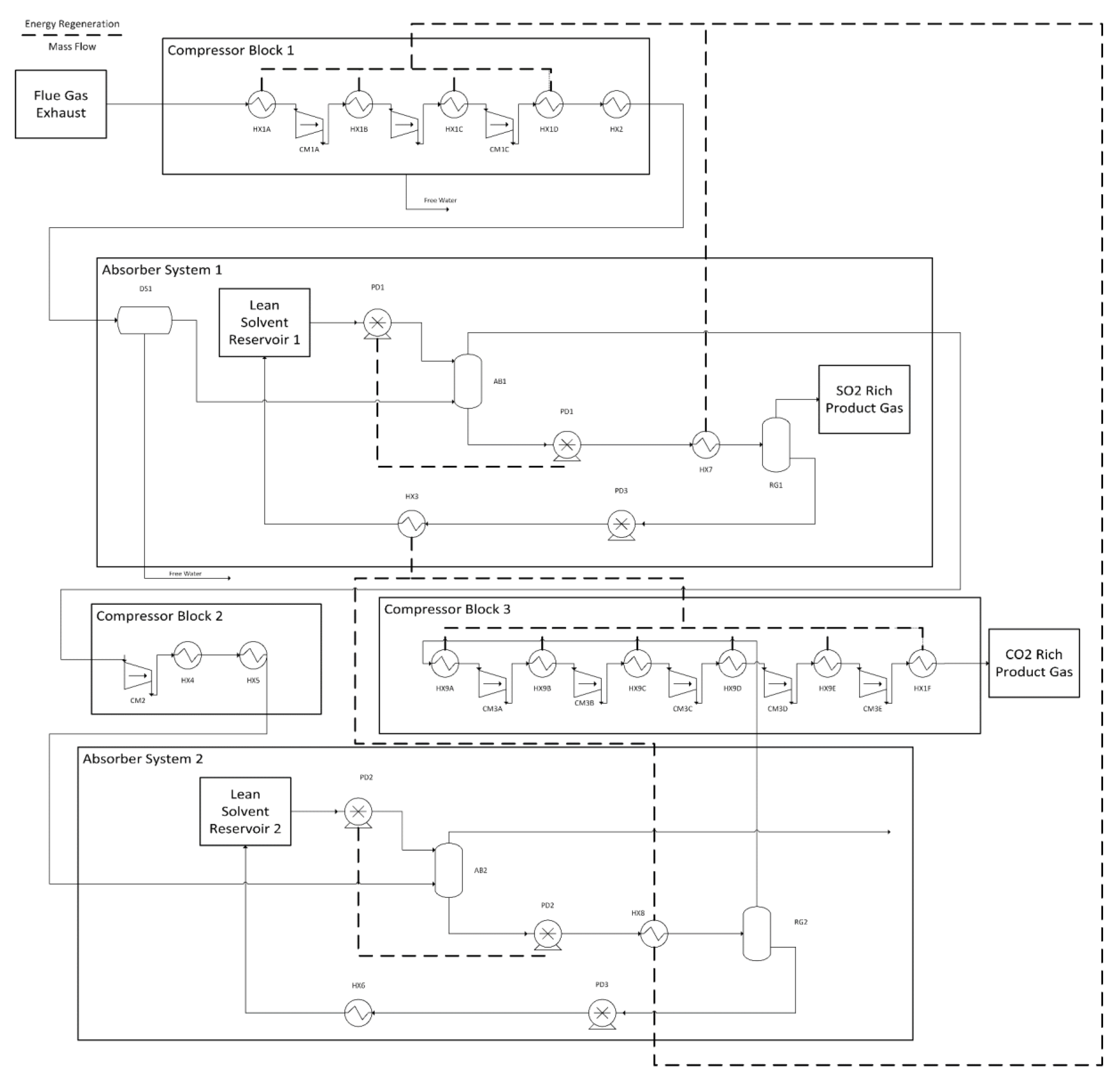
Dual-Stage DES-Based Absorption System
- Henry’s law is valid for all absorption and desorption steps. This is justified by keeping the mole% of SO2 and CO2 below 3%.
- Absorber 2 operates at twice the pressure of absorber 1. This ratio was selected as it was found to be the most efficient in regards to energy demand over a wide variety of CO2 removal rates.
2.4. Process Economics
2.5. Parameters for Sensitivity Analysis
3. Results and Discussion
3.1. DES Solvent Properties
3.2. Process Design of Dual-Stage CO2 Absorption in DES
3.3. Process Economics of Dual-Stage CO2 Absorption in DES
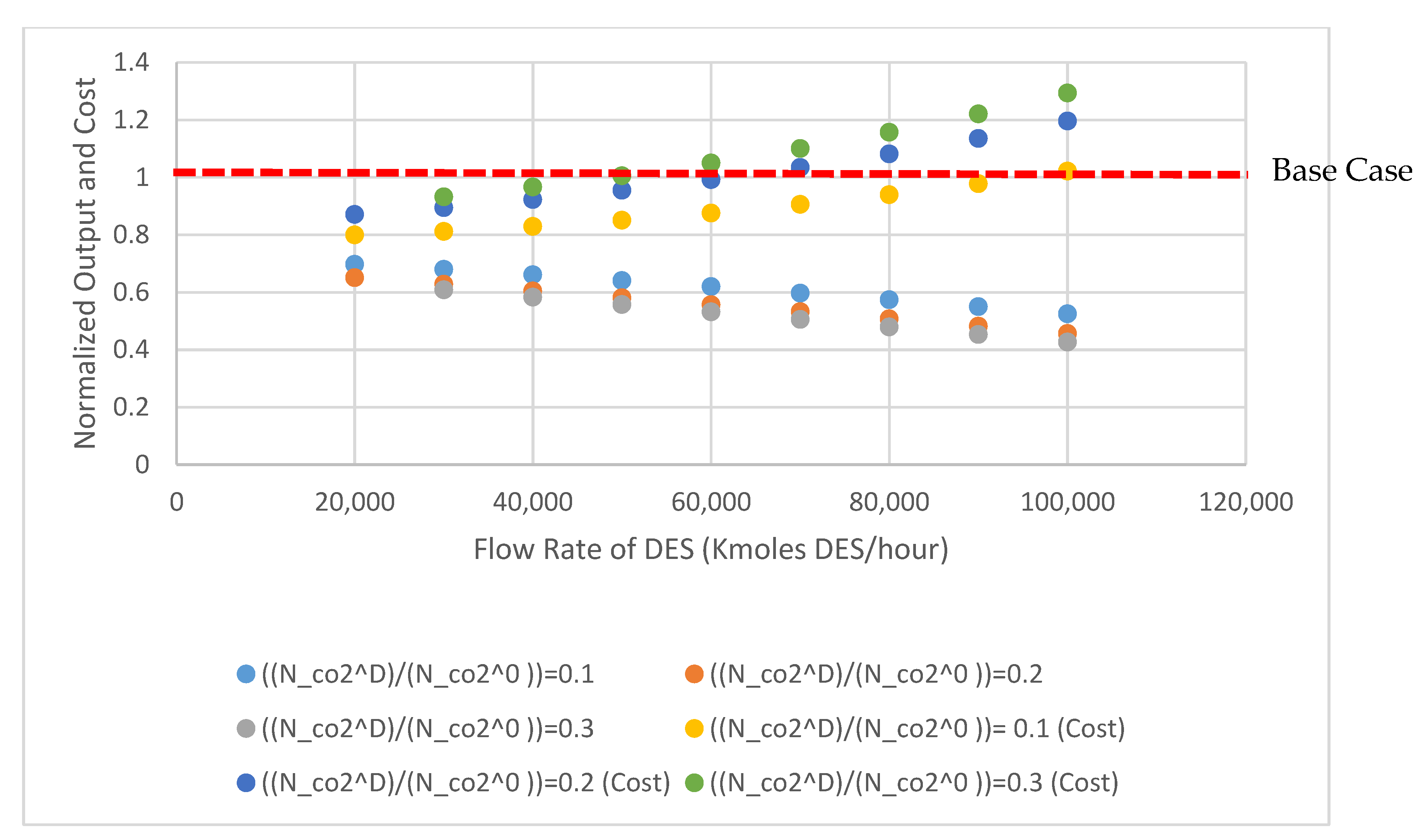
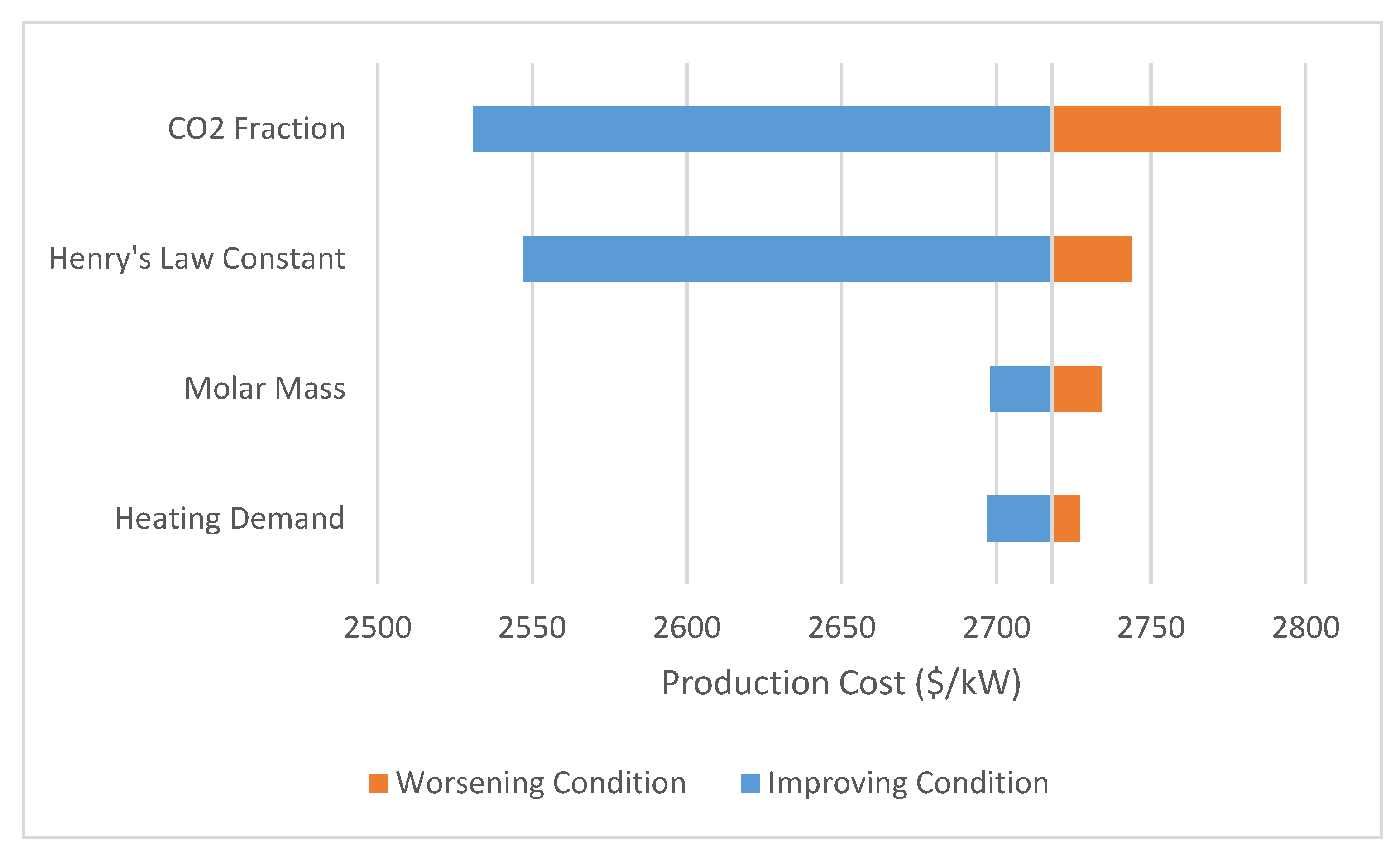
3.4. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, A.B.; Rubin, E.S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef]
- Fout, T.; Zoelle, A.; Keairns, D.; Turner, M.; Woods, M.; Kuehn, N.; Shah, V.; Chou, V.; Pinkerton, L. Cost and Performance Baseline for Fossil Energy Plants Volume 1a: Bituminous Coal (PC) and Natural Gas to Electricity Revision 3; Technical Report No. DOE/NETL-2015/1723; NETL: Pittsburgh, PA, USA, 2015. [Google Scholar]
- Lv, B.; Guo, B.; Zhou, Z.; Jing, G. Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes. Environ. Sci. Technol. 2015, 49, 10728–10735. [Google Scholar] [CrossRef]
- Gouedard, C.; Picq, D.; Launay, F.; Carrette, P.-L. Amine degradation in CO2 capture. I. A review. Int. J. Greenh. Gas Control 2012, 10, 244–270. [Google Scholar] [CrossRef]
- Sexton, A.J.; Rochelle, G.T. Reaction products from the oxidative degradation of monoethanolamine. Ind. Eng. Chem. Res. 2010, 50, 667–673. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Kareem, M.A.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. Phosphonium-based ionic liquids analogues and their physical properties. J. Chem. Eng. Data 2010, 55, 4632–4637. [Google Scholar] [CrossRef]
- Sze, L.L.; Pandey, S.; Ravula, S.; Pandey, S.; Zhao, H.; Baker, G.A.; Baker, S.N. Ternary deep eutectic solvents tasked for carbon dioxide capture. ACS Sustain. Chem. Eng. 2014, 2, 2117–2123. [Google Scholar] [CrossRef]
- Chen, Y.; Ai, N.; Li, G.; Shan, H.; Cui, Y.; Deng, D. Solubilities of carbon dioxide in eutectic mixtures of choline chloride and dihydric alcohols. J. Chem. Eng. Data 2014, 59, 1247–1253. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep eutectic solvents: Physicochemical properties and gas separation applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Sarmad, S.; Mikkola, J.-P.; Ji, X. Carbon dioxide capture with ionic liquids and deep eutectic solvents: A new generation of sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, C.; Lu, X.; Ji, X. Techno-economic analysis and performance comparison of aqueous deep eutectic solvent and other physical absorbents for biogas upgrading. Appl. Energy 2018, 225, 437–447. [Google Scholar] [CrossRef]
- Rinaldi, R. Plant biomass fractionation meets catalysis. Angew. Chem. Int. Ed. 2014, 53, 8559–8560. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Hou, M.; Ning, H.; Zhang, J.; Ma, J.; Yang, G.; Han, B. Efficient SO2 absorption by renewable choline chloride–glycerol deep eutectic solvents. Green Chem. 2013, 15, 2261–2265. [Google Scholar] [CrossRef]
- Yang, D.; Hou, M.; Ning, H.; Ma, J.; Kang, X.; Zhang, J.; Han, B. Reversible capture of SO2 through functionalized ionic liquids. ChemSusChem 2013, 6, 1191–1195. [Google Scholar] [CrossRef]
- Deng, D.; Liu, X.; Gao, B. Physicochemical properties and investigation of azole-based deep eutectic solvents as efficient and reversible SO2 absorbents. Ind. Eng. Chem. Res. 2017, 56, 13850–13856. [Google Scholar] [CrossRef]
- Leron, R.B.; Caparanga, A.; Li, M.-H. Carbon dioxide solubility in a deep eutectic solvent based on choline chloride and urea at T = 303.15–343.15 K and moderate pressures. J. Taiwan Inst. Chem. Eng. 2013, 44, 879–885. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Leron, R.B.; Li, M.-H. Molar heat capacities of choline chloride-based deep eutectic solvents and their binary mixtures with water. Thermochim. Acta 2012, 530, 52–57. [Google Scholar] [CrossRef]
- Fang, Y.; Osama, M.; Rashmi, W.; Shahbaz, K.; Khalid, M.; Mjalli, F.; Farid, M. Synthesis and thermo-physical properties of deep eutectic solvent-based graphene nanofluids. Nanotechnology 2016, 27, 075702. [Google Scholar] [CrossRef] [PubMed]
- Shaoyang, S.; Yanxia, N.; Qiang, X.; Zuchen, S.; Xionghui, W. Efficient SO2 absorptions by four kinds of deep eutectic solvents based on choline chloride. Ind. Eng. Chem. Res. 2015, 54, 8019–8024. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Zubeir, L.F.; van den Bruinhorst, A.; Rocha, M.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Leron, R.B.; Li, M.-H. High-pressure density measurements for choline chloride: Urea deep eutectic solvent and its aqueous mixtures at T = (298.15 to 323.15) K and up to 50 MPa. J. Chem. Thermodyn. 2012, 54, 293–301. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.; Whiting, W.; Shaeiwitz, J.; Bhattacharyya, D. Analysis, Synthesis, and Design of Chemical Process, 4th ed.; Prentice Hall International Series in the Physical and Chemical Engineering Sciences; Prentice Hall: Ann Arbor, MI, USA, 2012; ISBN 978-0-13-261812-0. [Google Scholar]
- Leron, R.B.; Li, M.-H. Solubility of carbon dioxide in a choline chloride–ethylene glycol based deep eutectic solvent. Thermochim. Acta 2013, 551, 14–19. [Google Scholar] [CrossRef]
- Last, G.V.; Schmick, M.T. Identification and Selection of Major Carbon Dioxide Stream Compositions; Technical Report No. PNNL-20493; Pacific Northwest National Laboratory: Richland, WA, USA, 2011. [Google Scholar]


| Variable | METPB:EG | ChCl:Urea |
|---|---|---|
| C (Component Moles/Mol Solvent) | 4 | 3 |
| Molar Mass of DES (kg/kmol) | 543 | 260 |
| Thermal Demand (kj/kg) | 44.2 [22] | 24.2 [21] |
| H(CO2) (bar/mole frac) | 169 | 175 [18] |
| Ρ (kg/m3) | 1240 | 1186 [21] |
| Parameter | Baseline | Improved Value | Worsened Value |
|---|---|---|---|
| Henry’s law constant (Bar/mole Frac) | 175 | 100 | 200 |
| CO2 Fraction in Flue Gas | 13.76% | 25% | 8% |
| Molar Mass (g/mole DES) | 260 | 200 | 350 |
| Heating Demand (kj/kg) | 24.2 | 18 | 30 |
| Fraction Dissolved ( | 0.1 | ||
| Pressure (bar; 2nd Stage) | Plant Output (kW) | Cost ($/kW) | |
| 20,000 | 13.0 | 383,193 | 2771 |
| 30,000 | 8.7 | 373,740 | 2814 |
| 40,000 | 6.5 | 363,433 | 2874 |
| 50,000 | 5.2 | 352,369 | 2948 |
| 60,000 | 4.3 | 340,637 | 3036 |
| 70,000 | 3.7 | 328,316 | 3139 |
| 80,000 | 3.2 | 315,474 | 3256 |
| 90,000 | 2.9 | 302,171 | 3389 |
| 100,000 | 2.6 | 288,461 | 3541 |
| Fraction Dissolved ( | 0.2 | ||
| Pressure (bar; 2nd Stage) | Plant Output (kW) | Cost ($/kW) | |
| 20,000 | 26.6 | 357,713 | 3019 |
| 30,000 | 17.7 | 345,128 | 3100 |
| 40,000 | 13.3 | 332,296 | 3198 |
| 50,000 | 10.6 | 319,231 | 3311 |
| 60,000 | 8.9 | 305,946 | 3440 |
| 70,000 | 7.6 | 292,455 | 3586 |
| 80,000 | 6.6 | 278,770 | 3750 |
| 90,000 | 5.9 | 264,902 | 3935 |
| 100,000 | 5.3 | 250,862 | 4145 |
| Fraction Dissolved ( | 0.3 | ||
| Pressure (bar; 2nd Stage) | Plant Output (kW) | Cost ($/kW) | |
| 20,000 * | 40.8 * | 348,454 * | 3131 * |
| 30,000 | 27.2 | 334,553 | 3231 |
| 40,000 | 20.4 | 320,539 | 3350 |
| 50,000 | 16.3 | 306,418 | 3486 |
| 60,000 | 13.6 | 292,193 | 3640 |
| 70,000 | 11.6 | 277,868 | 3814 |
| 80,000 | 10.2 | 263,449 | 4010 |
| 90,000 | 9.1 | 248,937 | 4232 |
| 100,000 | 8.2 | 234,337 | 4485 |
| Fraction Dissolved | 0.1 | ||
| Pressure (bar; 2nd Stage) | Plant Output (kW) | Cost ($/kW) | |
| 20,000 | 17.9 | 393,542 | 2718 |
| 30,000 | 12.0 | 394,394 | 2686 |
| 40,000 | 9.0 | 394,748 | 2666 |
| 50,000 | 7.2 | 394,648 | 2652 |
| 60,000 | 6.0 | 394,133 | 2644 |
| 70,000 | 5.1 | 393,238 | 2641 |
| 80,000 | 4.5 | 391,996 | 2640 |
| 90,000 | 4.0 | 390,436 | 2643 |
| 100,000 | 3.6 | 388,587 | 2649 |
| Fraction Dissolved | 0.2 | ||
| Pressure (bar; 2nd Stage) | Plant Output (kW) | Cost ($/kW) | |
| 20,000 * | 36.7 * | 374,130 * | 2907 * |
| 30,000 | 24.4 | 372,370 | 2894 |
| 40,000 | 18.3 | 370,474 | 2889 |
| 50,000 | 14.7 | 368,446 | 2890 |
| 60,000 | 12.2 | 366,294 | 2895 |
| 70,000 | 10.5 | 364,022 | 2902 |
| 80,000 | 9.2 | 361,636 | 2912 |
| 90,000 | 8.1 | 359,139 | 2924 |
| Fraction Dissolved | 0.3 | ||
| Pressure (bar; 2nd Stage) | Plant Output (kW) | Cost ($/kW) | |
| 20,000 * | 56.3 * | 367,237 * | 2992 * |
| 30,000 * | 37.5 * | 364,450 * | 2987 * |
| 40,000 | 28.1 | 361,604 | 2991 |
| 50,000 | 22.5 | 358,698 | 2999 |
| 60,000 | 18.8 | 355,735 | 3011 |
| 70,000 | 16.1 | 352,716 | 3026 |
| 80,000 | 14.1 | 349,643 | 3043 |
| 90,000 | 12.5 | 346,517 | 3062 |
| 100,000 | 11.3 | 343,341 | 3083 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGaughy, K.; Reza, M.T. Systems Analysis of SO2-CO2 Co-Capture from a Post-Combustion Coal-Fired Power Plant in Deep Eutectic Solvents. Energies 2020, 13, 438. https://doi.org/10.3390/en13020438
McGaughy K, Reza MT. Systems Analysis of SO2-CO2 Co-Capture from a Post-Combustion Coal-Fired Power Plant in Deep Eutectic Solvents. Energies. 2020; 13(2):438. https://doi.org/10.3390/en13020438
Chicago/Turabian StyleMcGaughy, Kyle, and M. Toufiq Reza. 2020. "Systems Analysis of SO2-CO2 Co-Capture from a Post-Combustion Coal-Fired Power Plant in Deep Eutectic Solvents" Energies 13, no. 2: 438. https://doi.org/10.3390/en13020438
APA StyleMcGaughy, K., & Reza, M. T. (2020). Systems Analysis of SO2-CO2 Co-Capture from a Post-Combustion Coal-Fired Power Plant in Deep Eutectic Solvents. Energies, 13(2), 438. https://doi.org/10.3390/en13020438







