Soil Bioremediation: Overview of Technologies and Trends
Abstract
1. Introduction
2. Main Soil Contaminants
- In Agriculture: reductions of soil fertility and nitrogen fixation, increase in soil erosion and nutrient depletion, sludge storage, reduction of agricultural production, and imbalance between vegetable and animal life in the soil.
- In the Environment: unavailability or low productivity of the land for crops intended for food/feed consumption, and changes in soil composition and microflora.
- In the Urban Environment: problems in waste management and issues of public health such as potable water contamination.
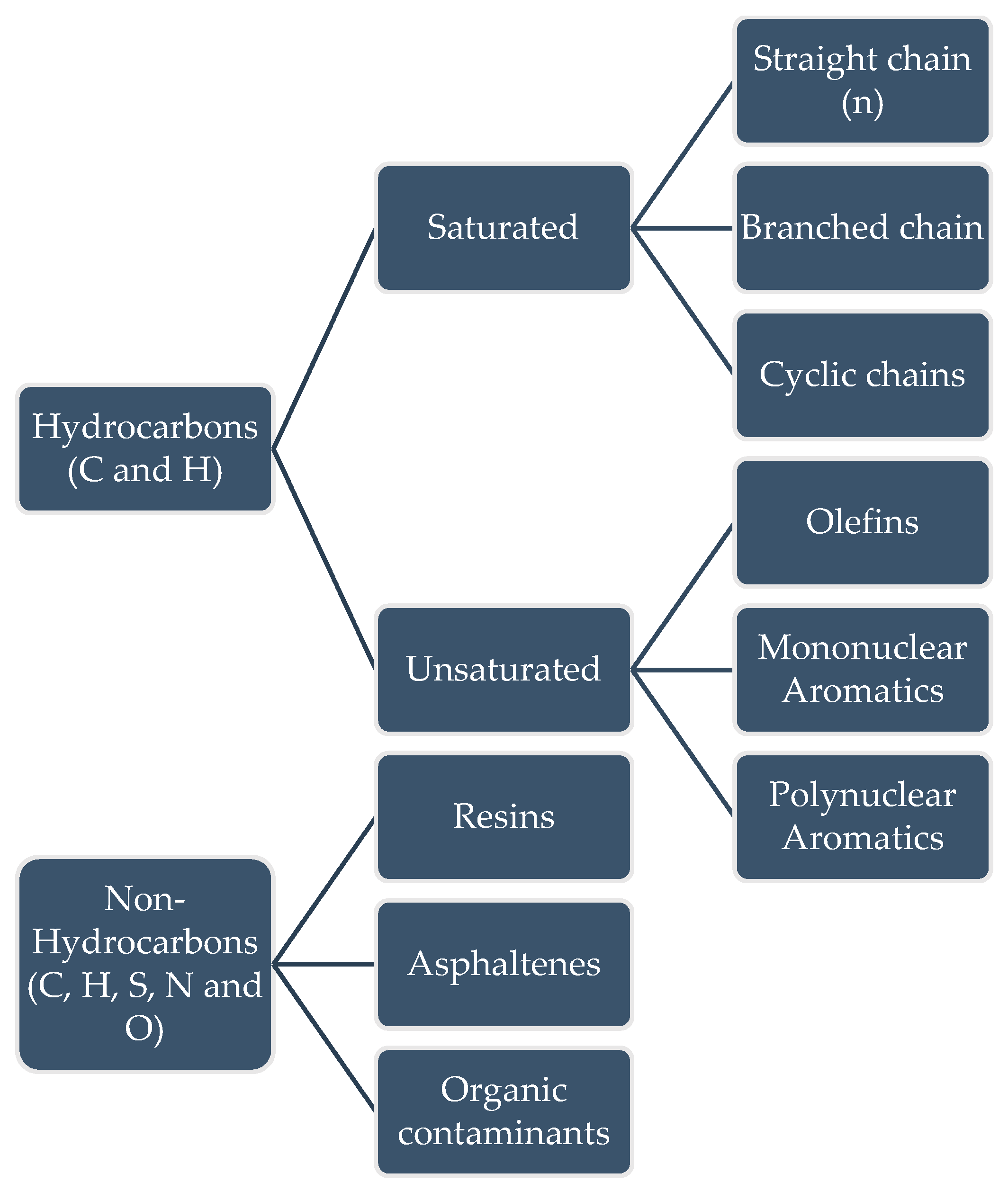
2.1. Petroleum and Derivatives
2.1.1. Pollution by Petroleum Derivatives
2.1.2. Ecotoxicity of Petroleum Derivatives
2.1.3. The BTEX Group
2.2. Heavy Metals
3. Bioremediation
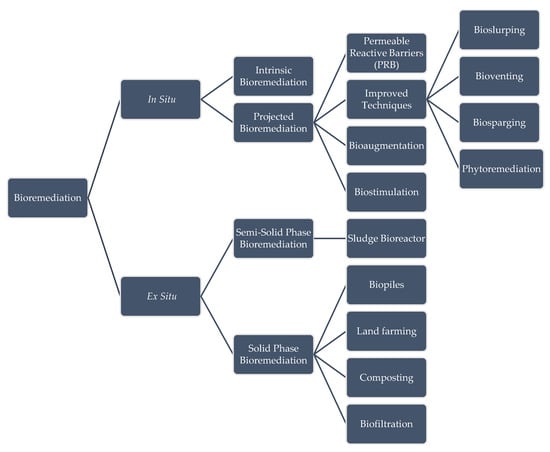
3.1. Classification
3.2. In-Situ Techniques
3.2.1. Intrinsic Bioremediation
- suited population of biodegrading microorganisms in the polluted site;
- optimum environmental conditions (temperature, pH, moisture degree, O2 concentration);
- available carbon and nitrogen sources to sustain microbial activity and growth;
- sufficient time for microorganisms to convert pollutants into less harmful products.
3.2.2. Projected Bioremediation
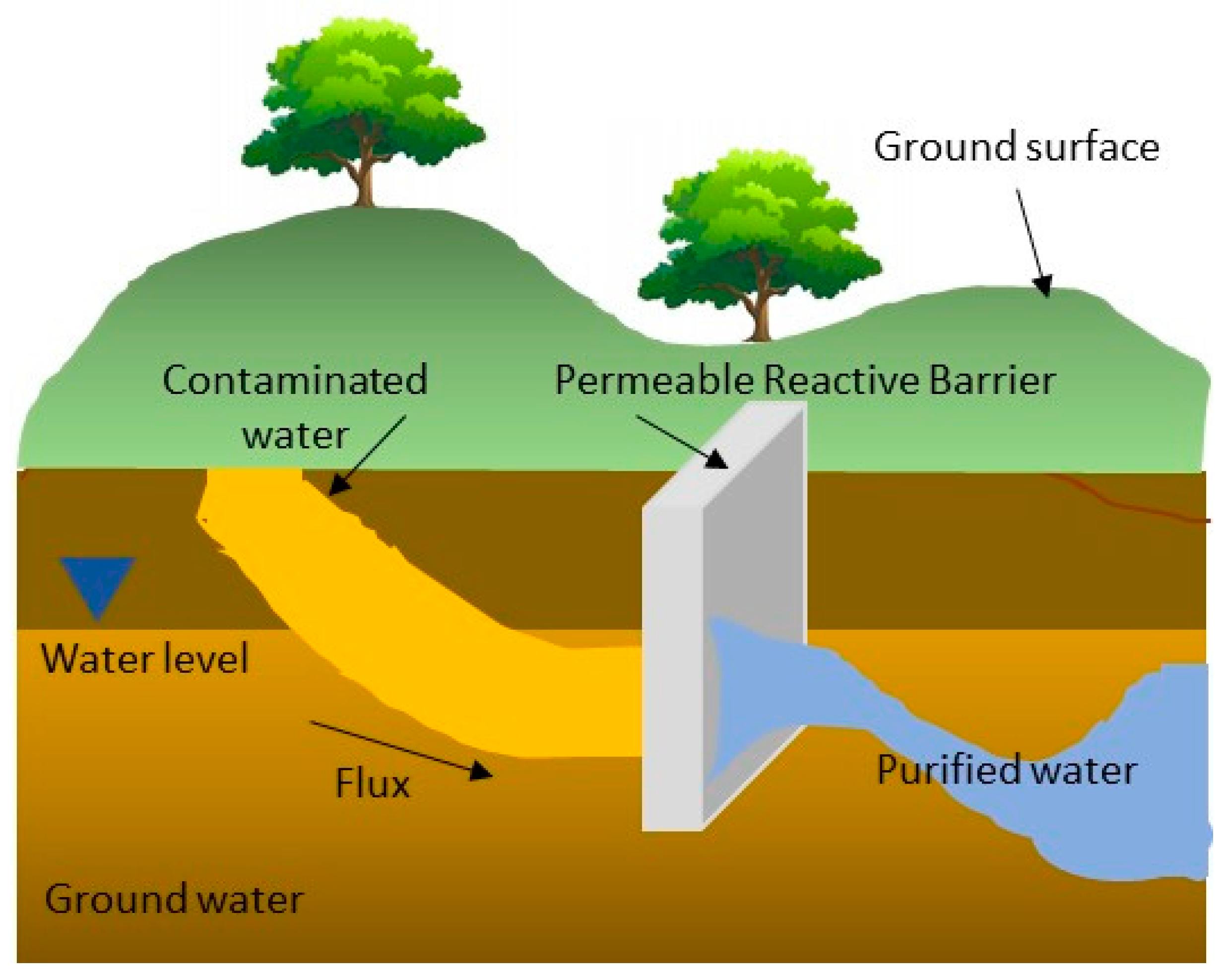
Permeable Reactive Barriers
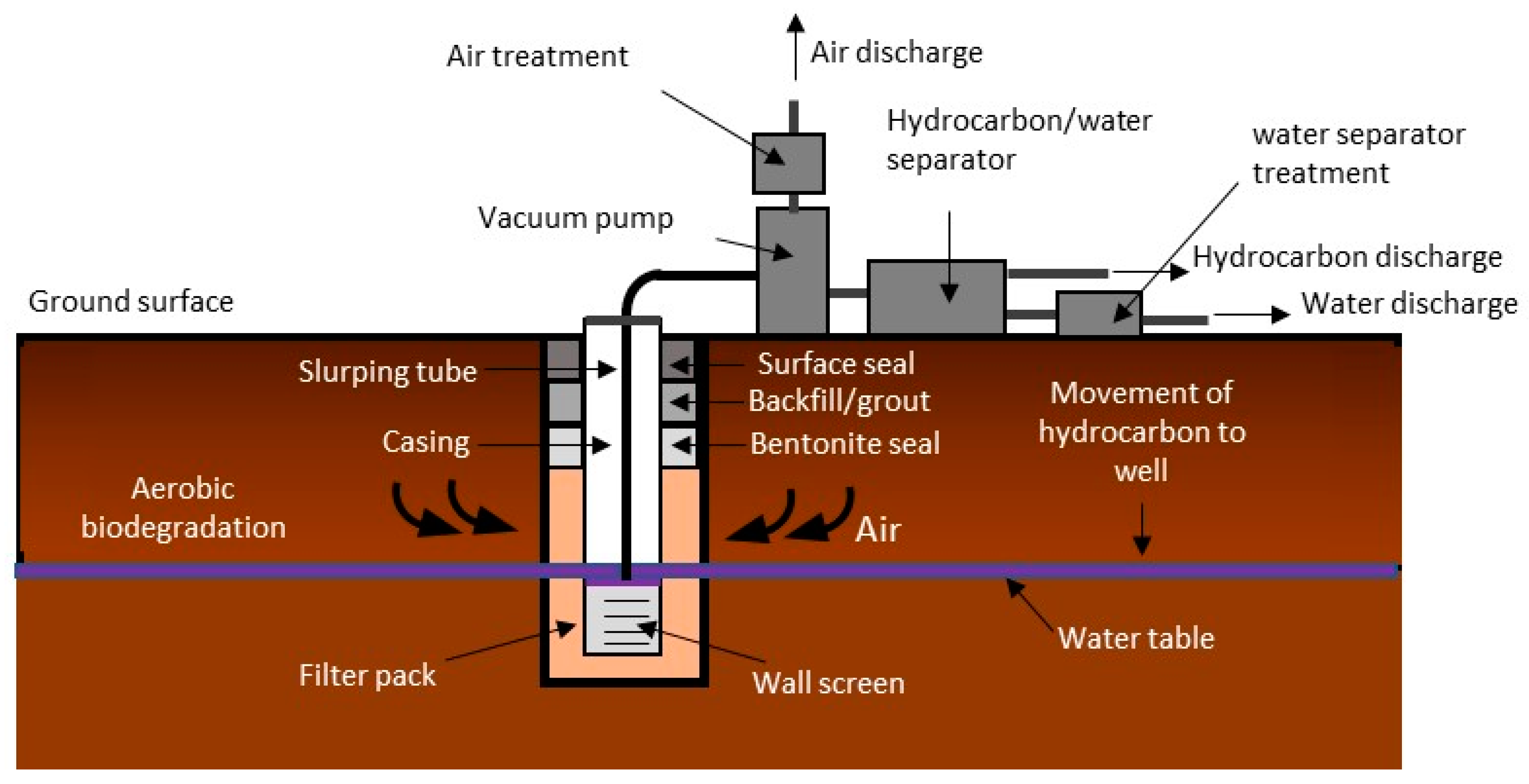
Bioslurping
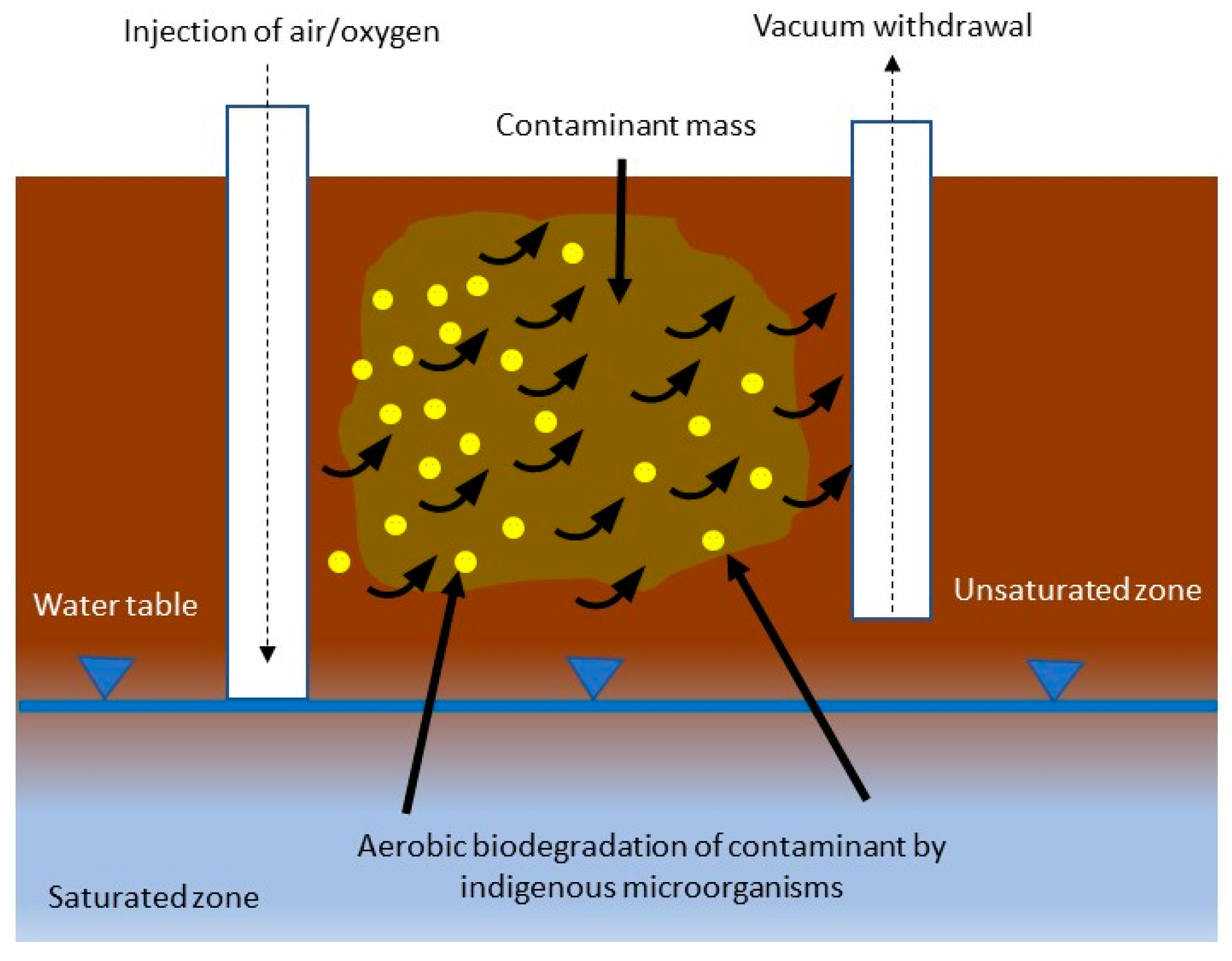
Bioventing
Biosparging
Phytoremediation
- Phytoextraction: This mechanism involves removal of the contaminant from the soil and its accumulation in some part of the plant (e.g., root, stem or leaf) [65].
- Phytotransformation or Phytodegradation: After the absorption of the contaminant by the plant, it is transformed into a less toxic form. Such a transformation can also occur on the plant’s own surface [66].
- Phytovolatilization: In this process, the plant removes the pollutant from the soil and then manages to convert it into a volatile product, releasing it into the atmosphere [67].
- Phytostimulation: Plant roots release substances (exudates) that can accelerate degradation of certain pollutants. When microorganisms contribute to degradation, the whole process is called phytostimulation [68].
- Phytostabilization: This process consists in the immobilization of pollutants in the soil, thus avoiding erosive processes and allowing the association with humus and lignin [69].
- Rhizofiltration: It is the process in which plant roots promote absorption, precipitation and removal of organic and inorganic contaminants from wastewater, groundwater and surface water [70].
- Rhizodegradation: The metabolic activity of microorganisms in the rhizosphere (fungi, bacteria, and yeasts) is 10 to 100 times greater than in soil, which allows them to effectively degrade contaminants together with natural organic nutrients [71].
Bioaugmentation and Biostimulation
3.3. Ex-Situ Techniques
3.3.1. Bioreactors
3.3.2. Biopiles
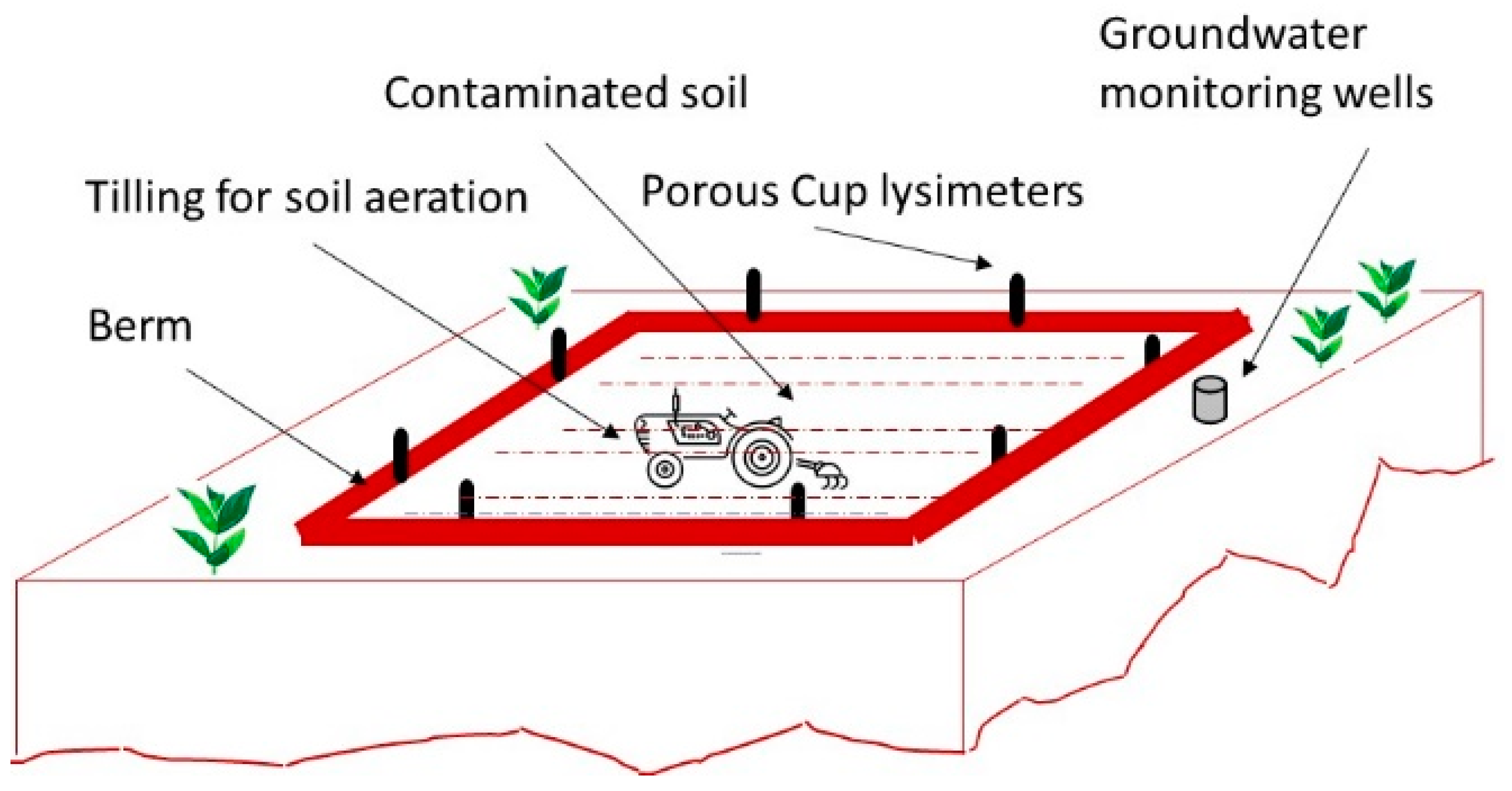
3.3.3. Landfarming
3.3.4. Composting
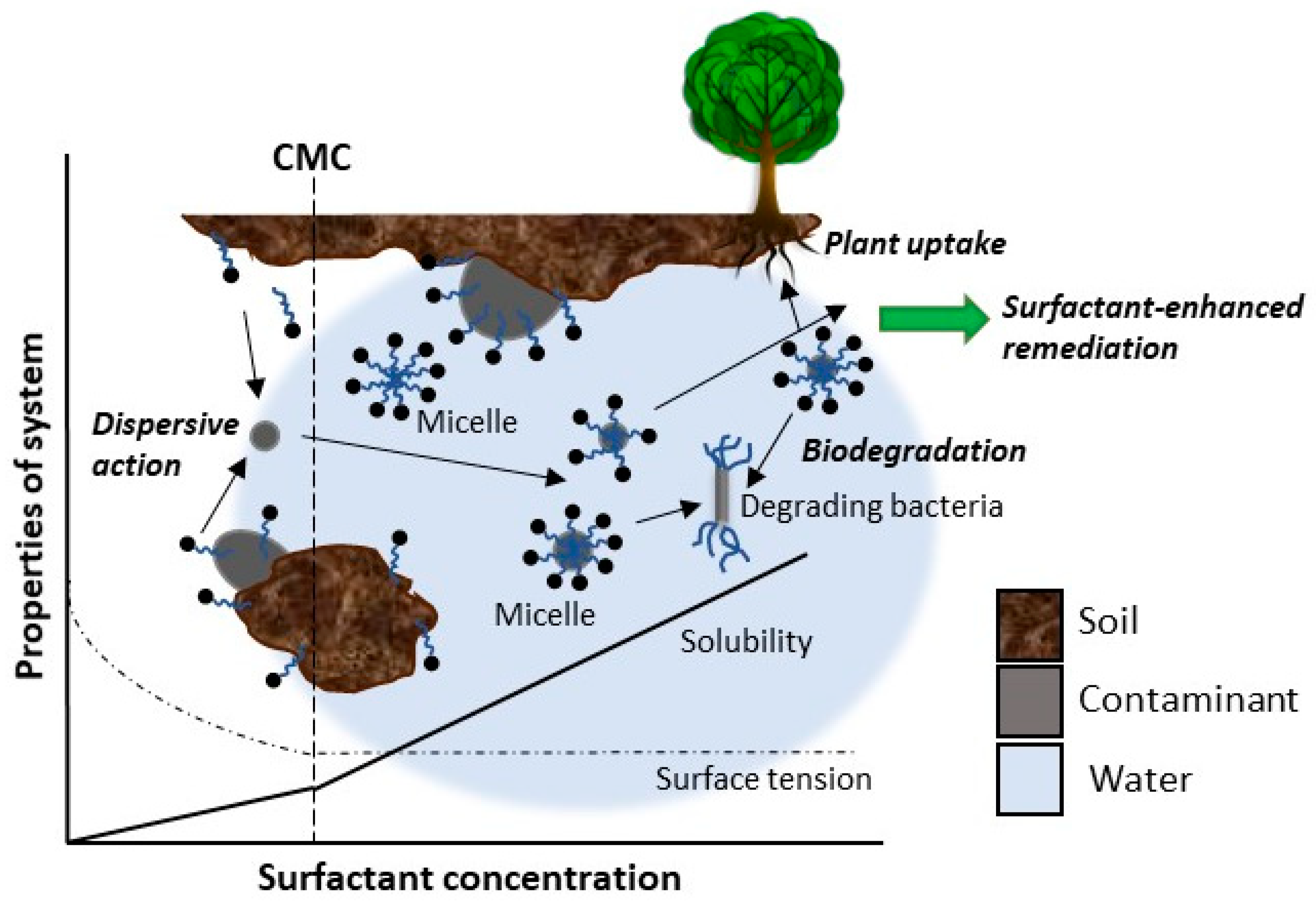
3.4. Surfactant-Enhanced Soil Bioremediation
3.4.1. Soil Washing Enhanced by Surfactants
3.4.2. Synthetic Surfactants
3.4.3. Biosurfactants
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quintella, C.M.; Mata, A.M.T.; Lima, L.C.P. Overview of bioremediation with technology assessment and emphasis on fungal bioremediation of oil contaminated soils. J. Environ. Manag. 2019, 241, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Ecological impacts of total petroleum hydrocarbons. In Total Petroleum Hydrocarbons; Springer International Publishing: Cham, Switzerland, 2020; Volume 1, pp. 95–138. [Google Scholar]
- Kumar, V.; Shahi, S.K.; Singh, S. Bioremediation: An eco-sustainable approach for restoration of contaminated sites. In Microbial Bioprospecting for Sustainable Development; Springer: Singapore, 2018; pp. 115–136. ISBN 9789811300530. [Google Scholar]
- Soleimani, M. Comparison of biological and thermal remediation methods in decontamination of oil polluted soils. J. Bioremed. Biodegrad. 2014, 5. [Google Scholar] [CrossRef]
- Okrent, D. On intergenerational equity and its clash with intragenerational equity and on the need for policies to guide the regulation of disposal of wastes and other activities posing very long-term risks. Risk Anal. 1999, 19, 877–901. [Google Scholar] [CrossRef]
- Mareddy, A.R. Environmental Impact Assessment. Theory and Practice; Technology in EIA; Butterworth-Heinemann: Oxford, UK, 2017; Chapter 13; ISBN 9780128111390. [Google Scholar]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- CETESB. Qualidade do Solo. Available online: https://cetesb.sp.gov.br/solo/poluicao/ (accessed on 30 March 2020).
- FAO. Status of the World’s Soil Resources; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; ISBN 9789251090046. [Google Scholar]
- FAO. Be the Solution to Soil. Available online: http://www.fao.org/3/Ca1087en/Ca1087en.pdf (accessed on 30 March 2020).
- BBC. The True Toll of the Chernobyl Disaster. Available online: https://www.bbc.com/future/article/20190725-will-we-ever-know-chernobyls-true-death-toll (accessed on 30 March 2020).
- Van Liedekerke, M. Proceedings of the Global Symposium on Soil Pollution 2018. In Regional Status of Soil Pollution: Europe; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; p. 976. [Google Scholar]
- Ashraf, M.A.; Maah, M.J.; Yusoff, I. Soil contamination, risk assessment and remediation. In Environmental Risk Assessment of Soil Contamination; IntechOpen: London, UK, 2014; Chapter 1; pp. 1–56. Available online: https://www.intechopen.com/books/environmental-risk-assessment-of-soil-contamination (accessed on 4 September 2020). [CrossRef]
- ANP. Petróleo e Derivados. Available online: http://www.anp.gov.br/Carregamento-Comercializacao-Autoprodutor-Autoimportador-Consumo-Em-Refinarias-E-Fafens/2-Uncategorised/709-Petroleo-E-Derivados (accessed on 30 March 2020).
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K.; Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. An overview of total petroleum hydrocarbons. In Total Petroleum Hydrocarbons; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 1–27. [Google Scholar]
- Farah, M.A. Petróleo e Seus Derivados, 1st ed.; LTC: São Paulo, Brazil, 2012; ISBN 9788521620525. [Google Scholar]
- Triggia, A.A.; Thomas, J.E. Fundamentos de Engenharia de Petróleo, 2nd ed.; Interciência: São Paulo, Brazil, 2004; Volume 1, ISBN 9788571930995. [Google Scholar]
- Umar, A.A.; Saaid, I.B.M.; Sulaimon, A.A.; Pilus, R.B.M. A review of petroleum emulsions and recent progress on water-in-crude oil emulsions stabilized by natural surfactants and solids. J. Pet. Sci. Eng. 2018, 165, 673–690. [Google Scholar] [CrossRef]
- Neto, A.P. Classificação da Qualidade de Ensaios de Planejamentos Experimentais de Processos de Biodegradação de Petroderivados Usando Lógica Fuzzy. Master’s Thesis, Universidade Católica de Pernambuco, Recife, Brazil, 2019. [Google Scholar]
- De Almeida, D.G.; Soares Da Silva, R.D.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, e1718. [Google Scholar] [CrossRef]
- Al-Turki, A.I. Assessment of effluent quality of tertiary wastewater treatment plant at buraidah city and its reuse in irrigation. J. Appl. Sci. 2010, 10, 1723–1731. [Google Scholar] [CrossRef]
- Rajabi, H.; Sharifipour, M. Geotechnical properties of hydrocarbon-contaminated soils: A comprehensive review. Bull. Eng. Geol. Environ. 2019, 78, 3685–3717. [Google Scholar] [CrossRef]
- Truskewycz, A.; Gundry, T.D.; Khudur, L.S.; Kolobaric, A.; Taha, M.; Aburto-Medina, A.; Ball, A.S.; Shahsavari, E. Petroleum hydrocarbon contamination in terrestrial ecosystems—fate and microbial responses. Molecules 2019, 24, 3400. [Google Scholar] [CrossRef]
- Izdebska-Mucha, D.; TrzcińSk, J.; Żbik, M.S.; Frost, R.L. Influence of hydrocarbon contamination on clay soil microstructure. Clay Miner. 2011, 46, 47–58. [Google Scholar] [CrossRef]
- Gao, W.; Yin, X.; Mi, T.; Zhang, Y.; Lin, F.; Han, B.; Zhao, X.; Luan, X.; Cui, Z.; Zheng, L. Microbial diversity and ecotoxicity of sediments 3 years after the Jiaozhou Bay oil spill. AMB Express 2018, 8, e79. [Google Scholar] [CrossRef] [PubMed]
- Gross, E. Chemical ecology and ecotoxicology. In Ecotoxicology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 1–31. [Google Scholar]
- Amaral, I.C.C.; de Carvalho, L.V.B.; da Silva Pimentel, J.N.; Pereira, A.C.; Vieira, J.A.; de Castro, V.S.; Borges, R.M.; Alves, S.R.; Nogueira, S.M.; Tabalipa, M.; et al. Avaliação ambiental de BTEX (benzeno, tolueno, etilbenzeno, xilenos) e biomarcadores de genotoxicidade em trabalhadores de postos de combustíveis. Rev. Bras. Saúde Ocup. 2017, 42, e8s. [Google Scholar] [CrossRef][Green Version]
- Varjani, S.J.; Upasani, V.N. A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int. Biodeterior. Biodegrad. 2017, 120, 71–83. [Google Scholar] [CrossRef]
- Hesham, A.E.L.; Alrumman, S.A.; ALQahtani, A.D.S. Degradation of toluene hydrocarbon by isolated yeast strains: Molecular Genetic approaches for identification and characterization. Russ. J. Genet. 2018, 54, 933–943. [Google Scholar] [CrossRef]
- Nasrollahpour, S.; Kebria, D.Y.; Ghavami, M.; Ghasemi-Fare, O. Application of organically modified clay in removing BTEX from produced water. In Proceedings of the Geo-Congress 2020, Minneapolis, MN, USA, 25–28 February 2020; Geotechnical Special Publication. American Society of Civil Engineers (ASCE): Reston, VA, USA, 2020; pp. 275–283. [Google Scholar]
- Alencar, T.D.S.; Santos de Oliveira, V.D.P.; De Oliveira, M.M.; Saraiva, V.B. Contaminação por metais pesados e hidrocarbonetos de petróleo: Uma ameaça para os manguezais. Bol. do Obs. Ambient. Alberto Ribeiro Lamego 2016, 10, 7–24. [Google Scholar] [CrossRef]
- Akpoveta, O.V.; Osakwe, S.A. Determination of heavy metal contents in refined petroleum products. IOSR J. Appl. Chem. 2014, 7, 1–2. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Hembrom, S.; Singh, B.; Gupta, S.K.; Nema, A.K. A Comprehensive evaluation of heavy metal contamination in foodstuff and associated human health risk: A global perspective. In Contemporary Environmental Issues and Challenges in Era of Climate Change; Springer: Singapore, 2020; pp. 33–63. ISBN 978-981-32-9594-0. [Google Scholar]
- Gupta, V. Vehicle-generated heavy metal pollution in an urban environment and its distribution into various environmental components. In Environmental Concerns and Sustainable Development; Springer: Singapore, 2020; pp. 113–127. ISBN 978-981-13-5888-3. [Google Scholar]
- Sarubbo, L.A.; Rocha, R.B.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M. Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem. Ecol. 2015, 31, 707–723. [Google Scholar] [CrossRef]
- Saxena, G.; Bharagava, R.N. Bioremediation of Industrial Waste for Environmental Safety: Volume I: Industrial Waste and Its Management; Saxena, G., Bharagava, R.N., Eds.; Springer: Singapore, 2020; ISBN 978-981-13-1891-7. [Google Scholar]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K.; Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Approaches for remediation of sites contaminated with total petroleum hydrocarbons. In Total Petroleum Hydrocarbons; Springer International Publishing: Cham, Switzerland, 2020; Volume 1, pp. 167–205. ISBN 9783030240356. [Google Scholar]
- Banerjee, A.; Roy, A.; Dutta, S.; Mondal, S. Bioremediation of hydrocarbon—A review. Int. J. Adv. Res. 2016, 4, 1303–1313. [Google Scholar] [CrossRef]
- De La Cueva, S.C.; Rodríguez, C.H.; Cruz, N.O.S.; Contreras, J.A.R.; Miranda, J.L. Changes in bacterial populations during bioremediation of soil contaminated with petroleum hydrocarbons. Water Air Soil Pollut. 2016, 227, e91. [Google Scholar] [CrossRef]
- Lindeberg, M.R.; Maselko, J.; Heintz, R.A.; Fugate, C.J.; Holland, L. Conditions of persistent oil on beaches in Prince William Sound 26 years after the Exxon Valdez spill. Deep. Res. Part. II Top. Stud. Oceanogr. 2018, 147, 9–19. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; Li, Y.; Klassen, W. Potential approaches to improving biodegradation of hydrocarbons for bioremediation of crude oil pollution. J. Environ. Prot. (Irvine Calif). 2011, 2, 47–55. [Google Scholar] [CrossRef]
- Bagi, A. Effect of Low Temperature on Hydrocarbon Biodegradation in Marine Environments; Faculty of Science and Technology: Stavanger, Norway, 2013. [Google Scholar]
- Al Disi, Z.; Jaoua, S.; Al-Thani, D.; Al-Meer, S.; Zouari, N. Considering the specific impact of harsh conditions and oil weathering on diversity, adaptation, and activity of hydrocarbon-degrading bacteria in strategies of bioremediation of harsh oily-polluted soils. BioMed Res. Int. 2017, 2017, e8649350. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pratap, R.; Vaibhav, S. Contemporary Environmental Issues and Challenges in Era of Climate Change; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9789813295940. [Google Scholar]
- Ray, S. Bioremediation of pesticides: A case study. In Microbial Biodegradation and Bioremediation; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 514–520. ISBN 9780128004821. [Google Scholar]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, e180. [Google Scholar] [CrossRef] [PubMed]
- Hazen, T.C. In situ: Groundwater bioremediation. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 1, pp. 2583–2596. ISBN 978-3-540-77584-3. [Google Scholar]
- Sharma, J. Advantages and limitations of in situ methods of bioremediation. Recent Adv. Biol. Med. 2019, 5, 1. [Google Scholar] [CrossRef]
- Cascade, A. Deeper Look at Permeable Reactive Barriers. Available online: https://www.cascade-env.com/resources/blogs/archive/a-deeper-look-at-permeable-reactive-barriers/ (accessed on 4 September 2020).
- Zhou, D.; Li, Y.; Zhang, Y.; Zhang, C.; Li, X.X.; Chen, Z.; Huang, J.; Li, X.X.; Flores, G.; Kamon, M. Column test-based optimization of the permeable reactive barrier (PRB) technique for remediating groundwater contaminated by landfill leachates. J. Contam. Hydrol. 2014, 168, 1–16. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef]
- De Pourcq, K.; Ayora, C.; García-Gutiérrez, M.; Missana, T.; Carrera, J. A clay permeable reactive barrier to remove Cs-137 from groundwater: Column experiments. J. Environ. Radioact. 2015, 149, 36–42. [Google Scholar] [CrossRef]
- Liu, Y.; Mou, H.; Chen, L.; Mirza, Z.A.; Liu, L. Cr(VI)-contaminated groundwater remediation with simulated permeable reactive barrier (PRB) filled with natural pyrite as reactive material: Environmental factors and effectiveness. J. Hazard. Mater. 2015, 298, 83–90. [Google Scholar] [CrossRef]
- Ramírez, E.M.; Jiménez, C.S.; Camacho, J.V.; Rodrigo, M.A.R.; Cañizares, P. Feasibility of coupling permeable bio-barriers and electrokinetics for the treatment of diesel hydrocarbons polluted soils. Electrochim. Acta 2015, 181, 192–199. [Google Scholar] [CrossRef]
- Miller, R.R. Ground-Water Remediation Technologies Analysis Center Prepared For.: Technology Overview Report; Ground-Water Remediation Technologies Analysis Center: Pittsburgh, PA, USA, 1996.
- Kim, S.; Krajmalnik-Brown, R.; Kim, J.O.; Chung, J. Remediation of petroleum hydrocarbon-contaminated sites by DNA diagnosis-based bioslurping technology. Sci. Total Environ. 2014, 497–498, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Godheja, J.; Modi, D.R.; Kolla, V.; Pereira, A.M.; Bajpai, R.; Mishra, M.; Sharma, S.V.; Sinha, K.; Shekhar, S.K. Environmental remediation: Microbial and nonmicrobial prospects. In Microbial Interventions in Agriculture and Environment; Springer: Singapore, 2019; Volume 1, pp. 379–409. ISBN 9789811383830. [Google Scholar]
- Brown, L.D.; Cologgi, D.L.; Gee, K.F.; Ulrich, A.C. Bioremediation of Oil Spills on Land; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9781856179430. [Google Scholar]
- Höhener, P.; Ponsin, V. In situ vadose zone bioremediation. Curr. Opin. Biotechnol. 2014, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.M.; Chen, C.Y.; Chen, S.C.; Chien, H.Y.; Chen, Y.L. Application of in situ biosparging to remediate a petroleum-hydrocarbon spill site: Field and microbial evaluation. Chemosphere 2008, 70, 1492–1499. [Google Scholar] [CrossRef]
- Bioclear Biosparging: Bacterial Removal of Mineral Oil and Aromatic Compounds. Available online: https://bioclearearth.com/#Technieken (accessed on 28 April 2020).
- Kuiper, I.; Lagendijk, E.L.; Bloemberg, G.V.; Lugtenberg, B.J.J. Rhizoremediation: A beneficial plant-microbe interaction. Mol. Plant. Microbe Interact. 2004, 17, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Favas, P.J.C.; Pratas, J.; Varun, M.; D’Souza, R.; Paul, M.S. Phytoremediation of soils contaminated with metals and metalloids at mining areas: Potential of native flora. In Environmental Risk Assessment of Soil Contamination; InTechOpen: London, UK, 2014; ISBN 978-953-51-1235-8. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Bali, A.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Ethylenediamine disuccinic acid enhanced phytoextraction of nickel from contaminated soils using Coronopus didymus (L.) Sm. Chemosphere 2018, 205, 234–243. [Google Scholar] [CrossRef]
- Park, S.; Kim, K.S.; Kim, J.T.; Kang, D.; Sung, K. Effects of humic acid on phytodegradation of petroleum hydrocarbons in soil simultaneously contaminated with heavy metals. J. Environ. Sci. 2011, 23, 2034–2041. [Google Scholar] [CrossRef]
- The Interstate Technology and Regulatory Cooperation Work Group Phytoremediation Work Team: Phytoremediation Decision Tree. 1999. Available online: https://clu-in.org/download/partner/phytotree.pdf (accessed on 5 September 2020).
- Borriss, R. Phytostimulation and Biocontrol by the Plant-Associated Bacillus amyloliquefaciens FZB42: An Update. In Phyto-Microbiome in Stress Regulation; Springer: Singapore, 2020; Volume 1, pp. 1–20. [Google Scholar]
- Shackira, A.M.; Puthur, J.T. Phytostabilization of Heavy Metals: Understanding of Principles and Practices. In Plant-Metal Interactions; Springer International Publishing: Cham, Switzerland, 2019; pp. 263–282. [Google Scholar]
- Awa, S.H.; Hadibarata, T. Removal of heavy metals in contaminated soil by phytoremediation mechanism: A review. Water, Air, Soil Pollut. 2020, 231, e47. [Google Scholar] [CrossRef]
- Cristaldi, A.; Oliveri Conti, G.; Cosentino, S.L.; Mauromicale, G.; Copat, C.; Grasso, A.; Zuccarello, P.; Fiore, M.; Restuccia, C.; Ferrante, M. Phytoremediation potential of Arundo donax (Giant Reed) in contaminated soil by heavy metals. Environ. Res. 2020, 185, e109427. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Gupta, D.; Singh, L.K.; Gupta, A.D.; Babu, V. Phytoremediation: An efficient approach for bioremediation of organic and metallic ions pollutants. In Bioremediation and Sustainability: Research and Applications; Wiley: Hoboken, NJ, USA, 2012; Chapter 5; pp. 213–240. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Role of hyperaccumulators in phytoextraction of metals from contaminated mining sites: A review. Crit. Rev. Environ. Sci. Technol. 2010, 41, 168–214. [Google Scholar] [CrossRef]
- Clay, L.; Pichtel, J. Treatment of simulated oil and gas produced water via pilot-scale rhizofiltration and constructed wetlands. Int. J. Environ. Res. 2019, 13, 185–198. [Google Scholar] [CrossRef]
- Limmer, M.; Burken, J. Phytovolatilization of organic contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Phytoremediation for the elimination of metals, pesticides, PAHs, and other pollutants from wastewater and soil. In Phytobiont and Ecosystem Restitution; Kumar, V., Kumar, M., Prasad, R., Eds.; Springer: Singapore, 2018; ISBN 978-981-13-1186-4. [Google Scholar]
- Woźniak-Karczewska, M.; Lisiecki, P.; Białas, W.; Owsianiak, M.; Piotrowska-Cyplik, A.; Wolko, Ł.; Ławniczak, Ł.; Heipieper, H.J.; Gutierrez, T.; Chrzanowski, Ł. Effect of bioaugmentation on long-term biodegradation of diesel/biodiesel blends in soil microcosms. Sci. Total. Environ. 2019, 671, 948–958. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar] [CrossRef]
- Zeneli, A.; Kastanaki, E.; Simantiraki, F.; Gidarakos, E. Monitoring the biodegradation of TPH and PAHs in refinery solid waste by biostimulation and bioaugmentation. J. Environ. Chem. Eng. 2019, 7, e103054. [Google Scholar] [CrossRef]
- Mapelli, F.; Scoma, A.; Michoud, G.; Aulenta, F.; Boon, N.; Borin, S.; Kalogerakis, N.; Daffonchio, D. Biotechnologies for marine oil spill cleanup: Indissoluble ties with microorganisms. Trends Biotechnol. 2017, 35, 860–870. [Google Scholar] [CrossRef]
- Roy, A.; Dutta, A.; Pal, S.; Gupta, A.; Sarkar, J.; Chatterjee, A.; Saha, A.; Sarkar, P.; Sar, P.; Kazy, S.K. Biostimulation and bioaugmentation of native microbial community accelerated bioremediation of oil refinery sludge. Bioresour. Technol. 2018, 253, 22–32. [Google Scholar] [CrossRef]
- Speight, J.G. Environmental Organic Chemistry for Engineers; Elsevier Ltd.: Butterworth-Heinemann, UK, 2018; Volume 1, ISBN 9780128006689. [Google Scholar]
- BioRangers. Reliable Remediation Approach. Available online: https://www.bri.co.jp/english/teq/index.html (accessed on 29 August 2020).
- Bodor, A.; Petrovszki, P.; Erdeiné Kis, Á.; Vincze, G.E.; Laczi, K.; Bounedjoum, N.; Szilágyi, Á.; Szalontai, B.; Feigl, G.; Kovács, K.L.; et al. Intensification of ex situ bioremediation of soils polluted with used lubricant oils: A comparison of biostimulation and bioaugmentation with a special focus on the type and size of the inoculum. Int. J. Environ. Res. Public Health 2020, 17, 4106. [Google Scholar] [CrossRef]
- Zaneti, I.C.B.B.; Silva, G.O. Sustentabilidade urbana e gestão de resíduos sólidos: O caso do Distrito Federal. In Proceedings of the Forum Internacional de Resíduos Sólidos-Anais, Curitiba, Brazil, 12–14 June 2017; p. 10. [Google Scholar]
- Cristorean, C.; Micle, V.; Sur, I.M. A critical analysis of ex-situ bioremediation technologies of hydrocarbon polluted soils. ECOTERRA J. Environ. Res. Prot. 2016, 13, 17–29. [Google Scholar]
- Salehi, M.A.; Hakimghiasi, N. Hydrodynamics and mass transfer inthree-phase airlift reactors for activated carbon and sludge filtration. Adv. Environ. Technol. 2017, 2, 179–184. [Google Scholar] [CrossRef]
- Balseiro-Romero, M.; Monterroso, C.; Kidd, P.S.; Lu-Chau, T.A.; Gkorezis, P.; Vangronsveld, J.; Casares, J.J. Modelling the ex situ bioremediation of diesel-contaminated soil in a slurry bioreactor using a hydrocarbon-degrading inoculant. J. Environ. Manag. 2019, 246, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Whelan, M.J.; Coulon, F.; Hince, G.; Rayner, J.; McWatters, R.; Spedding, T.; Snape, I. Fate and transport of petroleum hydrocarbons in engineered biopiles in polar regions. Chemosphere 2015, 131, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.S.; Puustinen, J.; Suortti, A.M. Bioremediation of petroleum hydrocarbon-contaminated soil by composting in biopiles. Environ. Pollut. 2000, 107, 245–254. [Google Scholar] [CrossRef]
- Tecnohidro. Tecnohidro—Biopilhas. Available online: http://tecnohidro.com.br/ (accessed on 28 April 2020).
- Pavel, L.; Gavrilescu, M. Overview of ex situ decontamination techniques for soil cleanup. Environ. Eng. Manag. J. 2008, 7, 815–834. [Google Scholar] [CrossRef]
- Macaulay, B.; Rees, D. Bioremediation of oil spills: A review of challenges for research advancement. Ann. Environ. Sci. 2014, 8, 9–37. [Google Scholar]
- Ortega, M.F.; Guerrero, D.E.; García-Martínez, M.J.; Bolonio, D.; Llamas, J.F.; Canoira, L.; Gallego, J.L.R. Optimization of landfarming amendments based on soil texture and crude oil concentration. Water Air Soil Pollut. 2018, 229, e234. [Google Scholar] [CrossRef]
- Guerin, T.F. Long-term performance of a land treatment facility for the bioremediation of non-volatile oily wastes. Resour. Conserv. Recycl. 2000, 28, 105–120. [Google Scholar] [CrossRef]
- Nedeef, V.; Chițimuș, A.; Moșneguțu, E.; Lazăr, G. Technologies for Soil Remediation, Methods and Techniques for Environmental Protection; Alma Mater: Sibiu, Romania, 2012. [Google Scholar]
- Narayan Chadar, S. Composting as an eco-friendly method to recycle organic waste. Prog. Petrochem. Sci. 2018, 2, 252–254. [Google Scholar] [CrossRef]
- Rosca, M.; Hlihor, R.-M.; Gavrilescu, M. Bioremediation of Persistent Toxic Substances: From conventional to new approaches in using microorganisms and plants. In Microbial Technology for the Welfare of Society; Springer: Singapore, 2019; pp. 289–312. ISBN 978-981-13-8843-9. [Google Scholar]
- Aguelmous, A.; El Fels, L.; Souabi, S.; Zamama, M.; Hafidi, M. The fate of total petroleum hydrocarbons during oily sludge composting: A critical review. Rev. Environ. Sci. Bio/Technol. 2019, 18, 473–493. [Google Scholar] [CrossRef]
- Sayara, T.; Sánchez, A. Bioremediation of PAH-contaminated soils: Process enhancement through composting/compost. Appl. Sci. 2020, 10, 3684. [Google Scholar] [CrossRef]
- Grasserová, A.; Hanč, A.; Innemanová, P.; Cajthaml, T. Composting and vermicomposting used to break down and remove pollutants from organic waste: A mini review. Eur. J. Environ. Sci. 2020, 10, 9–14. [Google Scholar] [CrossRef]
- Loick, N.; Hobbs, P.J.; Hale, M.D.C.; Jones, D.L. Bioremediation of poly-aromatic hydrocarbon (PAH)-contaminated soil by composting. Crit. Rev. Environ. Sci. Technol. 2009, 39, 271–332. [Google Scholar] [CrossRef]
- Sayara, T.; Borràs, E.; Caminal, G.; Sarrà, M.; Sánchez, A. Bioremediation of PAHs-contaminated soil through composting: Influence of bioaugmentation and biostimulation on contaminant biodegradation. Int. Biodeterior. Biodegrad. 2011, 65, 859–865. [Google Scholar] [CrossRef]
- Yamashita, Y.; Miyahara, R.; Sakamoto, K. Emulsion and emulsification technology. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 489–506. ISBN 9780128020548. [Google Scholar]
- Pichot, R. Stability and Characterisation of Emulsions in the Presence of Colloidal Particles and Surfactants. Ph.D. Thesis, The University of Birmingham, Birmingham, UK, 2012. [Google Scholar]
- Lebdioua, K.; Aimable, A.; Cerbelaud, M.; Videcoq, A.; Peyratout, C. Influence of different surfactants on Pickering emulsions stabilized by submicronic silica particles. J. Colloid Interface Sci. 2018, 520, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef]
- Chaprão, M.J.; Ferreira, I.N.S.; Correa, P.F.; Rufino, R.D.; Luna, J.M.; Silva, E.J.; Sarubbo, L.A. Application of bacterial and yeast biosurfactants for enhanced removal and biodegradation of motor oil from contaminated sand. Electron. J. Biotechnol. 2015, 18, 471–479. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Surfactant-enhanced remediation of contaminated soil: A review. Eng. Geol. 2001, 60, 371–380. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Saponins and microbial biosurfactants: Potential raw materials for the formulation of cosmetics. Biotechnol. Prog. 2018, 34, 1482–1493. [Google Scholar] [CrossRef]
- Kim, B.K.; Baek, K.; Ko, S.H.; Yang, J.W. Research and field experiences on electrokinetic remediation in South Korea. Sep. Purif. Technol. 2011, 79, 116–123. [Google Scholar] [CrossRef]
- Paria, S. Surfactant-enhanced remediation of organic contaminated soil and water. Adv. Colloid Interface Sci. 2008, 138, 24–58. [Google Scholar] [CrossRef]
- Jia, L.-Q.; Ou, Z.-Q.; Ouyang, Z. Ecological behavior of linear alkylbenzene sulfonate (LAS) in soil-plant systems. Pedosphere 2005, 15, 216–224. [Google Scholar]
- Cserháti, T.; Forgács, E.; Oros, G. Biological activity and environmental impact of anionic surfactants. Environ. Int. 2002, 28, 337–348. [Google Scholar] [CrossRef]
- Santonicola, M.G.; Lenhoff, A.M.; Kaler, E.W. Binding of alkyl polyglucoside surfactants to bacteriorhodopsin and its relation to protein stability. Biophys. J. 2008, 94, 3647–3658. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.L.; Tilton, F.; Jansik, D.P.; Ergas, S.J.; Marshall, M.J.; Miracle, A.L.; Wellman, D.M. Growth inhibition and stimulation of Shewanella oneidensis MR-1 by surfactants and calcium polysulfide. Ecotoxicol. Environ. Saf. 2012, 80, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, F.J.; Lowery, B.; Kelling, K.A. Surfactant impact on nitrogen utilization and leaching in potatoes. Am. J. Potato Res. 2009, 86, 383–390. [Google Scholar] [CrossRef]
- Ostroumov, S.A.; Solomonova, E.A. Phytotoxicity of a surfactant-containing product towards macrophytes. Russ. J. Gen. Chem. 2013, 83, 2614–2617. [Google Scholar] [CrossRef]
- Silva, R.D.C.F.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int. J. Mol. Sci. 2014, 15, 12523–12542. [Google Scholar] [CrossRef] [PubMed]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Fahr, A.; Liu, X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Dissolved air flotation combined to biosurfactants: A clean and efficient alternative to treat industrial oily water. Rev. Environ. Sci. Biotechnol. 2018, 17, 591–602. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, D.; Kumar, G.; Sharma, N.R. Microbial Bioprospecting for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2018; Volume II, pp. 1–397. [Google Scholar] [CrossRef]
- Hmidet, N.; Jemil, N.; Nasri, M. Simultaneous production of alkaline amylase and biosurfactant by Bacillus methylotrophicus DCS1: Application as detergent additive. Biodegradation 2019, 30, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Rocha e Silva, N.M.P.; Meira, H.M.; Almeida, F.C.G.; Soares da Silva, R.D.C.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Natural surfactants and their applications for heavy oil removal in industry. Sep. Purif. Rev. 2019, 48, 267–281. [Google Scholar] [CrossRef]
- Campos, J.M.; Stamford, T.L.M.; Sarubbo, L.A. Characterization and application of a biosurfactant isolated from Candida utilis in salad dressings. Biodegradation 2019, 30, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Invally, K.; Sancheti, A.; Ju, L.K. A new approach for downstream purification of rhamnolipid biosurfactants. Food Bioprod. Process. 2019, 114, 122–131. [Google Scholar] [CrossRef]
- Halecký, M.; Kozliak, E. Modern bioremediation approaches: Use of biosurfactants, emulsifiers, enzymes, biopesticides, GMOs. In Advanced Nano-Bio Technologies for Water and Soil Treatment; Springer: Cham, Switzerland, 2020; Volume 1, pp. 495–526. ISBN 978-3-030-29839-5. [Google Scholar]
- Fritsche, W.; Hofrichter, M. Aerobic degradation by microorganisms. In Biotechnology Set; Wiley: Hoboken, NJ, USA, 2008; pp. 144–167. [Google Scholar]
- Ławniczak, Ł.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Microbial degradation of hydrocarbons—Basic principles for bioremediation: A review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.D.; Luna, J.M.; Marinho, P.H.C.; Farias, C.B.B.; Ferreira, S.R.M.; Sarubbo, L.A. Removal of petroleum derivative adsorbed to soil by biosurfactant Rufisan produced by Candida lipolytica. J. Pet. Sci. Eng. 2013, 109, 117–122. [Google Scholar] [CrossRef]
- Silva, E.J.; Correa, P.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Recovery of contaminated marine environments by biosurfactant-enhanced bioremediation. Colloids Surf. B Biointerfaces 2018, 172, 127–135. [Google Scholar] [CrossRef]
- Rita de Cássia, F.; Almeida, D.G.; Meira, H.M.; Silva, E.J.; Farias, C.B.B.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Production and characterization of a new biosurfactant from Pseudomonas cepacia grown in low-cost fermentative medium and its application in the oil industry. Biocatal. Agric. Biotechnol. 2017, 12, 206–215. [Google Scholar] [CrossRef]
- Da Rocha Junior, R.B.; Meira, H.M.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Application of a low-cost biosurfactant in heavy metal remediation processes. Biodegradation 2019, 30, 215–233. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Biosurfactant from Candida sphaerica UCP0995 exhibiting heavy metal remediation properties. Process. Saf. Environ. Prot. 2016, 102, 558–566. [Google Scholar] [CrossRef]





| Petroleum Derivatives | Chemical War | Urban Source | Agrochemical Source | Biological Warfare |
|---|---|---|---|---|
| Exploration, production, refining, transport, and consumption. | Contaminants, toxic chemical compounds, and arms; ground contamination from Cold War army operations. | Energy generation emissions; soil pollution by transportation and manufacture; soil contamination by residues and sludge from wastewater treatment. | Insecticides, herbicides, fungicides, and fuel spills on farms. | Bacteria, viruses, fungi, and toxins. |
| Phytotechnology | Mechanism | Pollutants | Reference |
|---|---|---|---|
| Phytoextraction | Hyperaccumulation in collectable parts of the plants | Inorganics: Co, Cr, Ni, Pb, Zn, Au, Hg, Mo, Ag, Cd Radionuclides: Sr, Cs, Pb, U. | [74] |
| Rhizofiltration | Rhizosphere accumulation through sorption, concentration, and precipitation | Organics/Inorganics: Metals like Cd, Cu, Ni, Zn, Cr Radionuclides. | [75] |
| Phytovolatilization | Volatilization by leaves through transpiration. | Organics/Inorganics: Chlorinated solvents, Inorganics (Se, Hg, As). | [76] |
| Phytodegradation | Pollutant eradication | Organic compounds, Chlorinated solvents, Phenols, Herbicides, Munitions. | [77] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales da Silva, I.G.; Gomes de Almeida, F.C.; Padilha da Rocha e Silva, N.M.; Casazza, A.A.; Converti, A.; Asfora Sarubbo, L. Soil Bioremediation: Overview of Technologies and Trends. Energies 2020, 13, 4664. https://doi.org/10.3390/en13184664
Sales da Silva IG, Gomes de Almeida FC, Padilha da Rocha e Silva NM, Casazza AA, Converti A, Asfora Sarubbo L. Soil Bioremediation: Overview of Technologies and Trends. Energies. 2020; 13(18):4664. https://doi.org/10.3390/en13184664
Chicago/Turabian StyleSales da Silva, Israel Gonçalves, Fabíola Carolina Gomes de Almeida, Nathália Maria Padilha da Rocha e Silva, Alessandro Alberto Casazza, Attilio Converti, and Leonie Asfora Sarubbo. 2020. "Soil Bioremediation: Overview of Technologies and Trends" Energies 13, no. 18: 4664. https://doi.org/10.3390/en13184664
APA StyleSales da Silva, I. G., Gomes de Almeida, F. C., Padilha da Rocha e Silva, N. M., Casazza, A. A., Converti, A., & Asfora Sarubbo, L. (2020). Soil Bioremediation: Overview of Technologies and Trends. Energies, 13(18), 4664. https://doi.org/10.3390/en13184664