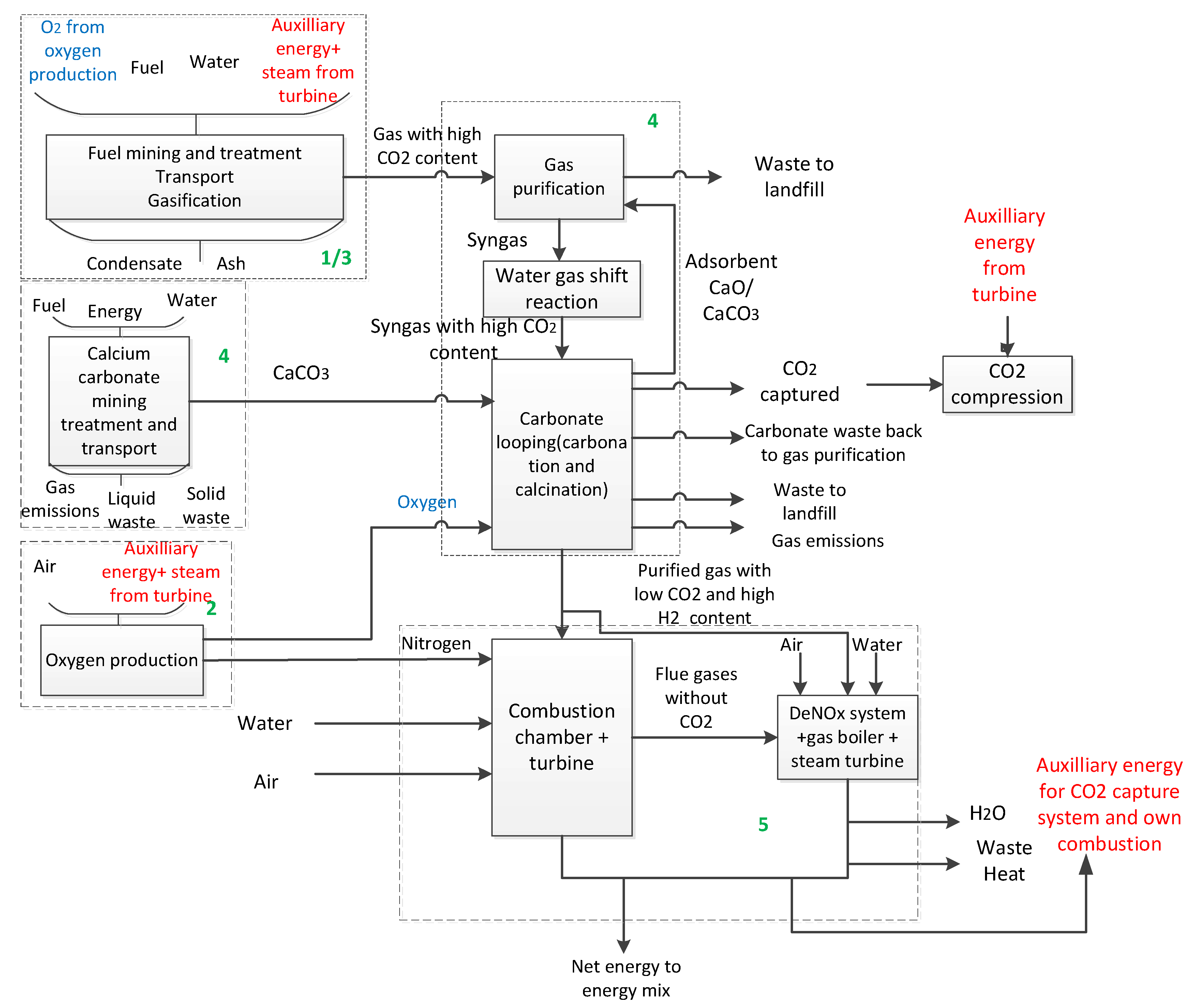
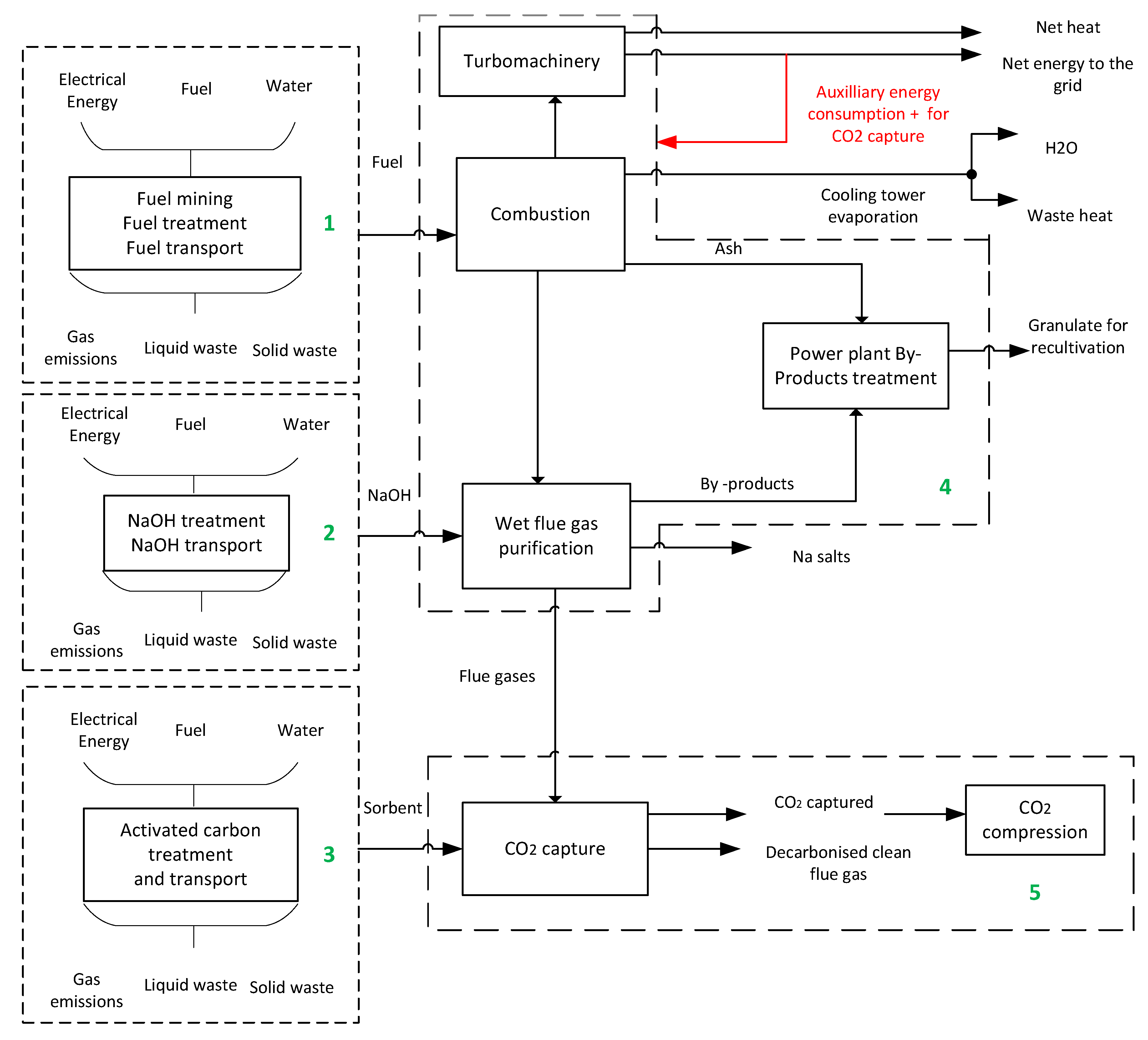
1. Introduction
Energy self-sufficiency and low-carbon-economy transition are the concepts currently forcing the coal-based energy industry to significantly decrease its produced emissions. The annual consumption of coal (in coal-based industries) in the Czech Republic is over 60 million tons per year, ranking Czech Republic at 17th in worldwide consumption [
1]. The Czech energy industry is also dependent on imports of 97% of oil and gas [
1]. Several measures were adopted to reduce energy import dependence, including implementation of a higher share of renewable energy sources and more efficient use of fossil fuels such as brown coal [
1]. However, the transition process cannot be sudden and must be gradually implemented by viable investments. One such investment, which seems to be feasible for the current Czech energy industry but also usable for steel industries, is carbon capture technologies (CCTs). These technologies must be carefully assessed and planned for the specific conditions of a given country. There are three parameters that must be considered for the feasibility assessment of CCT—(i) technological feasibility, (ii) economic performance, and (iii) environmental performance. This paper considers each of these three parameters in a new combined analysis.
In the conditions of the Czech operational power units, several CCT options were considered, such as post-combustion technologies of ammonia scrubbing, activated carbon adsorption (PCC-A), and pre-combustion integrated gasification gas cycle with integrated carbonate loop (IGCC-CaL). These technologies are the subject of intensive research and optimization to achieve their implementation into the operational power units. The decision-making process for the choice of suitable technologies may be significantly influenced by environmental performance consideration via comprehensive methodology. Life cycle assessment (LCA) is one of the best certified methods to create environmental models of the considered systems. LCA allows the comparison of the assessed systems among each other [
2].
The ammonia scrubbing process of LCA was already performed [
3]. The ammonia scrubbing process increased the impact of fossils depletion and mineral resource depletion in comparison with the power unit (250 MWe) without CCT. That is caused by a large amount of additional energy consumption for ammonia solvent preparation. Moreover, energy efficiency of the power unit decreased from 38% to 27%. The environmental problem occurs with the treatment of ammonia salts, currently considered as non-utilized waste. On the other hand, CO
2 was captured in a ratio of 90%.
An LCA for the PCC-A system for Czech conditions was recently published by the authors of this paper [
4]. The LCA model in the study considers a functional unit nominal power output of 250 MWe. The paper concludes that adding such technology would increase the energy demand (an additional 1133 MJ for hard coal activation) and fossils depletion. The reason for this is the resource consumption of hard coal (additional fresh carbon 23 kg/h) in the production chain of the activated carbon.
IGCC-CaL was not previously assessed for the Czech conditions from the environmental point of view. However, several studies were made for the IGCC systems integration and its environmental assessment. A summary of the following studies can be found in
Table 1.
The extensive study by Singh et al. [
5] compares the environmental results for 400 MWe power plants with post, pre, and oxy-fuel combustion capture systems. For pre-combustion systems with IGCC based on Selexol absorption, the CO
2 capture ratio is 90%, with an energy efficiency of 37.6%. Comparative LCA was made by hybrid LCA approach, using input–output analysis together with the ReCiPe 2008 version 1.02 method. Environmental results of a pre-combustion system show a reduction of 78% in the category of global warming potential (GWP), the highest reduction in comparison with the aforementioned systems. On the other hand, IGCC system contributes to increase of 120% in category of freshwater eutrophicaton (FE), influenced mainly by infrastructure development.
Cormos C. [
6] evaluated the techno-economic and environmental performance of IGCC system for power plant concepts of a net power output of about 400–500 MWe. The study states that the introduction of the CCT system decreased net plan energy efficiency by 7.1–9.5%. The environmental part of the study compared the IGCC systems based on the physical solvent Selexol. Environmental impacts refer to the production of 1 kWh of electricity. However, the impacts were categorized in normalized mass and energy flows where the integration of the IGCC systems caused an increase of coal (25%), oxygen (24%), and cooling water (22%) consumption, and the ratio of captured CO
2 was modelled at 90%.
In another study [
7], three following three systems were compared: (i) a conventional supercritical coal power system, (ii) an IGCC-CCS system based on Selexol solvent system, (iii) and an IGCC without CCS. The systems were chosen according to equal coal consumption rather than equal electricity production. The environmental results for IGCC systems per 1 MWh are based on mass–energy balances and show higher water consumption due to the gasification process and the shift reaction in comparison to the power unit without CCT. The IGCC system with CO
2 capture was designed for 81% CO
2 capture ratio. The IGCC-CCS system reduced net energy efficiency from 32.1% (IGCC without CCS) to 26.1%.
The work of Petrescu et al. [
8] compares IGCC power plant with gross electric output of 570.61 MWe and 2 IGCC-CCS systems. Two compared IGCC-CCS systems are based on Ca-based (IGCC-CaL) sorbents and iron-based oxygen carriers (IGCC-FeL). The used method for LCA analysis was CML 2001 using GaBi software. In both scenarios, the highest values refer to GWP, where the majority (85%) comes from coal mining and extraction. The results show that the highest carbon capture rate happens with IGCC-FeL (99.45%), with a net electrical efficiency drop from 45.09% to 38.76%. Energy efficiency dropped for IGCC-CaL from 45.09% to 36.44%, and the capture rate was 91.56%.
Regarding CaO looping, some studies were done for post-combustion CO
2 capture. One study [
9] considers 600 MWe supercritical pulverized coal power plant as a basis for CCT. According to the study [
9], net energy efficiency drops from 39% to 32% due to CaO looping. LCA analysis was done at the endpoint level via SimaPro v8.3 software. The results indicate an increase in resources depletion, ozone depletion, and toxicities. The climate change impact was reduced by 72%.
Clarens et al. [
10] compared a sub-critical coal power plant without CCT (500 MWe; net plant efficiency 36.9%), post-combustion capture technologies based on amine absorption (Econamine and Econamine FG+), and CaO looping without capture. This study used the LCA method of ReCiPe v1.04 and Simapro software. The CaO looping results in the best environmental performance among all systems in the categories of ozone depletion, photochemical ozone formation, particulate matter, and water depletion impacts. The CO
2 capture ratio was 90% in each CCT systems. However, the net plant efficiency for the Econamine case dropped from 36.9% to 22.3%, for Econamine FG+ to 25.9%, and for CaO loop to 29.6%.
The literature survey clearly shows that very few environmental assessments were done on the subject of pre-combustion IGCC-CaL and none for comparison of IGCC-CaL and PCC-A. Moreover, the data in the studies is, in the majority, based on literature sources and heat-mass models rather than real case studies. Also, the selected environmental assessment methods were based on mass-energy flows analysis or methods such as hybrid LCA or CML.
The first part of the paper is focused on the environmental study that compares both CCT systems integrated into their reference power plants. The environmental study does not evaluate the reference power plants without CCT due to lack of data for a single IGCC system. Moreover, IGCC-CaL was designed as the one whole technology with an already integrated CCT system. Yet, in the case of PCC-A, a recent study [
4] published by the same authors compares the reference 250 MWe power unit and the same reference power unit with PCC-A.
The second part of the paper is the economic study of both systems. The economic part compares the investments of both systems (IGCC-CaL, PCC-A) with the case of the energy system without CCT based on the cost and market trend of CO2 allowances.
The third part of the paper combines environmental and economic results to determinate the specifics that can influence the decision-making process for the final technology selection.
The paper has several contributions:
Environmental performance of two innovative systems in the conditions of the Czech energy mix;
Comprehensive LCA model including decision-making processes of characterization, normalization, pareto analysis;
Identification of cost effectiveness of energy systems without CCT and with assessed CCT;
Selection of the most suitable technology via a combined analysis of the environmental and economic dimensions.
The paper is structured as follows:
Section 2.1 defines the LCA methodology,
Section 2.2 defines the economical assessment method,
Section 3.1 provides a technical description of case study 1,
Section 3.2 provides a technical description of case study 2,
Section 3.3 defines the systems boundaries,
Section 3.4 describes a life cycle inventory,
Section 3.5 defines the cost effectiveness parameters,
Section 4.1 presents the results of the life cycle impact assessment.
Section 4.2 presents the Pareto analysis,
Section 4.3 presents the results for cost effectiveness comparison, and
Section 5 and
Section 6 provide the discussion and conclusions.
5. Discussion
The discussion part follows the sections of the results. At first, the environmental assessment with processes analysis will be discussed. In the second part, the economical assessment will be analyzed. In the last part, the combination of environmental and economic results will be concluded.
5.1. Environmental Assessment
The results of the characterization, normalization, and relative contribution of the environmental impacts are shown in
Table 8. The absolute values of the characterization process enable the comparison of the same environmental impact category among scenarios. At first glance, scenario 1 has a lower characterization values in comparison with scenario 2 in almost all environmental categories except in the category of ionizing radiation. Ionizing radiation in scenario 1 is influenced by the process of oxygen production via cryogenic separation. This process is the database process and does not reflect the local impact. Moreover, the radiation impact has an insignificant contribution in comparison with other impacts (sc.1 0.16%; sc.2 0.06%).
When we aggregate the environmental impact categories after normalization (see
Table 8 sum of normalized values), the scenario 2 has a higher global environmental impact (0.00231) than scenario 1 (0.0042). However, it is important to stress out that the case studies considered within this manuscript are different in several parameters such as (i) different scale of REU, (ii) site specific case studies (iii) different power generation technology. Therefore, the environmental performance for scenario 2 might be improved regarding LCA results if the REU technology would have the same technological basis in both cases.
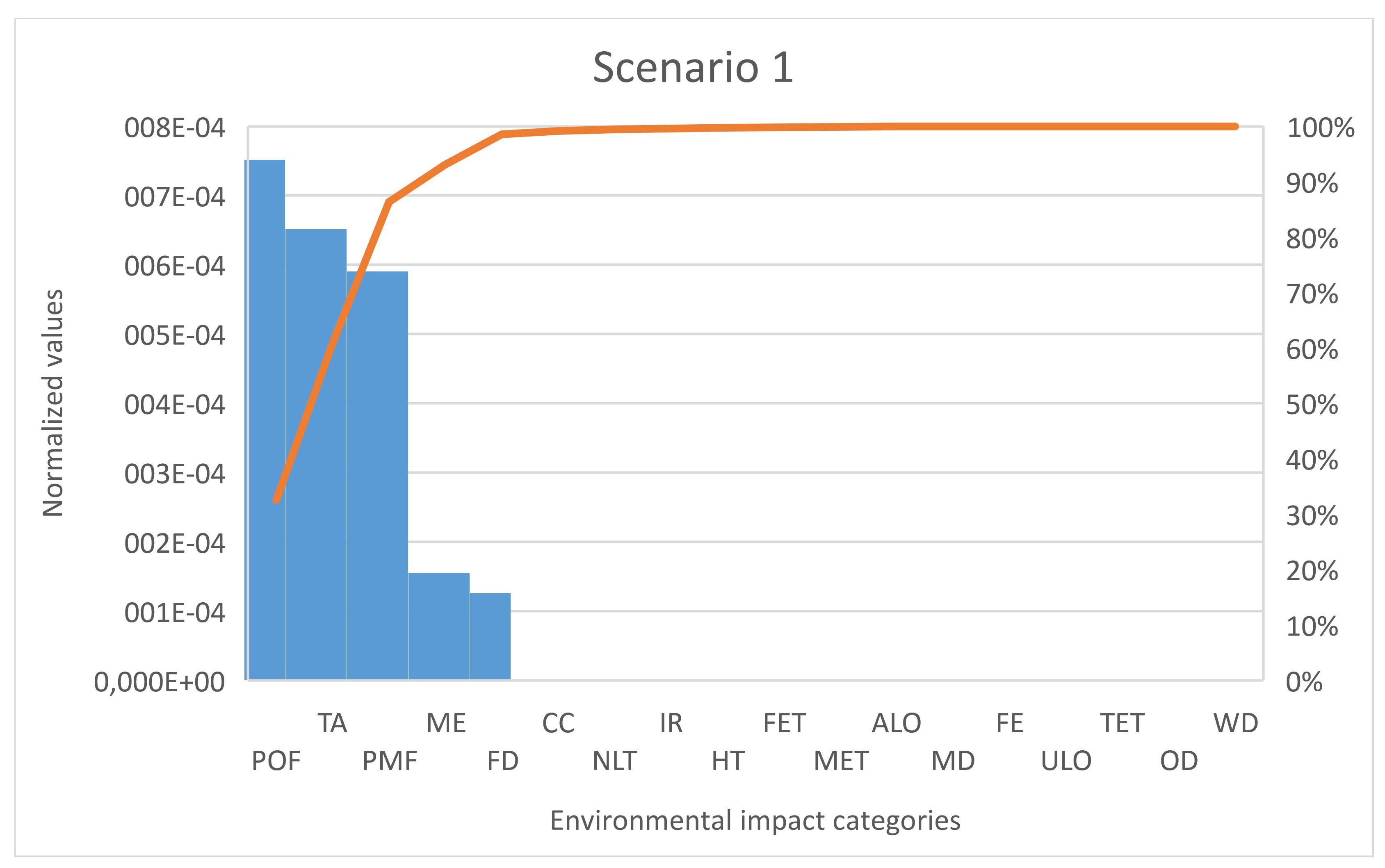
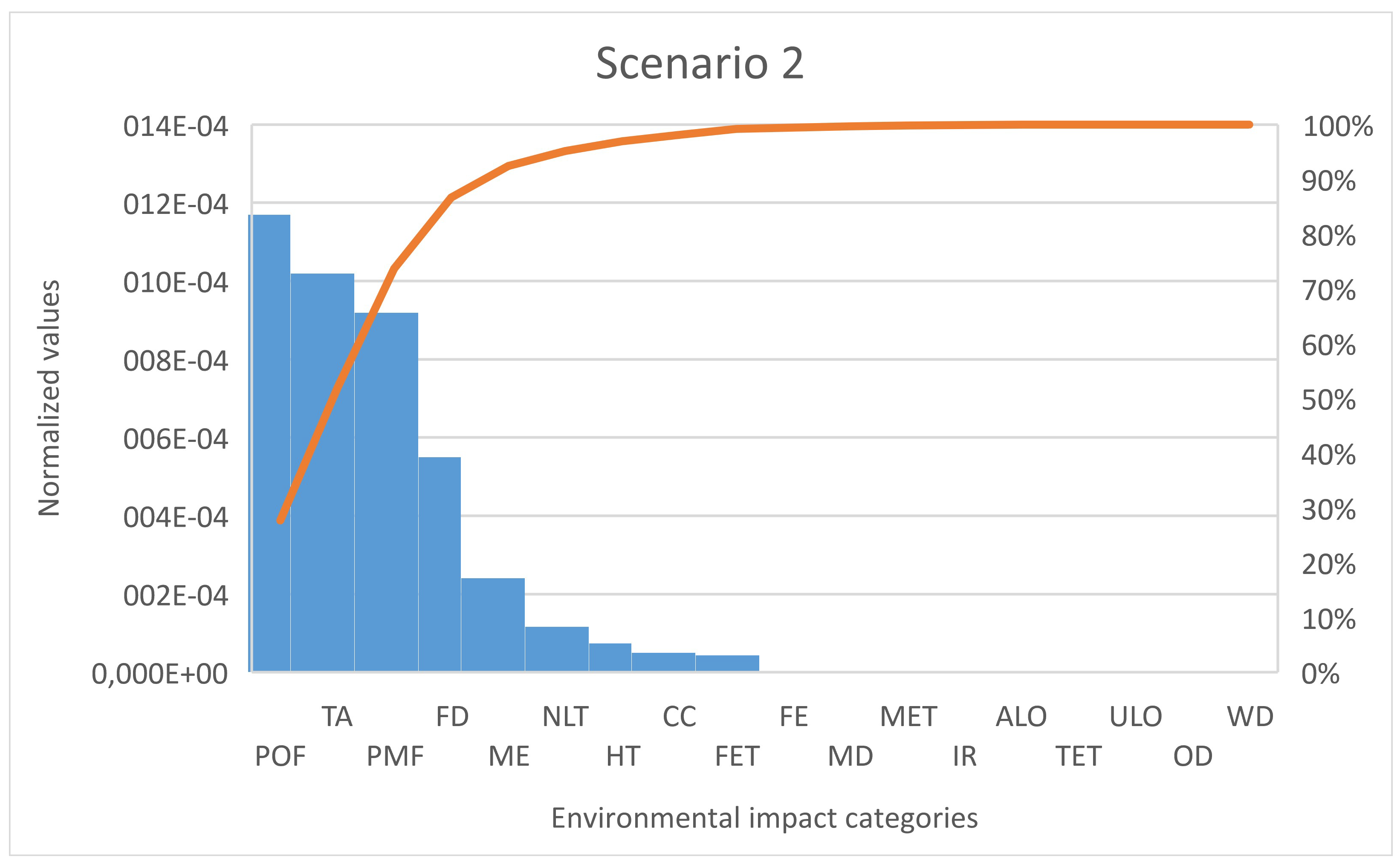
However, it is important to analyze the contribution of the individual impact categories in the total environmental impact. To analyze the highest significance of the impact categories of each scenario, the pareto analysis was chosen as a decision-making tool (
Figure 3 and
Figure 4). Both figures are confirming the relative contribution results that impacts category of POF, TA and PMF have the highest contribution to the sum of all normalized values. Nevertheless, in comparison to scenario 2, scenario 1 has in the category of ME a slightly higher contribution of 1%, and in the category of FD, it has a lower contribution of 7.57%. Another difference is seen in the category of NLT, where scenario 2 exceeds scenario 1 by 2.5%. As both scenarios have CO
2 capture as primary function, the environmental impact category of climate change (CC) (which measures the contribution of CO
2 and other compounds to the global warming) has a lower contribution to the total environmental impact. This was expected since both technologies capture the CO
2.
The impact categories are influenced by the environmental impact of the different processes in the life cycle.
Table 9 shows that the significant impact categories taken from the pareto analysis are influenced by specific processes in both scenarios. According to
Table 9, the categories of POF, TA, PMF, and ME are influenced in 95% to 99.6% by the emissions of CO
2 capture process (carbonate looping, adsorption) in both scenarios (
Table 2 and
Table 4). However, the characterization values, for instance in category POF, are very small (scenario 1—0.039 kg NMVOC eq./1 kWh; scenario 2—0.062 kg NMVOC eq./1 kWh).
In the category of FD, the production chain of the lignite from Slovak lignite mix (which has a similar thermal efficiency as Czech lignite of 11 MJ/kg [
12]) results as the most significant process in both scenarios. In scenario 1, the lignite mix for the power unit contributes almost 86.40% to FD (
Table 9). In scenario 2, the hard coal mix for active carbon production increases the FD contribution by 40.70%, whereas lignite mix for power unit contributes 19.10%. Moreover, in scenario 2, the category of FD is influenced 40% by the utilization of thermal energy from natural gas mix for activated carbon production.
An interesting result is shown for the category of NLT. In scenario 1, major land transformation would be impacted by the construction of an air separation unit for oxygen production. In scenario 2, the hard coal production chain with all the mining process necessary for active carbon production turns out to be the process with the highest impact to the natural land transformation. Moreover, in comparison with scenario 1, hard coal would need to be mined and transported to the power unit, which creates an additional environmental burden. In scenario 1, the air separation unit would need to be built right in the local area of the power unit.
The category of CC is mainly influenced by the ratio of captured CO2. It is obvious that adsorption process would require higher amount of active carbon to be able to capture 95% of CO2 such as an IGCC-CaL system. That would lead to the increase of the total environmental impact. Also, the thermal energy from the natural gas combustion as a primary energy for activated carbon production, is influencing category of CC in 72%.
The primary goal for the CCT solution under Czech energy conditions was to design and compare post combustion and pre combustion systems for the same REU. However, during the research, problems occurred with the technological requirements (such as quality of the lignite for each REU) of each system. Thus, the input parameters had to be optimized, which led to different scale of REU, different lignite quality and different technological segments. Therefore, the specific case studies considered in this manuscript do not have the same basis for fair comparison. Still, in LCA analysis relating all the environmental impacts to 1 kWhe, the aforementioned differences are still present in particular environmental impacts.
This paper considers the specific case of activated carbon production from hard coal. However, activated carbon can be produced in several options from biomass or other organic waste that would decrease total environmental performance of the process. Also, different adsorption process configurations may lead to different results. This specific case of the PCC-A points out the problem with Na salts production after flue gas purification, which are currently considered as waste material with no other use.
The main advantage of PCC-A against IGCC-CaL is the thermal efficiency of the whole system. For the process of the CO
2 capture and compression, PCC-A requires a consumption of 28 MWe from the power unit, whereas IGCC-CaL requires for the same process 119.31 MWe (
Table 5). Thus, PCC-A decreases the thermal efficiency of the power unit by 4.67%, and IGCC-CaL decreases the thermal efficiency of the power unit by 12.5%. The thermal efficiency decrease would require a higher energy production that might also influence the environmental performance of IGCC-CaL system as well. Moreover, the thermal efficiency decline may be significant for the further operational costs increase. On the other hand, the specific energy consumption (MWe/t CO
2) in
Table 5 states that PCC-A (1.3 MWe/t CO
2) would require a slightly higher energy demand than the IGCC-CaL process (0.9 MWe/t CO
2).
The following table (
Table 11) shows the comparison of this study with other studies of [
8,
9,
10]. The environmental results of this study for pre-combustion IGCC-CaL system shows a lower kg CO
2 eqv./MWh than in a similar study of Petrescu et al. [
8]. The lower impact of climate change of this study (global warming potential) corresponds to the smaller size of the reference energy power plant and higher capture ratio. For eutrophication potential this study is resulting in much lower values that are comparable with the study of Clarens et al. [
10]. However, the impacts of the acidification potential in this study are the highest among of all studies. This might be influenced by the production of the used sorbent in the form of CaCO
3 + CaSO
4 as non-utilized waste product of high-temperature desulphurization. Moreover, the study shows the highest drop in net energy efficiency due to CCT implementation. The reason might be that the IGCC-CaL design of this study does not consider utilizing the heat losses due to lack of commercially viable heat exchangers for such amount of heat. In the case of post-combustion capture, PCC-A has the lowest CO
2 capture rate among all the studies. It corresponds to higher values in climate change in comparison with similar study of Clarens et al. [
10]. Also, in this study of PCC-A, specific emissions are the highest, which refers to the low CO
2 capture rate.
5.2. Economical Assessment
The key economic parameter for CCT integration is capture cost. The comparison of capture costs (
Table 10) among assessed CCT systems states that the less expensive technology is PCC-A system. The difference is shown in the values of CAPEX, which for IGCC-CaL is higher by €167 million and in OPEX by €17 million annually. The main reason for this difference is that PCC-A does not require high technological adjustments when compared to the IGCC-CaL systems. The IGCC-CaL system requires an initial batch of lignite with higher quality for gasification process, therefore the technological components (such as boiler) would increase the initial CAPEX. Moreover, IGCC-CaL requires the construction of an additional segment with auxiliary systems of air separation unit that also increases the initial investment.
Another parameter—cost of electricity (COE)—is influenced by OPEX. The higher OPEX of the IGCC-CaL system increases the energy cost by €33/MWh in comparison with the PCC-A (
Table 7). However, both case CCT studies are showing a higher COE per MWh in comparison with the actual market price of electricity (
Table 6). That leads to the conclusion that both systems tailored for the current Czech conditions are currently not economically viable.
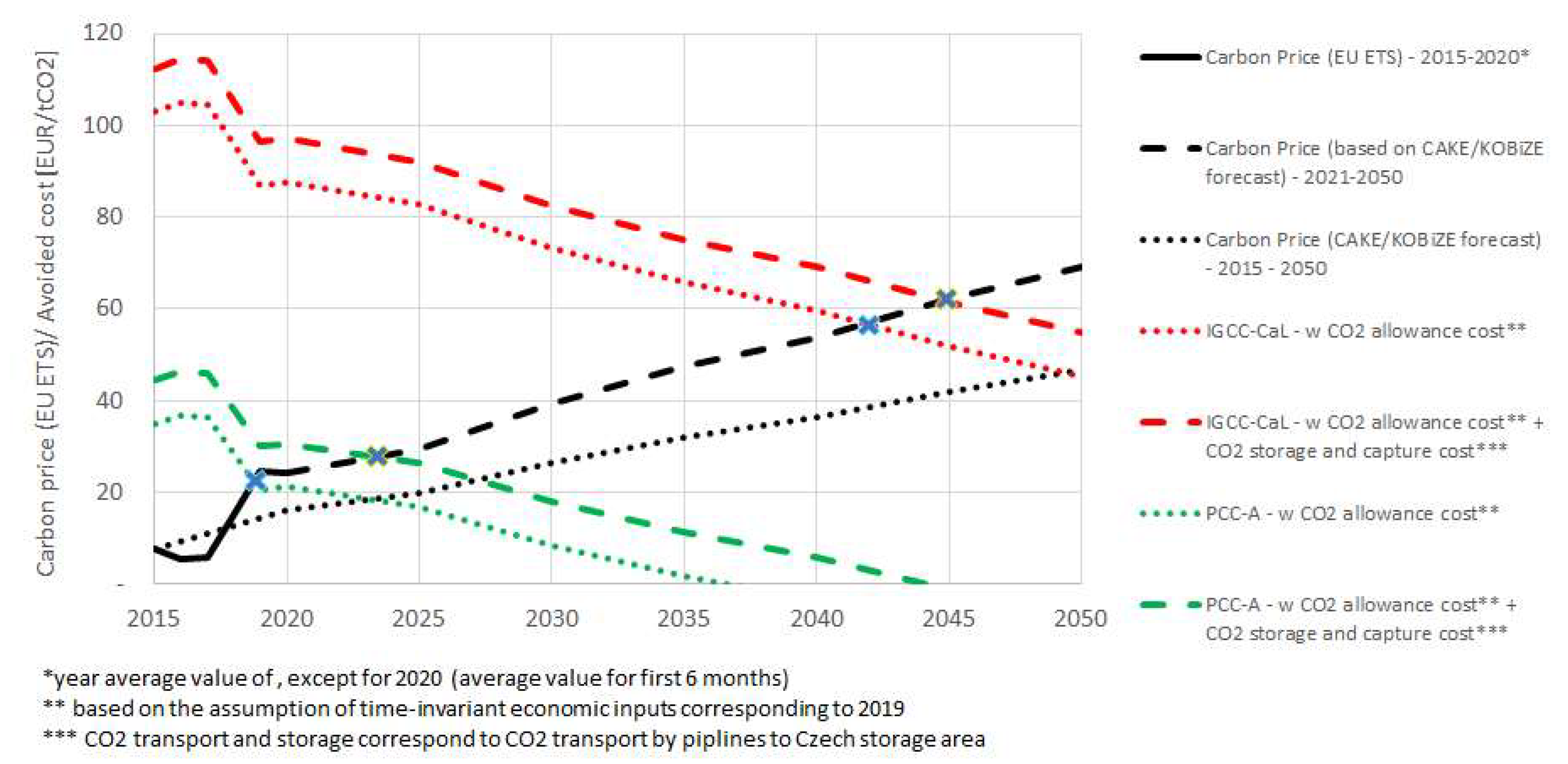
The economical assessment of this study is based on the comparison of the cost effectiveness of both CO
2 capture systems with the reference energy units. The results are shown in
Figure 3. The graph describes the rising trend of the price of CO
2 allowance throughout the years 2015–2050. The analyzed CCTs have the potential to achieve cost-effectivity in comparison to the power plant without CCT in the observed time frame. The PCC-A would be cost-competitive in the case the carbon price would be lower than €22.5/tCO
2 (for CCU case), or €26.3/tCO
2 (for CCS case). The IGCC-CaL would be cost-effective in the condition that the carbon price increases up to €58.7/tCO
2 (for CCU case) and €60.9/tCO
2 (for CCS case). PCC-A could achieve cost-effectiveness with carbon price at €24.1/tCO
2 or €20/tCO
2 under the condition of total CAPEX reduction by 5% and 15%, respectively.
It is important to note that the capital investments are decreasing over time for CCT, and therefore, total CCT costs will gradually decrease (dashed green and red lines in
Figure 5). These economic dynamics might be the subject of further comprehensive economic study on CCT.
It is assumed that the price of the CO
2 allowance will continue to rise (dashed black line in
Figure 3) in the current state of climate crisis and economy crisis. Therefore, the economic decision to invest into the CCT may be major but only in the first years (3–4 years of the CCT construction) but it will lead to cost savings after the payback period (six years according to Reference [
4]), as opposed to dealing with the inflating price of CO
2 allowance.
5.3. Environmental and Economic Combination Score
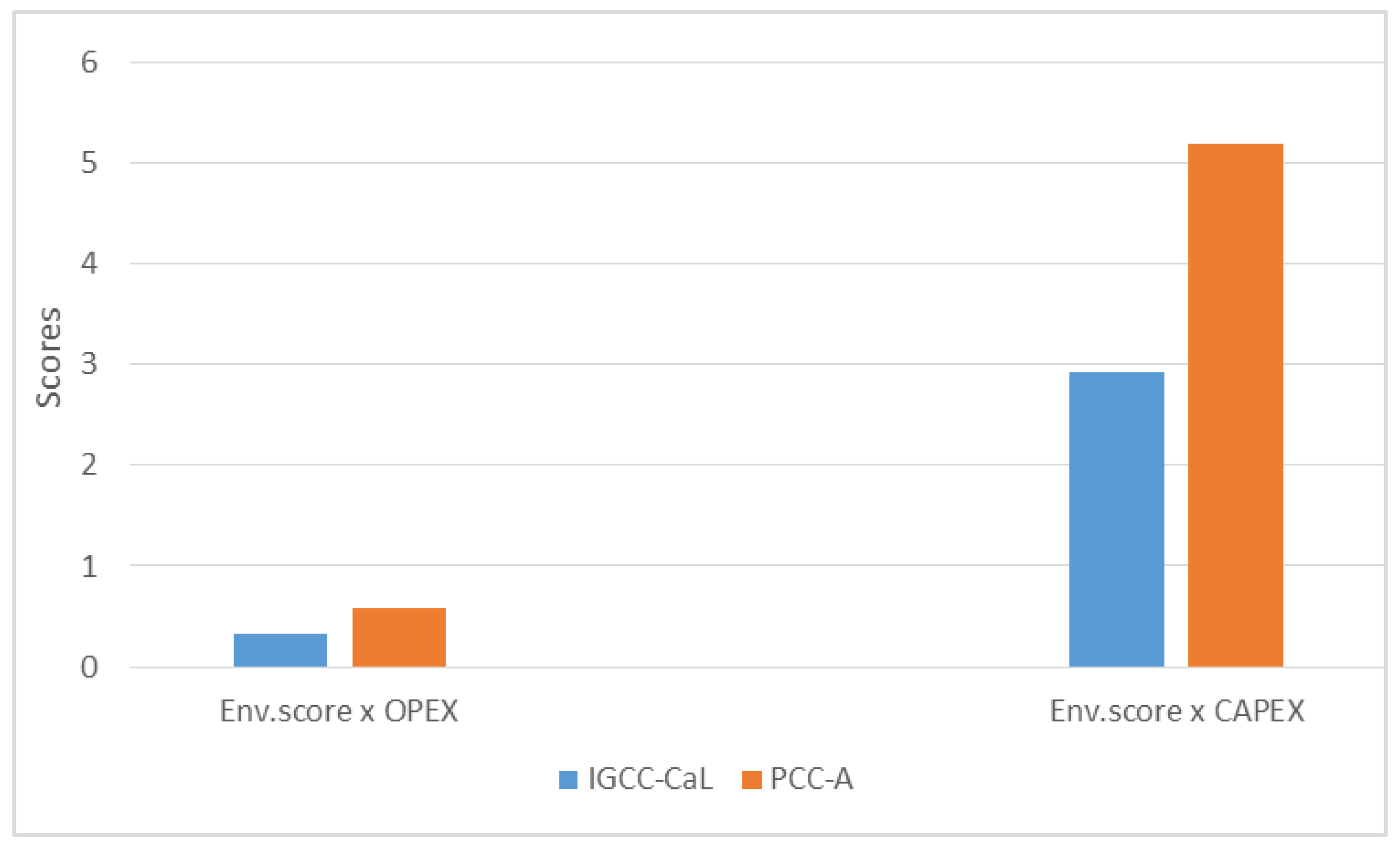
The decision-making process of the CCT integration into power units must be based on a complex assessment, where the environmental and economic scores combine. This combination can be done by simple multiplying the environmental score (sum of all normalized values) and values of OPEX or CAPEX. PCC-A in comparison with IGCC-CaL, has a worse environmental score (0.004) and lower OPEX (€123.06 million) and CAPEX values (€1.097 billion). The IGCC-CaL system has lower values for environmental score (0.0023) but higher values for OPEX (€140.3 million) and CAPEX (€1.2641 billion).
This decision conflict between environmental and economic performance can be resolved by the total product (multiplying the environmental score and CAPEX (or OPEX) value (
Figure 4). If the total value is low, it leads to the conclusion that the combination of environmental and economic performance is more favorable for the chosen technology.
The product of environmental score by CAPEX and OPEX can be seen in
Figure 6. The graph shows that the IGCC-CaL unit has a smaller total score for both CAPEX and OPEX combination with the environmental score. It concludes that even if the CAPEX and OPEX of IGCC are higher than in the PCC-A process, the environmental performance seems to lower the total combination score.
6. Conclusions
The study presents one of the possible options to select the suitable form of carbon capture technology (CCT) that would meet the sustainability indicators (technical, environmental, and economic) in the Czech energy industry. The presented paper combines environmental and economic variables to conclude the viable choice of carbon capture integration into the brown coal power units. The comparison of both systems concludes into the following points:
The specific case of IGCC with CaO looping integration has an overall better environmental performance with higher CO2 ratio capture of 95%; IGCC-CaL decreases the power unit thermal efficiency by 12.5%; IGCC-CaL has a higher capture cost and would become competitive if the carbon price increases up to €58.7/tCO2 (for CCU case) and €60.9/tCO2 (for CCS case).
The specific PCC-A case has an overall worse environmental performance with 75% of CO2 capture; PCC-A decreases the power unit thermal efficiency by 4.67%; Adsorption has lower capture costs and would become competitive if the carbon price is lower than €22.5/tCO2 (for CCU case) or €26.3/tCO2 (for CCS case).
The cost of electricity of both assessed carbon capture systems is exceeding the current market price.
The product of the environmental score by CAPEX, and the product of the environmental score by OPEX is lower for IGCC system with CaO looping.
Although it seems that IGCC-CaL integration would be the most suitable option for the carbon capture, thermal efficiency decrease can cause the additional increase in CAPEX and OPEX, which can also result in some environmental burden. On the other hand, the adsorption process can be optimized to enhance the environmental performance with relatively low investments.
It must be stressed that the decision-making process of CCT integration is affected by many other factors such as political decisions, social acceptance, or the economic statements of the energy industry and companies of the Czech Republic. This study presents how an environmental tool such as LCA and economical computation of cost effectiveness may help to contribute to the extensive feasibility study and complex decision-making process.
Further research of CCT technologies integrated into the Czech energy conditions could enhance this research and consider robust techno-economic and environmental analysis of all three considered technologies: post-combustion ammonia scrubbing, post-combustion capture by activated carbon adsorption, and pre-combustion IGCC-CaL.