Complex Investigation of High Efficiency and Reliable Heterojunction Solar Cell Based on an Improved Cu2O Absorber Layer
Abstract
1. Introduction
2. Numerical Modeling of Cu2O Heterojunction Solar Cell
2.1. Diagram of the Solar Cell Simulation Model
2.2. Electrical Modeling and Simulation Results for the Two Subcells of the Tandem Heterojunction Solar Cell
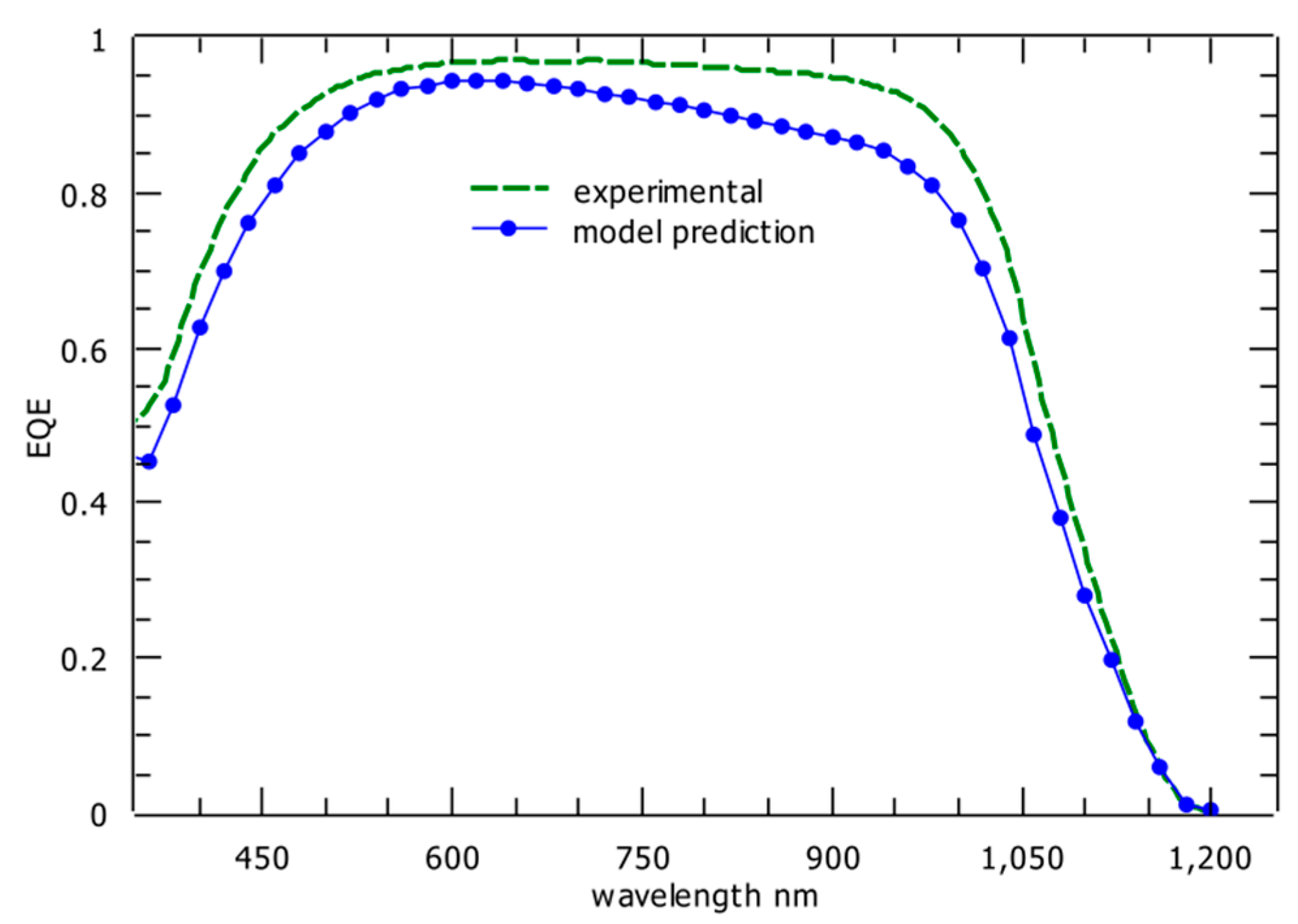
2.3. Optical Modeling and Simulation Results for the Top Subcell
2.4. Relevance of the Best Simulation Tools for Numerical Modeling and Optimization of Metal Oxide Solar Devices
3. Experimental Investigation of N-Doped Cu2O Absorber Layer
3.1. Cu2O Thin Film Fabrication and Overall Characterization
3.2. Morphological and Structural Characterization of the Cu2O:N Films
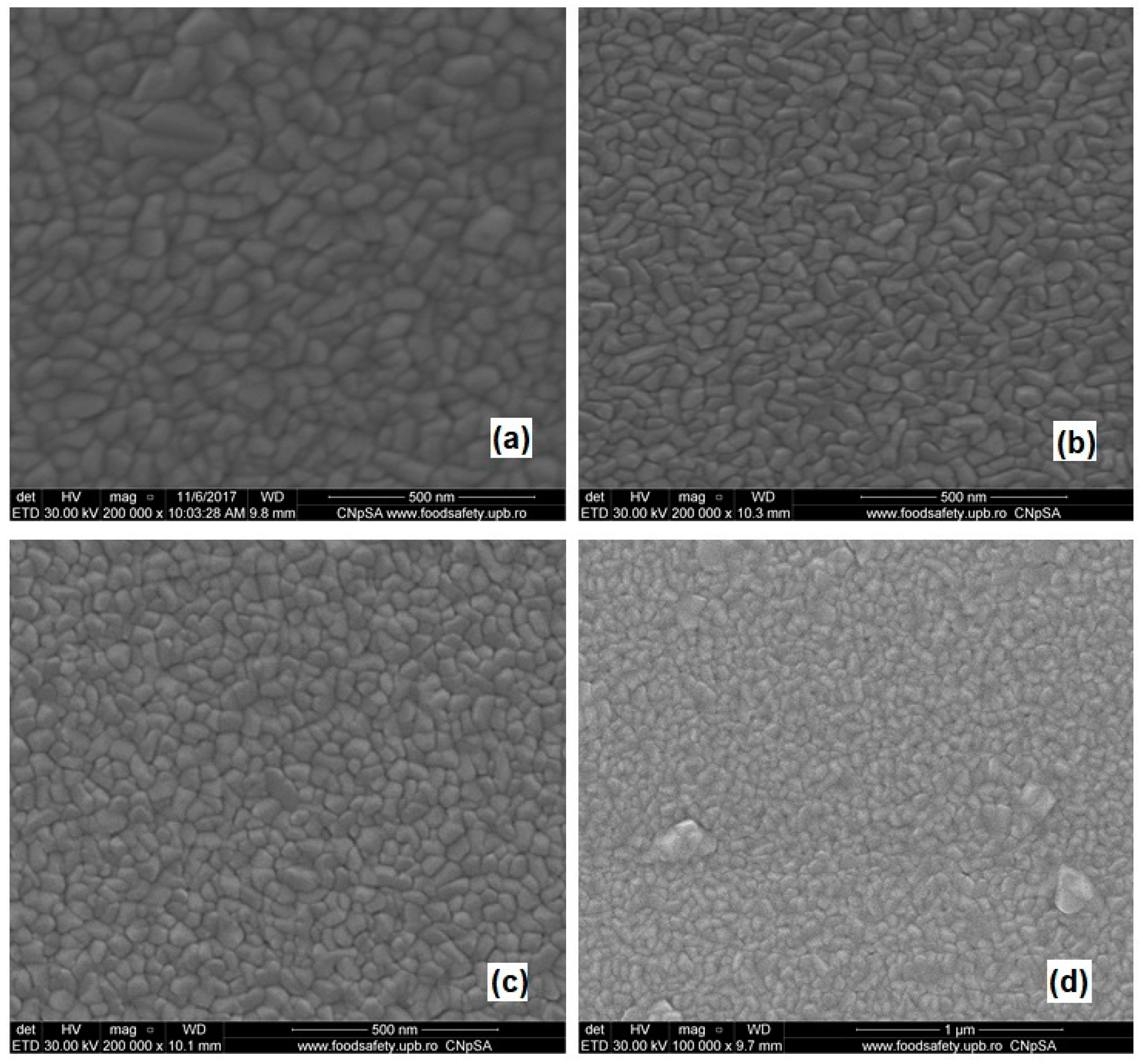
3.2.1. Scanning Electron Microscopy (SEM)
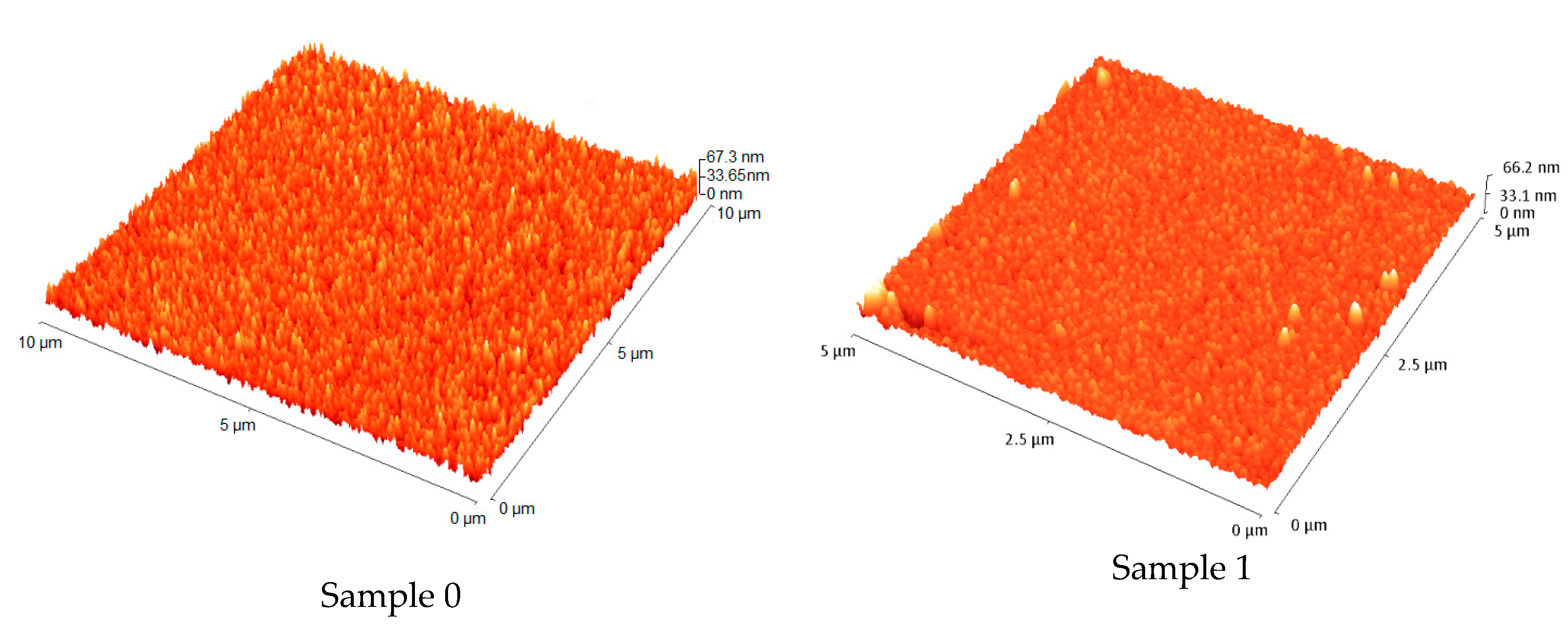
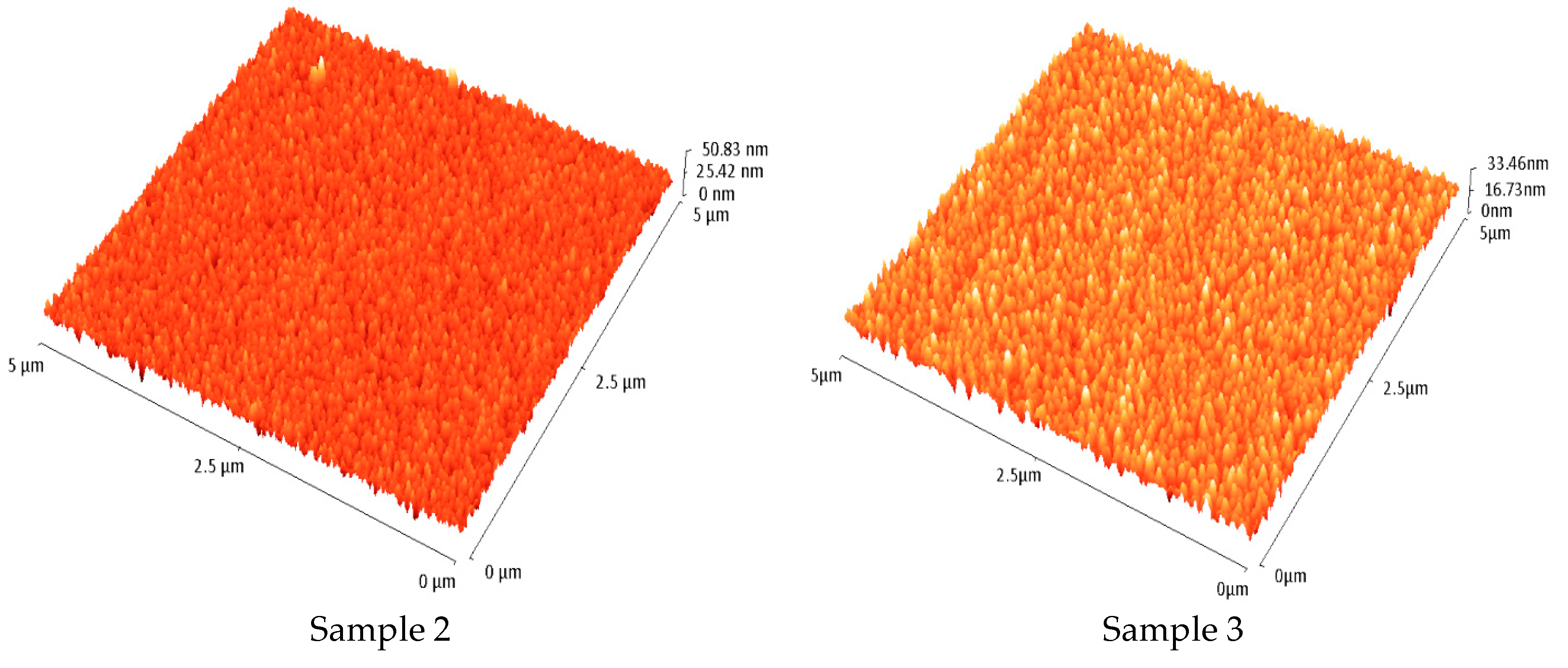
3.2.2. Atomic Force Microscopy (AFM)
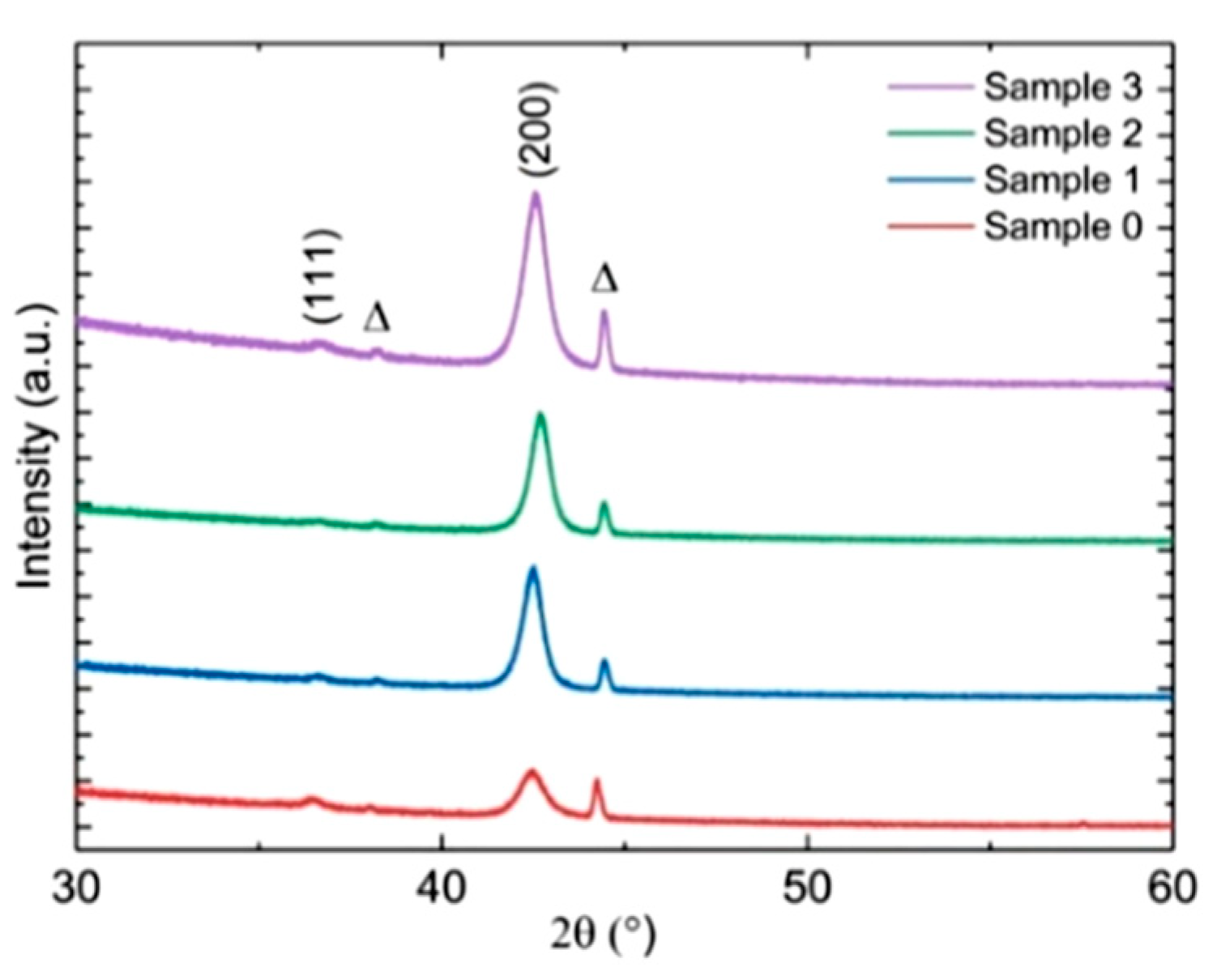
3.2.3. Structural Characterization by XRD
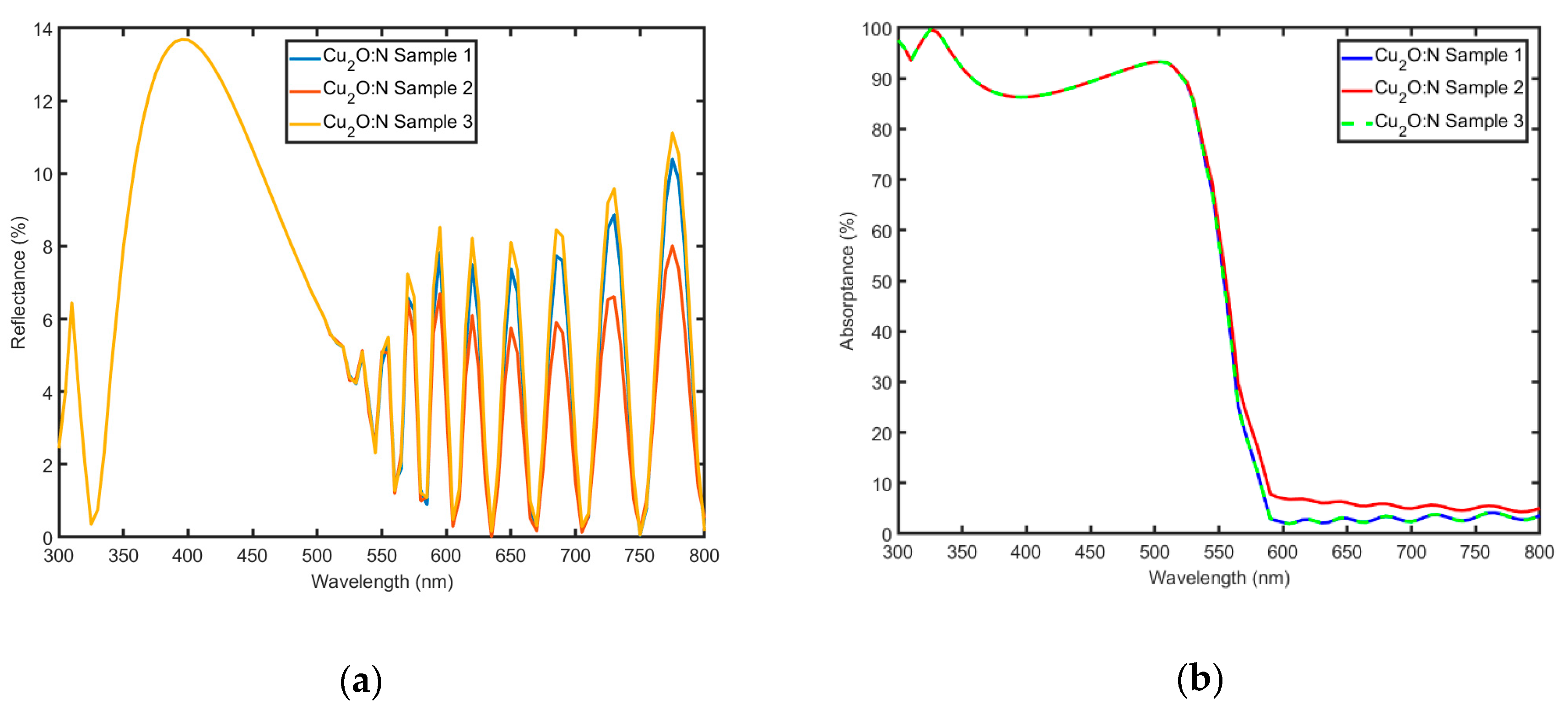
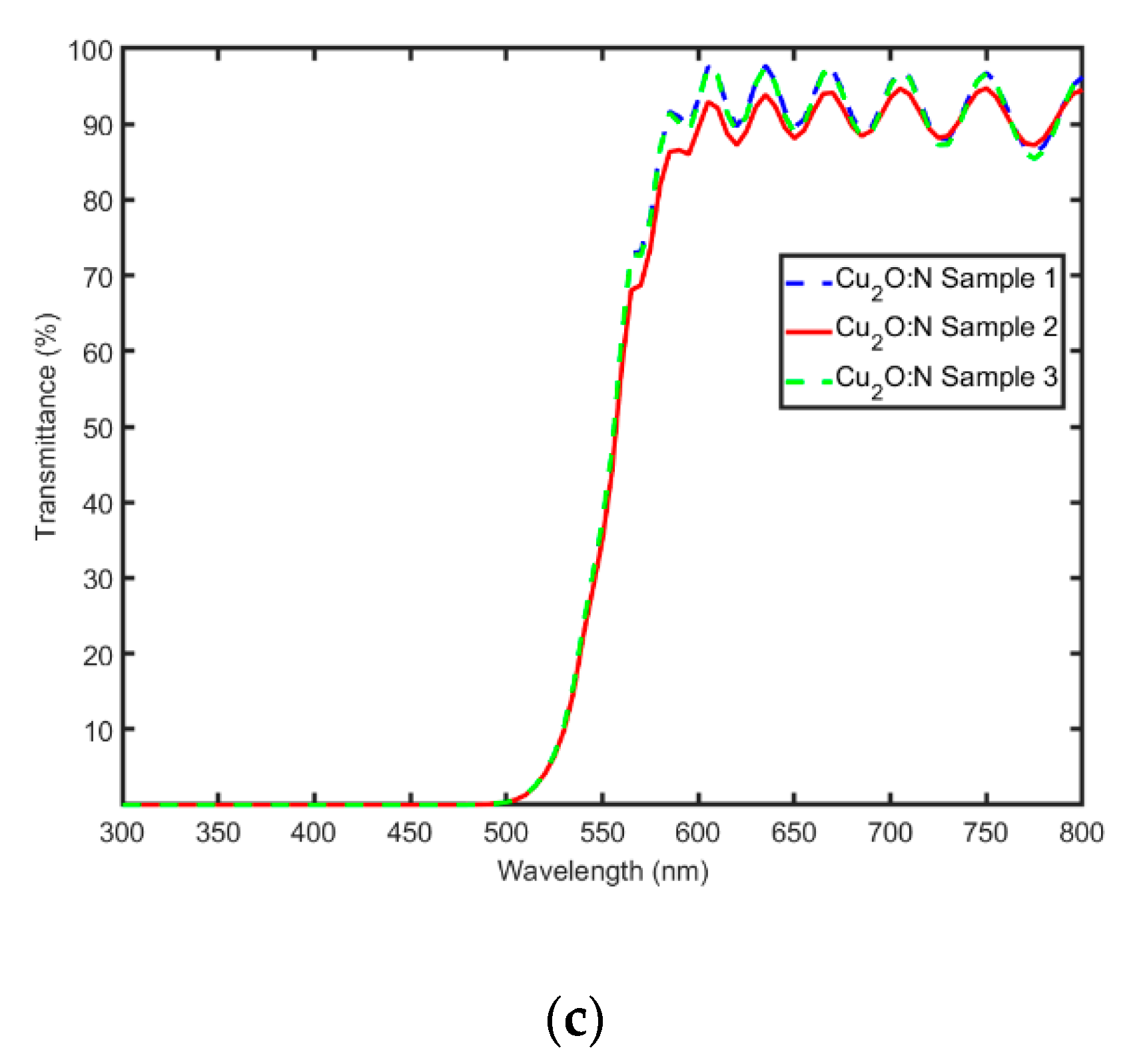
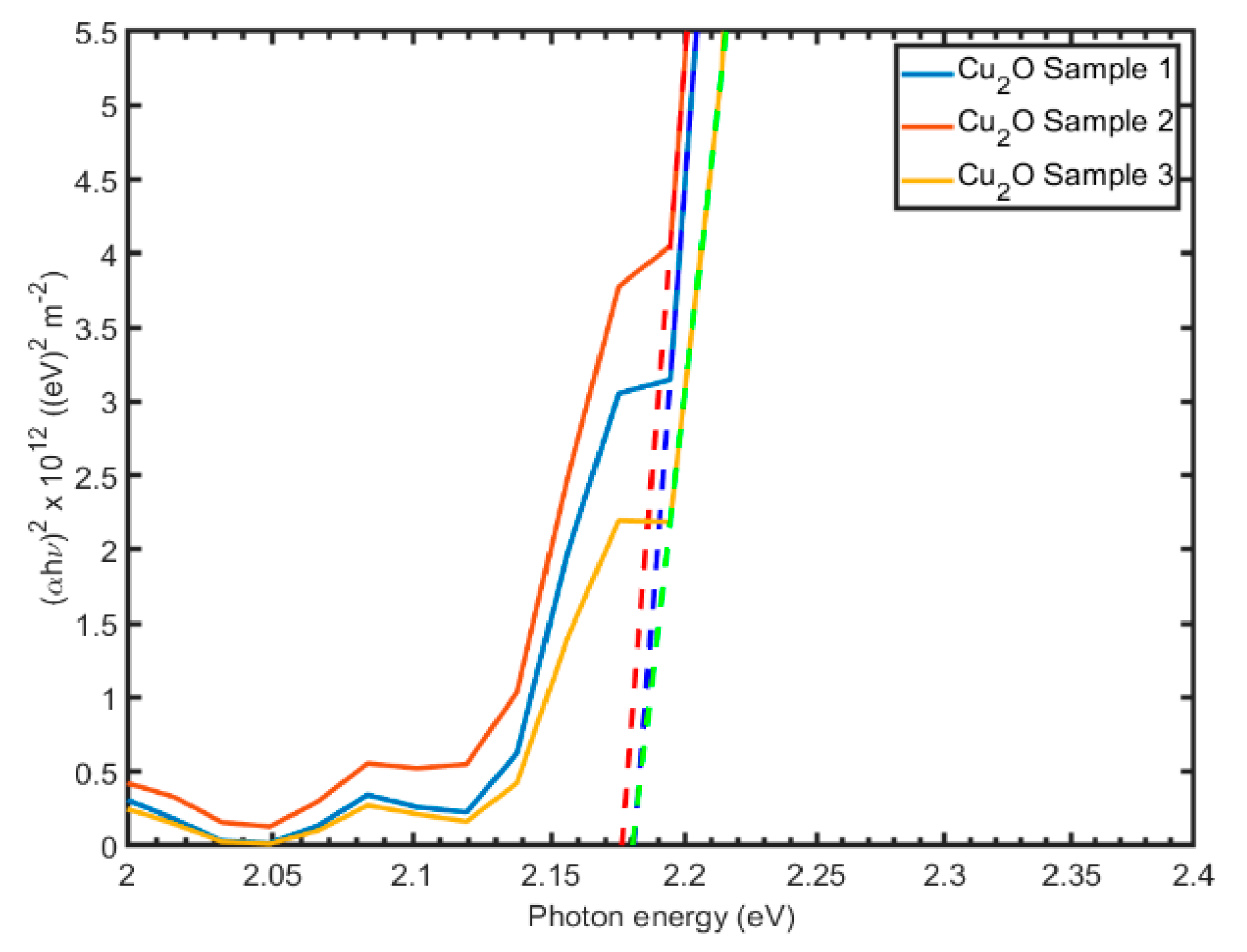
3.3. Optical Characterization
3.3.1. FTIR Spectroscopy
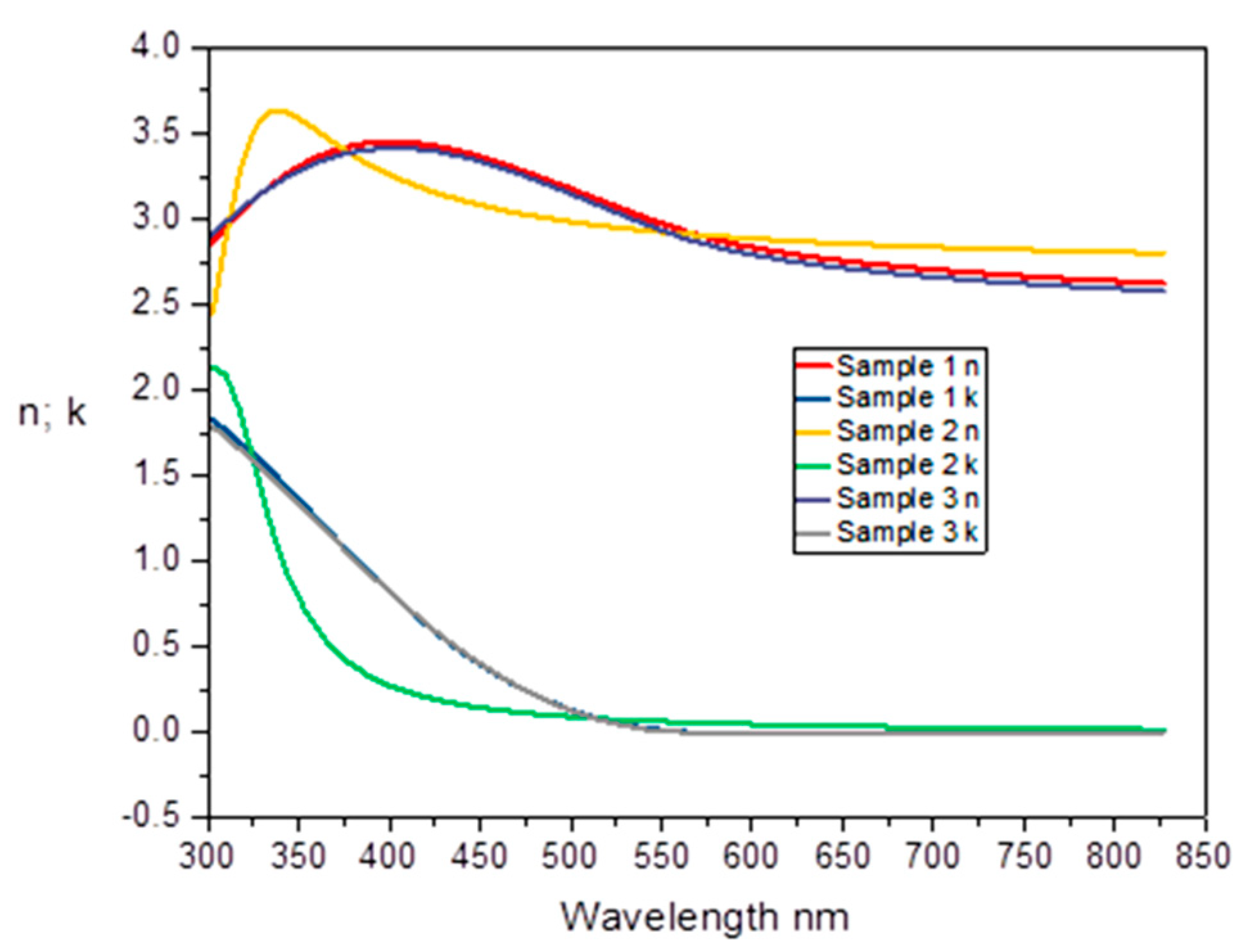
3.3.2. Spectroscopic Ellipsometry (SE)
3.4. General Comments on Experimental Investigation and Prospects of Heterojunction Cuprous Oxide Solar Cells with Nitrogen
- -
- The doped samples are characterized by higher whole carrier densities, lower carrier mobilities, and lower resistivities in comparison with the undoped sample;
- -
- The SEM images showed the improvement in the size uniformity of the deposited Cu2O layers due to N presence;
- -
- The AFM measurements determined that the roughness is slightly influenced by the N doping, which could affect the electrical properties of solar cells. The histograms derived from the AFM images showed the surface profile height for the studied Cu2O:N samples and emphasized their roughness deviation;
- -
- The X-ray analysis of Cu2O films put in evidence that all such films are polycrystalline and nitrogen doping does not show any significant change in the XRD pattern. A reduced resistivity was achieved in the N-doped samples compared to the undoped sample;
- -
- The FTIR spectra of the samples displayed peaks corresponding to the vibrational modes of Si-O belonging to the quartz substrate;
- -
- The SE analysis stressed the N influence over the optical properties of the Cu2O thin films. The changes of refraction and extinction coefficients were given by the Cu2O:N thin films associated with the structural change in Cu2O induced by N-doping.
- -
- regulation of the band gap enabling increased transmittance;
- -
- improved carrier lifetime and transport;
- -
- increased conversion efficiency of the solar cell device.
4. Reliability Study
4.1. Methodological Tools
- (1)
- Qualitative accelerated test: offers information regarding the solar cell malfunctions or failure ways;
- (2)
- High accelerated life test (HALT) offers information regarding the solar cell failure mechanisms and average lifetime;
- (3)
- High accelerated stress screening (HASS) offers information regarding the solar cell operating time and failure rate within the operating temperature range
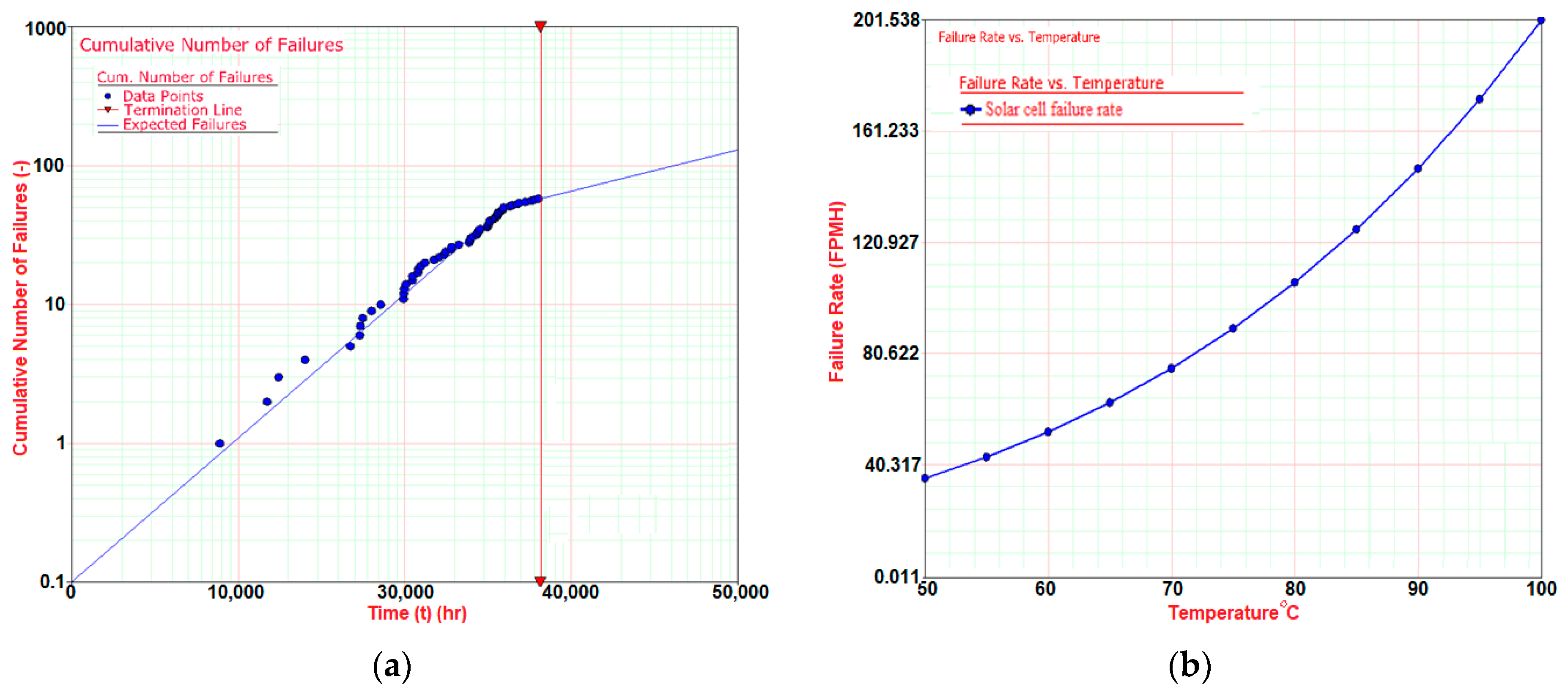
4.2. Solar Cells Thermal Stability Characteristics
4.3. Further Work
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Miska, P.; Pilloud, D.; Horwat, D.; Mücklich, F.; Pierson, J.F. Transmittance enhancement and optical band gap widening of Cu2O thin films after air annealing. J. Appl. Phys. 2014, 115, 073505. [Google Scholar] [CrossRef]
- Jung, Y.S.; Choi, H.W.; Kim, K.H. Properties of p-type N-doped Cu2O thin films prepared by reactive sputtering. Jpn. J. Appl. Phys. 2014, 53, 11RA10. [Google Scholar] [CrossRef]
- Siddiqui, H.; Parra, M.R.; Pandey, P.; Singh, N.; Qureshi, M.S.; Haque, F.Z. A review: Synthesis, characterization and cell performance of Cu2O based material for solar cells. Orient. J. Chem. 2012, 28, 1533–1545. [Google Scholar] [CrossRef]
- Wang, Y.; Ghanbaja, J.; Horwat, D.; Yu, L.; Pierson, J.F. Nitrogen chemical state in N-doped Cu2O thin films. Appl. Phys. Lett. 2017, 110, 131902. [Google Scholar] [CrossRef]
- Sberna, P.M.; Crupi, I.; Moscatelli, F.; Privitera, V.; Simone, F.; Miritello, M. Sputtered cuprous oxide thin films and nitrogen doping by ion implantation. Thin Solid Film. 2016, 600, 71–75. [Google Scholar] [CrossRef]
- Mitroi, R.M.; Ninulescu, V.; Fara, L. Tandem solar cells based on Cu2O and c-Si subcells in parallel configuration: Numerical simulation. Int. J. Photoenergy 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Takiguchi, Y.; Miyajima, S. Device simulation of cuprous oxide heterojunction solar cells. Jpn. J. Appl. Phys. 2015, 54, 112303. [Google Scholar] [CrossRef]
- Nordseth, O.; Fara, L.; Kumar, R.; Foss, S.E.; Dumitru, C.; Muscurel, V.F.; Drăgan, F.; Crăciunescu, D.; Bergum, K.; Haug, H.; et al. Electro-optical modeling of a ZnO/Cu2O subcell in a silicon-based tandem heterojunction solar cell. In Proceedings of the 33rd European Photovoltaic Solar Energy Conference and Exhibition, Amsterdam, The Netherlands, 25–29 September 2017; pp. 172–177. [Google Scholar] [CrossRef]
- Nordseth, O.; Haug, H.; Kumar, R.; Bergum, K.; Drăgan, F.; Craciunescu, D.; Fara, L.; Chilibon, I.; Monakhov, E.; Foss, S.E.; et al. Performance optimization of a four-terminal Cu2O/c-Si Tandem heterojunction solar cell. In Proceedings of the 35th European Photovoltaic Solar Energy Conference and Exhibition, Bruxelles, Belgium, 24–28 September 2018; pp. 29–34. [Google Scholar] [CrossRef]
- Dumitru, C.; Fara, L.; Nordseth, O.; Chilibon, I.; Kumar, R.; Svensson, B.G.; Dragan, F.; Muscurel, V.; Craciunescu, D.; Sterian, P. Electro-optical analysis and numerical modeling of Cu2O as the absorber layer in advanced solar cells. In Proceedings of the 2018 International Conference on Photovoltaic Science and Technologies (PVCon), Ankara, Turkey, 4–6 July 2018; pp. 1–7. [Google Scholar] [CrossRef]
- Chilibon, I.; Fara, L.; Nordseth, O.; Kumar, R.; Svensson, B.G.; Sterian, P.; Dumitru, C.; Dragan, F.; Muscurel, V.; Vasiliu, C. Structural and electrical analysis of Cu2O layers for solar cell application. Ann. Acad. Rom. Sci. 2018, 11, 53–60. [Google Scholar]
- Chilibon, I.; Fara, L.; Nordseth, O.; Kumar, R.; Svensson, B.G.; Sterian, P.; Dumitru, C.; Dragan, F.; Muscurel, V.; Vasiliu, C. Characterization of Cu2O thin films used in solar cells by fluorescence and FTIR spectroscopy. Ann. Acad. Rom. Sci. Ser. Sci. 2018, 11, 61–68. [Google Scholar]
- Lloyd, M.A.; Siah, S.C.; Brandt, R.E.; Serdy, J.; Johnson, S.W.; Lee, Y.S.; Buinassisi, T. Intrinsic defect engineering of cuprous oxide to enhance electrical transport properties for photovoltaic applications. In Proceedings of the IEEE 40th Photovoltaic Specialists Conference (PVSC), Denver, CO, USA, 8–13 June 2014; Volume 2016, pp. 3443–3445. [Google Scholar]
- Tolstova, Y.; Omelchenko, S.T.; Blackwell, R.E.; Shing, A.M. Polycrystalline Cu2O photovoltaic devices incorporating Zn (O, S) window layers. Sol. Energy Mat. Sol. Cells 2017, 160, 340–345. [Google Scholar] [CrossRef]
- Minami, T.; Nishi, Y.; Miyata, T. High-efficiency Cu2O-based heterojunction solar cells fabricated using a Ga2O3 thin film as n-type layer. Appl. Phys. Express 2013, 6, 044101. [Google Scholar] [CrossRef]
- Robertson, J.; Falabretti, B. Band offsets of high K gate oxides on III-V semiconductors. J. Appl. Phys. 2006, 100, 014111. [Google Scholar] [CrossRef]
- Brandt, R.E.; Young, M.; Hejin, H.P.; Dameron, A.; Chua, D.; Lee, S.Y.; Teeter, G.; Gordon, R.; Buonassisi, T. Band offsets of n-type electron-selective contacts on cuprous oxide (Cu2O) for photovoltaics. Appl. Phys. Lett. 2014, 105, 263901. [Google Scholar] [CrossRef]
- PV Lighthouse. Available online: https://www.pvlighthouse.com.au/ (accessed on 20 January 2020).
- Baker-Finch, S.C.; McIntosh, K.R. OPAL 2: Rapid optical simulation of silicon solar cells. In Proceedings of the 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 265–271. [Google Scholar] [CrossRef]
- Dumitru, C.; Muscurel, V.; Nordseth, O.; Fara, L.; Sterian, P. Optimization of electro-optical performance and material parameters for a tandem metal oxide solar cell. In Proceedings of the International Conference on Computational Science and Its Applications–ICCSA, Melbourne, Australia, 2–5 July 2018; pp. 573–582. [Google Scholar] [CrossRef]
- Murali, D.S.; Kumar, S.; Choudhary, R.J.; Wadikar, A.D.; Jain, M.K.; Subrahmanyam, A. Synthesis of Cu2O from CuO thin films: Optical and electrical properties. AIP Adv. 2015, 5, 047143. [Google Scholar] [CrossRef]
- Bennett, J.M.; Mattson, L. Introduction to Surface Roughness and Scattering; Optical Society of America: Washington, DC, USA, 1989. [Google Scholar]
- Nordseth, O.; Kumar, R.; Bergum, K.; Chilibon, I.; Fara, L.; Foss, S.E.; Monakhov, E. Nitrogen-doped Cu2O thin films for photovoltaic applications. Materials 2019, 12, 3038. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, Y.S.; Heo, J.; Siah, S.C.; Chua, D.; Brandt, R.; Kim, S.B.; Mailoa, J.P.; Buonassisi, T.; Gordon, R.G. Improved Cu2O-based solar cells using atomic layer deposition to control the Cu oxidation state at the p-n junction. Adv. Energy Mater. 2014, 4, 1301916. [Google Scholar] [CrossRef]
- JCPDS 78-2076 for Cu2O, JCPDS 03-0879 for Cu4O3, JCPDS 48-1548 for CuO, and JCPDS 85-1326 for Cu; International Centre for Diffraction Data-JCPDS: Newtown Square, PA, USA, 1996.
- Nakano, Y.; Saeki, S.; Morikawa, T. Optical bandgap widening of p-type Cu2O films by nitrogen doping. Appl. Phys. Lett. 2009, 94, 022111. [Google Scholar] [CrossRef]
- Reddy, M.H.P.; Pierson, J.F.; Uthanna, S. Structural, surface morphological, and optical properties of nanocrystalline Cu2O and CuO films formed by RF magnetron sputtering: Oxygen partial pressure effect. Phys. Status Solidi A 2012, 209, 1279–1286. [Google Scholar] [CrossRef]
- Ogwu, A.A.; Darma, T.H. A reactive magnetron sputtering route for attaining a controlled core-rim phase partitioning in Cu2O/CuO thin films with resistive switching potential. J. Appl. Phys. 2013, 113, 183522. [Google Scholar] [CrossRef]
- Su, J.; Niu, Q.; Sun, R.; An, X.; Zhang, Y. Investigation of Cu2O films sputtered with ceramic target: Effect of RF power. J. Optoelectron. Adv. M. 2018, 20, 441–444. [Google Scholar]
- Gong, J.B.; Dong, W.L.; Dai, R.C.; Wang, Z.P.; Zhang, Z.M.; Ding, Z.J. Thickness dependence of the optical constants of oxidized copper thin films based on ellipsometry and transmittance. Chin. Phys. B 2014, 23, 1–5. [Google Scholar] [CrossRef]
- Gan, J.; Venkatachalapathy, V.; Svensson, B.G.; Monakhov, E.V. Influence of target power on properties of CuxO thin films prepared by reactive radio frequency magnetron sputtering. Thin Solid Film. 2015, 594, 250–255. [Google Scholar] [CrossRef]
- Li, J.; Mei, Z.; Liu, L.; Liang, H.; Azarov, A.; Kuznetsov, A.; Liu, Y.; Ji, A.; Meng, Q.; Du, X. Probing defects in nitrogen-doped Cu2O. Sci. Rep. 2014, 4, 7240. [Google Scholar] [CrossRef] [PubMed]
- Reliability Engineering Software Products–ReliaSoft. Available online: https://www.reliasoft.com (accessed on 20 January 2019).
- Sharma, V.; Chandel, S.S. Performance and degradation analysis for long term reliability of solar photovoltaic systems: A review. Renew. Sustain. Energy Rev. 2013, 27, 753–767. [Google Scholar] [CrossRef]
- Guo, D.; Brinkman, D.; Shaik, A.R.; Ringhofer, C.; Vasileska, D. Metastability and reliability of CdTe solar cells. J. Phys. D Appl. Phys. 2018, 51, 15. [Google Scholar] [CrossRef]
- Fara, L.; Craciunescu, D. Reliability analysis of photovoltaic systems for specific applications. In Reliability and Ecological Aspects of Photovoltaic Modules; Gok, A., Ed.; Intech Open: London, UK, 2020; pp. 79–92. [Google Scholar]
- Ye, F.; Zeng, J.J.; Qiu, Y.B.; Cai, X.M.; Wang, B.; Wang, H.; Zhang, D.P.; Fan, P.; Roy, V.A.L. Deposition-rate controlled nitrogen-doping into cuprous oxide and its thermal stability. Thin Solid Film. 2019, 674, 44–51. [Google Scholar] [CrossRef]










| Parameter Name | Parameter Value |
|---|---|
| Open circuit voltage, VOC (mV) | 625.16 |
| Fill Factor, FF (%) | 76.83 |
| Series resistance, RS (ohm / cm2): | 0.196 |
| Shunt resistance, RSH (ohm / cm2): | 2382.8 |
| Sample Name | Gas Flow Conditions | Grains Size (nm) |
|---|---|---|
| Sample 0 | Ar/O2 was fixed at 42.5/7.5 sccm | 20–90 |
| Sample 1 | N-doped 1 sccm (Ar/O2 was fixed at 42.5/7.5 sccm) | 20–48 |
| Sample 2 | N-doped 3 sccm (Ar/O2 was fixed at 42.5/7.5 sccm) | 30–54 |
| Sample 3 | N-doped 1 sccm (Ar was varied at 41.5 sccm, O2 was fixed at 7.5 sccm) | 40–88 |
| Sample Deposited on Quartz | RRMS (nm) | Height Distribution Standard Deviation (nm) |
|---|---|---|
| Sample 0 | 6.3 | 7.8 |
| Sample 1 | 4.7 | 5.8 |
| Sample 2 | 4.2 | 4.5 |
| Sample 3 | 3.9 | 4.2 |
| Sample Name | νCu2O (cm−1) |
|---|---|
| Sample 1 | 608 |
| Sample 2 | 616 |
| Sample 3 | 615 |
| Sample Name | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|
| Band gap (eV): | 2.17 | 2.14 | 2.17 |
| Surface roughness (nm): | 15.9 | 19.6 | 17.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fara, L.; Chilibon, I.; Nordseth, Ø.; Craciunescu, D.; Savastru, D.; Vasiliu, C.; Baschir, L.; Fara, S.; Kumar, R.; Monakhov, E.; et al. Complex Investigation of High Efficiency and Reliable Heterojunction Solar Cell Based on an Improved Cu2O Absorber Layer. Energies 2020, 13, 4667. https://doi.org/10.3390/en13184667
Fara L, Chilibon I, Nordseth Ø, Craciunescu D, Savastru D, Vasiliu C, Baschir L, Fara S, Kumar R, Monakhov E, et al. Complex Investigation of High Efficiency and Reliable Heterojunction Solar Cell Based on an Improved Cu2O Absorber Layer. Energies. 2020; 13(18):4667. https://doi.org/10.3390/en13184667
Chicago/Turabian StyleFara, Laurentiu, Irinela Chilibon, Ørnulf Nordseth, Dan Craciunescu, Dan Savastru, Cristina Vasiliu, Laurentiu Baschir, Silvian Fara, Raj Kumar, Edouard Monakhov, and et al. 2020. "Complex Investigation of High Efficiency and Reliable Heterojunction Solar Cell Based on an Improved Cu2O Absorber Layer" Energies 13, no. 18: 4667. https://doi.org/10.3390/en13184667
APA StyleFara, L., Chilibon, I., Nordseth, Ø., Craciunescu, D., Savastru, D., Vasiliu, C., Baschir, L., Fara, S., Kumar, R., Monakhov, E., & Connolly, J. P. (2020). Complex Investigation of High Efficiency and Reliable Heterojunction Solar Cell Based on an Improved Cu2O Absorber Layer. Energies, 13(18), 4667. https://doi.org/10.3390/en13184667