Fatty Acid Methyl Esters from the Herbal Industry Wastes as a Potential Feedstock for Biodiesel Production
Abstract
1. Introduction
- quantify the content of FAMEs from herbal industry wastes and determine dominative ones;
- compare different herbal waste to determine the most suitable for biofuel production;
- estimate cetane number (CN) for different herbal wastes based on FAMEs content;
- compare fatty acid composition in herbal wastes with previously published data from other plant materials.
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Transesterification Procedure
2.4. Analytical Methods
2.5. Statistical Analysis
- the one-way analysis of variance (ANOVA) (‘stats’ package) followed by Tukey’s post-hoc test (‘laercio’ package [36]), which compared the mean values of fatty acids levels for different herbal wastes (n = 3, α = 0.05);
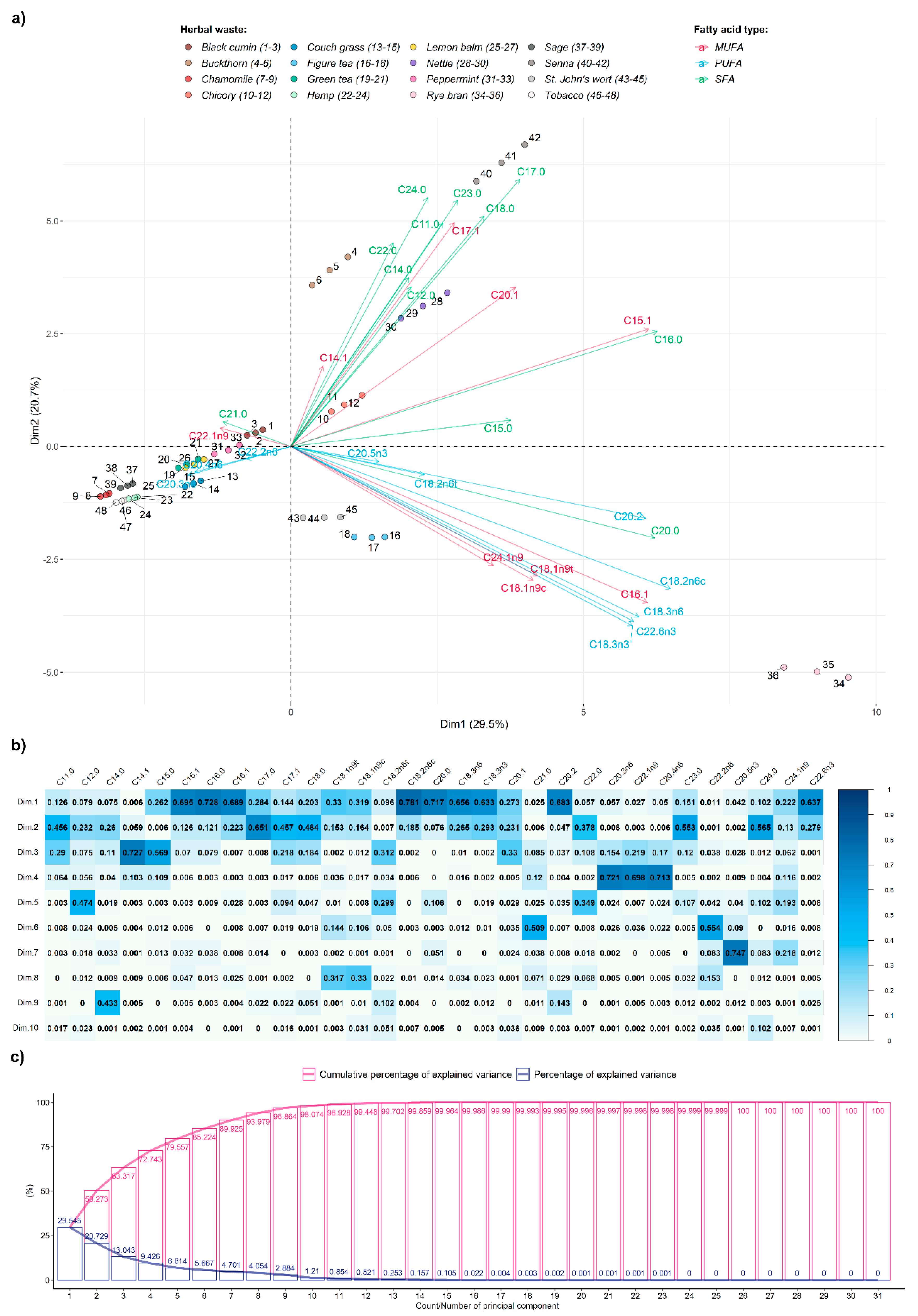
- principal component analysis (PCA) that transformed multivariate data into a reduced form and allowed simplified exploration of underlying relations in the original dataset.
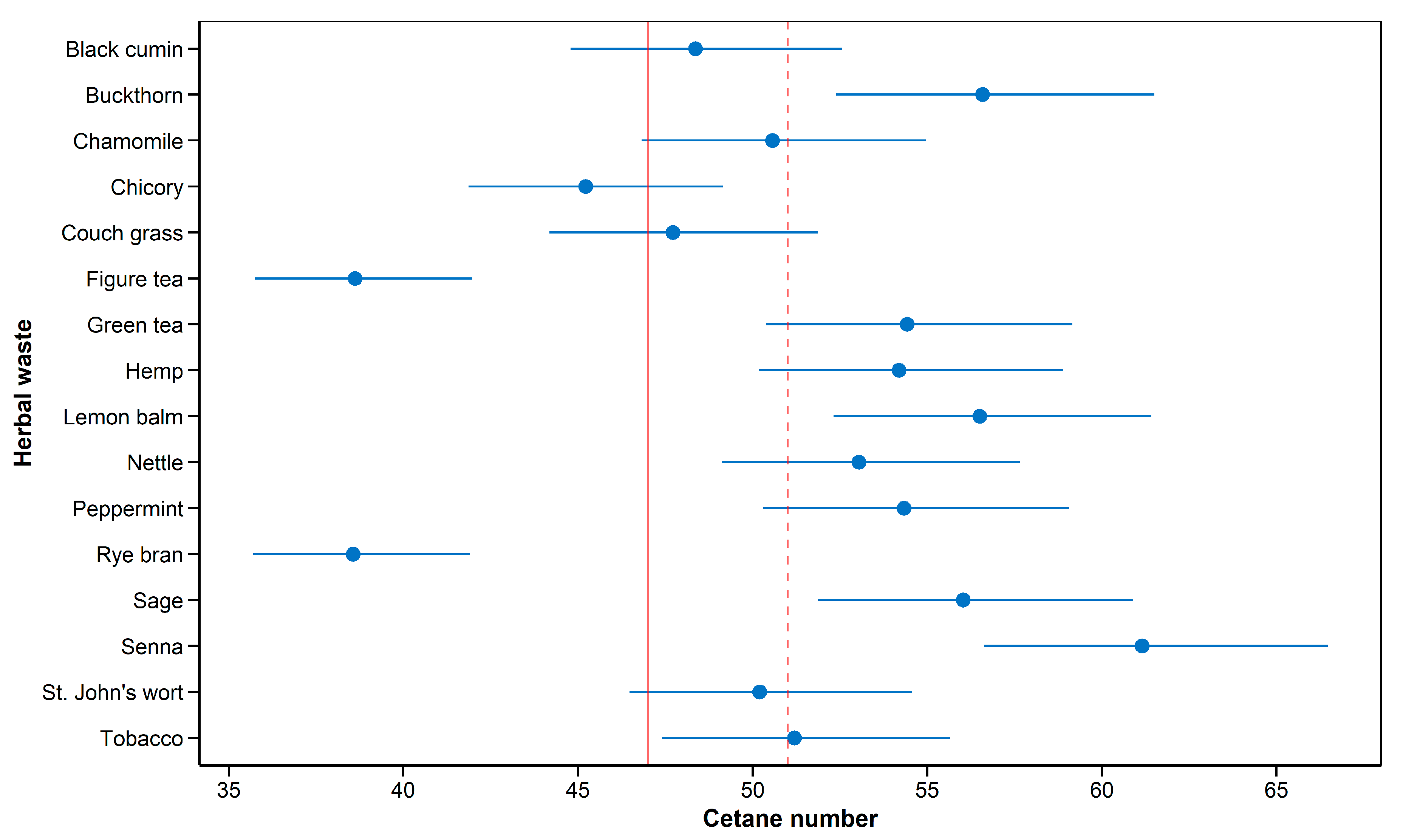
2.6. Cetane Number Prediction
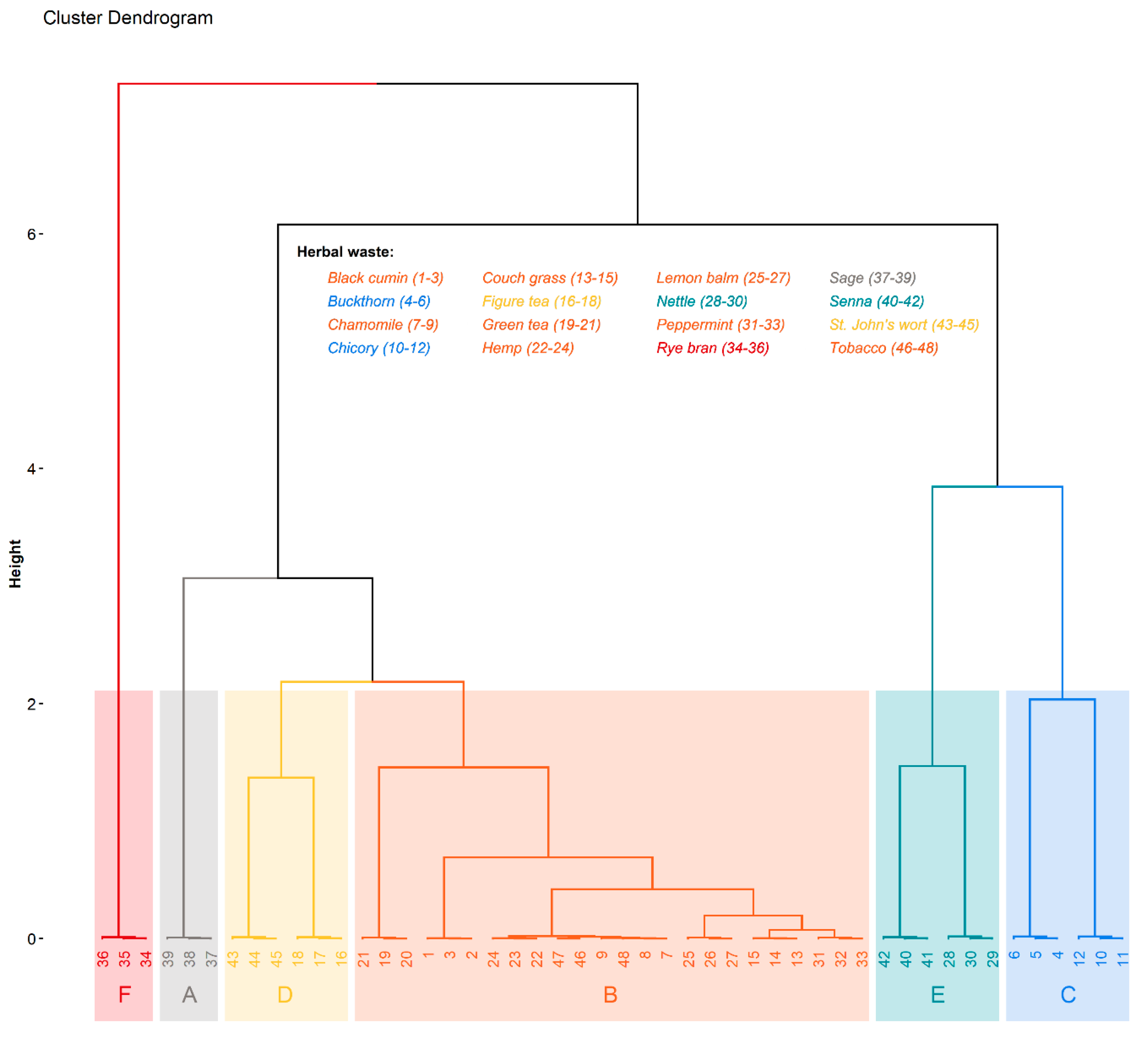
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.R.A.; Sulaiman, N.M.N. Refining technologies for the purification of crude biodiesel. Appl. Energy 2011, 88, 4239–4251. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. Production of biodiesel using high free fatty acid feedstocks. Renew. Sustain. Energy Rev. 2012, 16, 3275–3285. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Qu, J.; Mao, H.-Z.; Chen, W.; Gao, S.-Q.; Bai, Y.-N.; Sun, Y.-W.; Geng, Y.-F.; Ye, J. Development of marker-free transgenic Jatropha plants with increased levels of seed oleic acid. Biotechnol. Biofuels 2012, 5, 10. [Google Scholar] [CrossRef]
- Bunyakiat, K.; Makmee, S.; Sawangkeaw, R.; Ngamprasertsith, S. Continuous production of biodiesel via transesterification from vegetable oils in supercritical methanol. Energy Fuels 2006, 20, 812–817. [Google Scholar] [CrossRef]
- Nahian, M.R.; Islam, M.N.; Khan, S. Production of Biodiesel from Palm Oil and Performance Test with Diesel in CI Engine. In Proceedings of the International Conference on Mechanical, Industrial and Energy Engineering, Khulna, Bangladesh, 26–27 December 2016. [Google Scholar]
- Antolín, G.; Tinaut, F.V.; Briceño, Y.; Castaño, V.; Pérez, C.; Ramírez, A.I. Optimisation of biodiesel production by sunflower oil transesterification. Bioresour. Technol. 2002, 83, 111–114. [Google Scholar] [CrossRef]
- Dworakowska, S.; Bogdał, D.; Bednarz, S. Production of Biodiesel From Rapeseed Oil, Proceedings of the 1st World Sustainability Forum, Basel, Switzerland, 1–30 November 2011; Seijas, J.A., der Pilar, V., Tato, M., Eds.; MDPI: Basel, Switzerland, 2012. [Google Scholar] [CrossRef]
- Mallick, N.; Bagchi, S.K.; Koley, S.; Singh, A.K. Progress and challenges in microalgal biodiesel production. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Noor, M.M.; Wandel, A.P.; Yusaf, T. The simulation of biogas combustion in a mild burner. J. Mech. Eng. Sci. 2014, 6, 995–1013. [Google Scholar] [CrossRef]
- Bhuiya, M.M.K.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Azad, A.K.; Hazrat, M.A. Prospects of 2nd generation biodiesel as a sustainable fuel—Part 2: Properties, performance and emission characteristics. Renew. Sustain. Energy Rev. 2016, 55, 1129–1146. [Google Scholar] [CrossRef]
- Moon, G.; Lee, Y.; Choi, K.; Jeong, D. Emission characteristics of diesel, gas to liquid, and biodiesel-blended fuels in a diesel engine for passenger cars. Fuel 2010, 89, 3840–3846. [Google Scholar] [CrossRef]
- Ramos, L.; Fernandes, R.; Crispim, A.; Ramalho, E.; Caetano, N.; Silva, P. Biodiesel Production From Leather Industry Wastes. In Proceedings of the 10th International Chemical and Biological Engineering Conference, Braga, Portugal, 4–6 September 2008. [Google Scholar]
- Costa, J.F.; Almeida, M.F.; Alvim-Ferraz, M.C.M.; Dias, J.M. Biodiesel production using oil from fish canning industry wastes. Energy Convers. Manag. 2013, 74, 17–23. [Google Scholar] [CrossRef]
- Basso, D.; Patuzzi, F.; Castello, D.; Baratieri, M.; Rada, E.C.; Weiss-Hortala, E.; Fiori, L. Agro-industrial waste to solid biofuel through hydrothermal carbonization. Waste Manag. 2016, 47, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; O’Hara, I.M.; Mundree, S.; Gao, B.; Ball, A.S.; Zhu, N.; Bai, Z.; Jin, B. Biofuels from food processing wastes. Curr. Opin. Biotech. 2016, 38, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Żabiński, A.; Sadowska, U.; Wcisło, G. Options of use of waste biomass from herbal production for energy purposes. Agric. Eng. 2015, 4, 139–145. [Google Scholar] [CrossRef]
- Obidziński, S.; Joka, M.; Fijoł, O. Two-stage agglomeration of fine-grained herbal nettle waste. Int. Agrophys. 2017, 31, 515–523. [Google Scholar] [CrossRef]
- Obidziński, S. Charakterystyka aktywności wody i parametrów geometrycznych odpadów melisy w aspekcie ich wykorzystania jako dodatku do pasz. Acta Agrophys. 2013, 20, 113–124. [Google Scholar]
- Obidziński, S. Ocena właściwości energetycznych odpadów melisy. Zesz. Probl. Post. Nauk Roln. 2010, 546, 253–262. [Google Scholar]
- Kusmiyati, T.R.P.; Tri, W. Waste fish oil biodiesel production and its performace in diesel engine. ARPN J. Eng. Appl. Sci. 2016, 11, 1040–1044. [Google Scholar]
- Lewicki, A.; Pilarski, K.; Janczak, D.; Czekała, W.; Rodríguez Carmona, P.C.; Cieślik, M.; Witaszek, K.; Zbytek, Z. The biogas production from herbs and waste from herbal industry. J. Res. Appl. Agric. Eng. 2013, 58, 114–117. [Google Scholar]
- Obidziński, S. Pelletization process of postproduction plant waste. Int. Agrophys. 2012, 26, 279–284. [Google Scholar] [CrossRef]
- Kumari, M.; Kumar, S.; Chauhan, R.S.; Ravikanth, K. Bioconversion of herbal industry waste into vermicompost using an epigeic earthworm Eudrilus eugeniae. Waste Manag. Res. 2010, 29, 1205–1212. [Google Scholar] [CrossRef]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Chinese medicinal herbal residues as a bulking agent for food waste composting. Bioresour. Technol. 2018, 249, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Hanczakowska, E. Wpływ naturalnych przeciwutleniaczy w dawkach pokarmowych na wyniki tuczu i jakość mięsa tuczników. Rocz. Nauk. Zoot., Rozpr. Hab. 2004, 17, 1–73. [Google Scholar]
- Van Gerpen, J.; Knothe, G. Basics of the transesterification reaction. In The Biodiesel Handbook, 2nd ed.; Knothe, G., Krahl, J., Van Gerpen, J., Eds.; AOCS Press: Urbana, IL, USA, 2010; pp. 31–46. [Google Scholar]
- Sun, H.; Zhao, S. Determination of Fatty Acid Methyl Esters (FAMEs) in Milk Matrix Using an Agilent 5977E GC/MS. Agilent Technologies Application Note, Publication Number 5991–4867EN. Available online: https://www.agilent.com/cs/library/applications/5991-4867EN-D2.pdf (accessed on 13 April 2019).
- Wase, N.; Tu, B.; Allen, J.W.; Black, P.N.; DiRusso, C.C. Identification and metabolite profiling of chemical activators of lipid accumulation in green algae. Plant Physiol. 2017, 174, 2146–2165. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing (R version 4.0.0, Arbor Day). R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 24 April 2020).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. JOSS 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R package “corrplot”: Visualization of a Correlation Matrix (version 0.84). Available online: https://cran.r-project.org/web/packages/corrplot/ (accessed on 15 March 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Da Silva, L.J. Laercio-Package: Duncan Test, Tukey Test and Scott-Knott Test. Available online: https://CRAN.R-project.org/package=laercio (accessed on 20 February 2015).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Bartlett, M.S. Tests of significance in factor analysis. Br. J. Stat. Psychol. 1950, 3, 77–85. [Google Scholar] [CrossRef]
- Revelle, W. psych: Procedures for Personality and Psychological Research, Northwestern University, Evanston, Illinois, USA (R Package Version 1.8.10). Available online: https://CRAN.R-project.org/package=psych (accessed on 31 October 2018).
- Kaiser, H.F. An index of factorial simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses (R Package Version 1.0.6). Available online: https://CRAN.R-project.org/package=factoextra (accessed on 16 January 2020).
- Gopinath, A.; Puhan, S.; Nagarajan, G. Relating the cetane number of biodiesel fuels to their fatty acid composition: A critical study. Proc. Inst. Mech. Eng. D 2009, 223, 565–583. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty acid composition of edible oils and fats. J. Hyg. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- Sánchez, N.; Encinar, J.M.; Nogales, S.; González, J.F. Biodiesel production from castor oil by two-step catalytic transesterification: Optimization of the process and economic assessment. Catalysts 2019, 9, 864. [Google Scholar] [CrossRef]
- Martínez, G.; Sánchez, N.; Encinar, J.M.; González, J.F. Fuel properties of biodiesel from vegetable oils and oil mixtures. Influence of methyl esters distribution. Biomass Bioenergy 2014, 63, 22–32. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Yu, S.; Du, S.; Yuan, J.; Hu, Y. Fatty acid profile in the seeds and seed tissues of Paeonia L. species as new oil plant resources. Sci. Rep. 2016, 6, 26944. [Google Scholar] [CrossRef]
- Škrbić, B.; Cvejanov, J.; Ðurišić-Mladenović, N. Chemometric characterization of vegetable oils based on the fatty acid profiles for selection of potential feedstocks for biodiesel production. J. Biobased Mater. Bioenergy 2015, 9, 358–371. [Google Scholar] [CrossRef]
- Vizetto-Duarte, C.; Figueiredo, F.; Rodrigues, M.J.; Polo, C.; Rešek, E.; Custódio, L. Sustainable valorization of halophytes from the mediterranean area: A comprehensive evaluation of their fatty acid profile and implications for human and animal nutrition. Sustainability 2019, 11, 2197. [Google Scholar] [CrossRef]
- ASTM D975–20a. Standard Specification for Diesel Fuel; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- ASTM D6751–20. Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- BS EN 590:2013+A1:2017. Automotive Fuels. Diesel. Requirements and Test Methods; BSI Group: London, UK, 2013. [Google Scholar]
- BS EN 14214:2012+A2:2019. Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods; BSI Group: London, UK, 2019. [Google Scholar]
- Moser, B.R. Impact of fatty ester composition on low temperature properties of biodiesel-petroleum diesel blends. Fuel 2014, 115, 500–506. [Google Scholar] [CrossRef]
- Gopinath, A.; Sairam, K.; Velraj, R.; Kumaresan, G. Effects of the properties and the structural configurations of fatty acid methyl esters on the properties of biodiesel fuel: A review. Proc. Inst. Mech. Eng. D 2014, 229, 357–390. [Google Scholar] [CrossRef]
- Moser, B.R. Influence of blending canola, palm, soybean, and sunflower oil methyl esters on fuel properties of biodiesel. Energy Fuels 2008, 22, 4301–4306. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Marques, S.S.I.; Cabanelas, I.T.D.; Pereira, S.A.; Druzian, J.I.; de Souza, C.O.; Vich, D.V.; de Carvalho, G.C.; Nascimento, M.A. Screening microalgae strains for biodiesel production: Lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenergy Res. 2012, 6, 1–13. [Google Scholar] [CrossRef]
- Katre, G.; Joshi, C.; Khot, M.; Zinjarde, S.; Ravikumar, A. Evaluation of single cell oil (SCO) from a tropical marine yeast Yarrowia lipolytica NCIM 3589 as a potential feedstock for biodiesel. AMB Express 2012, 2, 36. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Kaur, S.; Sarkar, M.; Srivastava, R.B.; Gogoi, H.K.; Kalita, M.C. Fatty acid profiling and molecular characterization of some freshwater microalgae from India with potential for biodiesel production. New Biotechnol. 2012, 29, 332–344. [Google Scholar] [CrossRef]
- Pereira, G.G.; Garcia, R.K.A.; Ferreira, L.L.; Barrera-Arellano, D. Soybean and soybean/beef-tallow biodiesel: A comparative study on oxidative degradation during long-term storage. J. Am. Oil Chem. Soc. 2017, 94, 587–593. [Google Scholar] [CrossRef]
- Wyatt, V.T.; Hess, M.A.; Dunn, R.O.; Foglia, T.A.; Haas, M.J.; Marmer, W.N. Fuel properties and nitrogen oxide emission levels of biodiesel produced from animal fats. J. Am. Oil Chem. Soc. 2005, 82, 585–591. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Janulis, P. Oxidation stability of biodiesel fuel produced from fatty wastes. Pol. J. Environ. Stud. 2005, 14, 335–339. [Google Scholar]
- Avhad, M.R.; Marchetti, J.M. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Méndez Díaz, J.D.; Polo, M.S.; Utrilla, J.R. Selection of heterogeneous catalysts for biodiesel production from animal fat. Fuel 2012, 94, 418–425. [Google Scholar] [CrossRef]
- Encinar, J.M.; Sánchez, N.; Martínez, G.; García, L. Study of biodiesel production from animal fats with high free fatty acid content. Bioresour. Technol. 2011, 102, 10907–10914. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, G.; Iulianelli, A.; Sanna, A.; Basile, A. Glycerol production and transformation: A critical review with particular emphasis on glycerol reforming reaction for producing hydrogen in conventional and membrane reactors. Membranes 2017, 7, 17. [Google Scholar] [CrossRef]
- Pitt, F.D.; Domingos, A.M.; Barros, A.A.C. Purification of residual glycerol recovered from biodiesel production. S. Afr. J. Chem. Eng. 2019, 29, 42–51. [Google Scholar] [CrossRef]
- Raqeeb, M.A.; Bhargavi, R. Biodiesel production from waste cooking oil. J. Chem. Pharm. Res. 2015, 7, 670–681. [Google Scholar]




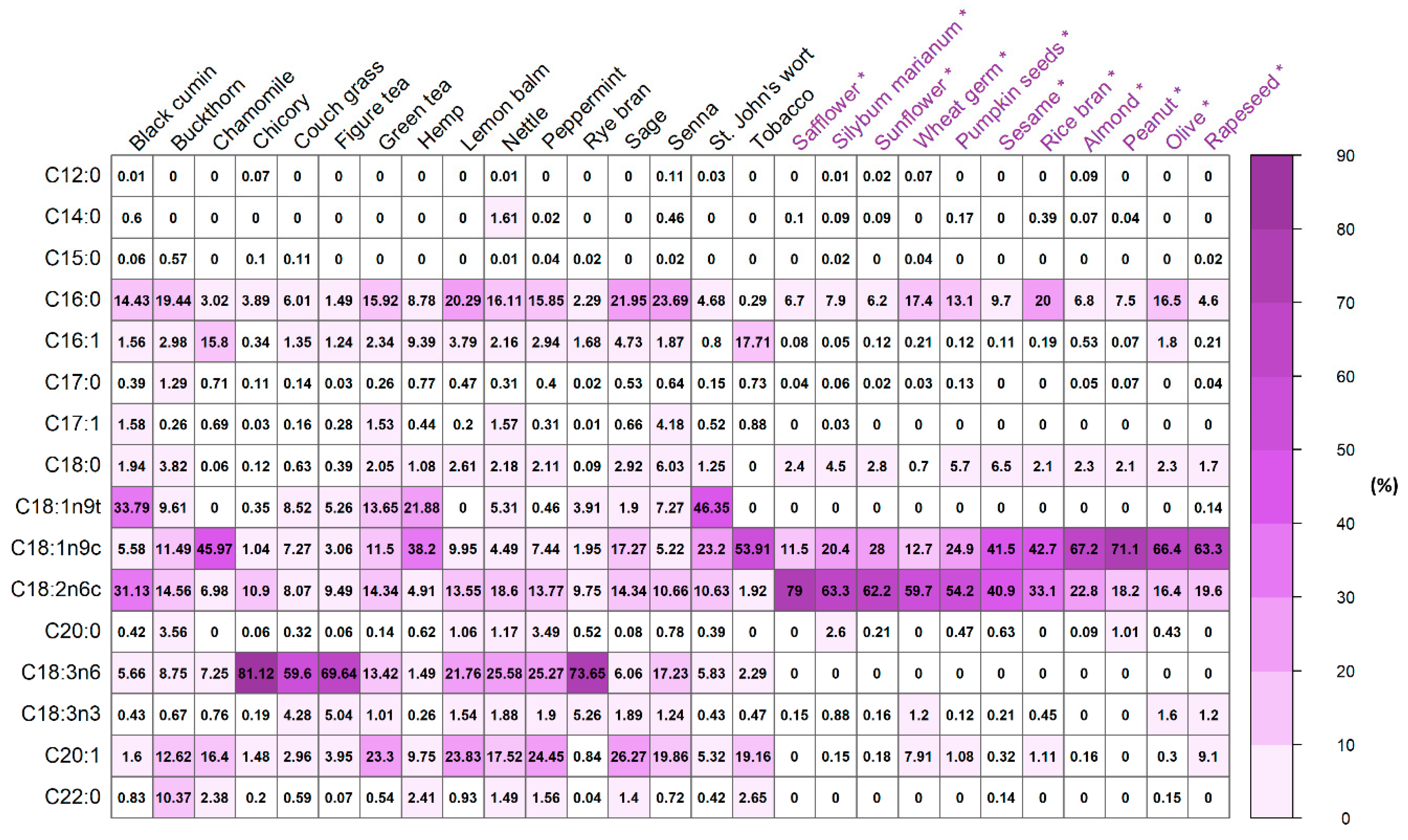
| Type of Fatty Acid | Systematic Name | The Common Name of FAME | Abbreviation * |
|---|---|---|---|
| MUFA | Myristoleic acid methyl ester | Myristoleic acid | C14:1 |
| cis-10-Pentadecanoic acid methyl ester | Pentadecanoic acid | C15:1 | |
| 9-Hexadecenoic acid methyl ester | Palmitoleic acid | C16:1 | |
| cis-10-Heptadecenoic acid methyl ester | Heptadecenoic acid | C17:1 | |
| trans-9-Octadecenoic acid methyl ester (Z) | Elaidic acid | C18:1n9t | |
| 9-Octadecenoic acid methyl ester (E) | Oleic acid | C18:1n9c | |
| cis-11-Eicosenoic acid methyl ester | Gondoic acid | C20:1 | |
| 13-Docosenoic acid methyl ester (Z) | Erucic acid | C22:1n9 | |
| 15-Tetracosenoic acid methyl ester (Z) | Nervonic acid | C24:1n9 | |
| PUFA | 9,12-Octadecadienoic acid methyl ester (E,E) | Linolelaidic acid | C18:2n6t |
| 9,12-Octadecadienoic acid methyl ester (Z,Z) | Linoleic acid | C18:2n6c | |
| all-cis-6,9,12-Octadecatrienoic acid | γ-Linolenic acid | C18:3n6 | |
| 9,12,15-Octadecatrienoic acid methyl ester (Z,Z,Z) | Linolenic acid | C18:3n3 | |
| cis-11,14-Eicosadienoic acid methyl ester | Eicosadienoic acid | C20:2 | |
| cis-11,14,17-Eicosatrienoic acid methyl ester | Eicosatrienoic acid | C20:3n3 | |
| cis-8,11,14-Eicosatrienoic acid methyl ester | Dihomo-γ-linolenic acid | C20:3n6 | |
| 5,8,11,14-Eicosatetraenoic acid methyl ester (all-Z) | Arachidonic acid | C20:4n6 | |
| cis-5,8,11,14,17-Eicosapentaenoic acid methyl ester | Eicosapentaenoic acid | C20:5n3 | |
| cis-13,16-Docasadienoic acid methyl ester | Docosadienoic acid | C22:2n6 | |
| 4,7,10,13,16,19-Docosahexaenoic acid methyl ester (all-Z) | Cervonic acid | C22:6n3 | |
| SFA | Butyric acid methyl ester | Butyric acid | C4:0 |
| Hexanoic acid methyl ester | Caproic acid | C6:0 | |
| Octanoic acid methyl ester | Caprylic acid | C8:0 | |
| Decanoic acid methyl ester | Capric acid | C10:0 | |
| Undecanoic acid methyl ester | Undecylic acid | C11:0 | |
| Dodecanoic acid methyl ester | Lauric acid | C12:0 | |
| Tridecanoic acid methyl ester | Tridecylic acid | C13:0 | |
| Tetradecanoic acid methyl ester | Myristic acid | C14:0 | |
| Pentadecanoic acid methyl ester | Pentadecylic acid | C15:0 | |
| Hexadecanoic acid methyl ester | Palmitic acid | C16:0 | |
| Heptadecanoic acid methyl ester | Margaric acid | C17:0 | |
| Octadecanoic acid methyl ester | Stearic acid | C18:0 | |
| Eicosanoic acid methyl ester | Arachidic acid | C20:0 | |
| Heneicosanoic acid methyl ester | Heneicosylic acid | C21:0 | |
| Docosanoic acid methyl ester | Behenic acid | C22:0 | |
| Tricosanoic acid methyl ester | Tricosylic acid | C23:0 | |
| Tetracosanoic acid methyl ester | Lignoceric acid | C24:0 |
| Herbal Waste | C14:1 | C15:1 | C16:1 | C17:1 | C18:1n9t | C18:1n9c | C20:1 | C22:1n9 | C24:1n9 |
|---|---|---|---|---|---|---|---|---|---|
| Black cumin | 15.97 ± 0.64 b | 157.68 ± 3.26 de | 26.75 ± 0.56 de | 27.21 ± 0.82 cd | 580.7 ± 17.42 c | 95.84 ± 4.98 fgh | 27.49 ± 1.43 j | 6.99 ± 0.28 c | 1.87 ± 0.06 g |
| Buckthorn | 18.53 ± 0.89 a | 118.23 ± 6.52 fg | 31.01 ± 2.17 de | 2.72 ± 0.13 e | 99.90 ± 2.07 ef | 119.5 ± 8.37 efg | 131.23 ± 6.83 h | 10.56 ± 0.53 c | 2.23 ± 0.10 fg |
| Chamomile | 13.88 ± 0.63 c | ND | 24.51 ± 0.86 e | 1.07 ± 0.07 e | ND | 71.32 ± 3.23 h | 25.45 ± 0.77 j | ND | 1.67 ± 0.05 g |
| Chicory | 19.79 ± 1.09 a | 174.16 ± 8.71 d | 25.73 ± 2.44 de | 1.99 ± 0.10 e | 26.80 ± 1.34 fg | 79.56 ± 3.98 gh | 112.63 ± 5.63 hi | ND | 10.57 ± 0.53 a |
| Couch grass | 14.96 ± 0.75 bc | 74.80 ± 2.99 h | 29.42 ± 1.18 de | 3.57 ± 0.27 e | 185.75 ± 7.43 de | 158.64 ± 3.17 de | 64.46 ± 2.58 ij | 0.49 ± 0.03 c | 3.43 ± 0.19 de |
| Figure tea | 14.00 ± 0.63 bc | 92.89 ± 3.78 gh | 136.48 ± 5.55 b | 31.04 ± 0.64 c | 577.23 ± 23.49 c | 336.21 ± 11.85 c | 434.31 ± 26.01 c | 37.13 ± 1.68 c | 11.22 ± 0.73 a |
| Green tea | 14.29 ± 0.86 bc | 124.89 ± 6.74 f | 32.81 ± 1.99 de | 21.48 ± 1.30 d | 191.34 ± 11.54 de | 161.19 ± 8.06 de | 326.55 ± 16.94 de | 0.43 ± 0.04 c | 2.82 ± 0.24 ef |
| Hemp | 13.96 ± 0.43 c | 10.62 ± 0.48 i | 24.84 ± 0.76 e | 1.17 ± 0.04 e | 57.85 ± 1.78 fg | 101.01 ± 2.02 fgh | 25.79 ± 0.94 j | 1.29 ± 0.06 c | 2.18 ± 0.08 fg |
| Lemon balm | 14.09 ± 0.84 bc | 128.93 ± 3.45 ef | 42.49 ± 1.07 d | 2.29 ± 0.14 e | ND | 111.50 ± 7.23 fgh | 266.92 ± 15.98 f | ND | 2.35 ± 0.12 fg |
| Nettle | 14.10 ± 0.43 bc | 341.67 ± 22.16 c | 76.82 ± 3.24 c | 55.82 ± 3.62 b | 189.08 ± 12.26 de | 159.86 ± 4.80 de | 623.50 ± 40.43 b | 4.73 ± 0.22 c | 2.76 ± 0.18 ef |
| Peppermint | 15.23 ± 0.55 bc | 129.30 ± 9.14 ef | 42.96 ± 3.04 d | 4.45 ± 0.31 e | 6.75 ± 0.52 fg | 108.60 ± 7.68 fgh | 356.80 ± 16.19 d | 9.79 ± 0.69 c | 2.80 ± 0.15 ef |
| Rye bran | 14.65 ± 0.51 bc | 475.78 ± 16.65 b | 586.29 ± 20.52 a | 5.03 ± 0.20 e | 1361.85 ± 47.67 b | 678.91 ± 22.12 b | 292.11 ± 13.22 ef | ND | 8.25 ± 0.25 b |
| Sage | 14.42 ± 0.51 bc | 92.08 ± 4.43 gh | 35.45 ± 2.31 de | 4.95 ± 0.39 e | 14.25 ± 0.45 fg | 129.38 ± 5.19 ef | 196.82 ± 11.94 g | 1066.26 ± 64.7 a | 4.52 ± 0.29 c |
| Senna | 13.99 ± 0.43 bc | 531.34 ± 24.59 a | 72.40 ± 4.00 c | 161.46 ± 8.92 a | 280.87 ± 15.52 d | 201.41 ± 11.13 d | 766.87 ± 27.93 a | 244.22 ± 14.65 b | 2.26 ± 0.15 fg |
| St. John’s wort | 14.78 ± 0.30 bc | 16.49 ± 1.17 i | 31.81 ± 2.25 de | 20.55 ± 1.57 d | 1834.95 ± 113.63 a | 918.29 ± 50.41 a | 210.50 ± 14.88 g | ND | 3.96 ± 0.20 cd |
| Tobacco | 15.59 ± 0.57 bc | ND | 23.58 ± 1.13 e | 1.17 ± 0.07 e | ND | 71.79 ± 1.44 h | 25.52 ± 1.28 j | 5.92 ± 0.36 c | 2.31 ± 0.11 fg |
| Herbal Waste | C18:2n6t | C18:2n6c | C18:3n6 | C18:3n3 | C20:2 | C20:3n6 | C20:4n6 | C22:2n6 | C20:5n3 | C22:6n3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Black cumin | 513.96 ± 17.99 b | 534.95 ± 16.05 e | 97.28 ± 2.92 f | 7.34 ± 0.22 ef | 3.81 ± 0.18 de | 3.57 ± 0.11 b | 2.89 ± 0.10 cd | 40.71 ± 0.85 c | 45.18 ± 1.36 bc | 4.72 ± 0.29 e |
| Buckthorn | 24.72 ± 1.73 fgh | 151.39 ± 4.93 g | 91.02 ± 6.37 f | 6.99 ± 0.35 ef | 3.10 ± 0.25 efg | ND | 2.49 ± 0.17 cd | 40.87 ± 2.30 c | 45.71 ± 2.12 bc | 6.27 ± 0.38 d |
| Chamomile | 3.36 ± 0.21 h | 10.83 ± 0.67 h | 11.25 ± 0.70 f | 1.17 ± 0.07 f | 2.16 ± 0.13 h | ND | ND | 40.32 ± 2.50 c | 43.84 ± 2.97 c | ND |
| Chicory | 809.03 ± 33.24 a | 829.61 ± 25.52 c | 6175.65 ± 165.04 c | 14.69 ± 0.73 ef | 2.83 ± 0.14 fgh | ND | 2.29 ± 0.13 cd | 42.09 ± 2.10 c | 45.74 ± 2.13 bc | 4.75 ± 0.15 e |
| Couch grass | 41.88 ± 1.68 fg | 175.92 ± 7.04 g | 1299.94 ± 52.00 d | 93.44 ± 4.67 c | 2.60 ± 0.14 fgh | ND | 2.39 ± 0.10 cd | 41.68 ± 3.12 c | 44.96 ± 1.80 bc | 5.11 ± 0.20 de |
| Figure tea | 120.49 ± 6.02 e | 1042.53 ± 31.28 b | 7648.76 ± 294.49 b | 553.56 ± 22.52 b | 4.28 ± 0.17 d | 1.59 ± 0.10 b | 17.02 ± 0.69 bc | 58.18 ± 3.19 b | 66.54 ± 2.71 a | 14.73 ± 0.77 b |
| Green tea | 43.30 ± 2.81 fg | 200.97 ± 12.12 g | 188.12 ± 12.46 ef | 14.17 ± 0.85 ef | 2.86 ± 0.23 fgh | ND | 5.21 ± 0.31 bcd | 284.24 ± 8.74 a | 45.39 ± 2.54 bc | 5.84 ± 0.18 de |
| Hemp | 3.45 ± 0.11 h | 12.99 ± 0.72 h | 3.95 ± 0.12 f | 0.68 ± 0.02 f | 2.43 ± 0.07 gh | ND | 5.88 ± 0.18 bcd | ND | 44.00 ± 2.38 c | 5.06 ± 0.24 de |
| Lemon balm | 21.39 ± 1.28 gh | 151.81 ± 3.14 g | 243.79 ± 14.60 ef | 17.3 ± 1.04 ef | 3.13 ± 0.20 efg | ND | 3.01 ± 0.20 cd | 41.79 ± 2.50 c | 51.49 ± 2.90 b | 5.21 ± 0.26 de |
| Nettle | 42.86 ± 2.78 fg | 661.80 ± 42.92 d | 910.30 ± 35.05 de | 66.71 ± 4.33 cd | 7.59 ± 0.45 b | 3.17 ± 0.17 b | 20.55 ± 1.33 b | 40.61 ± 2.19 c | 50.25 ± 3.26 bc | 5.09 ± 0.28 de |
| Peppermint | 21.34 ± 1.07 gh | 201.00 ± 14.21 g | 368.69 ± 18.62 ef | 27.68 ± 1.26 def | 2.87 ± 0.20 fgh | 1.56 ± 0.12 b | 4.25 ± 0.19 bcd | 66.92 ± 4.73 b | 44.13 ± 2.99 c | 5.00 ± 0.27 de |
| Rye bran | 278.51 ± 9.75 d | 3398.81 ± 118.97 a | 25,681.91 ± 898.92 a | 1832.6 ± 54.98 a | 9.16 ± 0.52 a | 0.10 ± 0.001 b | 3.48 ± 0.31 cd | 43.54 ± 2.70 c | 45.53 ± 1.15 bc | 27.04 ± 1.23 a |
| Sage | 16.66 ± 0.80 gh | 107.46 ± 6.52 gh | 45.44 ± 3.62 f | 14.13 ± 0.71 ef | 3.13 ± 0.19 efg | 496.99 ± 20.22 a | 411.18 ± 22.06 a | 44.47 ± 2.83 c | 44.45 ± 2.70 bc | 4.91 ± 0.12 e |
| Senna | 60.17 ± 2.54 f | 411.64 ± 18.50 f | 665.31 ± 39.92 def | 47.85 ± 2.64 de | 3.39 ± 0.19 ef | 1.63 ± 0.09 b | 6.72 ± 0.37 bcd | 41.83 ± 2.51 c | 46.46 ± 1.66 bc | 4.95 ± 0.20 e |
| St. John’s wort | 378.55 ± 26.77 c | 420.86 ± 27.88 f | 230.95 ± 16.33 ef | 17.08 ± 1.45 ef | 5.53 ± 0.50 c | 1.67 ± 0.08 b | 2.17 ± 0.18 cd | 41.50 ± 1.70 c | 43.85 ± 2.37 c | 8.93 ± 0.52 c |
| Tobacco | 2.97 ± 0.18 h | 2.55 ± 0.09 h | 3.05 ± 0.06 f | 0.63 ± 0.02 f | 2.12 ± 0.10 h | 4.76 ± 0.17 b | 2.35 ± 0.09 cd | 41.00 ± 1.34 c | 43.87 ± 2.11 c | 5.26 ± 0.19 de |
| Herbal Waste | C11:0 | C12:0 | C14:0 | C15:0 | C16:0 | C17:0 | C18:0 | C20:0 | C21:0 | C22:0 | C23:0 | C24:0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black cumin | ND | 0.10 ± 0.001 d | 10.23 ± 0.53 c | 1.10 ± 0.03 d | 247.90 ± 6.22 de | 6.70 ± 0.27 def | 33.33 ± 0.84 def | 7.27 ± 0.18 efg | 0.02 ± 0.001 i | 14.26 ± 0.43 de | 5.63 ± 0.17 ef | 282.70 ± 2.83 d |
| Buckthorn | 5.43 ± 0.38 a | ND | ND | 5.94 ± 0.42 b | 202.13 ± 12.27 efg | 13.39 ± 0.62 b | 39.77 ± 2.78 cde | 37.00 ± 2.59 cd | 6.15 ± 0.43 e | 107.79 ± 6.54 a | 14.42 ± 1.01 a | 525.09 ± 10.50 a |
| Chamomile | ND | ND | ND | ND | 4.68 ± 0.29 i | 1.10 ± 0.07 j | 0.09 ± 0.01 j | ND | 0.12 ± 0.01 i | 3.69 ± 0.17 h | 3.56 ± 0.12 i | 1.80 ± 0.11 g |
| Chicory * | 4.51 ± 0.27 b | 5.39 ± 0.32 a | ND | 7.32 ± 0.37 a | 296.46 ± 14.82 d | 8.06 ± 0.40 d | 9.31 ± 0.75 ij | 4.93 ± 0.25 fg | 9.63 ± 0.69 d | 15.29 ± 1.10 de | 7.36 ± 0.37 cd | 28.31 ± 1.42 f |
| Couch grass | ND | ND | ND | 2.35 ± 0.16 c | 131.01 ± 4.77 h | 3.01 ± 0.12 hi | 13.72 ± 0.55 hi | 7.04 ± 0.46 efg | 0.74 ± 0.05 hi | 12.94 ± 0.52 def | 6.06 ± 0.38 e | ND |
| Figure tea | 0.12 ± 0.001 f | ND | ND | ND | 163.72 ± 2.51 gh | 2.97 ± 0.12 hi | 42.39 ± 2.54 cd | 6.38 ± 0.26 efg | 3.01 ± 0.09 fg | 7.39 ± 0.37 fgh | 3.92 ± 0.23 hi | 177.34 ± 8.52 e |
| Green tea | ND | ND | ND | ND | 223.10 ± 13.45 efg | 3.7 ± 0.22 gh | 28.68 ± 1.02 fg | 1.97 ± 0.07 g | 32.81 ± 2.15 a | 7.63 ± 0.27 fgh | 5.06 ± 0.38 efgh | ND |
| Hemp | ND | ND | ND | ND | 23.21 ± 0.71 i | 2.04 ± 0.10 ij | 2.85 ± 0.09 j | 1.65 ± 0.05 g | 0.17 ± 0.001 i | 6.37 ± 0.20 gh | 4.24 ± 0.13 ghi | ND |
| Lemon balm | ND | ND | ND | ND | 227.25 ± 13.61 ef | 5.29 ± 0.32 fg | 29.26 ± 1.32 fg | 11.86 ± 0.71 ef | 26.48 ± 1.72 b | 10.44 ± 0.45 efg | 4.51 ± 0.27 fghi | 7.02 ± 0.42 g |
| Nettle | 2.05 ± 0.09 d | 0.30 ± 0.02 d | 57.2 ± 3.71 a | 0.29 ± 0.02 e | 573.32 ± 29.05 c | 11.00 ± 0.66 c | 77.49 ± 4.65 b | 41.64 ± 2.11 bc | 6.71 ± 0.47 e | 52.84 ± 3.43 b | 8.37 ± 0.54 c | 313.89 ± 11.06 c |
| Peppermint | ND | ND | 0.25 ± 0.02 d | 0.61 ± 0.04 d | 231.25 ± 9.25 ef | 5.82 ± 0.33 ef | 30.77 ± 2.18 efg | 50.90 ± 3.60 b | 2.77 ± 0.21 fgh | 22.71 ± 1.25 c | 6.16 ± 0.52 de | ND |
| Rye bran | 0.67 ± 0.04 e | ND | 0.08 ± 0.001 d | 5.50 ± 0.34 b | 798.44 ± 29.08 b | 7.03 ± 0.40 de | 30.4 ± 1.06 efg | 180.37 ± 11.77 a | 3.20 ± 0.11 f | 13.07 ± 0.59 def | 5.84 ± 0.20 e | 9.64 ± 0.54 g |
| Sage | ND | ND | ND | ND | 164.44 ± 8.22 gh | 3.98 ± 0.22 gh | 21.88 ± 1.33 gh | 0.59 ± 0.04 g | 18.93 ± 0.51 c | 10.48 ± 0.64 efg | 4.15 ± 0.25 ghi | ND |
| Senna | 3.05 ± 0.18 c | 4.25 ± 0.24 b | 17.86 ± 0.99 b | 0.92 ± 0.05 d | 914.69 ± 60.59 a | 24.74 ± 1.73 a | 232.95 ± 10.57 a | 30.08 ± 1.66 d | 6.20 ± 0.34 e | 27.96 ± 1.41 c | 10.08 ± 0.40s b | 367.34 ± 13.11 b |
| St. John’s wort | ND | 1.09 ± 0.08 c | ND | ND | 185.19 ± 6.53 fgh | 5.76 ± 0.41 ef | 49.62 ± 3.97 c | 15.47 ± 0.70 e | 0.92 ± 0.06 ghi | 16.55 ± 1.17 d | 5.38 ± 0.38 efg | ND |
| Tobacco | ND | ND | ND | ND | 0.38 ± 0.02 i | 0.97 ± 0.04 j | ND | ND | ND | 3.53 ± 0.21 h | 3.51 ± 0.13 i | ND |
| Herbal Waste | ∑ MUFAs ± SD | ∑ PUFAs ± SD | ∑ SFAs ± SD | ∑ FAMEs ± SD |
|---|---|---|---|---|
| Black cumin | 940.50 ± 18.51 | 1254.39 ± 24.34 | 609.23 ± 6.93 | 2804.11 ± 31.35 |
| Buckthorn | 533.91 ± 13.00 | 372.56 ± 8.84 | 957.11 ± 17.89 | 1863.58 ± 23.81 |
| Chamomile | 137.90 ± 3.49 | 112.93 ± 4.01 | 15.03 ± 0.38 | 265.86 ± 5.32 |
| Chicory | 451.23 ± 11.51 | 7926.68 ± 170.31 | 397.19 ± 14.99 | 8775.11 ± 171.35 |
| Couch grass | 535.51 ± 9.11 | 1707.93 ± 52.83 | 176.87 ± 4.87 | 2420.31 ± 53.83 |
| Figure tea | 1670.51 ± 37.65 | 9527.68 ± 297.09 | 407.23 ± 9.26 | 11,605.42 ± 299.61 |
| Green tea | 875.79 ± 23.17 | 790.10 ± 19.84 | 302.95 ± 13.67 | 1968.84 ± 33.43 |
| Hemp | 238.71 ± 3.02 | 78.45 ± 2.51 | 40.54 ± 0.77 | 357.70 ± 4.00 |
| Lemon balm | 568.58 ± 17.93 | 538.92 ± 15.51 | 322.12 ± 13.82 | 1429.62 ± 27.44 |
| Nettle | 1468.34 ± 48.20 | 1808.92 ± 55.80 | 1145.10 ± 31.92 | 4422.36 ± 80.35 |
| Peppermint | 676.70 ± 20.37 | 743.45 ± 24.14 | 351.23 ± 10.26 | 1771.38 ± 33.21 |
| Rye bran | 3422.87 ± 60.29 | 31,320.67 ± 908.48 | 1054.23 ± 31.41 | 35,797.77 ± 911.02 |
| Sage | 1558.14 ± 66.19 | 1188.81 ± 31.11 | 224.45 ± 8.37 | 2971.40 ± 73.62 |
| Senna | 2274.82 ± 45.39 | 1289.95 ± 44.25 | 1640.12 ± 62.96 | 5204.89 ± 89.34 |
| St. John’s wort | 3051.33 ± 125.24 | 1151.10 ± 42.09 | 279.97 ± 7.78 | 4482.40 ± 132.35 |
| Tobacco | 145.88 ± 2.33 | 108.55 ± 2.52 | 8.39 ± 0.25 | 262.82 ± 3.44 |
| Cluster | Variable | Mean in a Cluster | Overall Mean | V-Test | p-Value |
|---|---|---|---|---|---|
| A | C20:3n6 (P) | 496.99 ± 16.51 | 32.19 ± 120.09 | 6.85 | <0.00001 |
| A | C20:4n6 (P) | 411.18 ± 18.01 | 30.74 ± 98.48 | 6.84 | <0.00001 |
| A | C22:1n9 (M) | 1066.26 ± 52.83 | 86.74 ± 259.90 | 6.67 | <0.00001 |
| A | C21:0 (S) | 18.93 ± 0.41 | 7.37 ± 9.73 | 2.10 | 0.03553 |
| B | C18:2n6t (P) | 81.46 ± 164.27 | 148.91 ± 225.24 | −2.05 | 0.04005 |
| B | C22:1n9 (M) | 3.11 ± 3.62 | 86.74 ± 259.90 | −2.21 | 0.02740 |
| B | C18:3n3 (P) | 20.30 ± 29.10 | 169.75 ± 448.87 | −2.28 | 0.02246 |
| B | C20:0 (S) | 10.09 ± 15.95 | 24.82 ± 43.26 | −2.34 | 0.01953 |
| B | C16:1 (M) | 30.92 ± 7.47 | 77.71 ± 134.45 | −2.39 | 0.01704 |
| B | C17:1 (M) | 7.80 ± 9.74 | 21.62 ± 39.10 | –2.42 | 0.01537 |
| B | C18:3n6 (P) | 277.01 ± 405.99 | 2729.09 ± 6329.10 | −2.66 | 0.00791 |
| B | C18:1n9t (M) | 127.80 ± 187.55 | 337.96 ± 516.95 | −2.79 | 0.00532 |
| B | C15:0 (S) | 0.51 ± 0.80 | 1.50 ± 2.39 | −2.85 | 0.00434 |
| B | C22:0 (S) | 10.20 ± 6.03 | 20.81 ± 25.33 | −2.87 | 0.00408 |
| B | C18:0 (S) | 17.34 ± 13.85 | 40.16 ± 53.53 | −2.92 | 0.00347 |
| B | C24:0 (S) | 36.44 ± 93.11 | 107.07 ± 165.53 | −2.93 | 0.00344 |
| B | C12:0 (S) | 0.01 ± 0.03 | 0.70 ± 1.60 | −2.93 | 0.00334 |
| B | C22:6n3 (P) | 4.53 ± 1.75 | 7.05 ± 5.91 | −2.94 | 0.00333 |
| B | C18:2n6c (P) | 161.38 ± 163.30 | 519.69 ± 800.74 | −3.07 | 0.00216 |
| B | C23:0 (S) | 4.84 ± 1.02 | 6.14 ± 2.77 | −3.21 | 0.00133 |
| B | C18:1n9c (M) | 109.99 ± 32.36 | 218.94 ± 232.00 | −3.22 | 0.00128 |
| B | C20:1 (M) | 139.87 ± 139.68 | 242.93 ± 214.27 | −3.30 | 0.00098 |
| B | C15:1 (M) | 78.28 ± 61.85 | 154.30 ± 155.48 | −3.35 | 0.00080 |
| B | C17:0 (S) | 3.58 ± 2.05 | 6.60 ± 5.74 | −3.61 | 0.00031 |
| B | C16:0 (S) | 136.10 ± 103.82 | 274.20 ± 256.80 | −3.69 | 0.00023 |
| B | C24:1n9 (M) | 2.43 ± 0.55 | 4.08 ± 3.00 | −3.76 | 0.00017 |
| B | C20:2 (P) | 2.75 ± 0.54 | 3.81 ± 1.94 | −3.76 | 0.00017 |
| B | C11:0 (S) | 0 | 0.99 ± 1.74 | −3.90 | 0.00010 |
| C | C14:1 (M) | 19.16 ± 1.03 | 15.14 ± 1.73 | 6.01 | <0.00001 |
| C | C11:0 (S) | 4.97 ± 0.53 | 0.99 ± 1.74 | 5.93 | <0.00001 |
| C | C15:0 (S) | 6.63 ± 0.76 | 1.50 ± 2.39 | 5.56 | <0.00001 |
| C | C23:0 (S) | 10.89 ± 3.59 | 6.14 ± 2.77 | 4.44 | 0.00001 |
| C | C22:0 (S) | 61.54 ± 46.41 | 20.81 ± 25.33 | 4.17 | 0.00003 |
| C | C12:0 (S) | 2.69 ± 2.70 | 0.70 ± 1.60 | 3.24 | 0.00119 |
| C | C18:2n6t (P) | 416.88 ± 392.63 | 148.91 ± 225.24 | 3.08 | 0.00205 |
| C | C24:0 (S) | 276.70 ± 248.46 | 107.07 ± 165.53 | 2.66 | 0.00792 |
| C | C24:1n9 (M) | 6.40 ± 4.18 | 4.08 ± 3.00 | 2.01 | 0.04465 |
| D | C18:1n9c (M) | 627.25 ± 292.57 | 218.94 ± 232.00 | 4.56 | 0.00001 |
| D | C18:1n9t (M) | 1206.09 ± 632.42 | 337.96 ± 516.95 | 4.35 | 0.00001 |
| D | C20:5n3 (P) | 55.20 ± 11.53 | 46.96 ± 5.83 | 3.66 | 0.00025 |
| D | C24:1n9 (M) | 7.59 ± 3.66 | 4.08 ± 3.00 | 3.04 | 0.00240 |
| D | C22:6n3 (P) | 11.83 ± 2.95 | 7.05 ± 5.91 | 2.10 | 0.03615 |
| E | C14:0 (S) | 37.53 ± 19.79 | 5.35 ± 14.25 | 5.85 | <0.00001 |
| E | C17:1 (M) | 108.64 ± 53.12 | 21.62 ± 39.10 | 5.77 | <0.00001 |
| E | C18:0 (S) | 155.22 ± 78.02 | 40.16 ± 53.53 | 5.57 | <0.00001 |
| E | C20:1 (M) | 695.19 ± 77.09 | 242.93 ± 214.27 | 5.47 | <0.00001 |
| E | C17:0 (S) | 17.87 ± 6.95 | 6.60 ± 5.74 | 5.09 | <0.00001 |
| E | C16:0 (S) | 744.01 ± 175.03 | 274.20 ± 256.80 | 4.74 | <0.00001 |
| E | C15:1 (M) | 436.51 ± 96.74 | 154.30 ± 155.48 | 4.70 | <0.00001 |
| E | C24:0 (S) | 340.61 ± 28.50 | 107.07 ± 165.53 | 3.66 | 0.00026 |
| E | C23:0 (S) | 9.22 ± 0.94 | 6.14 ± 2.77 | 2.88 | 0.00397 |
| E | C12:0 (S) | 2.27 ± 1.98 | 0.70 ± 1.60 | 2.56 | 0.01037 |
| E | C11:0 (S) | 2.55 ± 0.51 | 0.99 ± 1.74 | 2.33 | 0.01971 |
| E | C20:2 (P) | 5.49 ± 2.12 | 3.81 ± 1.94 | 2.24 | 0.02525 |
| E | C22:0 (S) | 40.40 ± 12.62 | 20.81 ± 25.33 | 2.00 | 0.04509 |
| F | C16:1 (M) | 586.29 ± 16.76 | 77.71 ± 134.45 | 6.70 | <0.00001 |
| F | C18:3n3 (P) | 1832.60 ± 44.89 | 169.75 ± 448.87 | 6.56 | <0.00001 |
| F | C18:3n6 (P) | 25,681.91 ± 733.97 | 2729.09 ± 6329.10 | 6.42 | <0.00001 |
| F | C20:0 (S) | 180.37 ± 9.61 | 24.82 ± 43.26 | 6.37 | <0.00001 |
| F | C18:2n6c (P) | 3398.81 ± 97.14 | 519.69 ± 800.74 | 6.36 | <0.00001 |
| F | C22:6n3 (P) | 27.04 ± 1.00 | 7.05 ± 5.91 | 5.99 | <0.00001 |
| F | C20:2 (P) | 9.16 ± 0.42 | 3.81 ± 1.94 | 4.89 | <0.00001 |
| F | C15:1 (M) | 475.78 ± 13.60 | 154.30 ± 155.48 | 3.66 | 0.00025 |
| F | C16:0 (S) | 798.44 ± 23.74 | 274.20 ± 256.80 | 3.61 | 0.00030 |
| F | C18:1n9c (M) | 678.91 ± 18.06 | 218.94 ± 232.00 | 3.51 | 0.00045 |
| F | C18:1n9t (M) | 1361.85 ± 38.92 | 337.96 ± 516.95 | 3.51 | 0.00045 |
| F | C15:0 (S) | 5.50 ± 0.28 | 1.50 ± 2.39 | 2.96 | 0.00307 |
| F | C24:1n9 (M) | 8.25 ± 0.20 | 4.08 ± 3.00 | 2.46 | 0.01380 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sienkiewicz, A.; Piotrowska-Niczyporuk, A.; Bajguz, A. Fatty Acid Methyl Esters from the Herbal Industry Wastes as a Potential Feedstock for Biodiesel Production. Energies 2020, 13, 3702. https://doi.org/10.3390/en13143702
Sienkiewicz A, Piotrowska-Niczyporuk A, Bajguz A. Fatty Acid Methyl Esters from the Herbal Industry Wastes as a Potential Feedstock for Biodiesel Production. Energies. 2020; 13(14):3702. https://doi.org/10.3390/en13143702
Chicago/Turabian StyleSienkiewicz, Aneta, Alicja Piotrowska-Niczyporuk, and Andrzej Bajguz. 2020. "Fatty Acid Methyl Esters from the Herbal Industry Wastes as a Potential Feedstock for Biodiesel Production" Energies 13, no. 14: 3702. https://doi.org/10.3390/en13143702
APA StyleSienkiewicz, A., Piotrowska-Niczyporuk, A., & Bajguz, A. (2020). Fatty Acid Methyl Esters from the Herbal Industry Wastes as a Potential Feedstock for Biodiesel Production. Energies, 13(14), 3702. https://doi.org/10.3390/en13143702






