Testing the Durability of Anti-Soiling Coatings for Solar Cover Glass by Outdoor Exposure in Denmark
Abstract
1. Introduction
- To compare the anti-soiling properties of hydrophobic-coated and uncoated glass coupons when exposed to outdoor conditions for 24 weeks.
- To compare soiling of coupons that are manually cleaned every 4 weeks against coupons that are not cleaned.
2. Materials and Methods
2.1. Materials
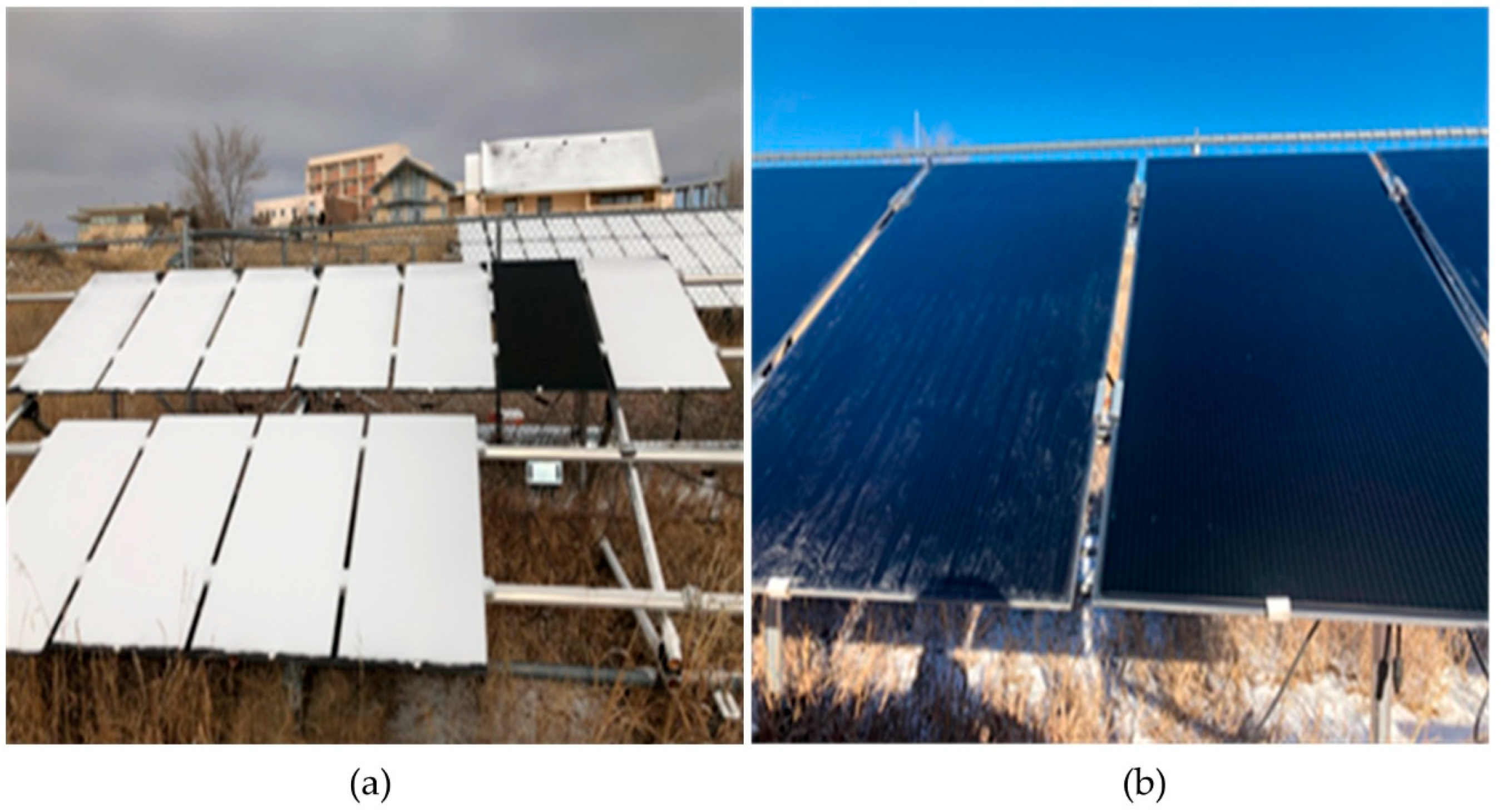
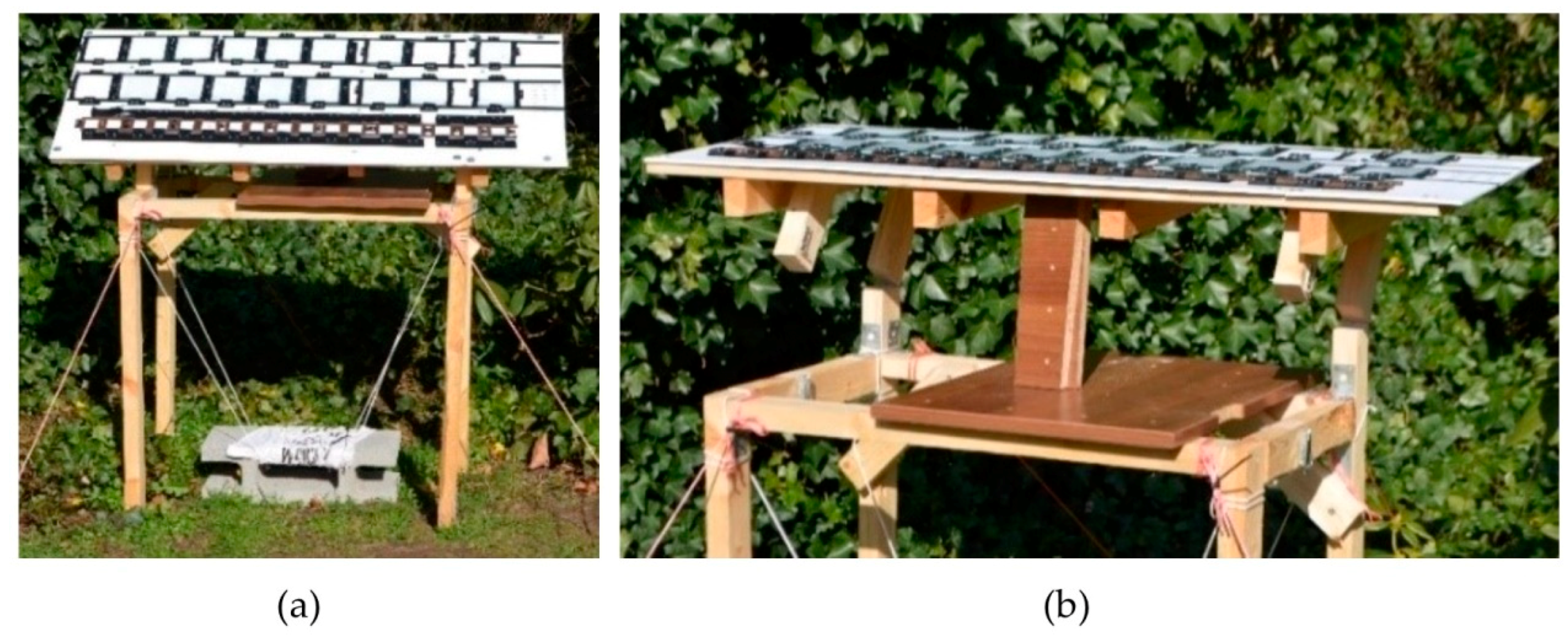
2.2. Outdoor Exposure
2.3. Weather and Environment
3. Results and Discussion
3.1. Coating Durability
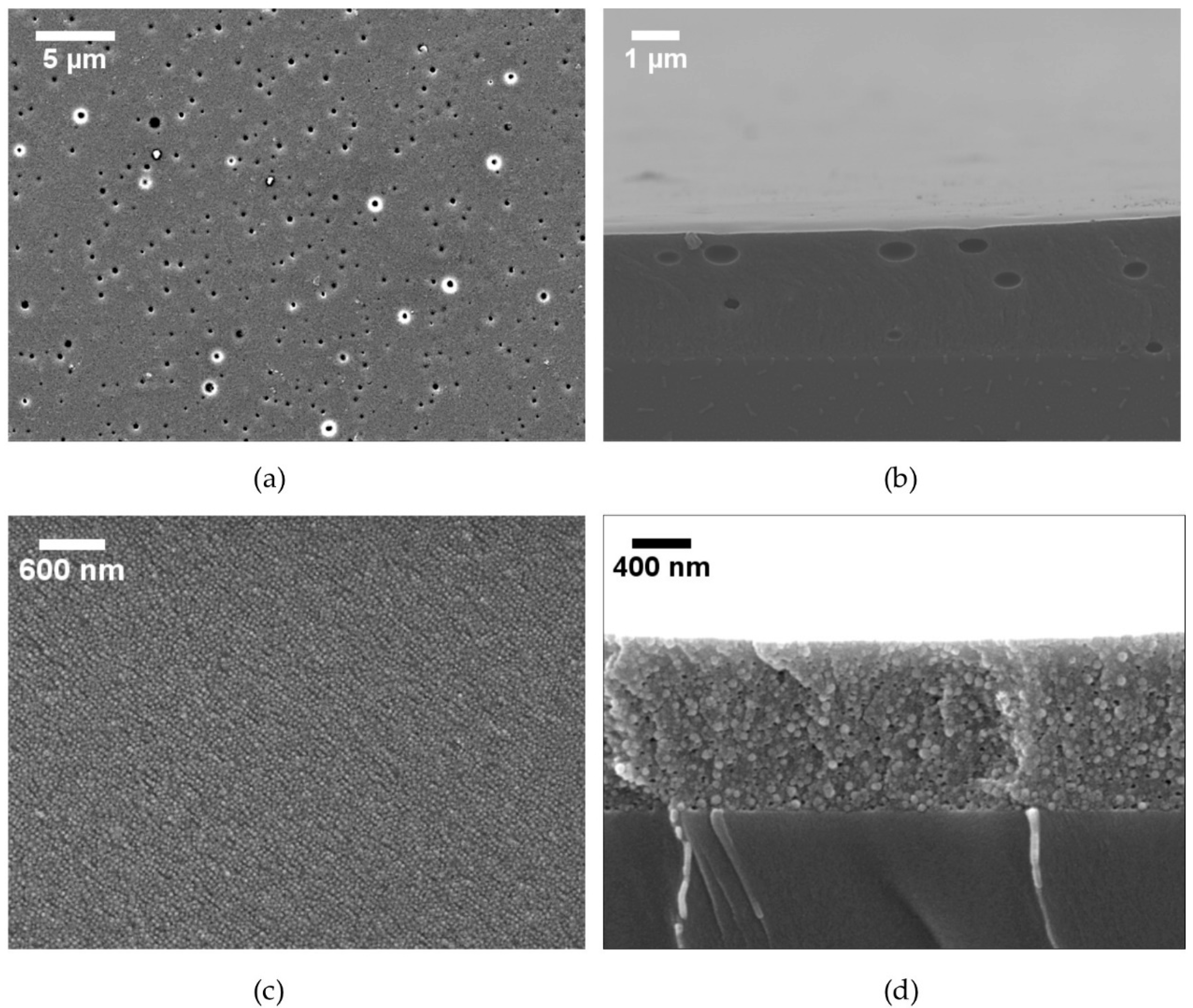
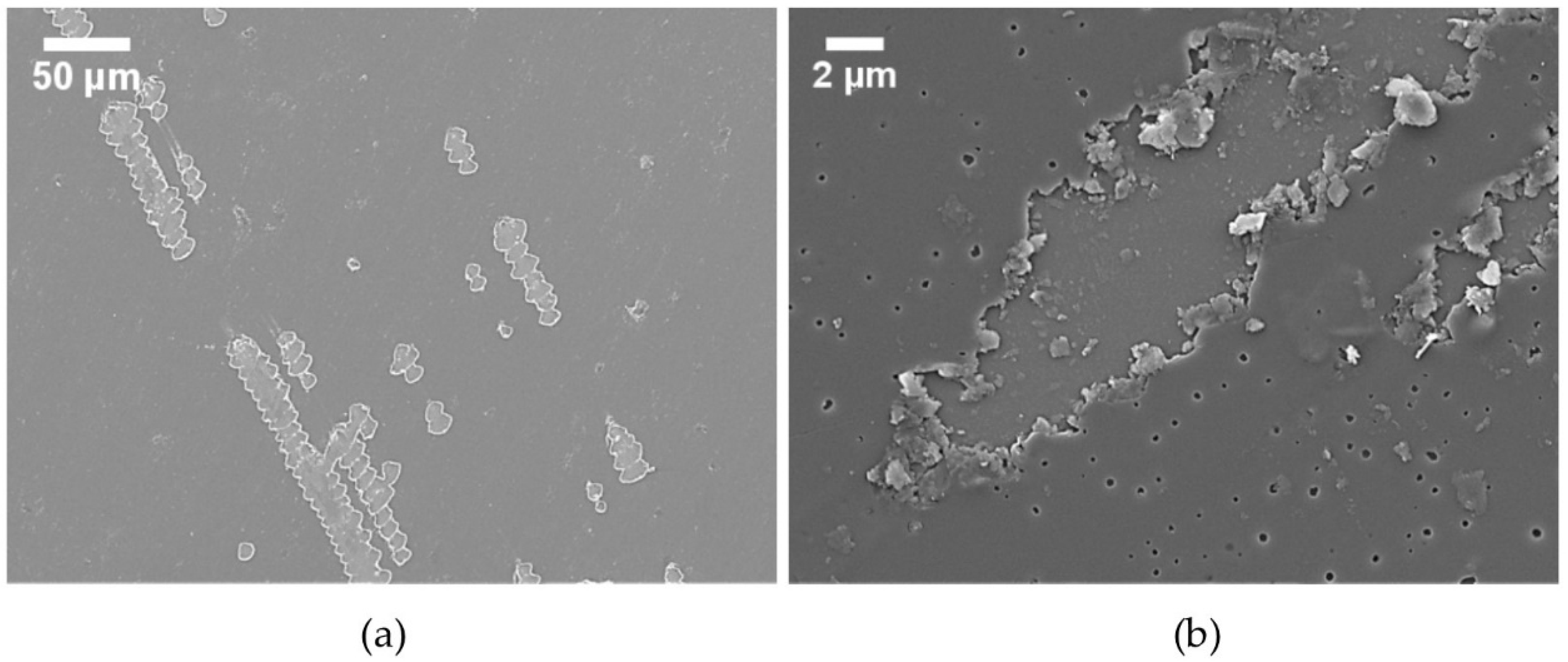
3.1.1. Coating Defects and Coating Failure
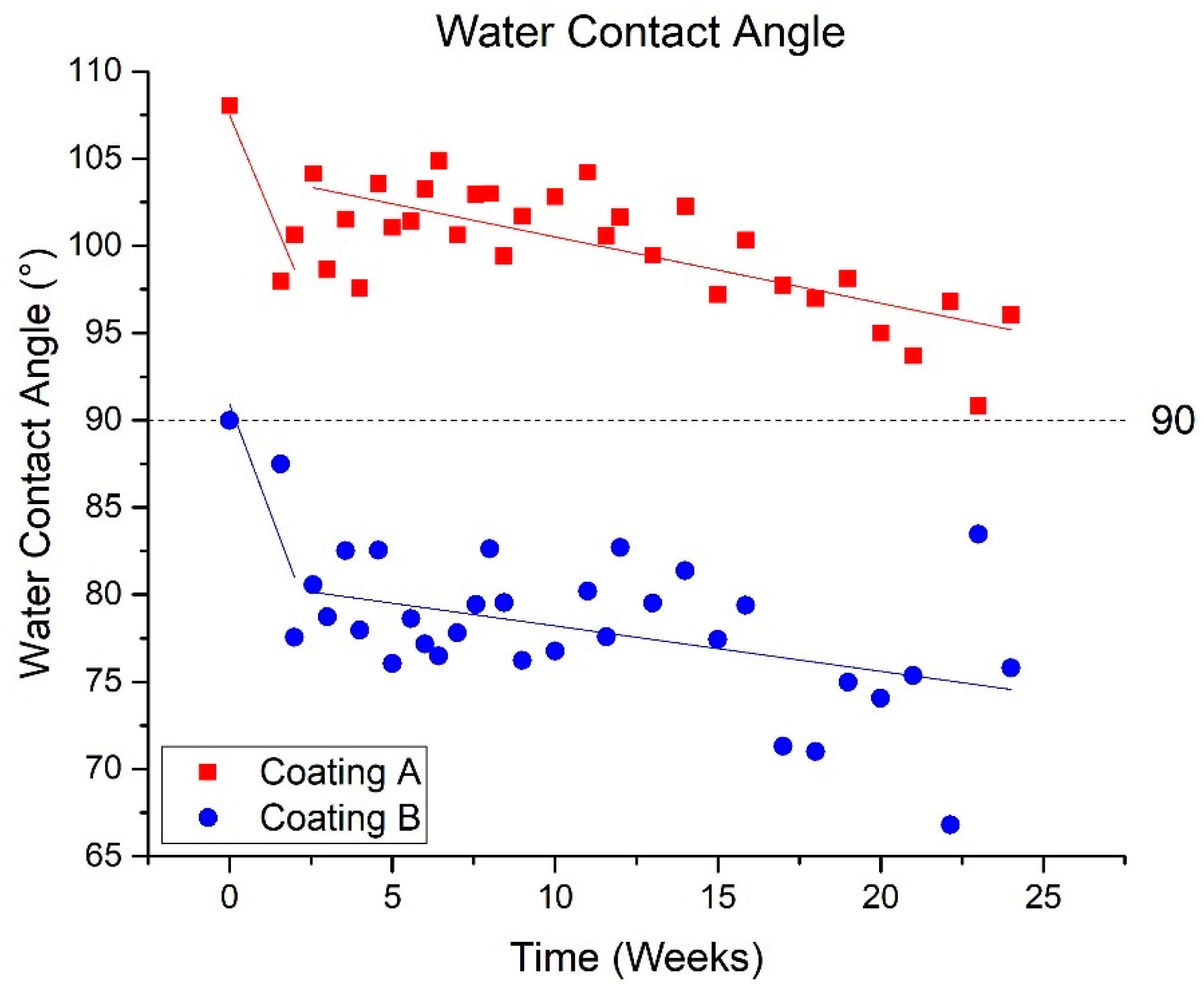
3.1.2. Water Contact Angle
3.2. Soiling
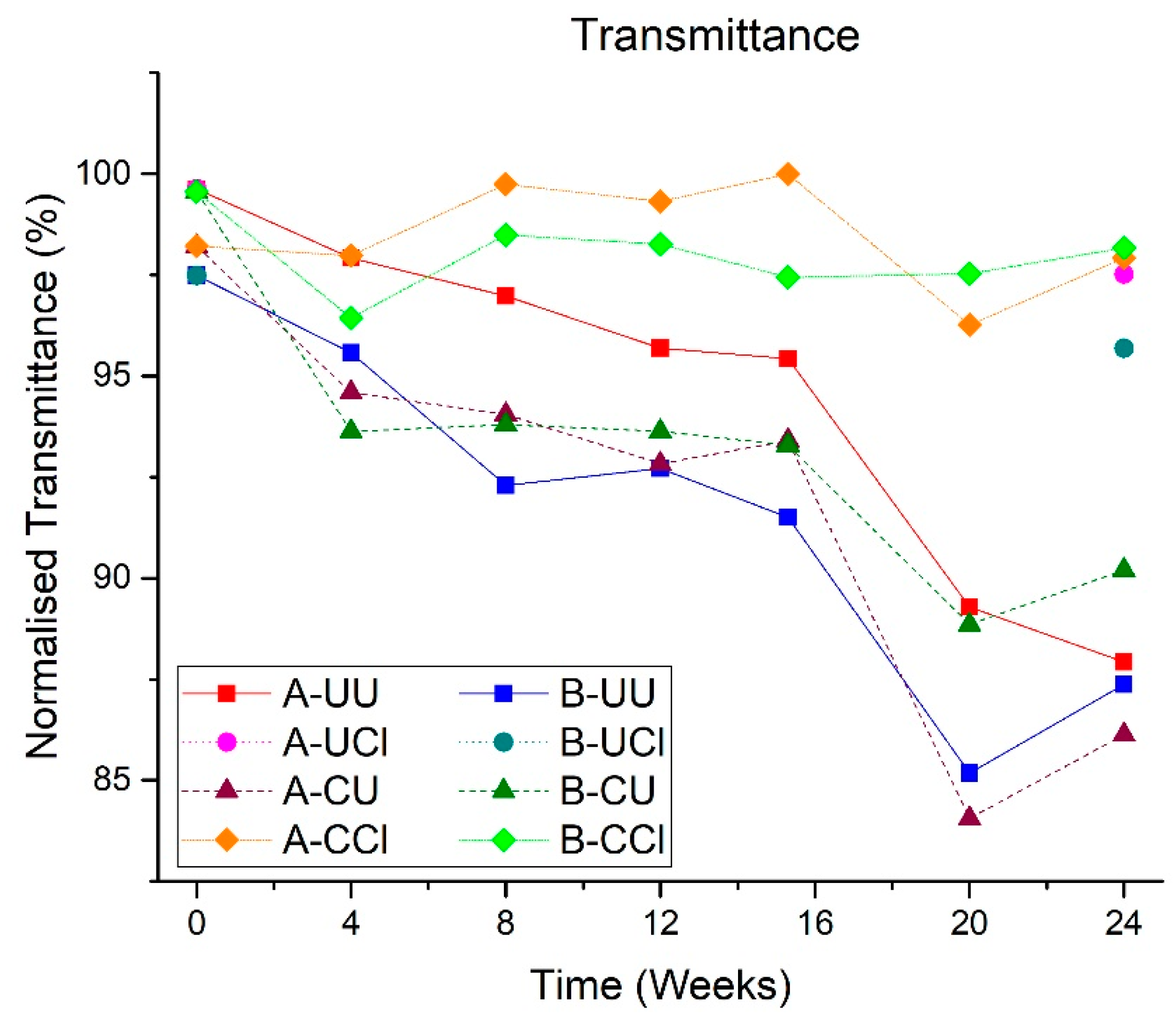
3.2.1. Transmittance
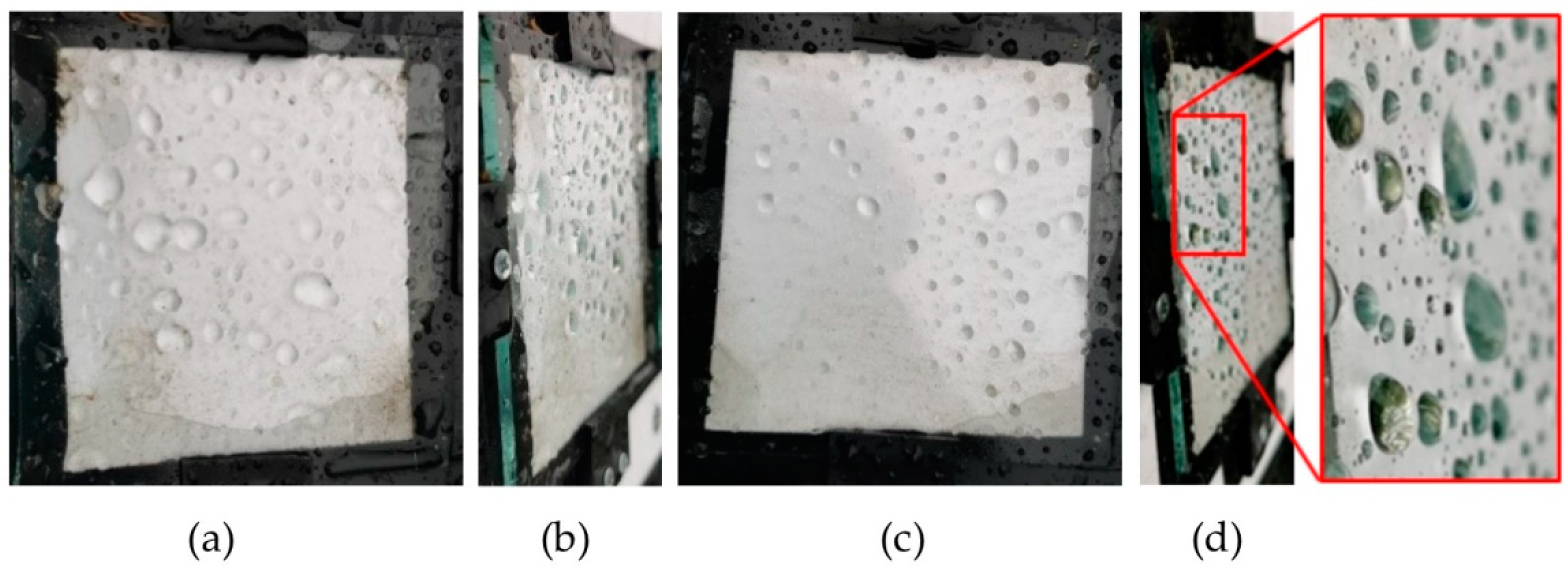
3.2.2. Droplet Retention
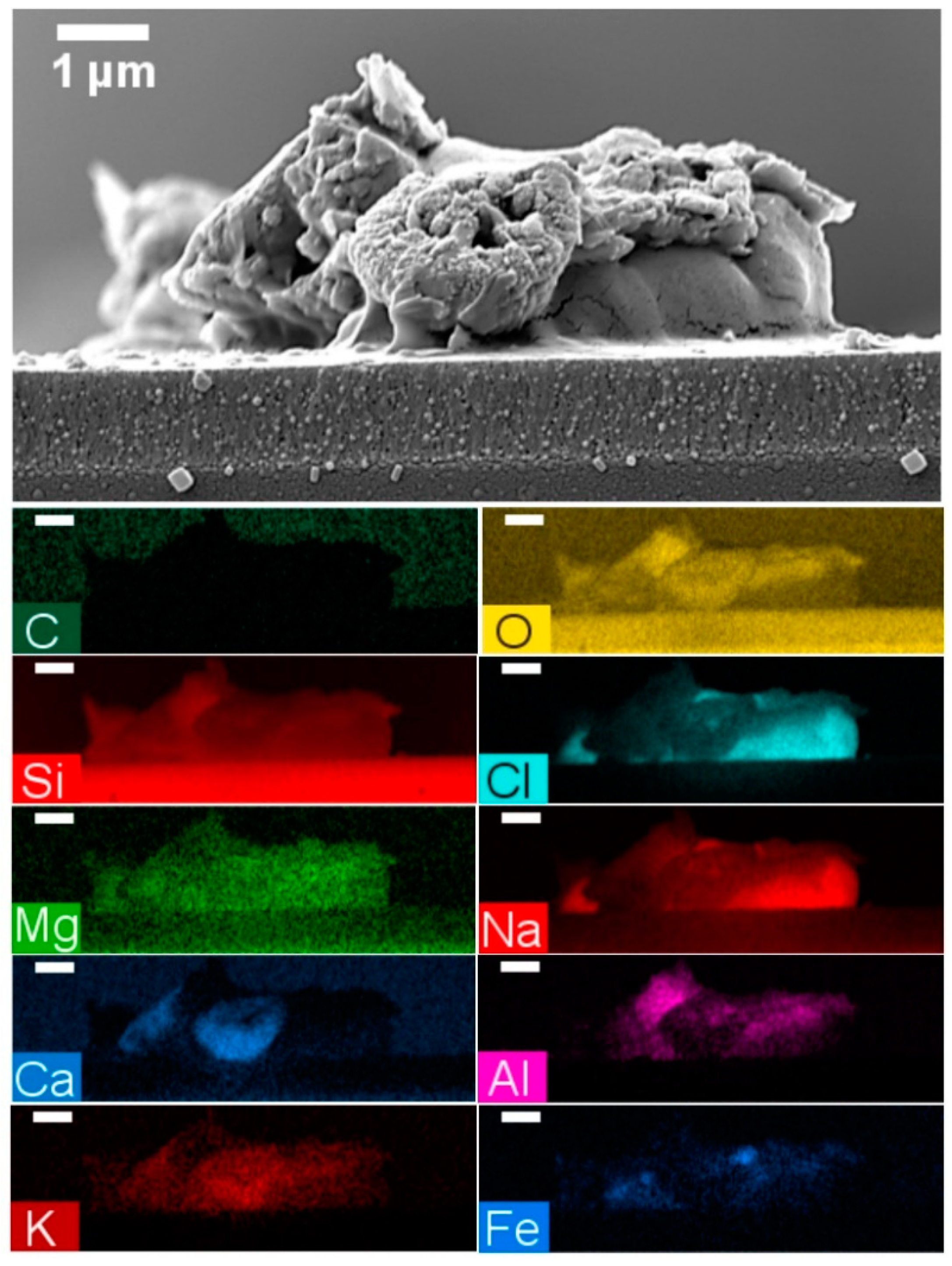
3.2.3. Cementation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sayyah, A.; Horenstein, M.N.; Mazumder, M.K. Energy yield loss caused by dust deposition on photovoltaic panels. Sol. Energy 2014, 107, 576–604. [Google Scholar] [CrossRef]
- Sarver, T.; Al-Qaraghuli, A.; Kazmerski, L.L. A comprehensive review of the impact of dust on the use of solar energy: History, investigations, results, literature, and mitigation approaches. Renew. Sustain. Energy Rev. 2013, 22, 698–733. [Google Scholar] [CrossRef]
- Bhaduri, S.; Zachariah, S.; Kazmerski, L.L.; Kavaipatti, B.; Kottantharayil, A. Soiling loss on PV modules at two locations in India studied using a water based artificial soiling method. In Proceedings of the IEEE 44th Photovoltaic Specialist Conference (PVSC 2017), Washington, DC, USA, 25–30 June 2017; pp. 2799–2803. [Google Scholar]
- Piliougine, M.; Cañete, C.; Moreno, R.; Carretero, J.; Hirose, J.; Ogawa, S.; Sidrach-de-Cardona, M. Comparative analysis of energy produced by photovoltaic modules with anti-soiling coated surface in arid climates. Appl. Energy 2013, 112, 626–634. [Google Scholar] [CrossRef]
- Brophy, B.; Abrams, Z.; Gonsalves, P.; Christy, K. Field performance and persistence of anti-soiling coatings on photovoltaic glass. In Proceedings of the 31st European Photovoltaic Solar Energy Conference and Exhibition (EU PVSEC 2015), Hamburg, Germany, 14–18 September 2015; pp. 2598–2602. [Google Scholar]
- Polizos, G.; Sharma, J.K.; Smith, D.B.; Tuncer, E.; Park, J.; Voylov, D.; Sokolov, A.P.; Meyer, H.M., III; Aman, M. Anti-soiling and highly transparent coatings with multi-scale features. Sol. Energy Mater. Sol. Cells 2018, 188, 255–262. [Google Scholar] [CrossRef]
- Quan, Y.Y.; Zhang, L.Z. Experimental investigation of the anti-dust effect of transparent hydrophobic coatings applied for solar cell covering glass. Sol. Energy Mater. Sol. Cells 2017, 160, 382–389. [Google Scholar] [CrossRef]
- Isbilir, K.; Lisco, F.; Womack, G.; Abbas, A.; Walls, J.M. Testing of an Anti-Soiling Coating for PV Module Cover Glass. In Proceedings of the 2018 IEEE 7th World Conference on Photovoltaic Energy Conversion (WCPEC 2018) (A Joint Conference of 45th IEEE PVSC, 28th PVSEC & 34th EU PVSEC), Waikoloa Village, HI, USA, 10–15 June 2018. [Google Scholar]
- Isbilir, K.; Maniscalco, B.; Gottschalg, R.; Walls, J.M. Test Methods for Hydrophobic Coatings on Solar Cover Glass. In Proceedings of the 44th IEEE Photovoltaic Specialist Conference (PVSC 44), Washington, DC, USA, 25–30 June 2017; pp. 2827–2832. [Google Scholar]
- Strauss, B.; Lisco, F.; Bukhari, F.; Walls, J.M.; Barth, K.L. Novel Hydrophobic Coatings for Soiling Mitigation in the PV Industry: Durability and Anti-Soiling Demonstrations. In Proceedings of the 46th IEEE Photovoltaic Specialists Conference (PVSC 46), Chicago, IL, USA, 16–21 June 2019. [Google Scholar]
- Bukhari, S.F.; Lisco, F.; Bozorgzad Moghim, T.; Taylor, A.; Walls, J.M. Development of a Hydrophobic, Anti-soiling coating for PV Module Cover Glass. In Proceedings of the 46th IEEE Photovoltaic Specialists Conference (PVSC 46), Chicago, IL, USA, 16–21 June 2019. [Google Scholar]
- Jacobson, M.Z.; Jadhav, V. World estimates of PV optimal tilt angles and ratios of sunlight incident upon tilted and tracked PV panels relative to horizontal panels. Sol. Energy 2018, 169, 55–66. [Google Scholar] [CrossRef]
- Danmarks Meteorologiske Institut. Vejrarkiv. Available online: https://www.dmi.dk/vejrarkiv/ (accessed on 23 August 2019).
- Strauss, B. Investigating the Suitability of Existing Commercial Hydrophobic Coatings for Soiling Mitigation in the Photovoltaic Industry. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2019. [Google Scholar]
- Grant, D. The Surface Resistivity of a Cooled Glass Surface at the Onset of Water Vapour Condensation. Ph.D. Thesis, Durham University, Durham, UK, May 1974. [Google Scholar]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Applied Surface Science Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Elminir, H.K.; Ghitas, A.E.; Hamid, R.H.; El-Hussainy, F.; Beheary, M.M.; Abdel-Moneim, K.M. Effect of dust on the transparent cover of solar collectors. Energy Convers. Manag. 2006, 47, 3192–3203. [Google Scholar] [CrossRef]
- Ilse, K.K.; Figgis, B.W.; Naumann, V.; Hagendorf, C.; Bagdahn, J. Fundamentals of soiling processes on photovoltaic modules. Renew. Sustain. Energy Rev. 2018, 98, 239–254. [Google Scholar] [CrossRef]
- Kimber, A.; Mitchell, L.; Nogradi, S.; Wenger, H. The effect of soiling on large grid-connected photovoltaic systems in California and the Southwest Region of the United States. In Proceedings of the Conference Record of the 2006 IEEE 4th World Conference on Photovoltaic Energy Conversion (WCPEC 4), Waikoloa, HI, USA, 7–12 May 2006. [Google Scholar]
- Cuddihy, E.F. Theoretical Considerations of Soil Retention. Sol. Energy Mater. 1980, 3, 21–33. [Google Scholar] [CrossRef]
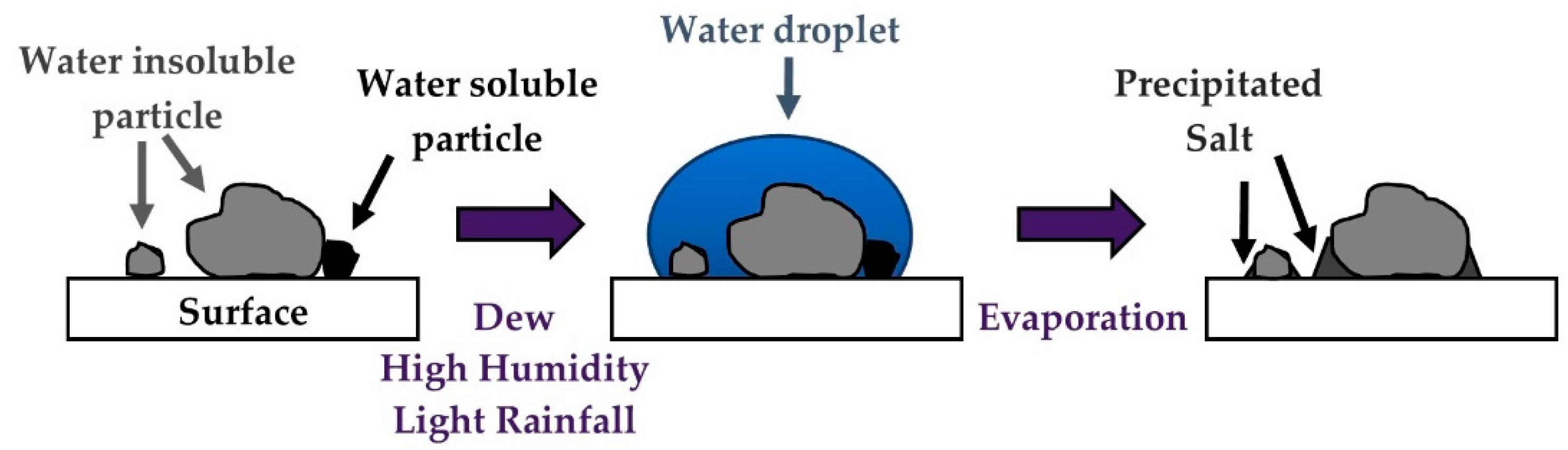
- Ilse, K.K.; Figgis, B.; Khan, M.Z.; Naumann, V.; Hagendorf, C. Dew as a Detrimental Influencing Factor for Soiling of PV Modules. IEEE J. Photovolt. 2019, 9, 287–294. [Google Scholar] [CrossRef]
- Ilse, K.K.; Figgis, B.; Werner, M.; Naumann, V.; Hagendorf, C.; Pöllmann, H.; Bagdahn, J. Comprehensive analysis of soiling and cementation processes on PV modules in Qatar. Sol. Energy Mater. Sol. Cells 2018, 186, 309–323. [Google Scholar] [CrossRef]
- Lombardo, T.; Ionescu, A.; Chabas, A.; Lefèvre, R.-A.; Ausset, P.; Candau, Y. Dose-response function for the soiling of silica-soda-lime glass due to dry deposition. Sci. Total Environ. 2010, 408, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Figgis, B.; Nouviaire, A.; Wubulikasimu, Y.; Javed, W.; Guo, B.; Ait-Mokhtar, A.; Belarbi, R.; Ahzi, S.; Rémond, Y.; Ennaoui, A. Investigation of factors affecting condensation on soiled PV modules. Sol. Energy 2018, 159, 488–500. [Google Scholar] [CrossRef]
- Isaifan, R.J.; Johnson, D.; Ackermann, L.; Figgis, B.; Ayoub, M. Evaluation of the adhesion forces between dust particles and photovoltaic module surfaces. Sol. Energy Mater. Sol. Cells 2019, 191, 413–421. [Google Scholar] [CrossRef]
- Hovmand, M.F.; Kystol, J. Atmospheric element deposition in southern Scandinavia. Atmos. Environ. 2013, 77, 482–489. [Google Scholar] [CrossRef]
- Ilse, K.K.; Rabanal, J.; Schönleber, L.; Khan, M.Z.; Naumann, V.; Hagendorf, C.; Bagdahn, J. Comparing indoor and outdoor soiling experiments for different glass coatings and microstructural analysis of particle caking processes. IEEE J. Photovolt. 2018, 8, 203–209. [Google Scholar] [CrossRef]
- Womack, G.; Isbilir, K.; Lisco, F.; Durand, G.; Taylor, A.; Walls, J.M. The performance and durability of single-layer sol-gel anti-reflection coatings applied to solar module cover glass. Surf. Coat. Technol. 2019, 358, 76–83. [Google Scholar] [CrossRef]
- Womack, G.; Kaminkski, P.M.; Abbas, A.; Isbilir, K.; Gottschalg, R.; Walls, J.M. Performance and durability of broadband antireflection coatings for thin film CdTe solar cells. J. Vac. Sci. Technol. A 2017, 35, 021201. [Google Scholar] [CrossRef]
- Kaminski, P.M.; Womack, G.; Walls, J.M. Broadband anti-reflection coatings for thin film photovoltaics. In Proceedings of the 40th IEEE Photovoltaic Specialist Conference (PVSC 2014), Denver, CO, USA, 8–13 June 2014; pp. 2778–2783. [Google Scholar]
- Bhaduri, S.; Alath, A.; Mallick, S.; Shiradkar, N.S.; Kottantharayil, A. Identification of stressors leading to degradation of anti-soiling coating in warm and humid climate zones. IEEE J. Photovolt. 2019, 10. [Google Scholar] [CrossRef]




| Week | Composition of Cleaned A (at%) | F reduction | ||||||
|---|---|---|---|---|---|---|---|---|
| F1s | O1s | C1s | Si2p | N1s | Cl2p | K2p | ||
| 0 | 28.2 | 22.9 | 36.4 | 10.8 | 1.7 | - | - | |
| 4 | 23.1 | 23.4 | 38.5 | 11.2 | 1.9 | 1.1 | 0.9 | 18.1% |
| 24 | 16.0 | 27.2 | 41.0 | 13.0 | 2.0 | 0.8 | - | 43.2% |
| Figure | Description | Composition (at%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Si | F | Na | Cl | Mg | Ca | Al | K | Fe | ||
| Figure 13 | Dust | 72.0 | 23.6 | 2.4 | 1.2 | 0.3 | 0.1 | 0.1 | 0.1 | |||
| Figure 14a | Pollen | 44.7 | 34.5 | 12.3 | 4.5 | 0.9 | 1.6 | 1.6 | 0.4 | 0.1 | ||
| Figure 14b | Pollen | 69.5 | 20.5 | 2.9 | 6.0 | 0.2 | 0.5 | 0.1 | 0.1 | 0.2 | ||
| Figure 14c | Dust | 64.7 | 24.8 | 3.2 | 5.2 | 0.7 | 0.2 | 0.1 | 0.1 | 0.9 | 0.1 | 0.1 |
| Figure 14d | Cluster | 62.9 | 28.6 | 5.8 | 1.7 | 0.4 | 0.3 | 0.2 | 0.1 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oehler, G.C.; Lisco, F.; Bukhari, F.; Uličná, S.; Strauss, B.; Barth, K.L.; Walls, J.M. Testing the Durability of Anti-Soiling Coatings for Solar Cover Glass by Outdoor Exposure in Denmark. Energies 2020, 13, 299. https://doi.org/10.3390/en13020299
Oehler GC, Lisco F, Bukhari F, Uličná S, Strauss B, Barth KL, Walls JM. Testing the Durability of Anti-Soiling Coatings for Solar Cover Glass by Outdoor Exposure in Denmark. Energies. 2020; 13(2):299. https://doi.org/10.3390/en13020299
Chicago/Turabian StyleOehler, Gizelle C., Fabiana Lisco, Farwah Bukhari, Soňa Uličná, Ben Strauss, Kurt L. Barth, and John M. Walls. 2020. "Testing the Durability of Anti-Soiling Coatings for Solar Cover Glass by Outdoor Exposure in Denmark" Energies 13, no. 2: 299. https://doi.org/10.3390/en13020299
APA StyleOehler, G. C., Lisco, F., Bukhari, F., Uličná, S., Strauss, B., Barth, K. L., & Walls, J. M. (2020). Testing the Durability of Anti-Soiling Coatings for Solar Cover Glass by Outdoor Exposure in Denmark. Energies, 13(2), 299. https://doi.org/10.3390/en13020299