Water Adsorption Effect on Carbon Molecular Sieve Membranes in H2-CH4 Mixture at High Pressure
Abstract
1. Introduction
- Knudsen diffusion occurs at high temperatures, where adsorption effects are attenuated and the permeance of pure gases through carbon membranes follows the molecular weight and temperature dependency expected for Knudsen diffusion, even though the pore size distribution lies demonstrably below 0.55 nm [18].
- Selective surface diffusion is governed by selective adsorption of the larger non-ideal components on the pore surface, followed by surface diffusion of the adsorbed molecules along the pore. In this case, the driving force for the separation is governed by the different affinity of the diffusing components on the pores. This means that a large driving force can be attained even with a small partial pressure difference for the permeating component.
- Molecular sieving is a separation based on molecular size caused by the passage of smaller molecules of a gas mixture through the pores while the larger molecules are retained. The pore size is usually within the range between 3–5 Å and molecular sieving is the preferred and dominating transport mechanism of these membranes. Therefore, they are commonly referred as CMSMs.
2. Experimental
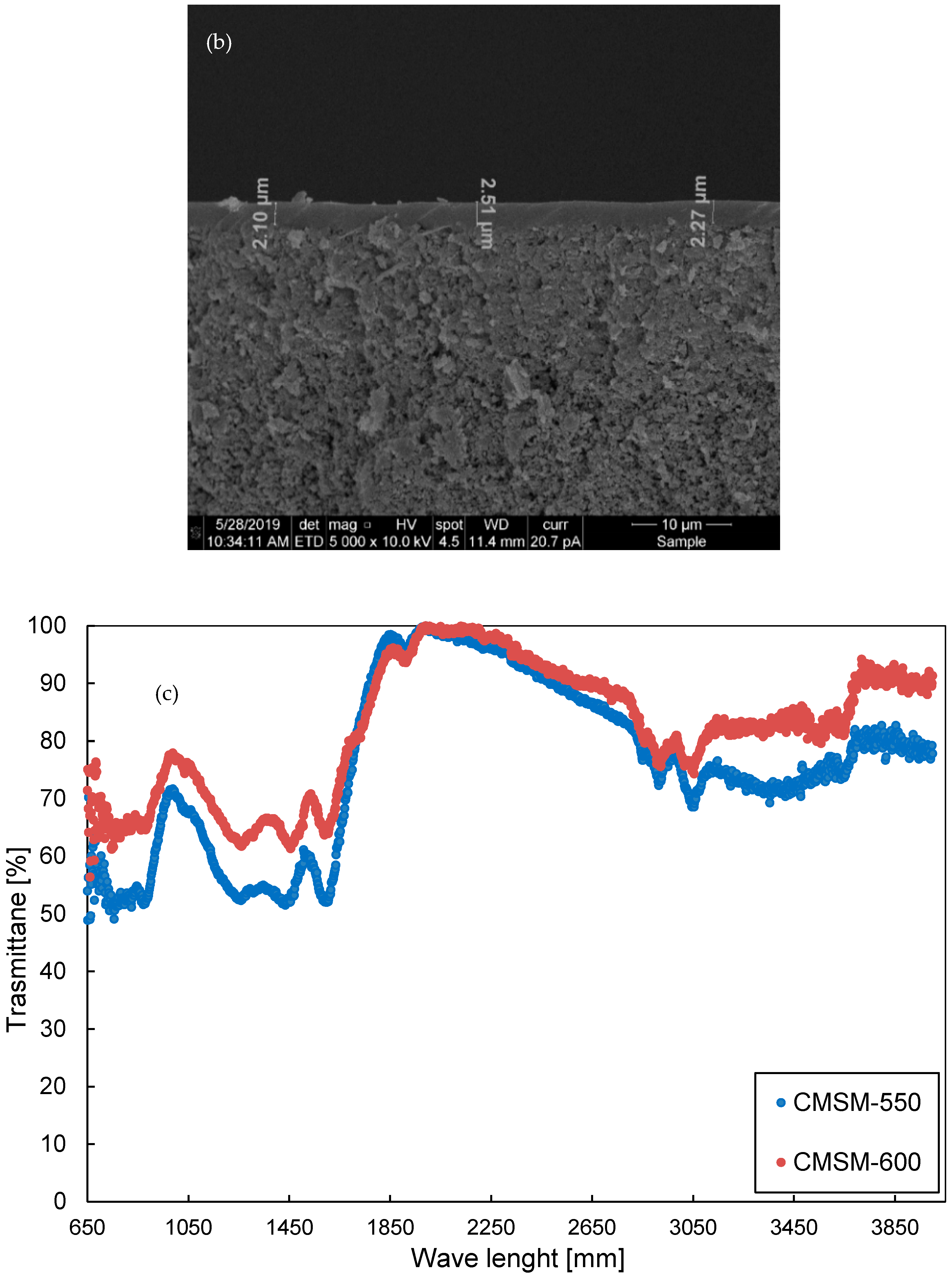
2.1. Membrane Preparation
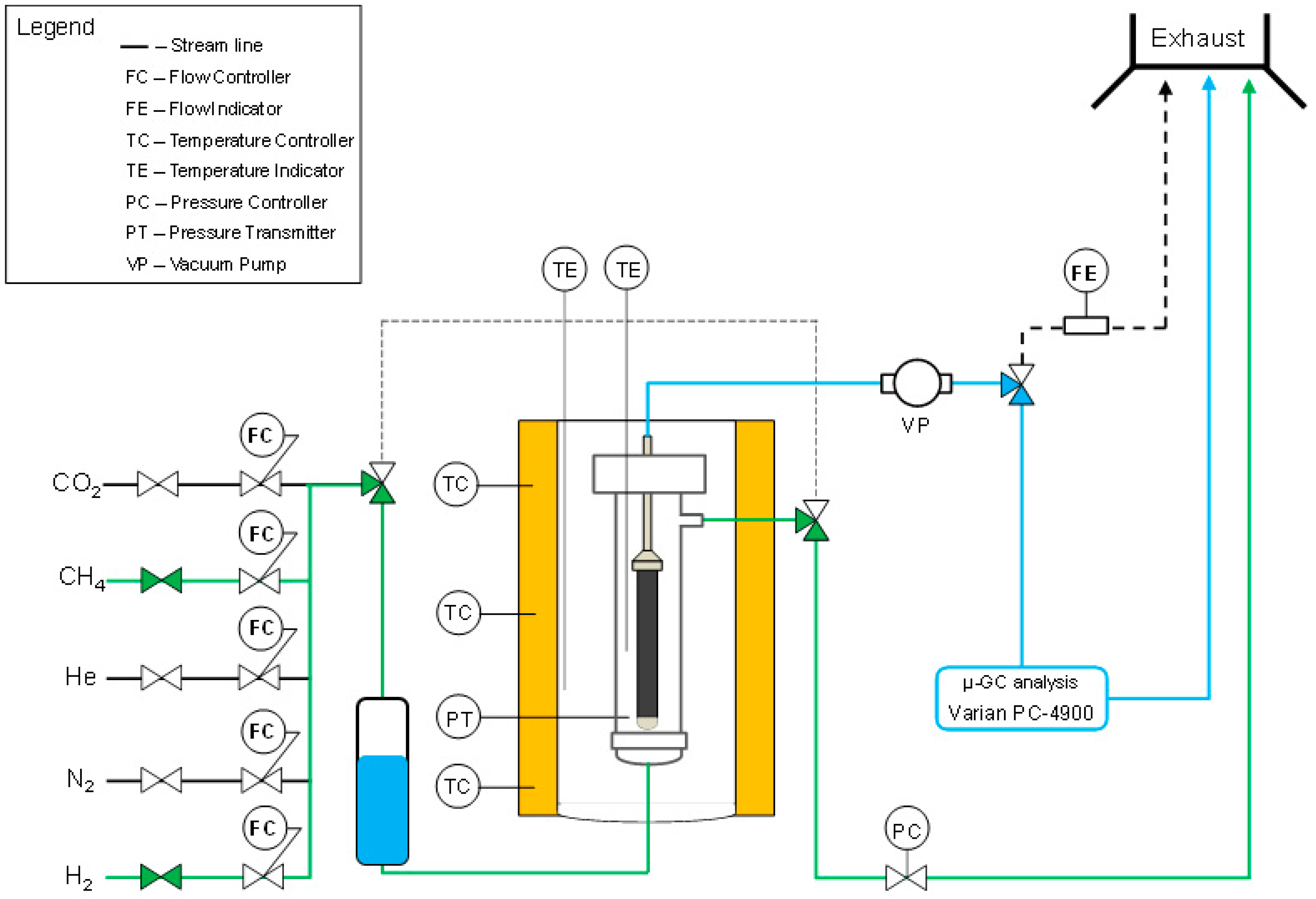
2.2. Permeation Setup and Experimental Tests
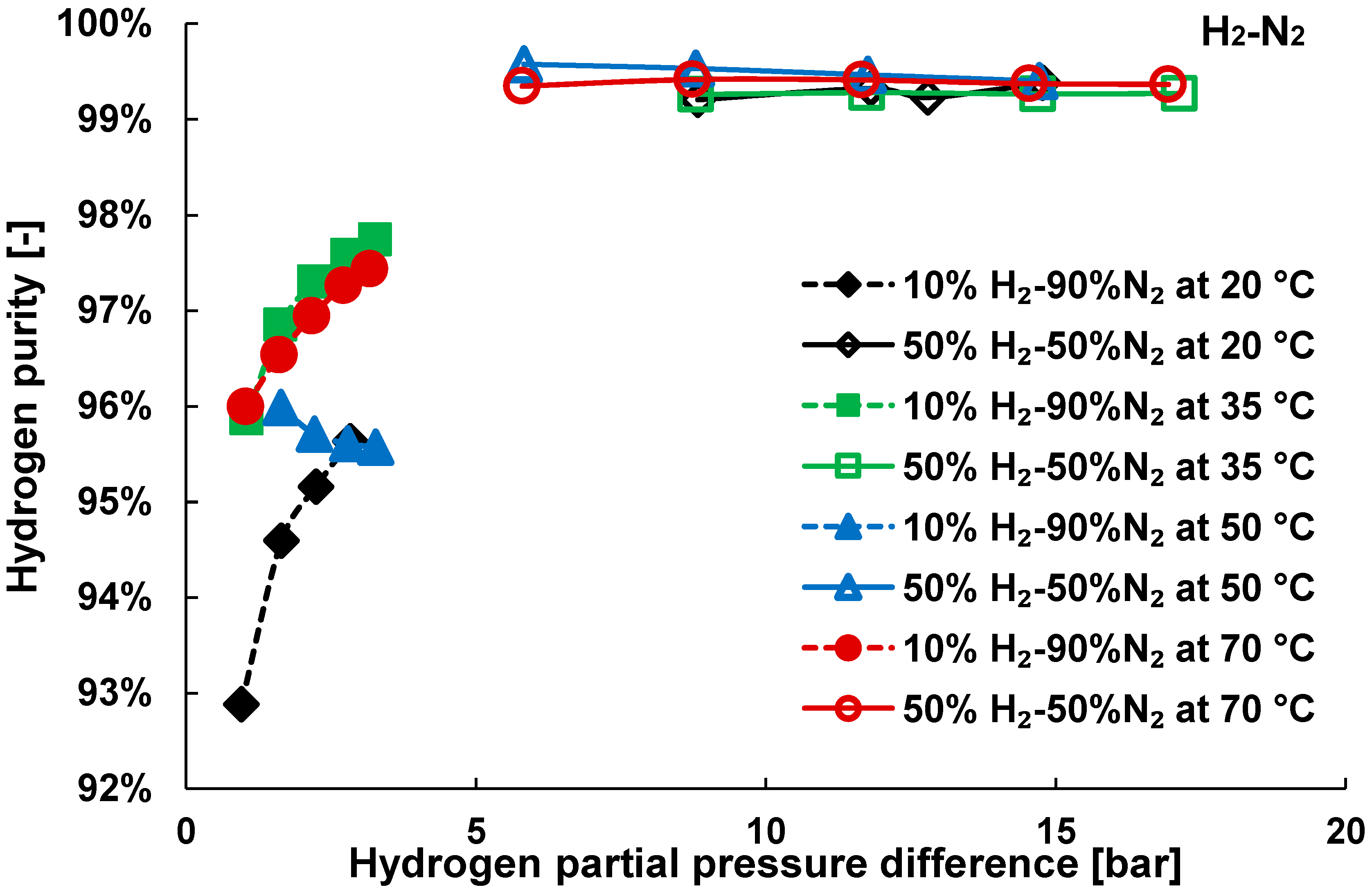
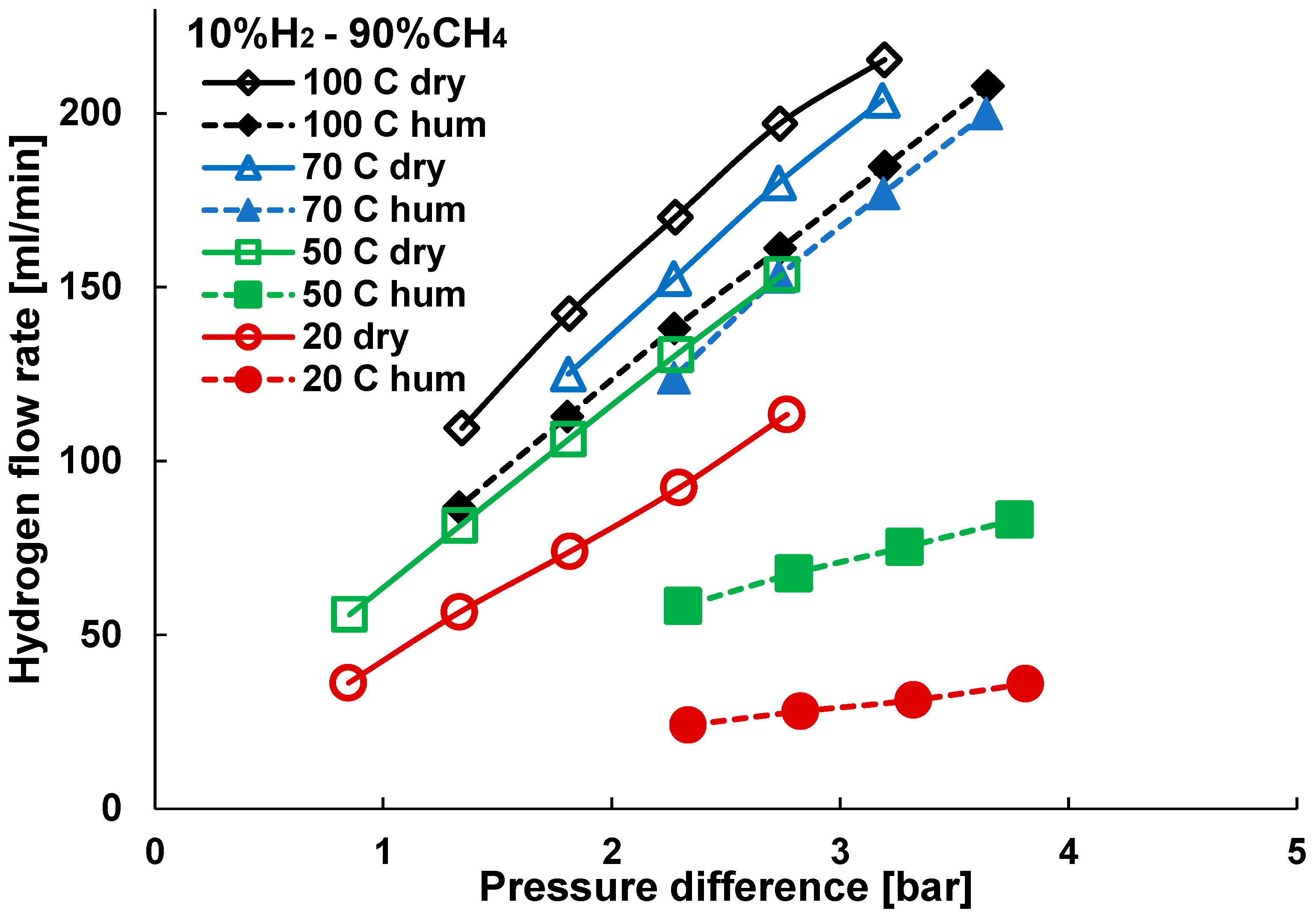
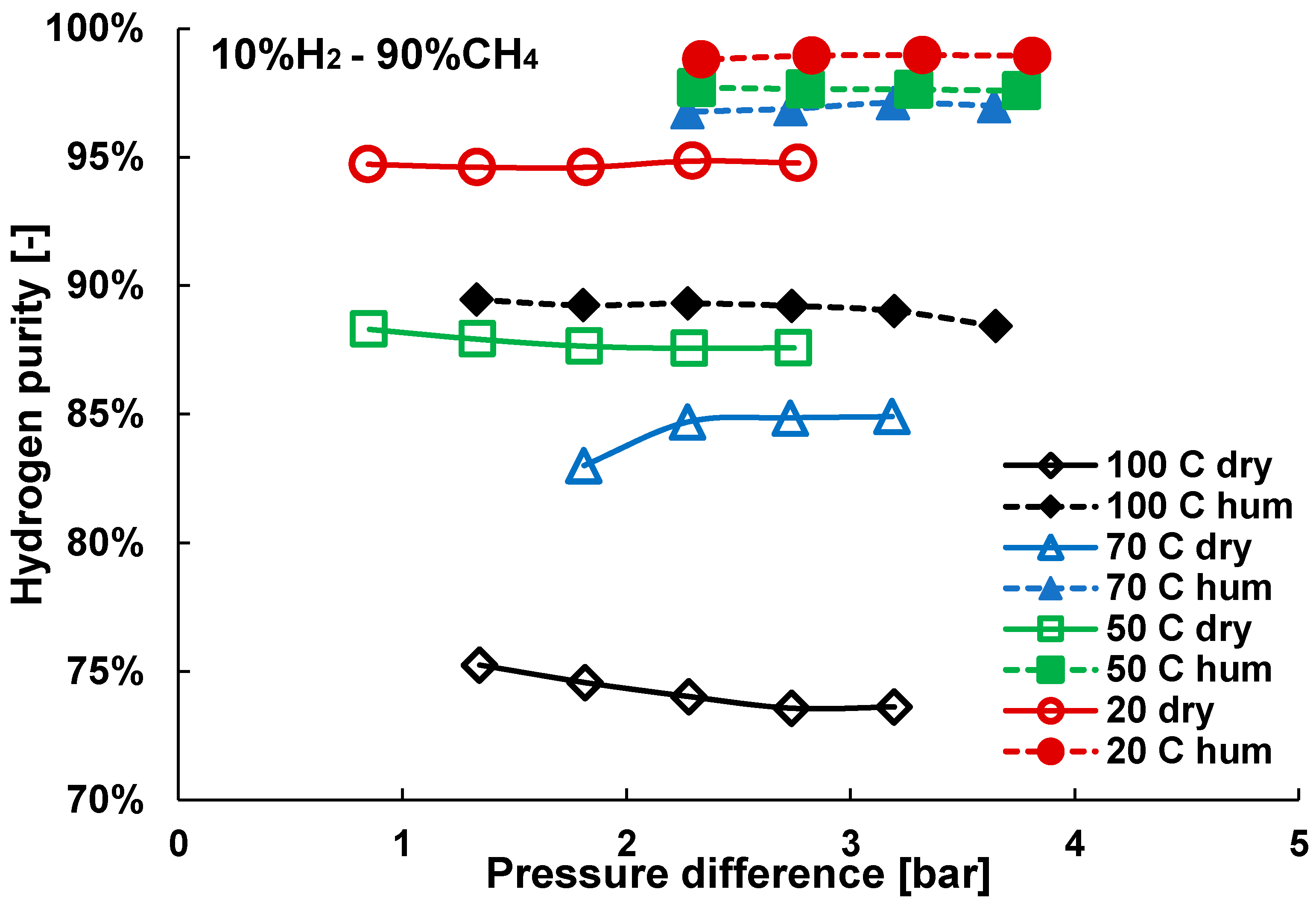
3. Results and Discussion
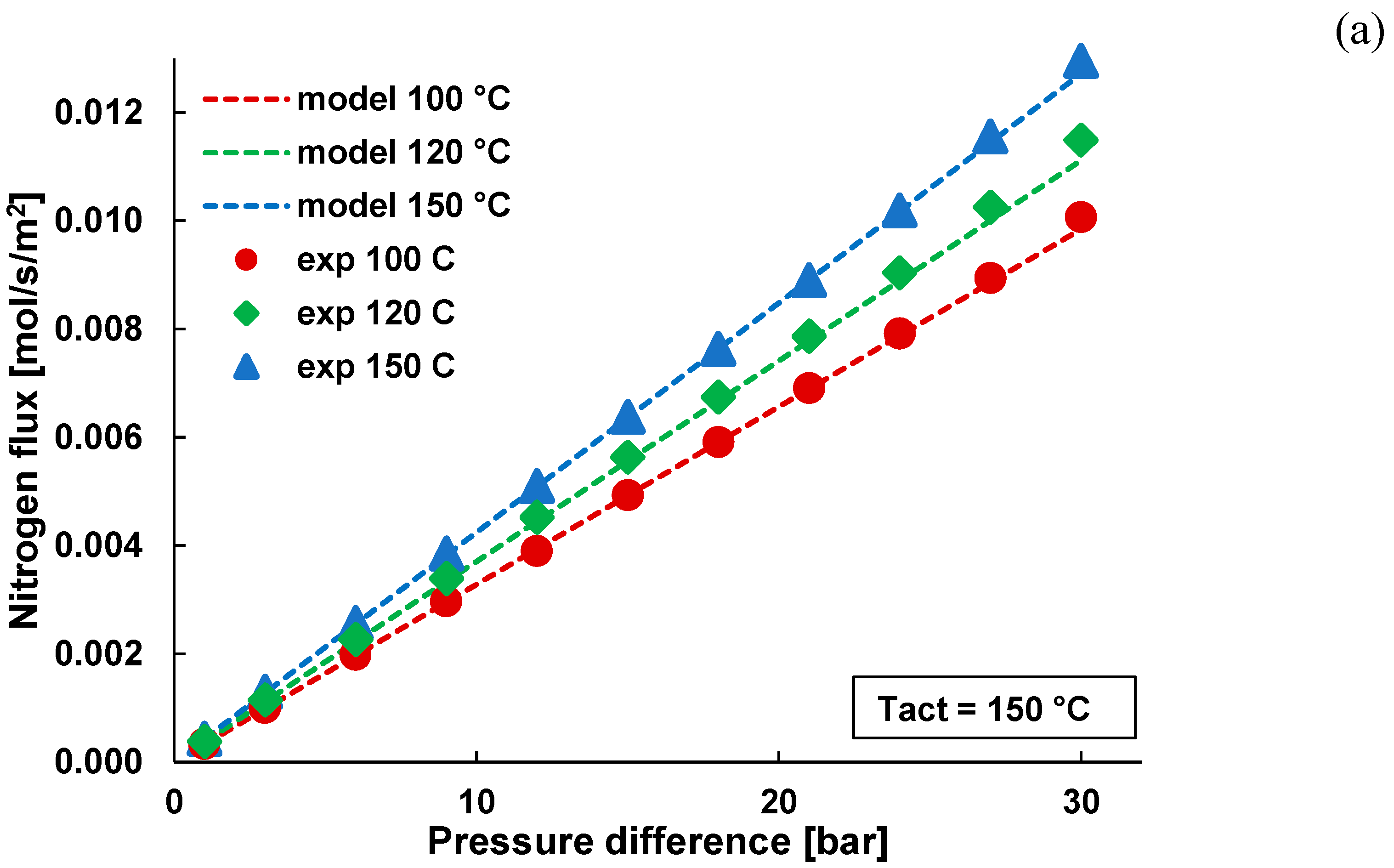
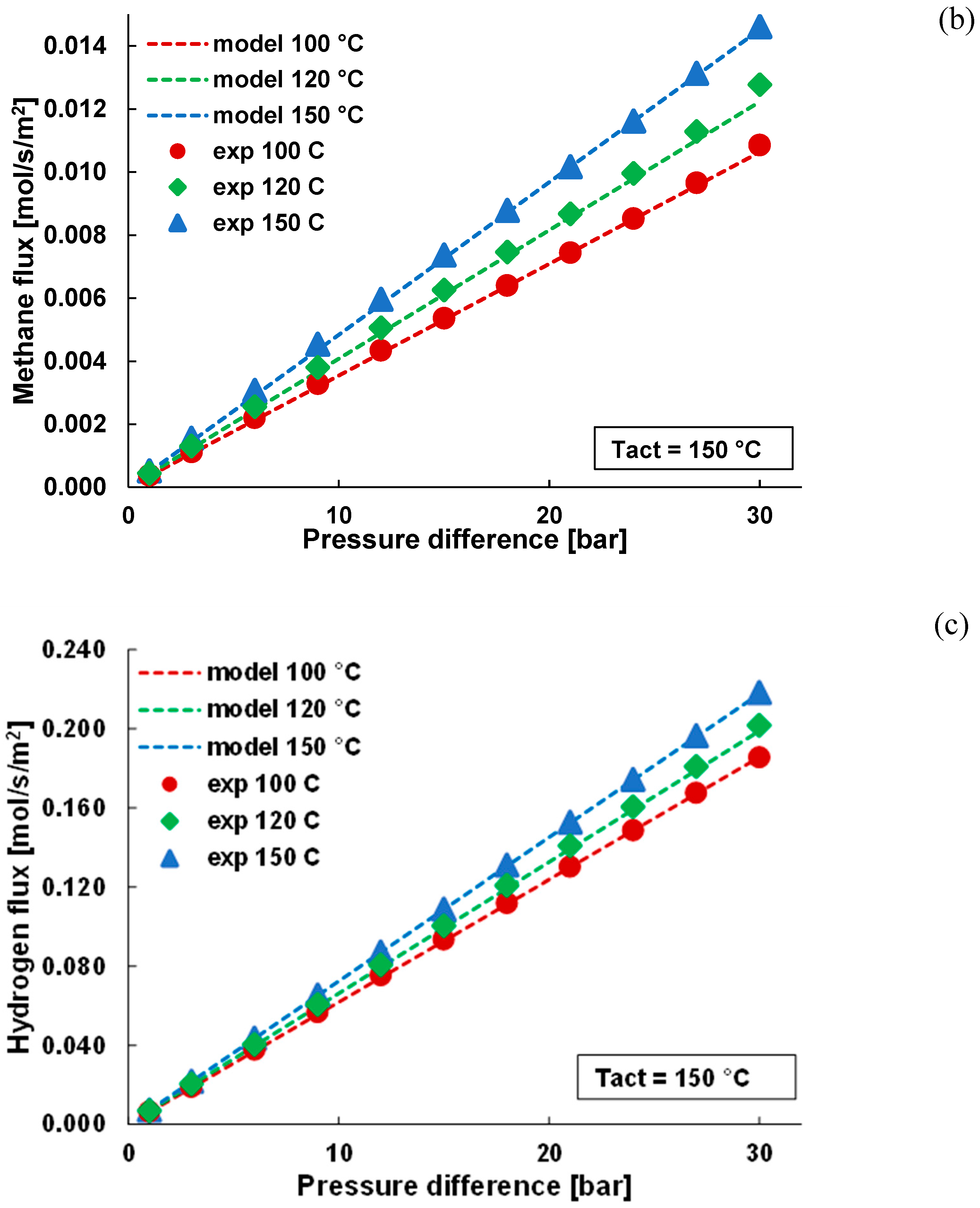
3.1. Pure Gas Tests
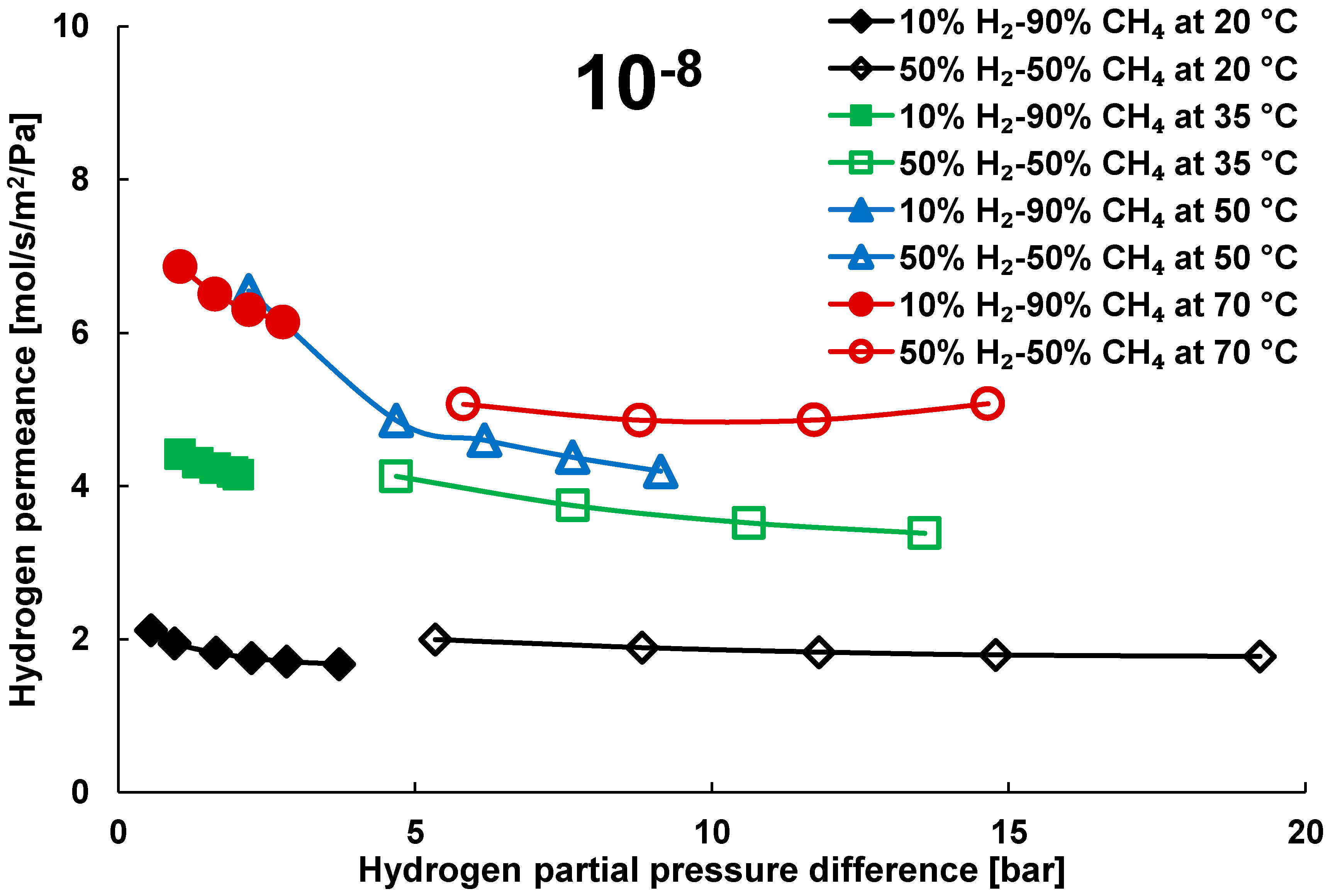
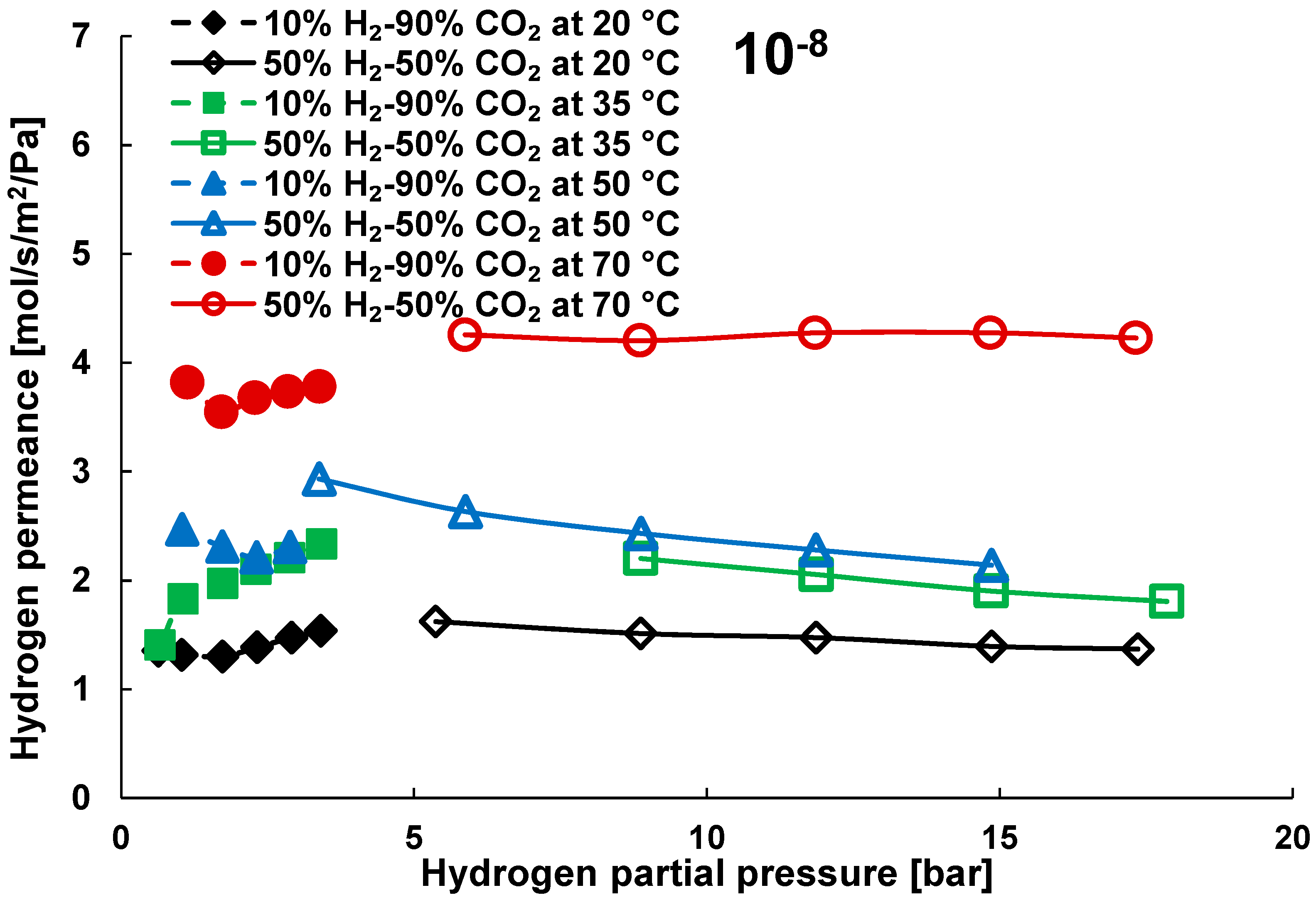
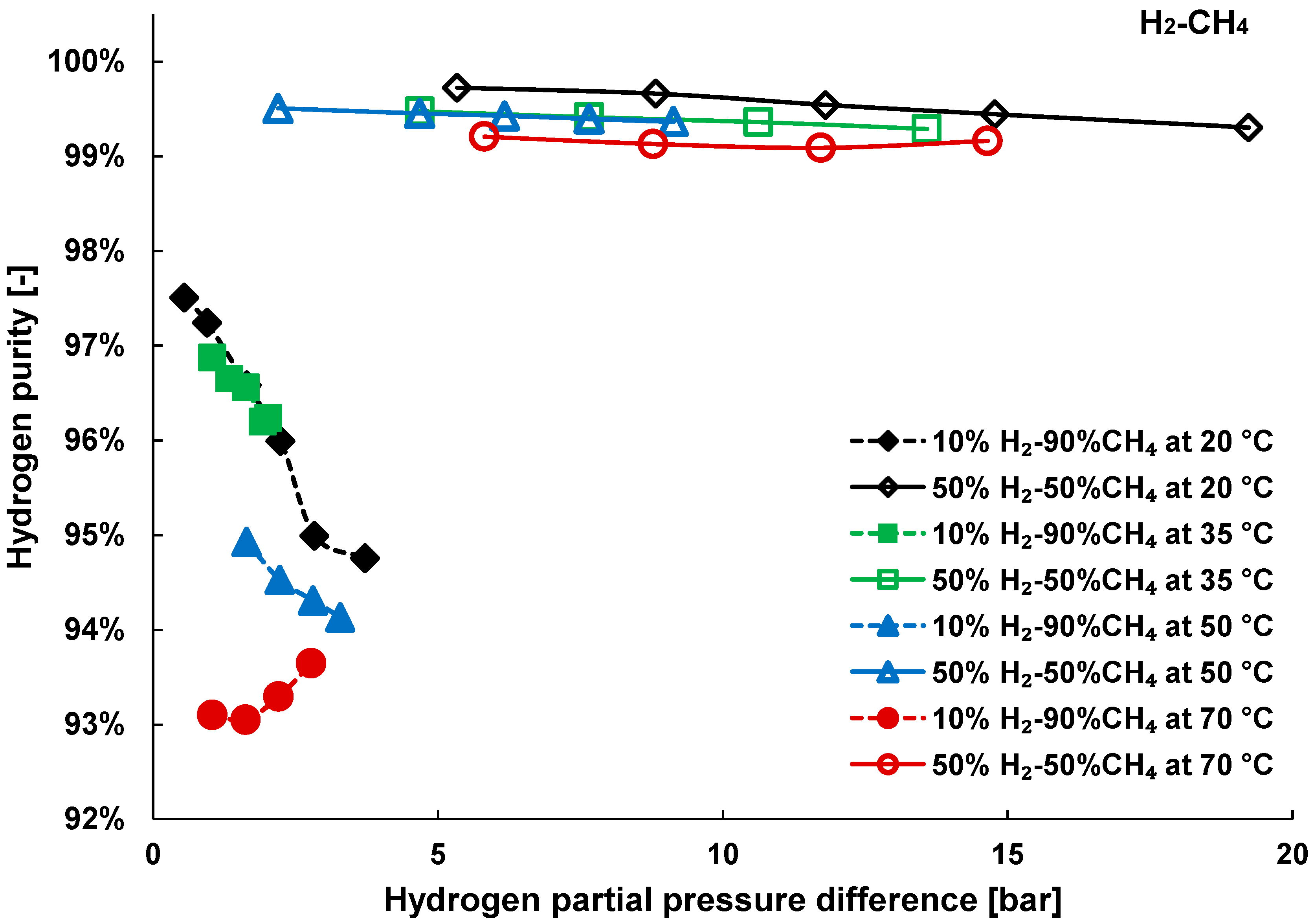
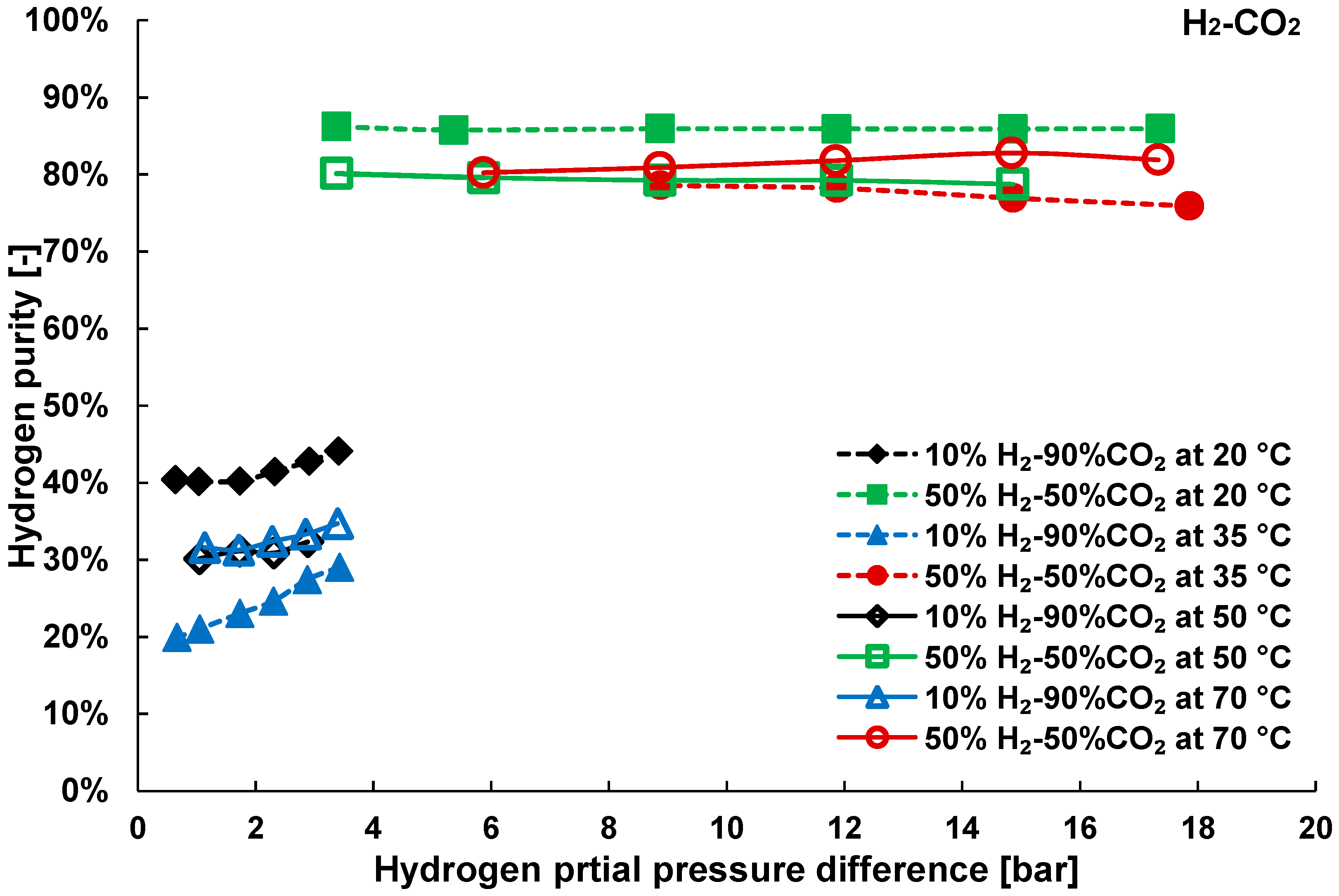
3.2. Mixture Tests with Dry Gas
3.3. Mixture Tests with Membrane Pre-Treated with Water Vapour
4. Conclusions
Author Contributions
Funding

Conflicts of Interest
Appendix A

References
- Baker, R. Future directions of membrane gas-separation technology. Membr. Technol. 2001, 2001, 5–10. [Google Scholar] [CrossRef]
- Iaquaniello, G. Hydrogen Palladium Selective Membranes: An Economic Perspective. In Membrane Reactors for Hydrogen Production Processes; Marcello, D.F., Luigi Marrelli, G.I., Eds.; Springer: London, UK, 2011; ISBN 9780857291509. [Google Scholar]
- Ulleberg, Ø.; Hancke, R. Techno-economic calculations of small-scale hydrogen supply systems for zero emission transport in Norway. Int. J. Hydrog. Energy 2020, 45, 1201–1211. [Google Scholar] [CrossRef]
- Koresh, J.E.; Soffer, A. Mechanism of Permeation through Molecular-sieve Carbon Membrane. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 2057–2063. [Google Scholar] [CrossRef]
- Hägg, M.; Lie, J.O.N.A.; Lindbråthen, A. Carbon Molecular Sieve Membranes A Promising Alternative for Selected Industrial Applications. Ann. N. Y. Acad. Sci. 2003, 345, 329–345. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Whitley, R.; Mendes, A. Preparation and characterization of carbon molecular sieve membranes based on resorcinol-formaldehyde resin. J. Membr. Sci. 2014, 459, 207–216. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S. Hydrogen membrane separation techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Grainger, D. Development of Carbon Membranes for Hydrogen Recovery; NTNU: Trondheim, Norway, 2007; ISBN 9788247142974. Available online: https://ntnuopen.ntnu.no/ntnu-xmlui/handle/11250/248707 (accessed on 13 June 2020).
- Moreira, R.F.P.M.; José, H.J.; Rodrigues, A.E. Modification of pore size in activated carbon by polymer deposition and its effects on molecular sieve selectivity. Carbon 2001, 39, 2269–2276. [Google Scholar] [CrossRef]
- Qiu, W.; Zhang, K.; Li, F.S.; Zhang, K.; Koros, W.J. Gas separation performance of carbon molecular sieve membranes based on 6FDA-mPDA/DABA (3:2) polyimide. ChemSusChem 2014, 7, 1186–1194. [Google Scholar] [CrossRef]
- Jones, C.W.; Koros, W.J. Carbon molecular sieve gas separation membranes-I. Preparation and characterization based on polyimide precursors. Carbon 1994, 32, 1419–1425. [Google Scholar] [CrossRef]
- Kiyono, M.; Williams, P.J.; Koros, W.J. Effect of polymer precursors on carbon molecular sieve structure and separation performance properties. Carbon 2010, 48, 4432–4441. [Google Scholar] [CrossRef]
- Jones, C.W.; Koros, W.J. Characterization of Ultramicroporous Carbon Membranes with Humidified Feeds. Ind. Eng. Chem. Res. 1995, 34, 158–163. [Google Scholar] [CrossRef]
- Lagorsse, S.; Campo, M.C.; Magalhães, F.D.; Mendes, A. Water adsorption on carbon molecular sieve membranes: Experimental data and isotherm model. Carbon 2005, 43, 2769–2779. [Google Scholar] [CrossRef]
- Llosa Tanco, M.A.; Pacheco Tanaka, D.A.; Mendes, A. Composite-alumina-carbon molecular sieve membranes prepared from novolac resin and boehmite. Part II: Effect of the carbonization temperature on the gas permeation properties. Int. J. Hydrog. Energy 2014, 40, 3485–3496. [Google Scholar] [CrossRef]
- Lagorsse, S.; Magalhães, F.D.; Mendes, A. Aging study of carbon molecular sieve membranes. J. Membr. Sci. 2008, 310, 494–502. [Google Scholar] [CrossRef]
- Hamm, J.; Ambrosi, A.; Griebeler, J.; Marcilio, N.; Tessaro, I.; Pollo, L. Recent advances in the development of supported carbon membranes for gas separation. Int. J. Hydrog. Energy 2017, 42, 24830–24845. [Google Scholar] [CrossRef]
- Gilron, J.; Soffer, A. Knudsen diffusion in microporous carbon membranes with molecular sieving character. J. Membr. Sci. 2002, 209, 339–352. [Google Scholar] [CrossRef]
- Llosa Tanco, M.A.; Pacheco Tanaka, D.A.; Rodrigues, S.C.; Texeira, M.; Mendes, A. Composite-alumina-carbon molecular sieve membranes prepared from novolac resin and boehmite. Part I: Preparation, characterization and gas permeation studies. Int. J. Hydrog. Energy 2015, 40, 5653–5663. [Google Scholar] [CrossRef]
- Von Kienle, H.; Kunze, N.; Mertens, D.H. The use of activated carbon in the removal of VOC’s. Stud. Environ. Sci. 1994, 61, 321–329. [Google Scholar]
- Gil, A.; Grange, P. Application of the Dubinin-Radushkevich and Dubinin-Astakhov equations in the characterization of microporous solids. Colloids Surf. A Physicochem. Eng. Asp. 1996, 113, 39–50. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Caravella, A.; Barbieri, G.; Drioli, E. Effect of the concentration polarization on the hydrogen permeation through pd-based membranes. Chem. Eng. Trans. 2009, 17, 1681–1686. [Google Scholar]
- Nordio, M.; Soresi, S.; Manzolini, G.; Melendez, J.; Van Sint Annaland, M.; Pacheco Tanaka, D.A.; Gallucci, F. Effect of sweep gas on hydrogen permeation of supported Pd membranes: Experimental and modeling. Int. J. Hydrog. Energy 2019, 44, 4228–4239. [Google Scholar] [CrossRef]
- Boon, J.; Li, H.; Dijkstra, J.W.; Pieterse, J.A.Z. 2-Dimensional membrane separator modelling: Mass transfer by convection and diffusion. Energy Procedia 2011, 4, 699–706. [Google Scholar] [CrossRef]
- Nakajima, T.; Kume, T.; Ikeda, Y.; Shiraki, M.; Kurokawa, H.; Iseki, T.; Kajitani, M.; Tanaka, H.; Hikosaka, H.; Takagi, Y.; et al. Effect of concentration polarization on hydrogen production performance of ceramic-supported Pd membrane module. Int. J. Hydrog. Energy 2015, 40, 11451–11456. [Google Scholar] [CrossRef]
- De Nooijer, N.; Gallucci, F.; Pellizzari, E.; Melendez, J.; Pacheco Tanaka, D.A.; Manzolini, G.; van Sint Annaland, M. On concentration polarisation in a fluidized bed membrane reactor for biogas steam reforming: Modelling and experimental validation. Chem. Eng. J. 2018, 348, 232–243. [Google Scholar] [CrossRef]
- Shelekhin, A.B.; Dixon, A.G.; Ma, Y.H. Theory of gas diffusion and permeation in inorganic molecular sieve membranes. AIChE J. 1995, 41, 58–67. [Google Scholar] [CrossRef]


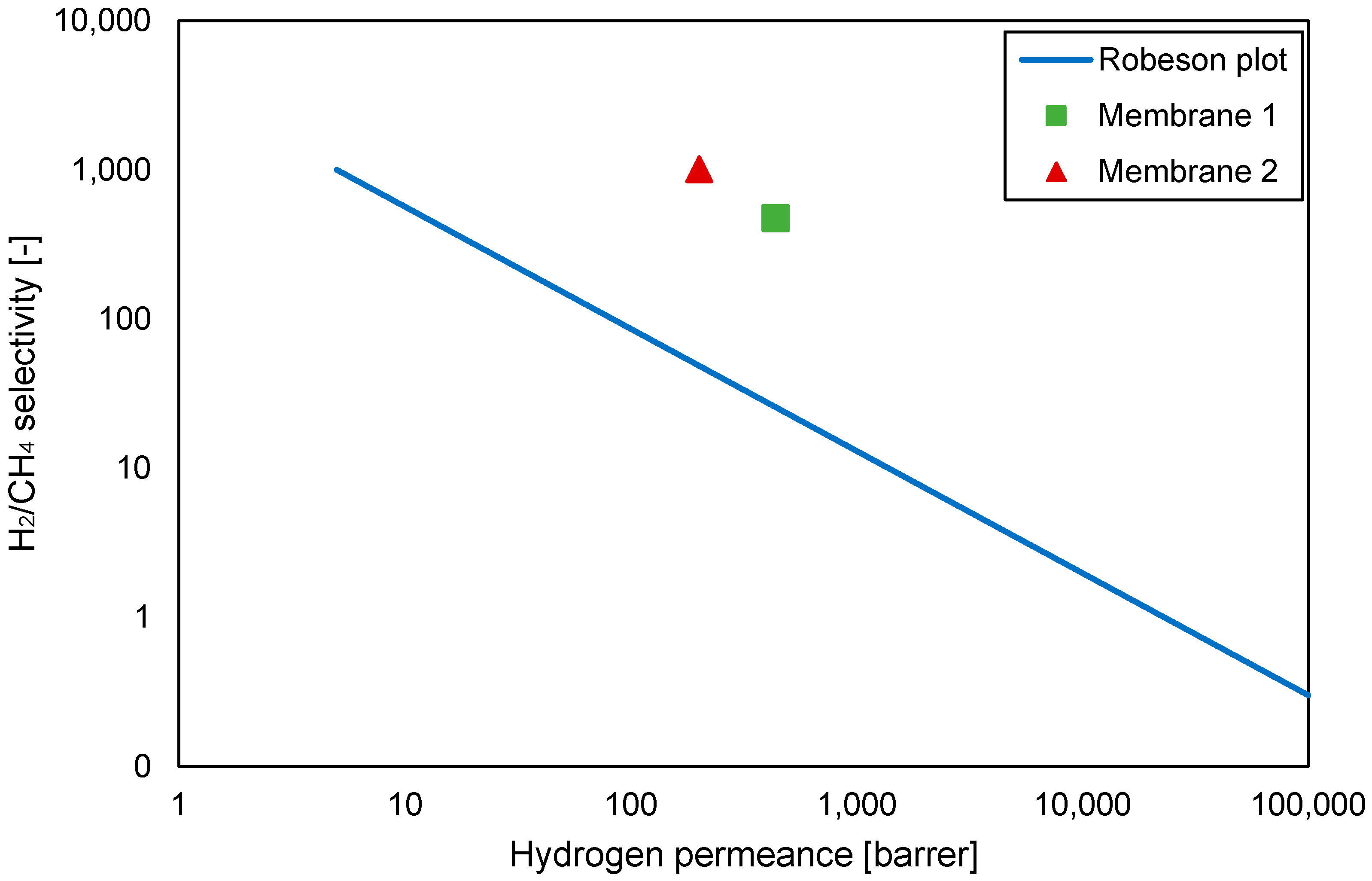
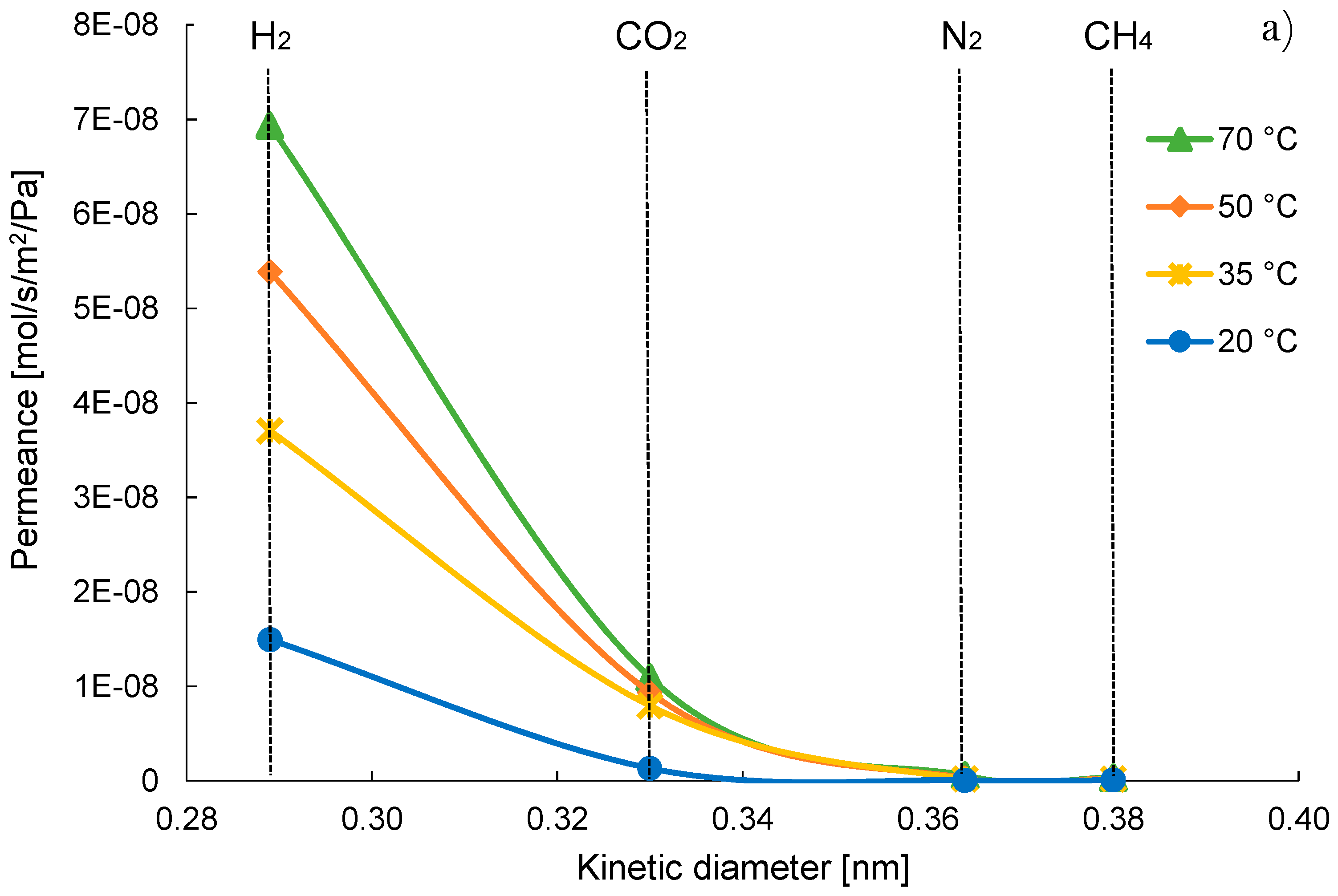
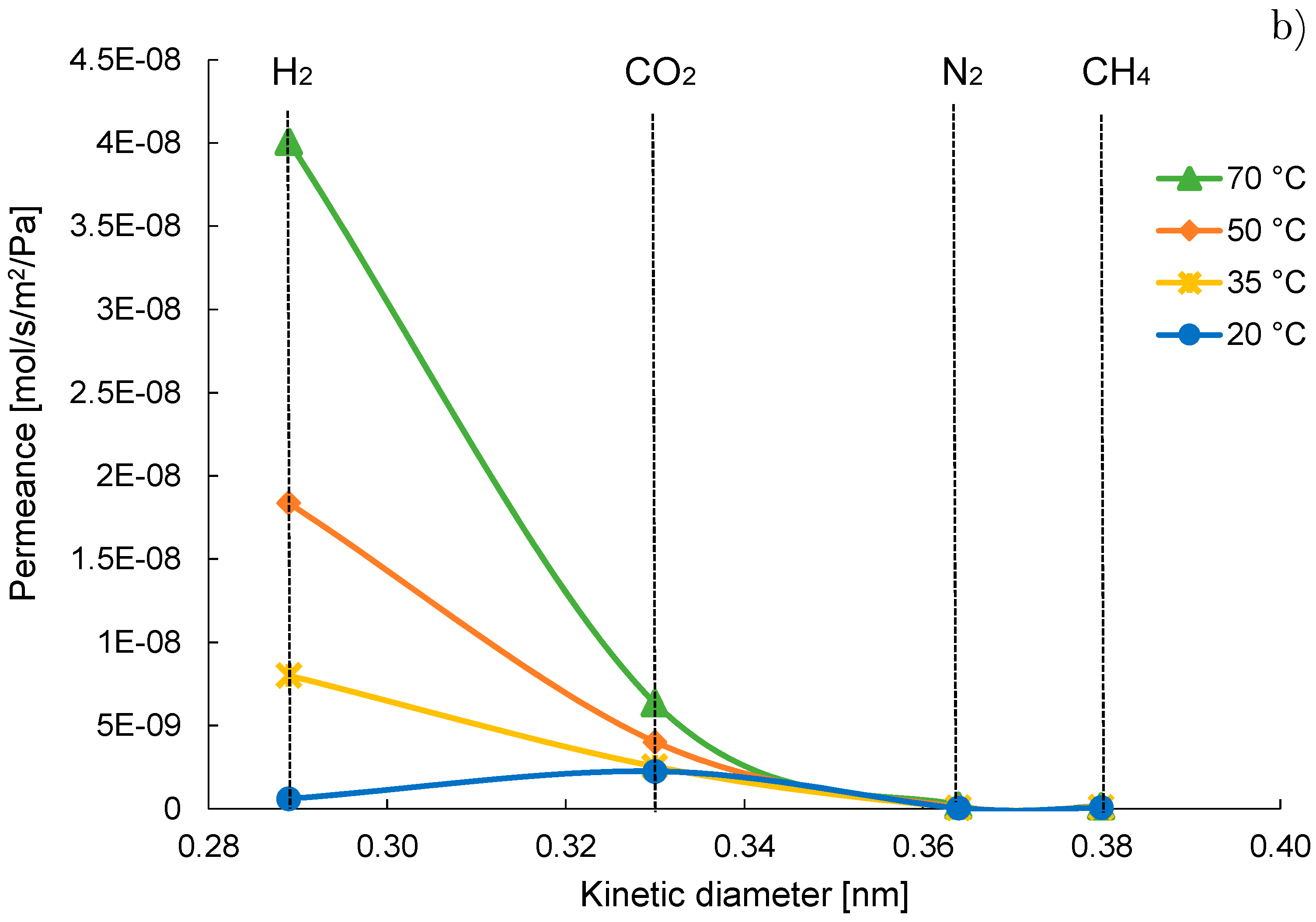
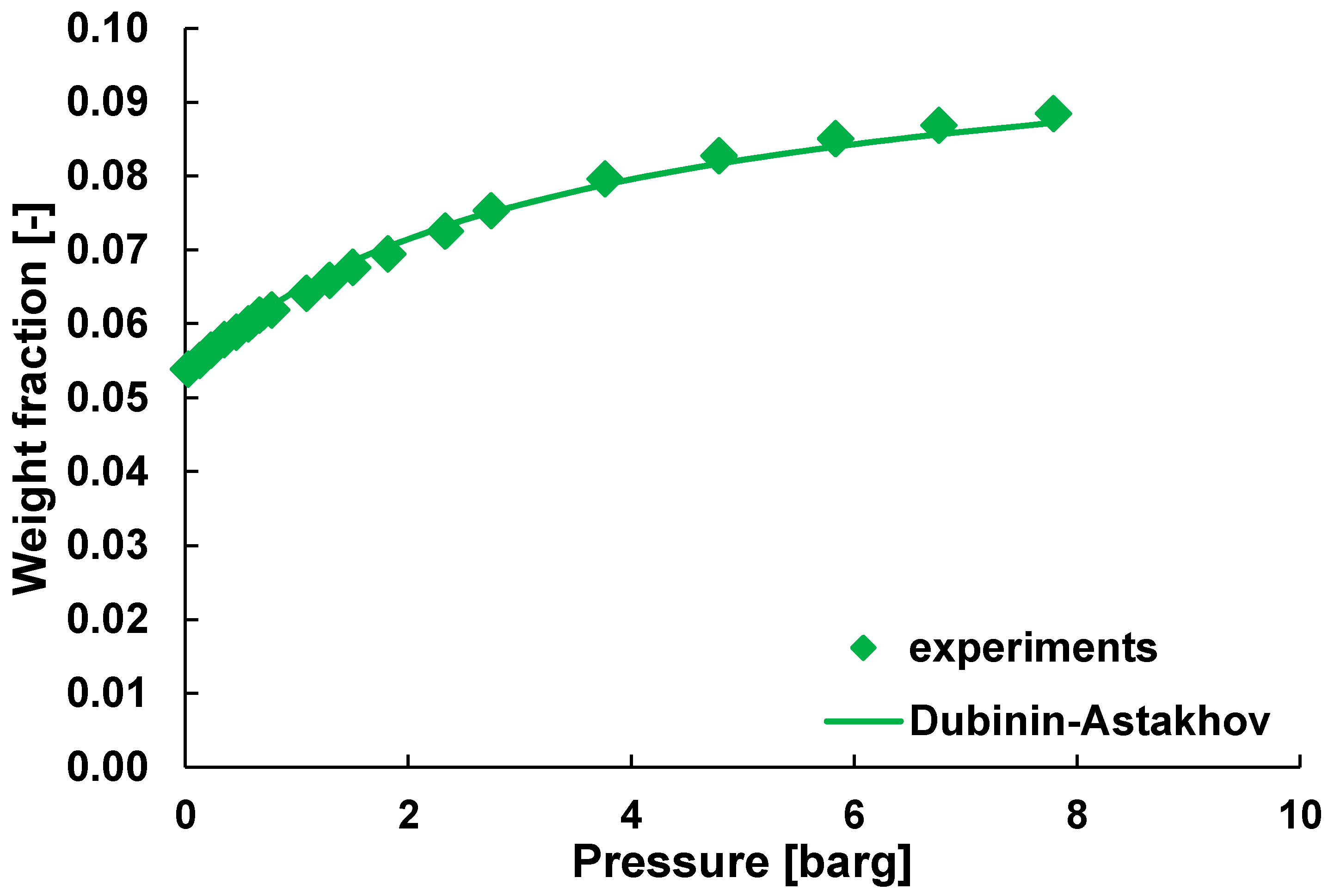
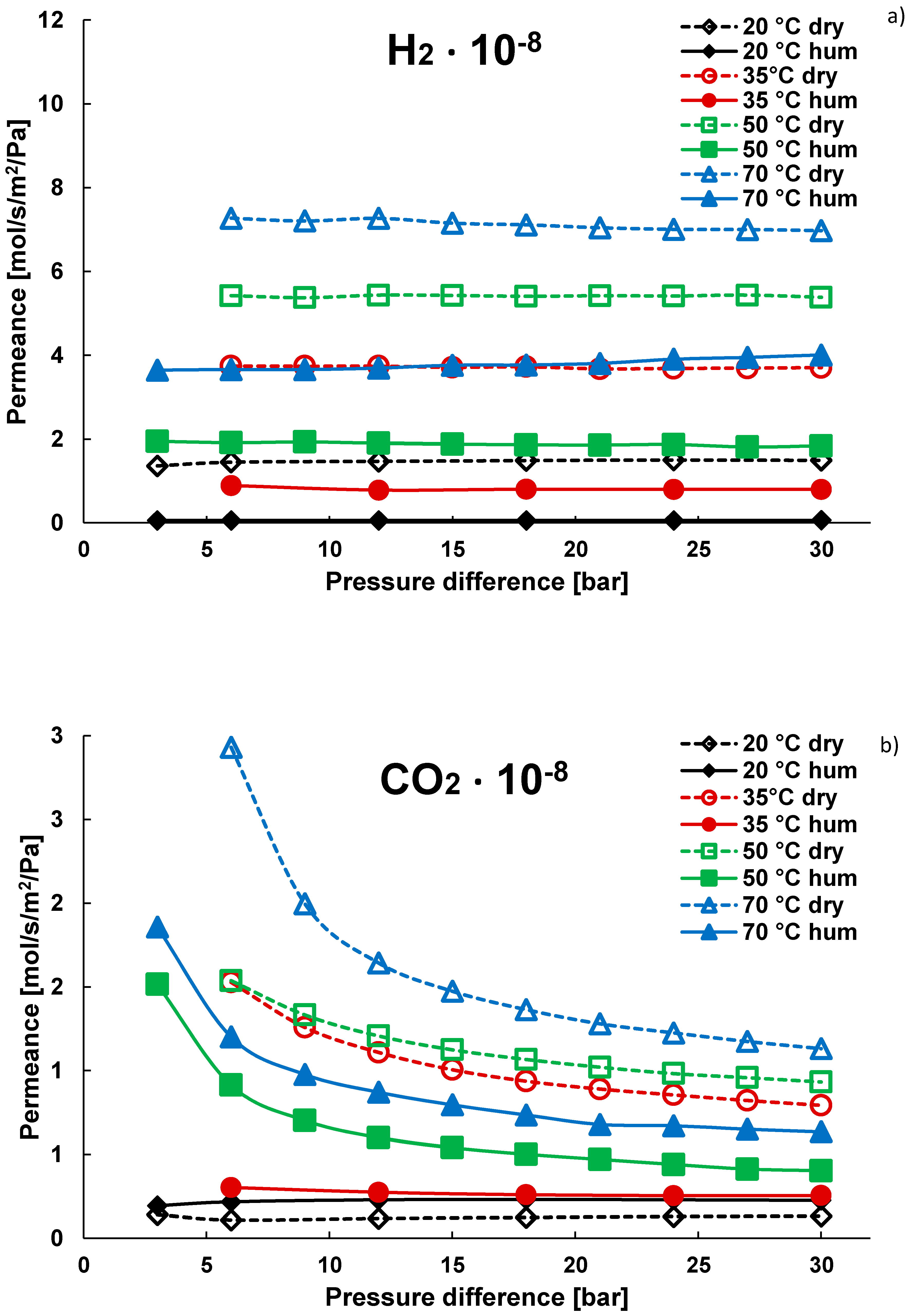
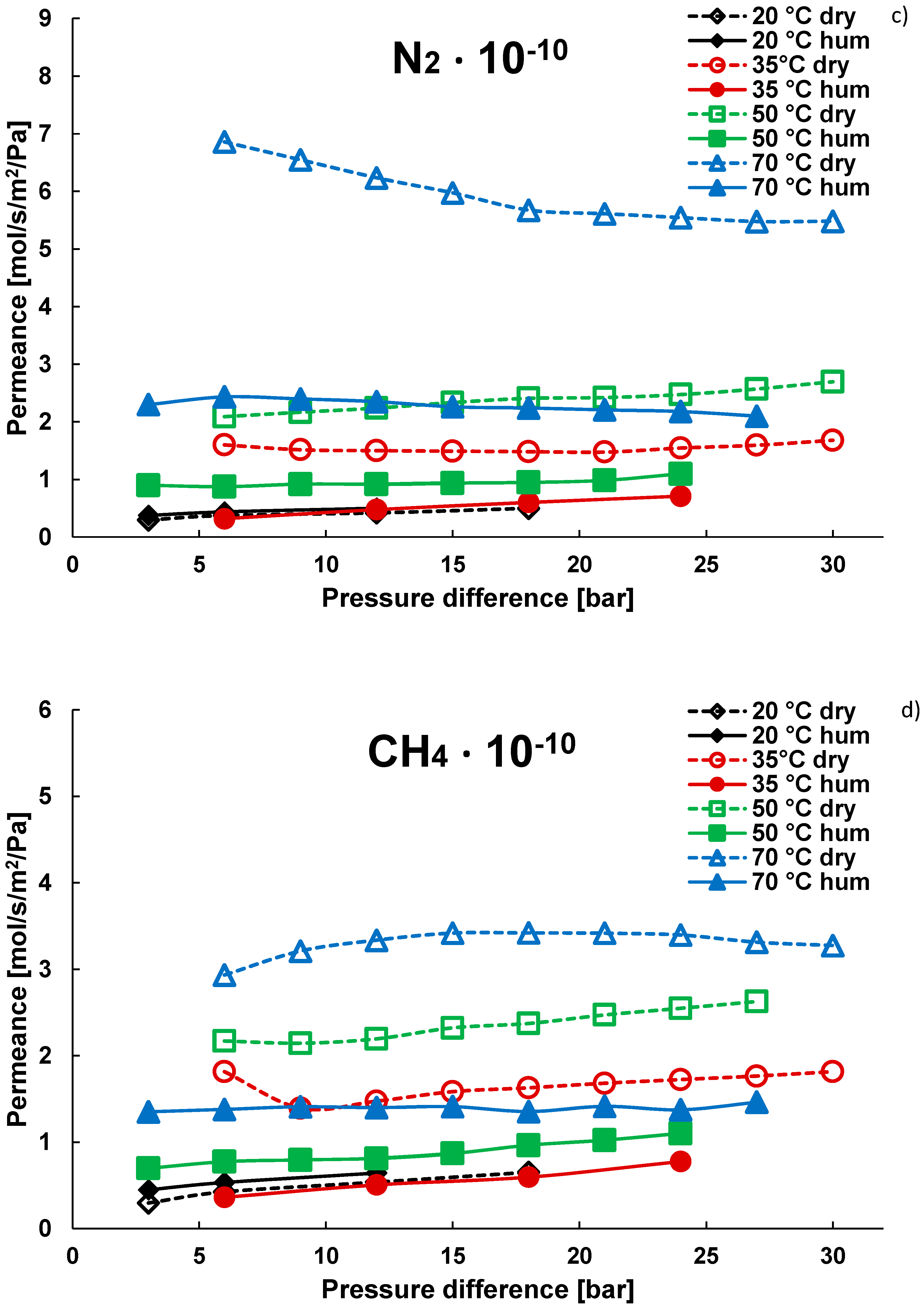
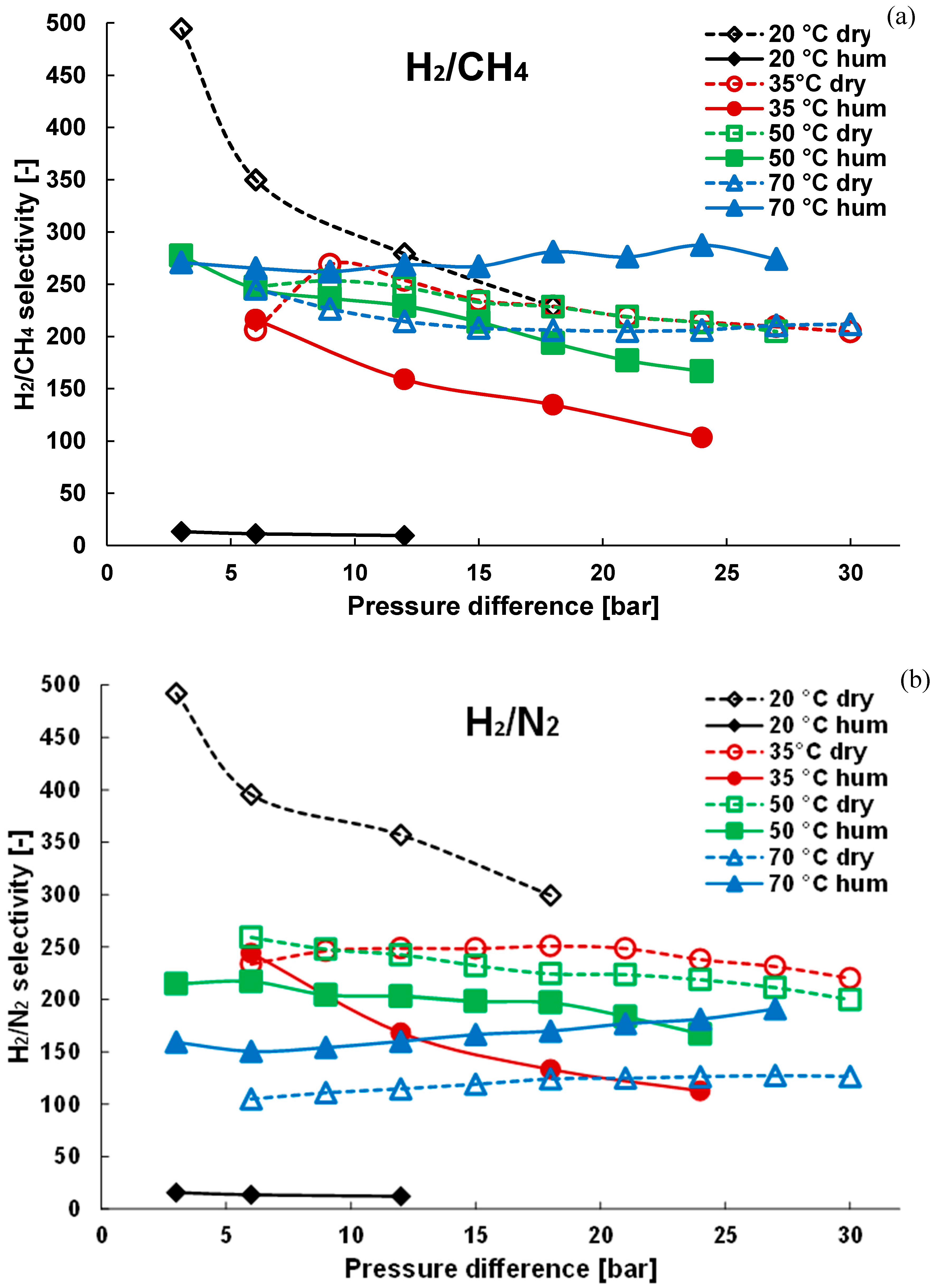
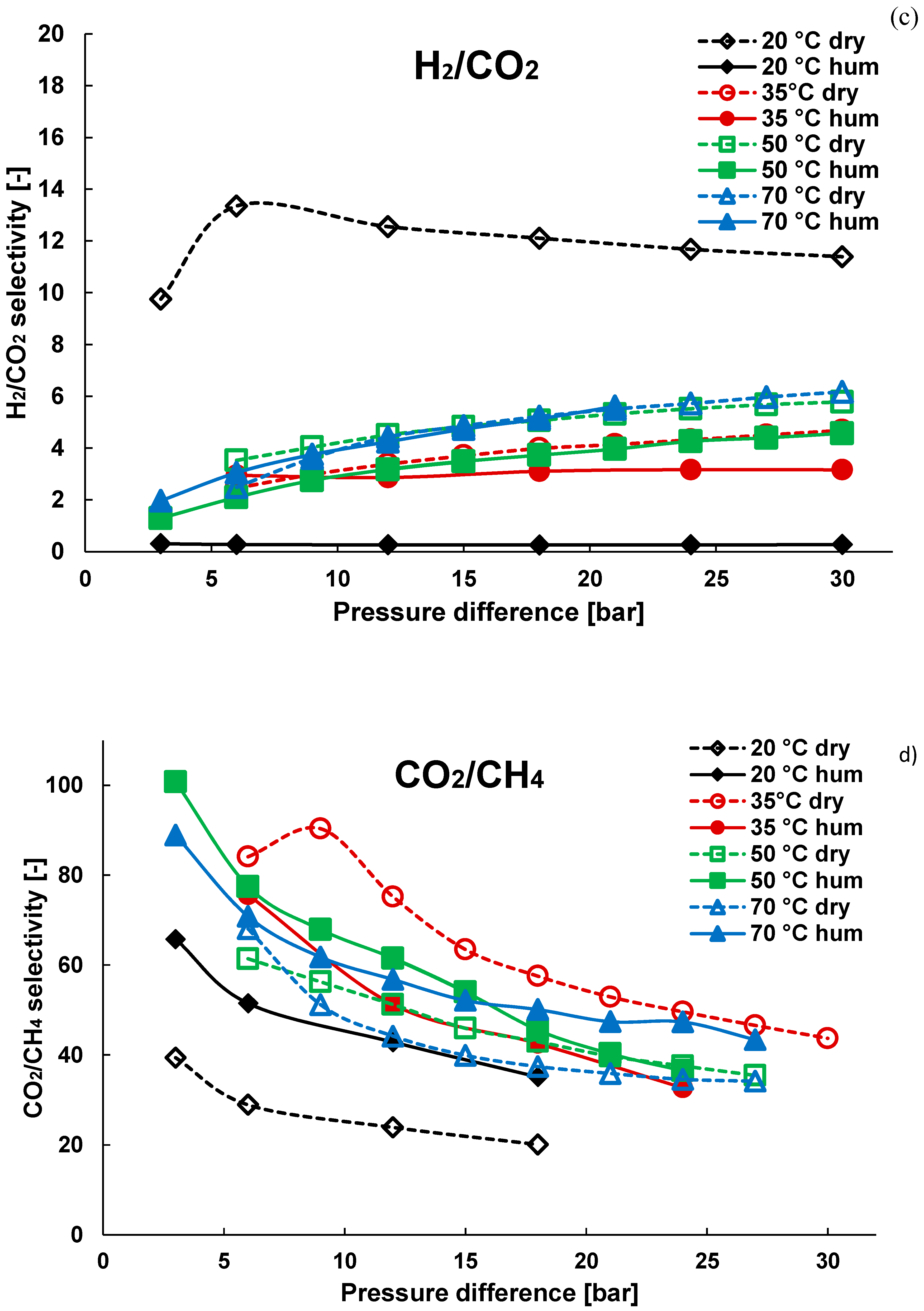

| Gas | Water Solubility [g/kg water] @ 20 °C | Gas Diffusivity in Water·10−9 [m2/s] @ 20 °C | Kinetic Diameter [pm] |
|---|---|---|---|
| carbon dioxide | 1.65 | 1.71 | 330 |
| hydrogen | 0.0016 | 4.5 | 289 |
| nitrogen | 0.019 | 1.75 | 364 |
| methane | 0.024 | 1.64 | 380 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordio, M.L.V.; Medrano, J.A.; van Sint Annaland, M.; Pacheco Tanaka, D.A.; Llosa Tanco, M.; Gallucci, F. Water Adsorption Effect on Carbon Molecular Sieve Membranes in H2-CH4 Mixture at High Pressure. Energies 2020, 13, 3577. https://doi.org/10.3390/en13143577
Nordio MLV, Medrano JA, van Sint Annaland M, Pacheco Tanaka DA, Llosa Tanco M, Gallucci F. Water Adsorption Effect on Carbon Molecular Sieve Membranes in H2-CH4 Mixture at High Pressure. Energies. 2020; 13(14):3577. https://doi.org/10.3390/en13143577
Chicago/Turabian StyleNordio, Maria L. V., José A. Medrano, Martin van Sint Annaland, David Alfredo Pacheco Tanaka, Margot Llosa Tanco, and Fausto Gallucci. 2020. "Water Adsorption Effect on Carbon Molecular Sieve Membranes in H2-CH4 Mixture at High Pressure" Energies 13, no. 14: 3577. https://doi.org/10.3390/en13143577
APA StyleNordio, M. L. V., Medrano, J. A., van Sint Annaland, M., Pacheco Tanaka, D. A., Llosa Tanco, M., & Gallucci, F. (2020). Water Adsorption Effect on Carbon Molecular Sieve Membranes in H2-CH4 Mixture at High Pressure. Energies, 13(14), 3577. https://doi.org/10.3390/en13143577









