The Latest Method for Surface Tension Determination: Experimental Validation
Abstract
1. Introduction
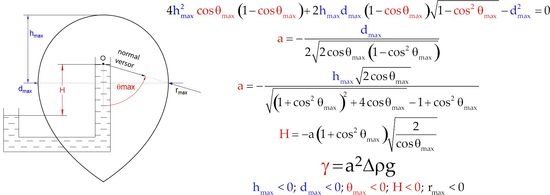
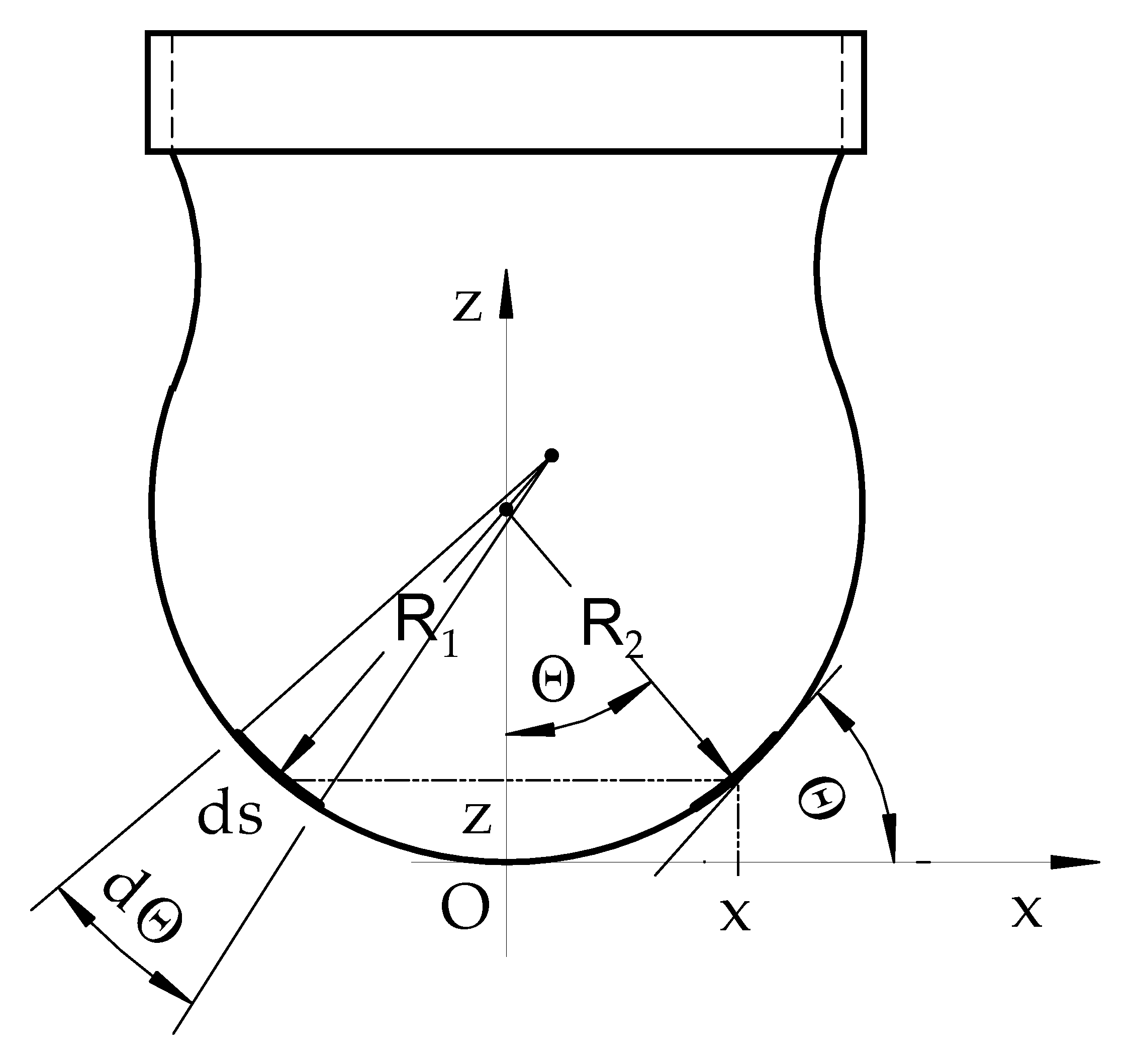
2. Contemporary Interface Model
3. The Experiment
4. Determining Specific Gravity of the Fluids
Fluid Densities Determination
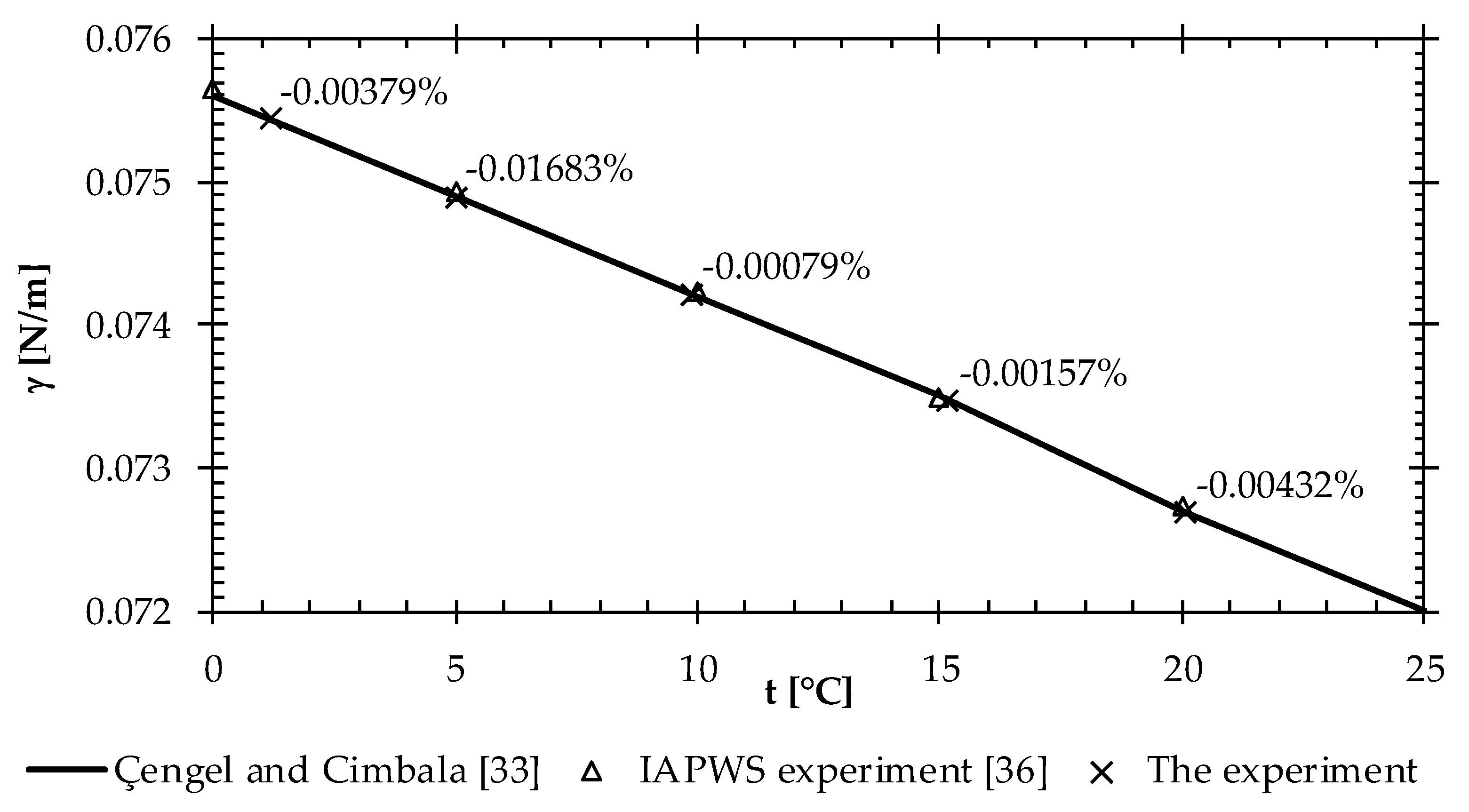
5. Results and Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gil, B.; Rogala, Z.; Dorosz, P. Pool Boiling Heat Transfer Coefficient of Low-Pressure Glow Plasma Treated Water at Atmospheric and Reduced Pressure. Energies 2020, 13, 69. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Ma, P.; Wang, B. Spray Combustion Characteristics and Soot Emission Reduction of Hydrous Ethanol Diesel Emulsion Fuel Using Color-Ratio Pyrometry. Energies 2017, 10, 2062. [Google Scholar] [CrossRef]
- Tziourtzioumis, D.N.; Stamatelos, A.M. Experimental Investigation of the Effect of Biodiesel Blends on a DI Diesel Engine’s Injection and Combustion. Energies 2017, 10, 970. [Google Scholar] [CrossRef]
- Na, M.M.; Aziz, A.R.A.; Hagos, F.Y.; Noor, M.M.; Kadirgama, K.; Mamat, R.; Abdullah, A.A. The Influence of Formulation Ratio and Emulsifying Settings on Tri-Fuel (Diesel–Ethanol–Biodiesel) Emulsion Properties. Energies 2019, 12, 1708. [Google Scholar] [CrossRef]
- Hossain, F.M.; Nabi, M.N.; Rahman, M.M.; Bari, S.; Van, T.C.; Rahman, S.M.A.; Rainey, T.J.; Bodisco, T.A.; Suara, K.; Ristovski, Z.; et al. Experimental Investigation of Diesel Engine Performance, Combustion and Emissions Using a Novel Series of Dioctyl Phthalate (DOP) BiofuelsDerived from Microalgae. Energies 2019, 12, 1964. [Google Scholar] [CrossRef]
- Belhaj, F.; Elraies, K.A.; Alnarabiji, M.S.; Shuhli, J.A.B.M.; Mahmood, S.M.; Ern, L.W. Experimental Investigation of Surfactant Partitioning in Pre-CMC and Post-CMC Regimes for Enhanced Oil Recovery Application. Energies 2019, 12, 2319. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Kamal, M.S.; Murtaza, M. Synthesis of Novel Ethoxylated Quaternary Ammonium Gemini Surfactants for Enhanced Oil Recovery Application. Energies 2019, 12, 1731. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.-H.; Park, S. Comprehensive Spray Characteristics ofWater in Port Fuel Injection Injector. Energies 2020, 13, 396. [Google Scholar] [CrossRef]
- Pruppacher, H.R.; Klett, J.D. Microphysics of Clouds and Precipitation; Springer: Berlin/Heidelberg, Germany, 2010; p. 954. [Google Scholar]
- Khvorostyanov, V.I.; Curry, J.A. Thermodynamics, Kinetics, and Microphysics of Clouds, 1st ed.; Cambridge University Press: Cambridge, UK, 2014; p. 782. [Google Scholar]
- Hellmuth, O.; Khvorostyanov, V.I.; Curry, J.A.; Shchekin, A.K.; Schmelzer, J.W.P.; Feistel, R.; Djikaev, Y.S.; Baidakov, V.G. Selected Aspects of Atmospheric Ice and Salt Crystallisation; Schmelzer, J.W.P., Hellmuth, O., Eds.; Joint Institute for Nuclear Research: Dubna, Russia, 2013; Volume 1, p. 513. [Google Scholar]
- Hellmuth, O.; AShchekin, K.; Feistel, R.; Schmelzer, J.W.P.; Abyzov, A.S. Physical interpretation of the ice contact angles, fitted to experimental data on immersion freezing of kaolinite particles. Interfacial Phenom. Heat Transf. 2018, 6, 37–74. [Google Scholar] [CrossRef]
- Vinš, V.; Hykl, J.; Hrubý, J.; Blahut, A.; Celný, D.; Čenský, M.; Prokopová, O. Possible Anomaly in the Surface Tension of Supercooled Water: New Experiments at Extreme Supercooling down to –31.4 °C. J. Phys. Chem. Lett. 2020, 11, 4443–4447. [Google Scholar]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65. [Google Scholar]
- Adamson, W. Physical Chemistry of Surfaces; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Bashforth, F.; Adams, J.C. Chapter III. On the calculation of the theoretical forms of drops of fluid, under the influence of capillary action, when such drops are bounded by surfaces of revolution which meet their respective axes at right angles. In An Attempt to Test the Theories of Capillary Action by Comparing the Theoretical and Measured Forms of Drops of Fluid; The University Press: Cambridge, UK, 1883; pp. 13–54. [Google Scholar]
- Hartland, S.; Hartley, R.W. Axisymmetric Fluid-Liquid Interfaces. Tables Giving the Shape of Sessile and Pendant Drops and External Menisci, with Examples of their Use; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Rotenberg, Y.; Boruvka, L.; Neumann, A.W. Determination of surface tension and contact angle from the shapes of axisymmetric fluid interfaces. J. Colloid Interface Sci. 1983, 93, 169–183. [Google Scholar] [CrossRef]
- Cheng, P.; Li, D.; Boruvka, L.; Rotenberg, Y.; Neumann, A.W. Automation of axisymmetric drop shape analysis for measurements of interfacial tensions and contact angles. Colloids Surfaces 1990, 43, 151–167. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Vollhardt, D.; Miller, R.; Li, D.; Neumann, A.W. Axisymmetric drop shape analysis as a film balance. Colloids Surfaces Physicochem. Eng. Asp. 1994, 88, 51–58. [Google Scholar] [CrossRef]
- Li, J.; Miller, R.; Möhwald, H. Phospholipid monolayers and their dynamic interfacial behaviour studied by axisymmetric drop shape analysis. Thin Solid Film 1996, 25, 284–285. [Google Scholar] [CrossRef]
- Passandideh-Fard, M.; Chen, P.C.; Mostaghimi, J.T.; Neumann, A.W. The generalized Laplace equation of capillarity I. Thermodynamic and hydrostatic considerations of the fundamental equation for interfaces. Adv. Colloid Interface Sci. 1996, 63, 151–177. [Google Scholar] [CrossRef]
- Del Río, I.; Neumann, A.W. Axisymmetric Drop Shape Analysis: Computational Methods for the Measurement of Interfacial Properties from the Shape and Dimensions of Pendant and Sessile Drops. J. Colloid Interface Sci. 1997, 196, 136–147. [Google Scholar]
- Juza, J. The pendant drop method of surface tension measurement: Equation interpolating the shape factor tables for several selected planes. Czechoslov. J. Phys. 1997, 47, 351–357. [Google Scholar] [CrossRef]
- Kazayawoko, M.; Neumann, A.W.; Balatinecz, J.J. Estimating the wettability of wood by the Axisymmetric Drop Shape Analysis-contact Diameter method. Wood Sci. Technol. 1997, 31, 87–95. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Cheung, L.K.; Park, C.B.; Neumann, A.W. Study on the surface tensions of polymer melts using axisymmetric drop shape analysis. Polym. Eng. Sci. 1998, 38, 757–764. [Google Scholar] [CrossRef]
- Berry, J.D.; Neeson, M.J.; Dagastin, R.R.; Chan, D.Y.; Tabor, R.F. Axisymmetric drop shape analysis (ADSA) for the. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Hoorfar, M.; Neumann, A.W. Recent progress in Axisymmetric Drop Shape Analysis (ADSA). Adv. Colloid Interface Sci. 2006, 121, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, A. Liquid–gas interface under hydrostatic pressure. In WIT Transactions on Engineering Sciences; WIT Press: Split, Croatia, 2012; pp. 251–261. [Google Scholar]
- Gajewski, A. The Surface Tension Effects in Rivulets and Drops; Oficyna Wydawnicza Politechniki Białostockiej: Białystok, Poland, 2016. (In Polish) [Google Scholar]
- Gajewski, A. A couple of new ways of surface tension determination. Int. J. Heat Mass Transf. 2017, 115, 909–917. [Google Scholar] [CrossRef]
- Karolczak, N.D.; Jaworska, J. Encyclopaedia—Physics with Astronomy; Greg: Kraków, Poland, 2016. (In Polish) [Google Scholar]
- Çengel, Y.A.; Cimbala, J.M. Fluid Mechanics Fundamental and Applications, 3rd ed.; McGraw-Hill Education: Singapore, 2014. [Google Scholar]
- Çengel, Y.A.; Boles, M.A. Thermodynamics: An Engineering Approach; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Ferencowicz, J. Wentylacja, Klimatyzacja, 1st ed.; Arkady: Warszawa, Poland, 1962. [Google Scholar]
- The International Association for the Properties of Water and Steam, “IAPWS R1–76(2014) Revised Release on Surface Tension of Ordinary Water Substance. 2014. Available online: http://www.iapws.org/relguide/Surf-H2O-2014.pdf (accessed on 29 June 2020).
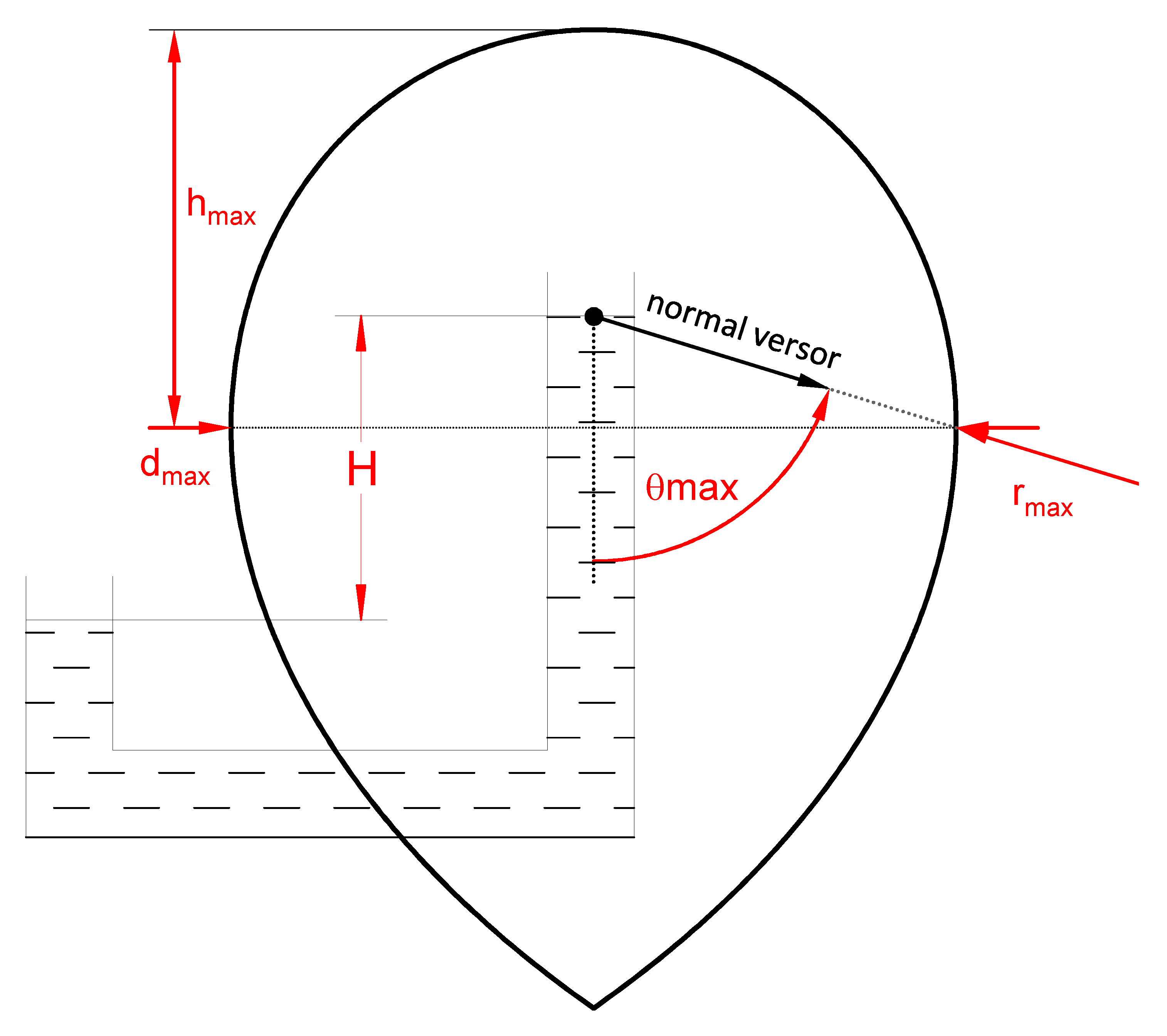


| Point Number | t [°C] | dmax [mm] | hmax [mm] |
|---|---|---|---|
| 1 | 1.20 | −4.076 | −2.256 |
| 2 | 5.10 | −4.062 | −2.248 |
| 3 | 9.90 | −4.043 | −2.238 |
| 4 | 15.20 | −4.024 | −2.227 |
| 5 | 20.10 | −4.005 | −2.217 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleszewski, T.J.; Gajewski, A. The Latest Method for Surface Tension Determination: Experimental Validation. Energies 2020, 13, 3629. https://doi.org/10.3390/en13143629
Teleszewski TJ, Gajewski A. The Latest Method for Surface Tension Determination: Experimental Validation. Energies. 2020; 13(14):3629. https://doi.org/10.3390/en13143629
Chicago/Turabian StyleTeleszewski, Tomasz Janusz, and Andrzej Gajewski. 2020. "The Latest Method for Surface Tension Determination: Experimental Validation" Energies 13, no. 14: 3629. https://doi.org/10.3390/en13143629
APA StyleTeleszewski, T. J., & Gajewski, A. (2020). The Latest Method for Surface Tension Determination: Experimental Validation. Energies, 13(14), 3629. https://doi.org/10.3390/en13143629