Exergetic Evaluation of an Ethylene Refrigeration Cycle
Abstract
1. Introduction
2. Methodology
2.1. Refrigeration Systems Applied to Olefin Plants
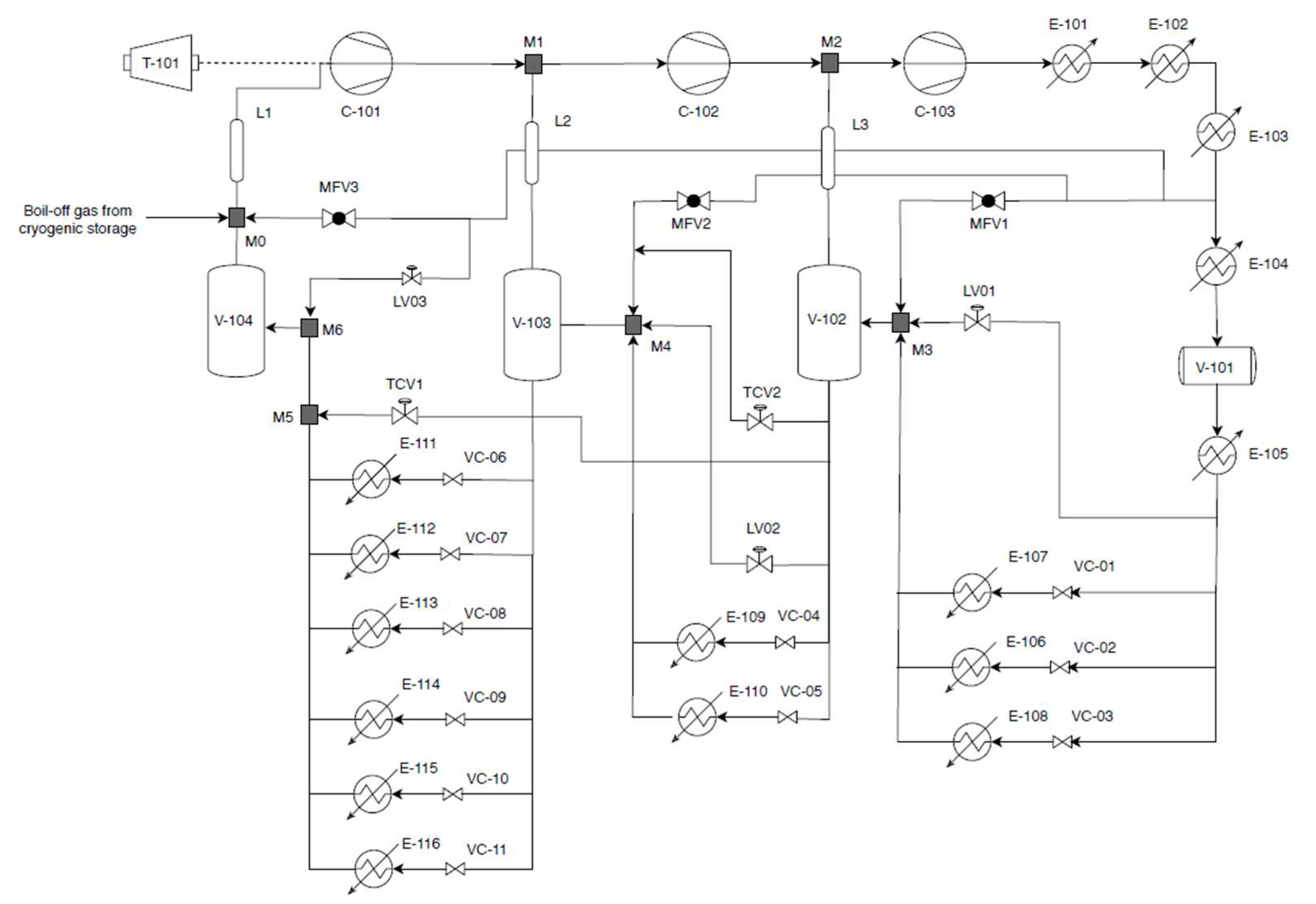
2.2. Modeling the Ethylene Refrigeration Cycle
2.3. Exergy Analys
2.3.1. Compressors
2.3.2. Heat Exchangers
2.3.3. Control Valves and Lines
2.3.4. Mixing Points
2.3.5. Vessels
2.3.6. Refrigeration Cycle
2.4. Methodological Approach
- Design case: This case considers the mass and energy balance of the unit project, retrieved from the process design book. The performance curves of the compressor were regressed and used in the model to determine the theoretical operating conditions of the machine.
- Operation case: This case represents the current refrigerant cycle operational condition. A stable operating period was selected, that had similar throughput and process conditions to the design case. The data were collected, treated, and inputted in the model. In this scenario, the performance of the refrigerant compressor is calculated using the real operating data.
3. Results and Discussion
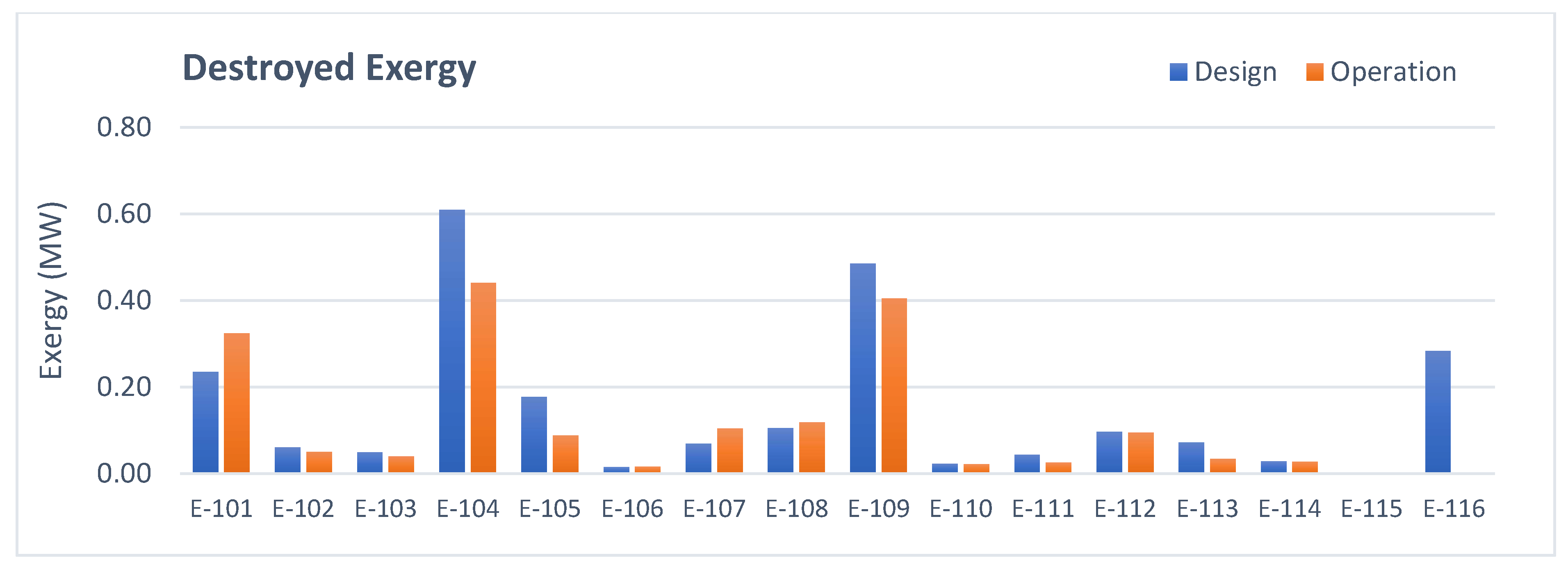
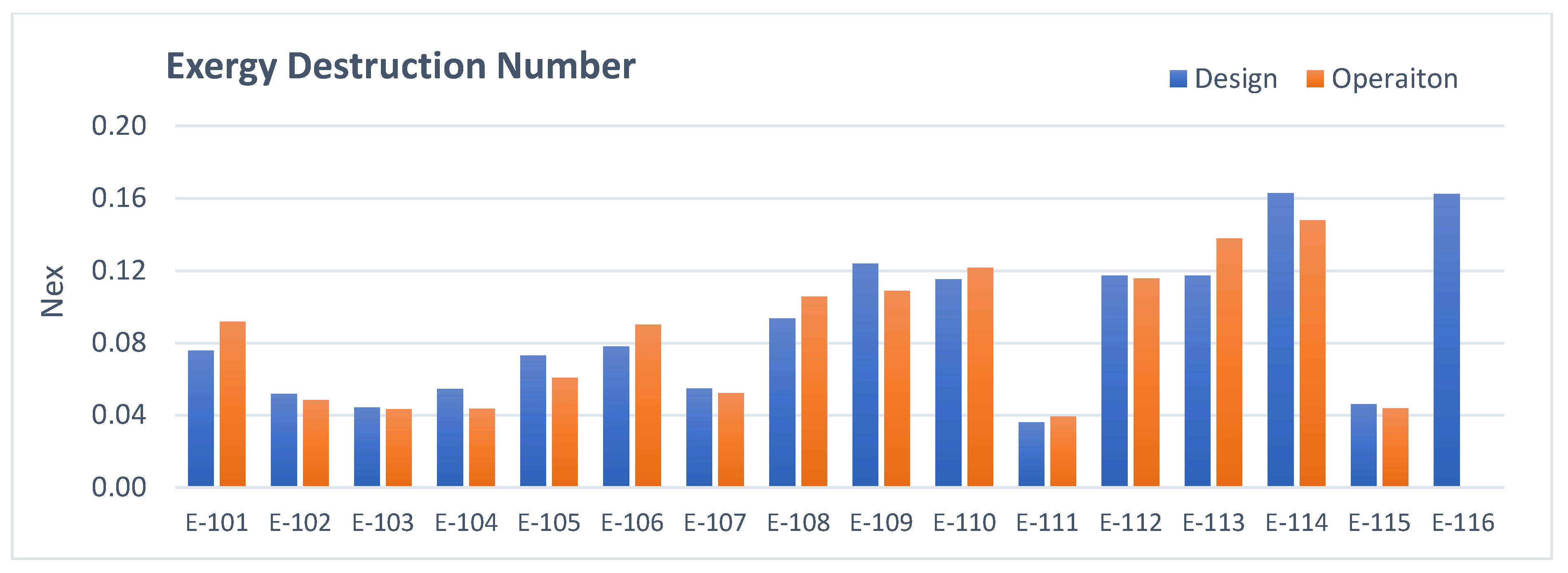
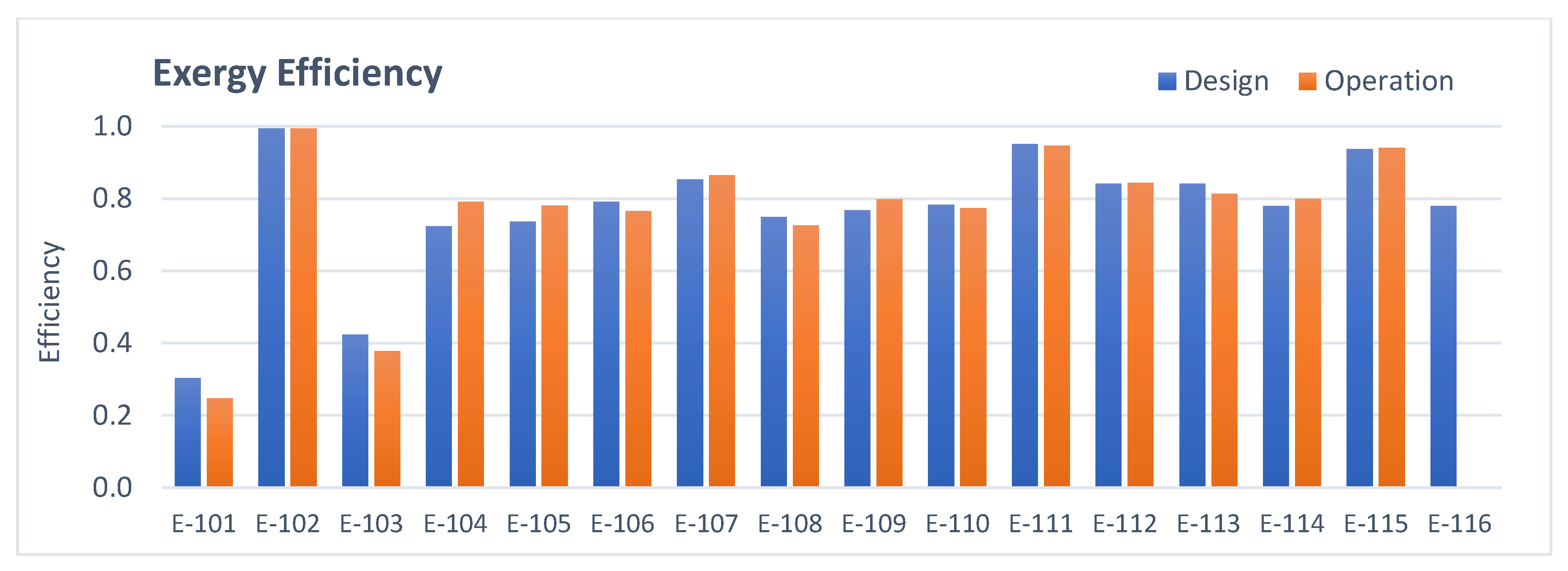
3.1. Heat Exchangers Analysis
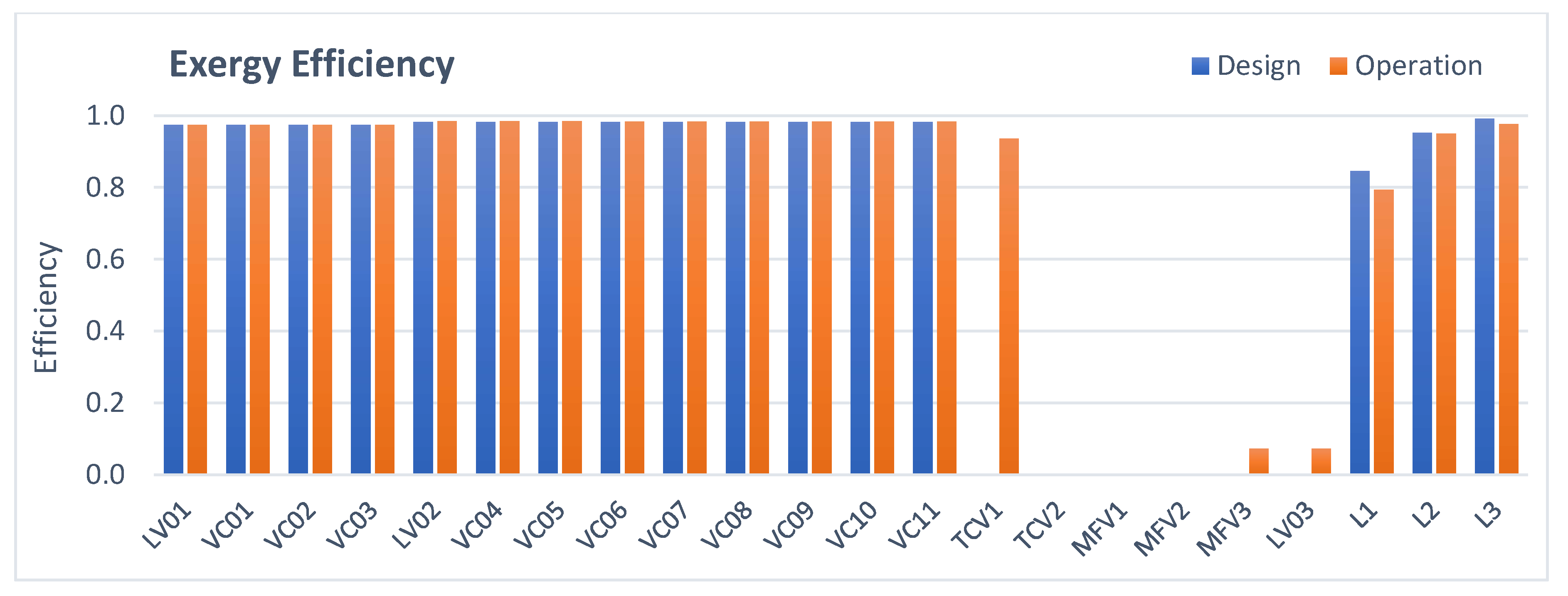
3.2. Valves and Lines Analysis
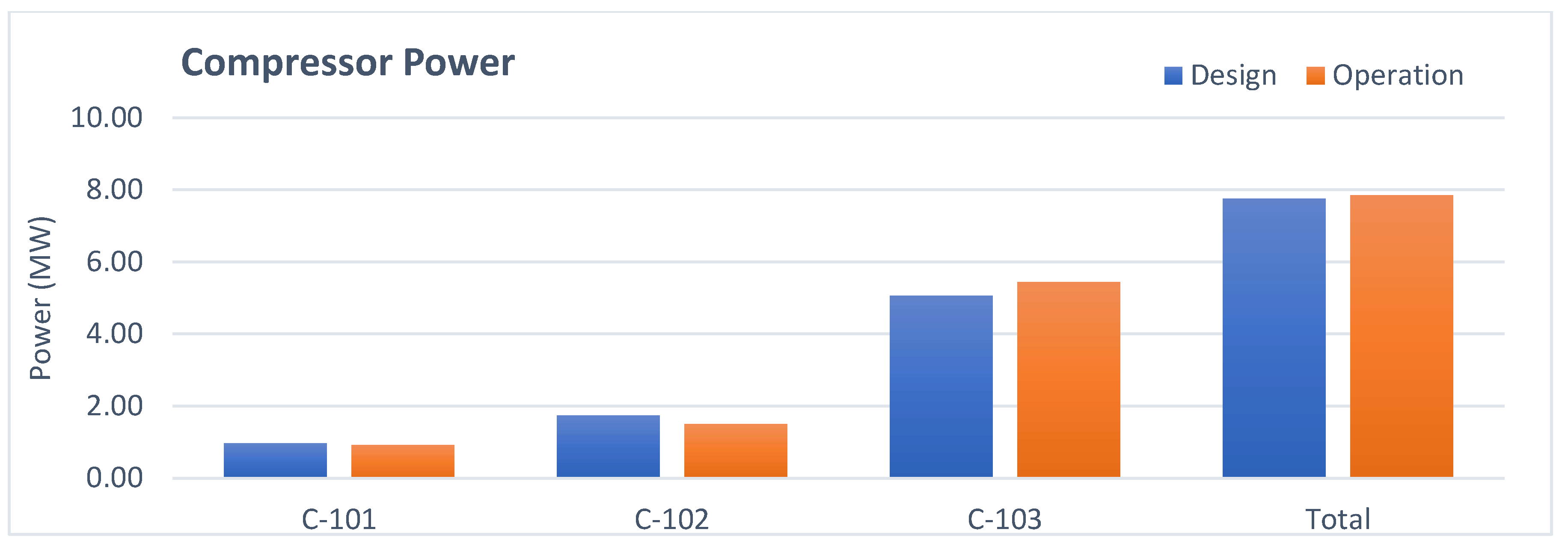
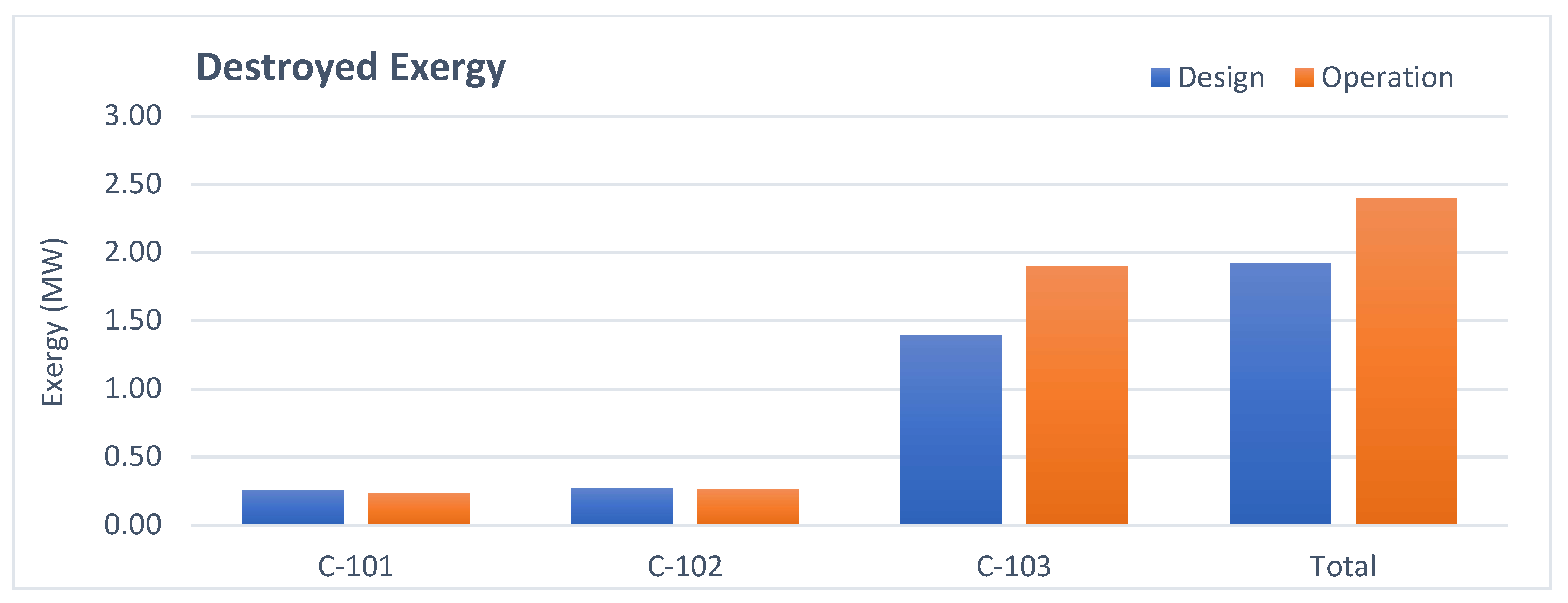
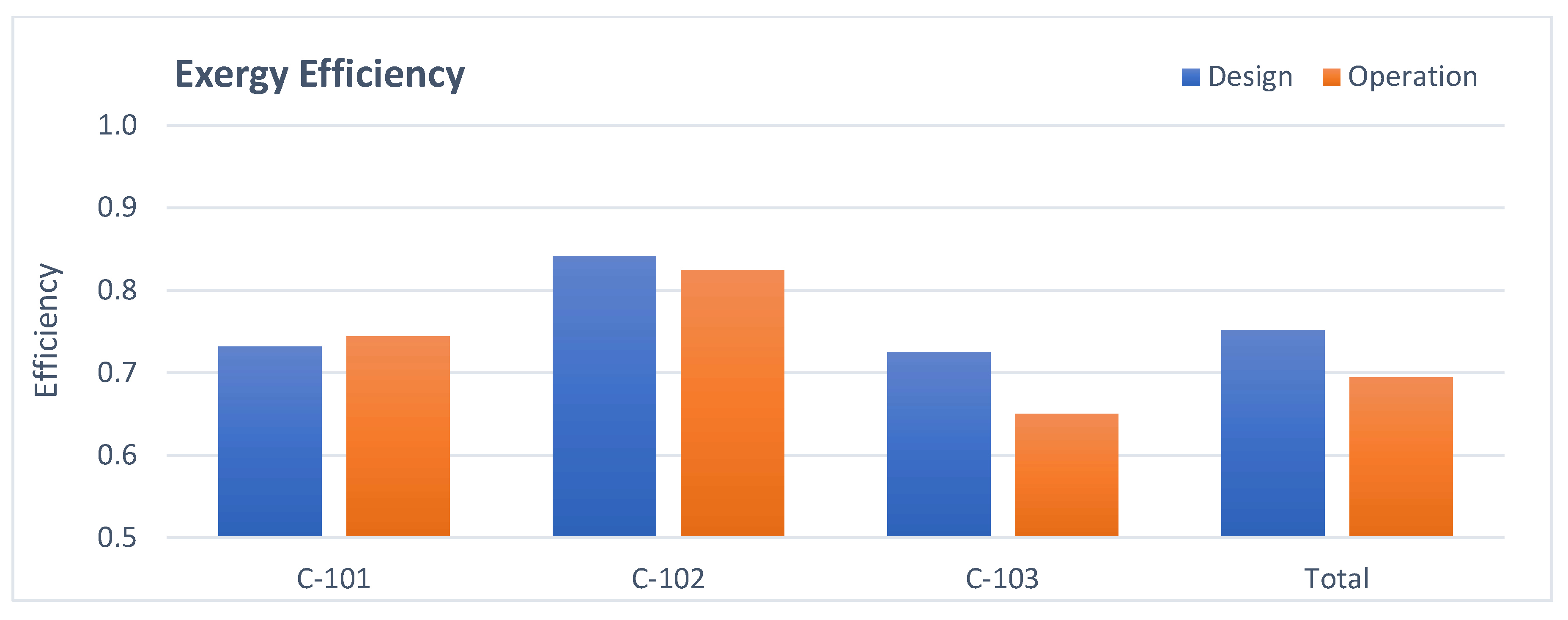
3.3. Refrigerant Compressor Analysis
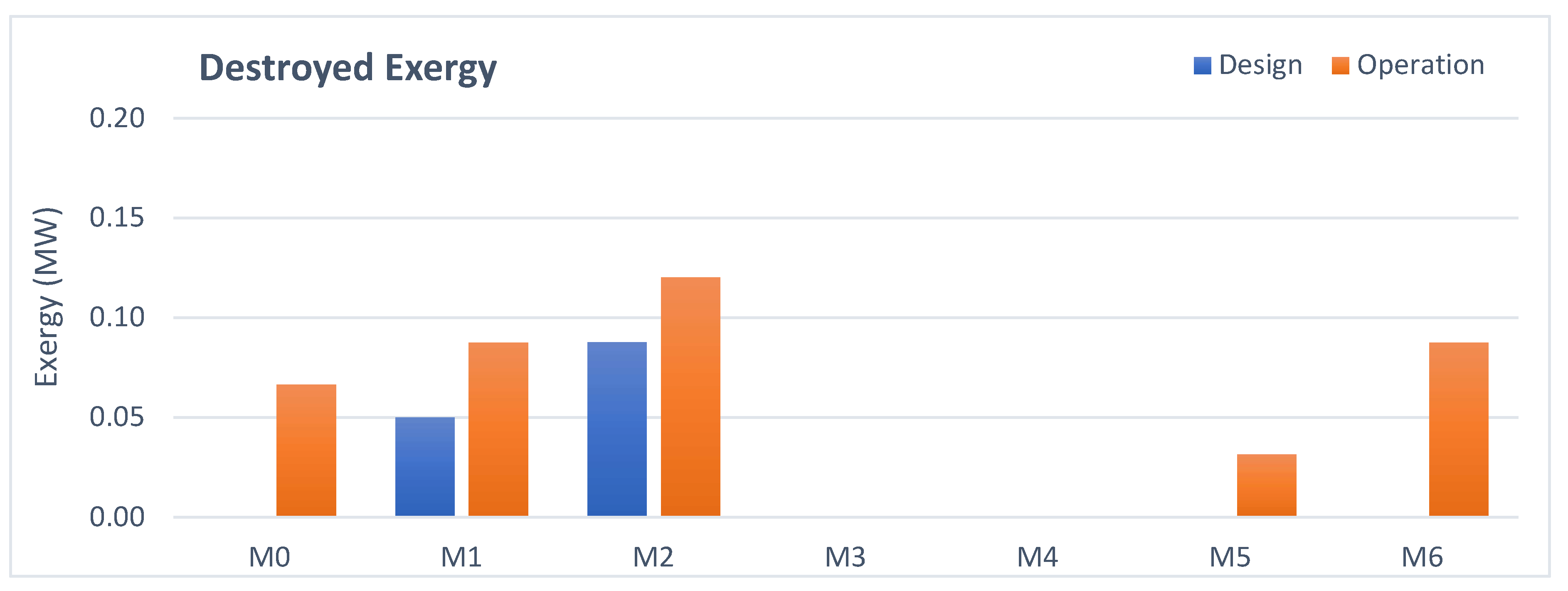
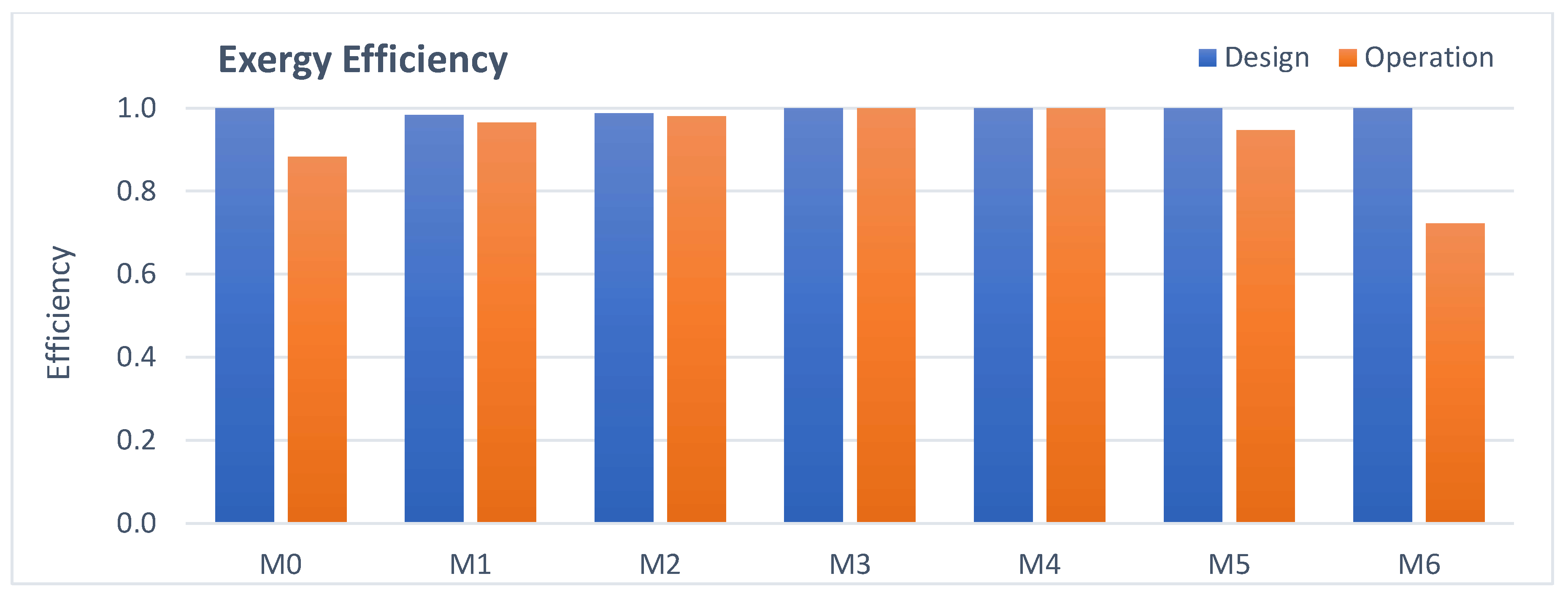
3.4. Mixing Points Analysis
3.5. Vessels Analysis
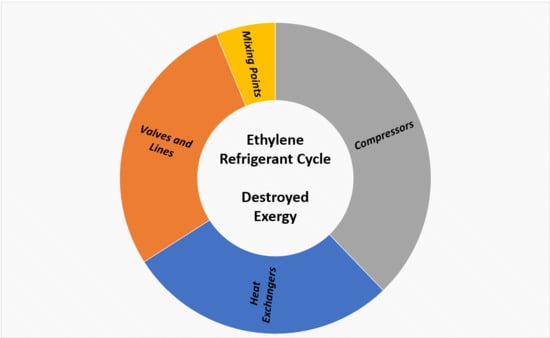
3.6. Refrigeration Cycle Analysis
- Heat exchangers: The total amount of irreversibilities is lower in operation mainly due to the lower energy consumption of the users and removal of E-116 from operation.
- Valves and lines: The higher amount of irreversibilities generated in operation is due to the action of the mini-flow valve (MFV3) and first stage level control valve.
- Compressor: The higher exergy destruction in operation is due to a lower polytropic efficiency.
- Mixing points: The higher exergy destruction in operation is due to the greater temperature difference between the streams at the suction of the first stage.
3.7. Identified Opportunities for Refrigeration Cycle Performance Improvement
- Refrigerant compressor efficiency recovery, whose value is below that predicted by the compressor performance curve. This can be achieved by performing general maintenance of this equipment in the next equipment turnaround.
- Closure of the first refrigeration stage mini-flow valve, through a better flow distribution among the TCV1, LV03, and MFV3 valves. With the removal of the E-116 exchanger from operation, it is necessary to maintain a recirculating flow to the first stage to ensure that the compressor does not operate close to the surge point. Currently, much of this circulation is performed by the anti-surge control valve, which causes ethylene to overheat at the compressor’s first stage suction. The use of the TCV1 and LV03 valves can increase the ethylene recirculation in the first stage to the point that it is no longer necessary to operate with the mini-flow valve open, which reduces the first stage suction temperature to values near to the saturation point.
- A third case (optimized operation) was simulated considering the implementation of the two identified opportunities. Figure 16 shows a comparison of the amount of exergy destroyed per device type for the operation case and for the optimized operation case. There was a reduction of approximately 4% in the total amount of exergy destroyed in the new case evaluated because of the better performance of the refrigerant compressor and the lower generation of irreversibilities at the mixing points.
4. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols | Units | |
| Specific exergy | kJ.kg−1 | |
| Specific enthalpy | kJ.kg−1 | |
| Specific entropy | kJ.kg−1.K−1 | |
| T | Temperature | K |
| V | Specific volume | m³.kg−1 |
| P | Pressure | kPa |
| g | Gravity | m.s2 |
| Polytropic coefficient | - | |
| Isentropic coefficient | - | |
| Polytropic head | m | |
| Work rate | MW | |
| Mass flow rate | kg.s−1 | |
| Exergy destruction rate of irreversibility | MW | |
| Exergetic efficiency | - | |
| Heat transfer rate | MW | |
| Exergy destruction number | - | |
| Specific energy consumption | kJ.kg−1 | |
Abbreviations
| Coefficient of performance | |
| SC | Steam cracker |
| C | Refrigerant compressor |
| E | Heat exchanger |
| VC | Control valves |
| LV | Level Control valves |
| MFV | Mini-flow valves |
| V | Vapor-liquid separators vessels |
| M | Mixing points |
| L | Compressor suction lines |
Subscripts
| 0 | Reference State |
| c | Compressor |
| HE | Heat Exchanger |
| In | Inlet |
| Out | Outlet |
| cv | Control Valve |
| M | Mixing Points |
| V | Vessel |
| Rev | Reversible |
References
- Matar, S.; Hatch, L.F. Chemistry of Petrochemical Process, 2nd ed.; Gulf Publishing Company: Houston, TX, USA, 2001. [Google Scholar]
- Pickett, T.M. Refrigeration Technology as Practiced in Olefins Plants. AIChE Spring Natl. Meet. 2005. Available online: https://epc.omnibooksonline.com/65235-epc-2005a-1.3718294/t008-1.3718512/f008-1.3718513/a031-1.3718523/ap031-1.3718524 (accessed on 10 April 2020).
- Fuentes, A. Brasil Piora em Ranking e Passa a ser o 6° com a Energia Mais Cara do Mundo. Available online: https://veja.abril.com.br/blog/impavido-colosso/brasil-piora-em-ranking-e-passa-a-ser-o-6-com-a-energia-mais-cara-do-mundo/ (accessed on 10 April 2020).
- Pfenninger, S.; Hawkes, A.; Keirstead, J. Energy systems modeling for twenty-first century energy challenges. Renew. Sustain. Energy Rev. 2014, 33, 74–86. [Google Scholar] [CrossRef]
- Zhou, N.; Fridley, D.; Khanna, N.Z.; Ke, J.; McNeil, M.; Levine, M. China’s energy and emissions outlook to 2050: Perspectives from bottom-up energy end-use model. Energy Policy 2013, 53, 51–62. [Google Scholar] [CrossRef]
- Catalán-Gil, J.; Sánchez, D.; Llopis, R.; Nebot-Andrés, L.; Cabello, R. Energy evaluation of multiple stage commercial refrigeration architectures adapted to F-gas regulation. Energies 2018, 11, 1915. [Google Scholar] [CrossRef]
- Rivero, R. Application of the exergy concept in the petroleum refining and petrochemical industry. Energy Convers. Manag. 2002, 43, 1199–1220. [Google Scholar] [CrossRef]
- Tsatsaronis, G. Recent developments in exergy analysis and exergoeconomics. Int. J. Exergy 2008, 5, 489. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, Y.; Kulacki, F.A. Energetic and exergetic analysis of a transcritical N2O refrigeration cycle with an expander. Entropy 2018, 20, 31. [Google Scholar] [CrossRef]
- Dokandari, D.A.; Mahmoudi, S.M.S.; Bidi, M.; Khoshkhoo, R.H.; Rosen, M.A. First and second law analyses of trans-critical N2O refrigeration cycle using an ejector. Sustainability 2018, 10, 1177. [Google Scholar] [CrossRef]
- Gullo, P. Advanced thermodynamic analysis of a transcritical R744 booster refrigerating unit with dedicated mechanical subcooling. Energies 2018, 11, 3058. [Google Scholar] [CrossRef]
- Ahamed, J.U.; Saidur, R.; Masjuki, H.H. A review on exergy analysis of vapor compression refrigeration system. Renew. Sustain. Energy Rev. 2011, 15, 1593–1600. [Google Scholar] [CrossRef]
- Jahromi, F.S.; Beheshti, M.; Rajabi, R.F. Comparison between differential evolution algorithms and response surface methodology in ethylene plant optimization based on an extended combined energy—Exergy analysis. Energy 2018, 164, 1114–1134. [Google Scholar] [CrossRef]
- Tirandazi, B.; Mehrpooya, M.; Vatani, A.; Moosavian, S.M.M.A.A. Exergy analysis of C2+ recovery plants refrigeration cycles. Chem. Eng. Res. Des. 2011, 89, 676–689. [Google Scholar] [CrossRef]
- Lobosco, R.J. Análise do Desempenho Termodinâmico e Ambiental de um ciclo de Refrigeração e Proposta de uma Função Global de Avaliação. Doctoral Dissertation, Universidade Estadual de Campinas, Campinas, Brazil, 2009. [Google Scholar]
- Sivakumar, M.; Somasundaram, P. Exergy and energy analysis of three stage auto refrigerating cascade system using Zeotropic mixture for sustainable development. Energy Convers. Manag. 2014, 84, 589–596. [Google Scholar] [CrossRef]
- Mafi, M.; Naeynian, S.M.M.; Amidpour, M. Exergy analysis of multistage cascade low temperature refrigeration systems used in olefin plants. Int. J. Refrig. 2009, 32, 279–294. [Google Scholar] [CrossRef]
- Fábrega, F.M.; Rossi, J.S.; D’Angelo, J.V.H. Exergetic analysis of the refrigeration system in ethylene and propylene production process. Energy 2010, 35, 1224–1231. [Google Scholar] [CrossRef]
- Mahabadipour, H.; Ghaebi, H. Development and comparison of two expander cycles used in refrigeration system of olefin plant based on exergy analysis. Appl. Therm. Eng. 2013, 50, 771–780. [Google Scholar] [CrossRef]
- Ghannadzadeh, A.; Sadeqzadeh, M. Exergy analysis as a scoping tool for cleaner production of chemicals: A case study of an ethylene production process. J. Clean. Prod. 2015, 129, 508–520. [Google Scholar] [CrossRef]
- Vilarinho, A.N.; Campos, J.B.L.M.; Pinho, C. Energy and exergy analysis of an aromatics plant. Case Stud. Therm. Eng. 2016, 8, 115–127. [Google Scholar] [CrossRef]
- Palizdar, A.; Sadrameli, S.M. Conventional and advanced exergoeconomic analyses applied to ethylene refrigeration system of an existing olefin plant. Energy Convers. Manag. 2017, 138, 474–485. [Google Scholar] [CrossRef]
- Harvey, S. Centrifugal compressors in ethylene plants. Chem. Eng. Prog. 2017, 113, 28–32. [Google Scholar]
- Schaider, L.; Desai, P. Trends in Monitoring and Control of Ethylene Plant Compressors. AIChE Ethyl. Prod. Conf. Proc. 2002, 23. [Google Scholar]
- Brown, J.S. Predicting performance of refrigerants using the Peng-Robinson Equation of State. Int. J. Refrig. 2007, 30, 1319–1328. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M.A. EXERGY: Energy, Environment and Sustainable Development; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 0080531350. [Google Scholar]
- Wark, K.J. Advanced Thermodynamics for Engineers; McGraw-Hill: New York, NY, USA, 1995. [Google Scholar]
- Montanez-Morantes, M.; Jobson, M.; Zhang, N. Operational optimisation of centrifugal compressors in multilevel refrigeration cycles. Comput. Chem. Eng. 2016, 85, 188–201. [Google Scholar] [CrossRef]






| Description | Unit | Design | Operation |
|---|---|---|---|
| 1st Stage Q users | MW | 4.6 | 1.9 |
| 2nd Stage Q users | MW | 4.1 | 3.9 |
| 3rd Stage Q users | MW | 2.6 | 3.3 |
| Q Coolers | MW | 19.0 | 17.0 |
| 1st Stage Suction Pressure | kPa | 103.3 | 103.3 |
| 2nd Stage Suction Pressure | kPa | 331.8 | 319.0 |
| 3rd Stage Suction Pressure | kPa | 844.6 | 760.3 |
| Discharge Pressure | kPa | 3004.1 | 2621.6 |
| 1st Stage Suction Temperature | °C | −102.0 | −84.0 |
| 2nd Stage Suction Temperature | °C | −58.7 | −49.0 |
| 3rd Stage Suction Temperature | °C | −19.9 | −19.2 |
| Discharge Temperature | °C | 82.8 | 92.8 |
| Name | Process Point |
|---|---|
| M0 | C-101 Suction |
| M1 | C-102 Suction |
| M2 | C-103 Suction |
| M3 | V-102 Inlet |
| M4 | V-103 Inlet |
| M5 | V-104 Inlet |
| M6 | V-104 Vaporization Point |
| Indicator | Type | Unit | Design | Operation | Variation |
|---|---|---|---|---|---|
| Specific energy consumption | 1st Law | kJ.kg−1 | 217.5 | 233.6 | 7.4% |
| COP | 1st Law | - | 1.45 | 1.16 | −20.0% |
| Reversible work | 2nd Law | MW | 2.59 | 1.51 | −41.8% |
| 2nd law efficiency | 2nd Law | - | 0.33 | 0.19 | −42.5% |
| Indicator | Unit | Design | Operation | Optimized Operation |
|---|---|---|---|---|
| Specific energy consumption | kJ.kg−1 | 217.5 | 233.6 | 224.9 |
| COP | - | 1.45 | 1.16 | 1.19 |
| Reversible work | MW | 2.59 | 1.51 | 1.56 |
| 2nd law efficiency | - | 0.33 | 0.19 | 0.20 |
| Compressor power | MW | 7.76 | 7.85 | 7.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, F.; Santos, A.; Calixto, E.; Pessoa, F.; Santana, D. Exergetic Evaluation of an Ethylene Refrigeration Cycle. Energies 2020, 13, 3753. https://doi.org/10.3390/en13143753
Amaral F, Santos A, Calixto E, Pessoa F, Santana D. Exergetic Evaluation of an Ethylene Refrigeration Cycle. Energies. 2020; 13(14):3753. https://doi.org/10.3390/en13143753
Chicago/Turabian StyleAmaral, Francisco, Alex Santos, Ewerton Calixto, Fernando Pessoa, and Delano Santana. 2020. "Exergetic Evaluation of an Ethylene Refrigeration Cycle" Energies 13, no. 14: 3753. https://doi.org/10.3390/en13143753
APA StyleAmaral, F., Santos, A., Calixto, E., Pessoa, F., & Santana, D. (2020). Exergetic Evaluation of an Ethylene Refrigeration Cycle. Energies, 13(14), 3753. https://doi.org/10.3390/en13143753