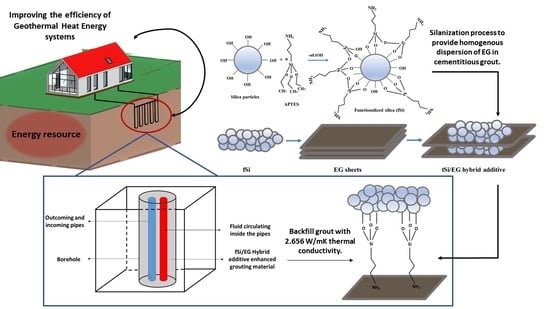
Synergistic Effect of Expanded Graphite-Silane Functionalized Silica as a Hybrid Additive in Improving the Thermal Conductivity of Cementitious Grouts with Controllable Water Uptake
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
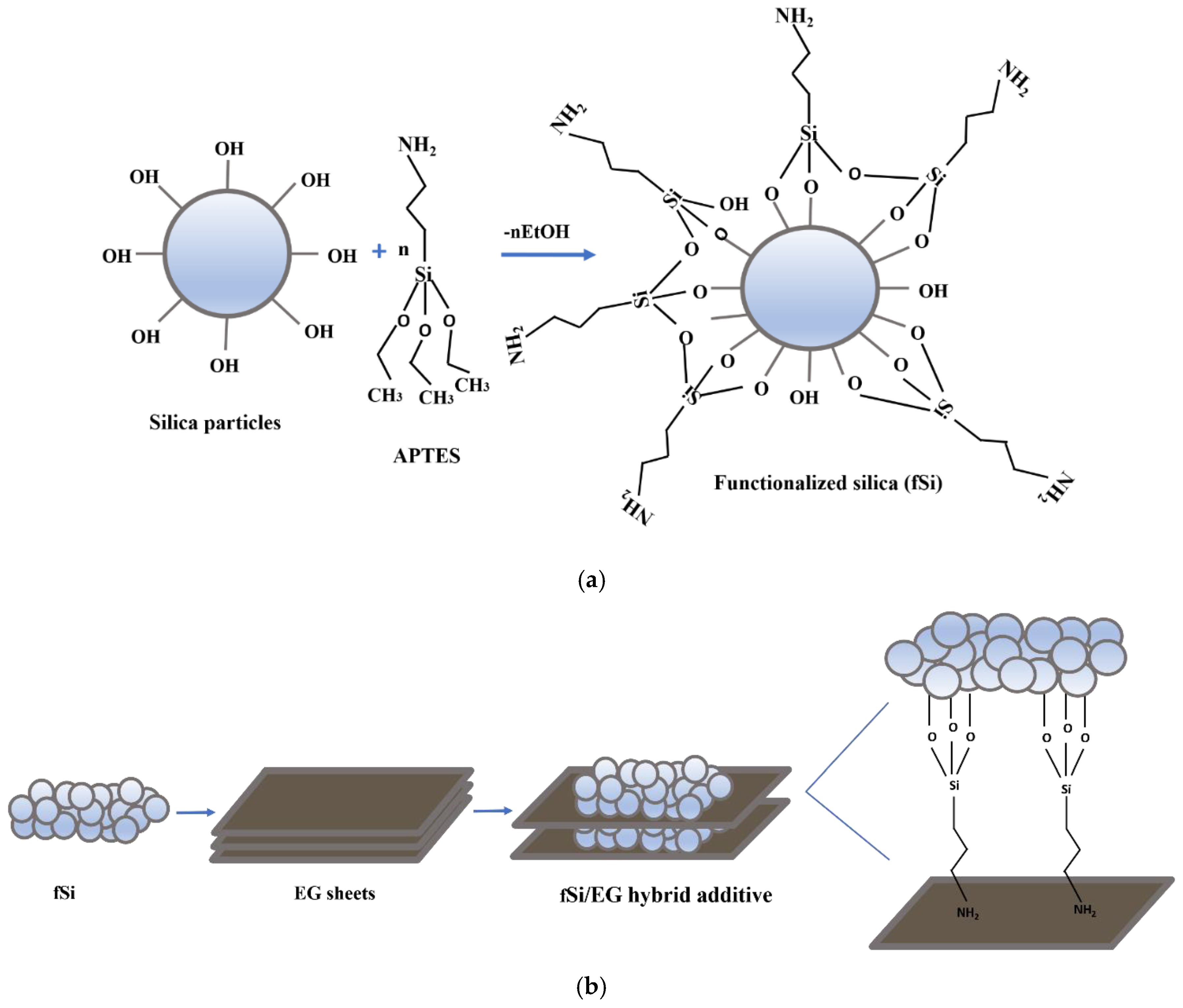
2.2. Synthesis of Functionalized Silica
2.3. Hybridization of EG with Functionalized Silica
2.4. Preparation of Grout Samples by Using Hybrid Additives
2.5. Characterization
3. Results and Discussion
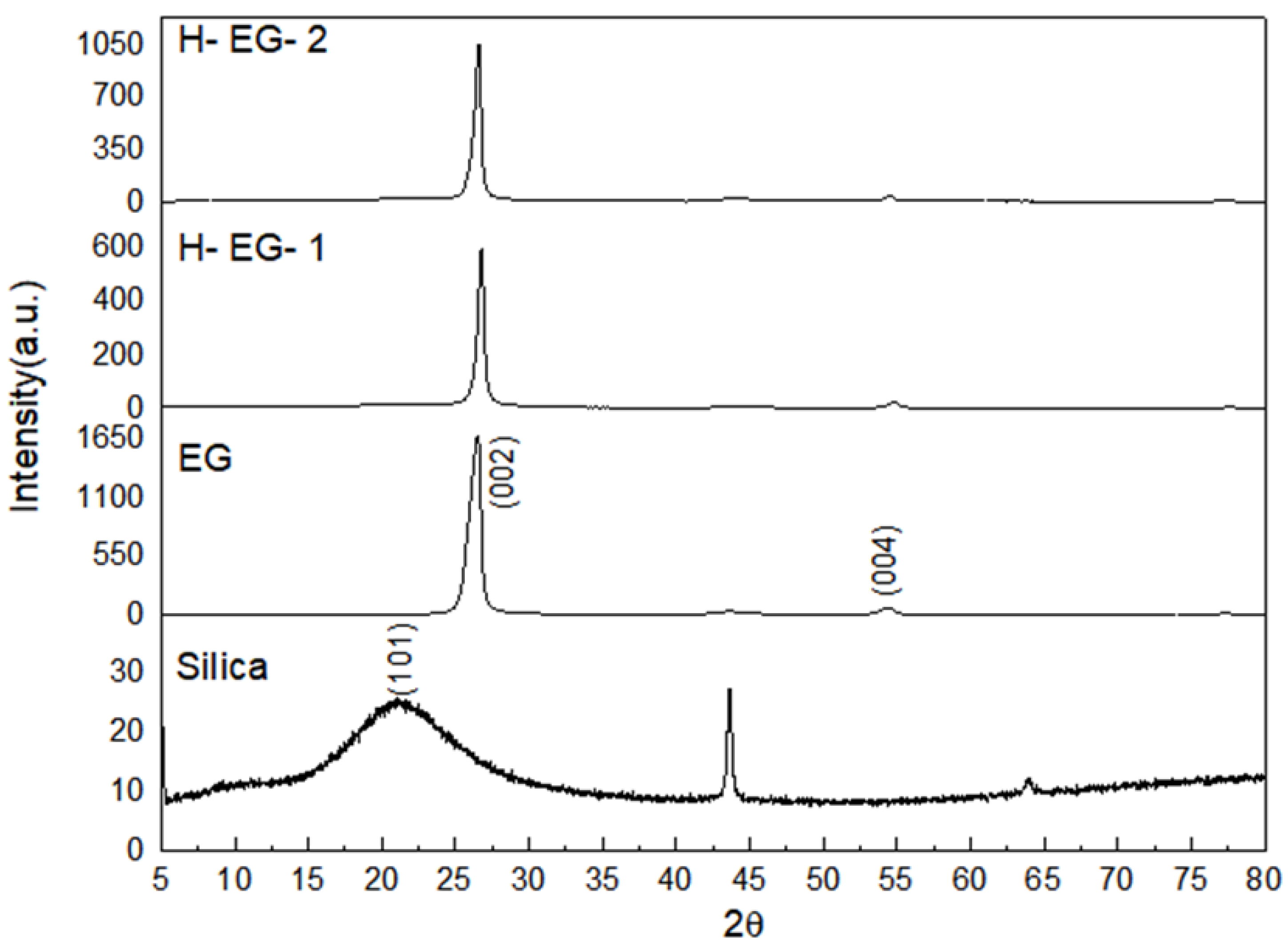
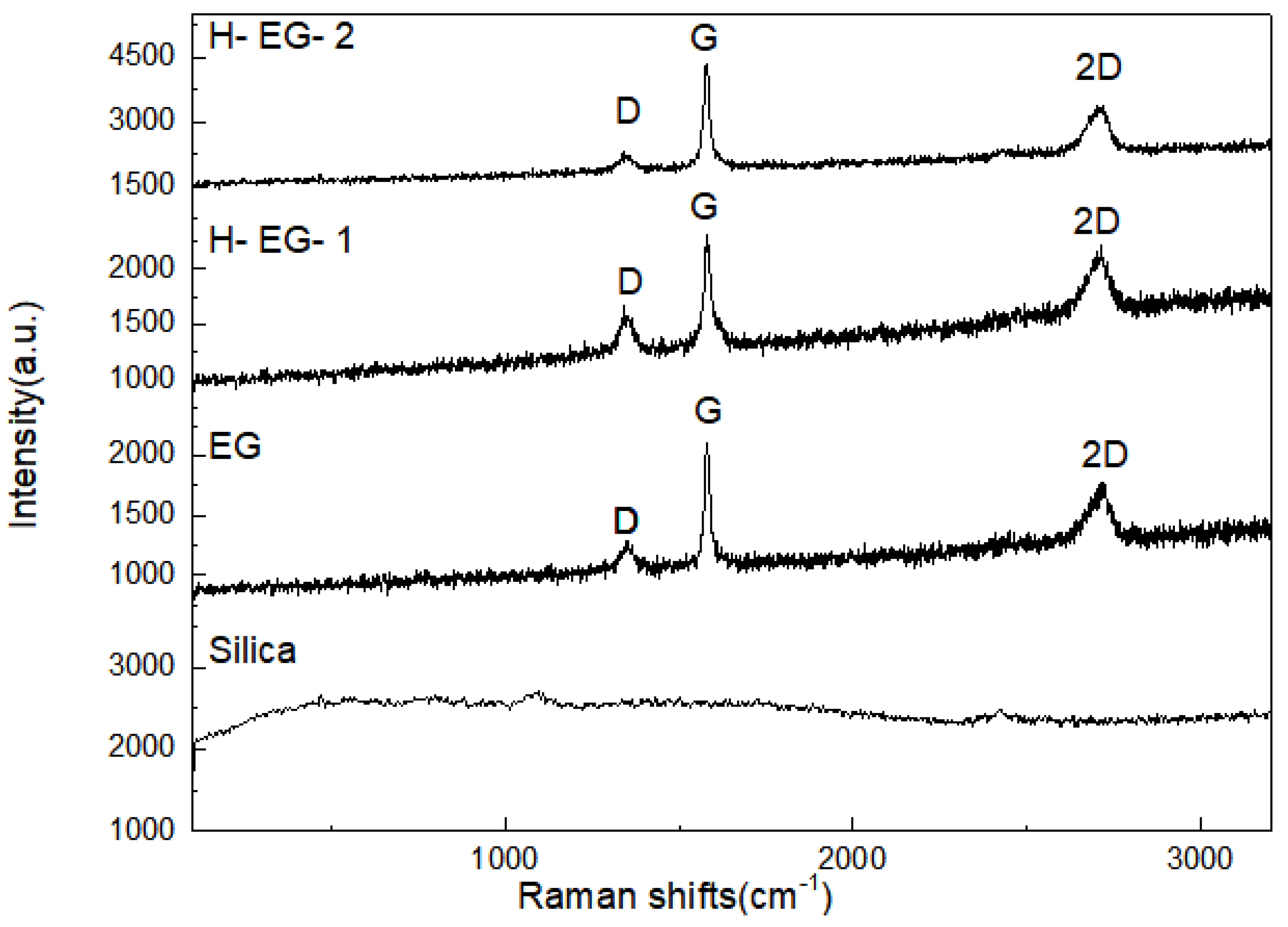
3.1. Structural Properties of Functionalized Silica and Silica-Modified Hybrid EG Additives
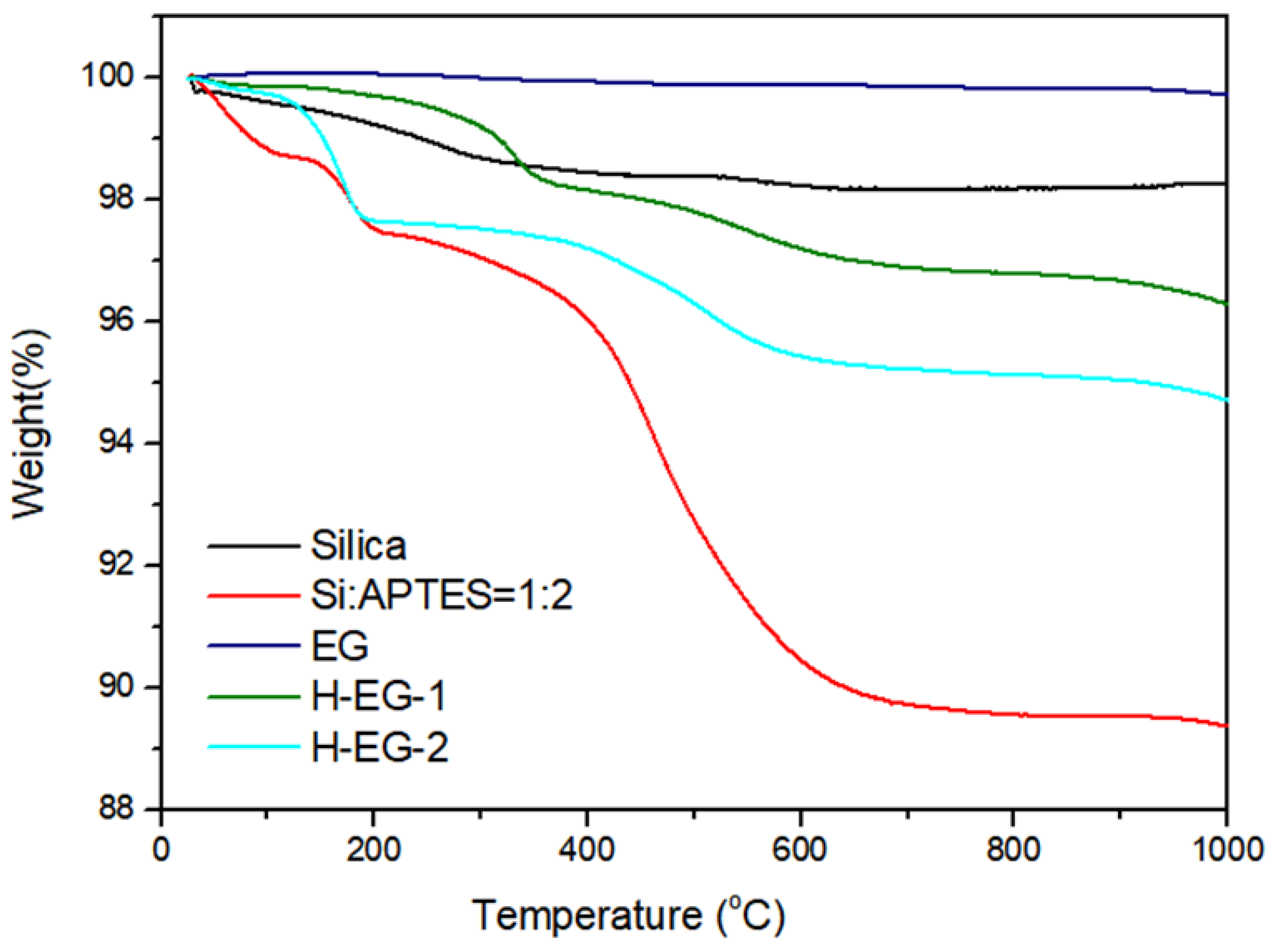
3.2. Thermal Degradation Behaviors of Neat and Silica-Modified EG Hybrid Additives
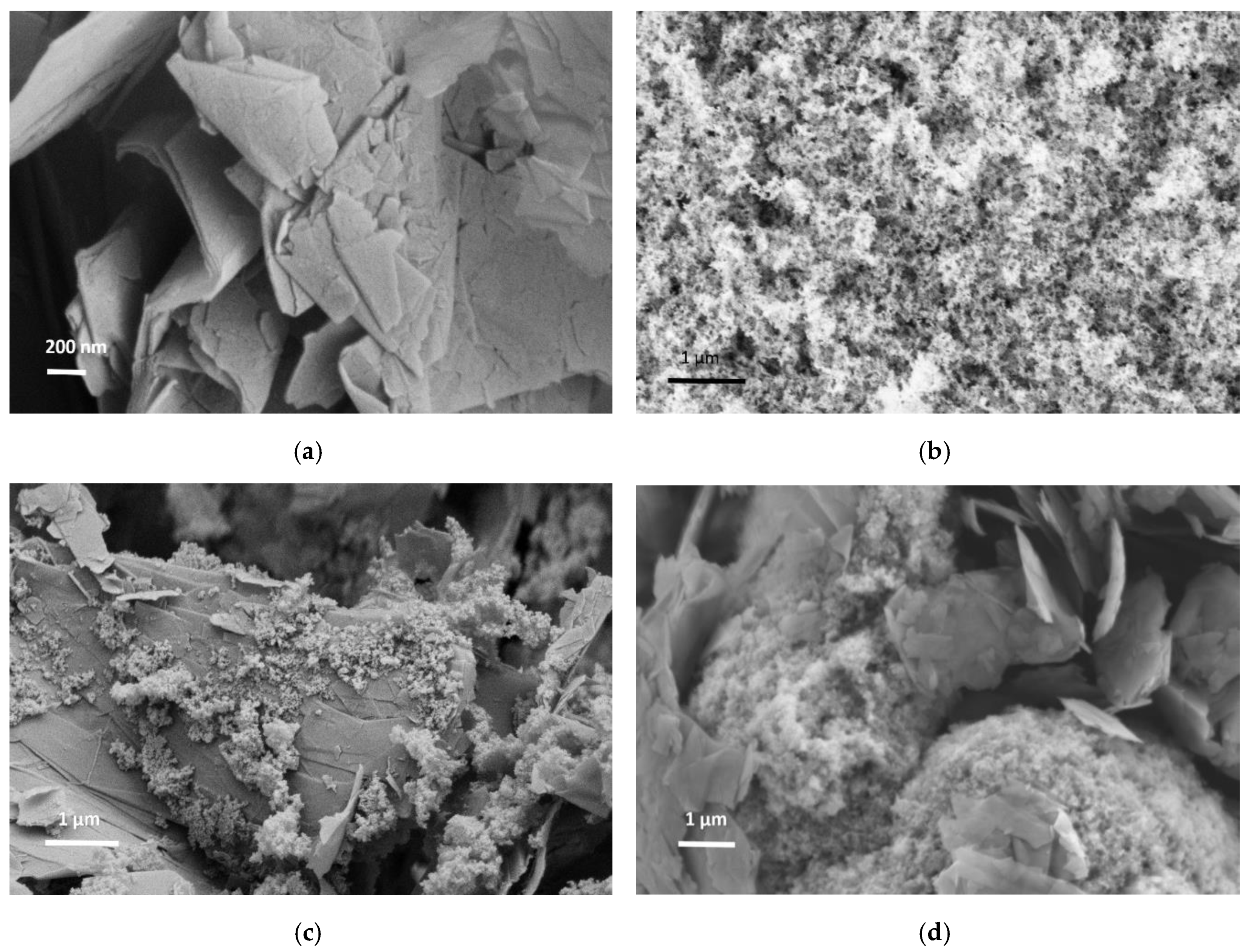
3.3. Morphological Properties of Neat and Hybrid Additives
3.4. Thermal Conductivity and Rheological Behaviors of Silica–EG Hybrid Additive Grout Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarbu, I.; Sebarchievici, C. Using Ground-Source Heat Pump Systems for Heating/Cooling of Buildings. In Advances in Geothermal Energy; Basel, I.I., Ed.; Rijeka, Croatia: Croatia, 2016. [Google Scholar]
- Yang, H.; Cui, P.; Fang, Z. Vertical-borehole ground-coupled heat pumps: A review of models and systems. Appl. Energy 2010, 87, 16–27. [Google Scholar] [CrossRef]
- Javadi, H.; Ajarostaghi, S.S.M.; Rosen, M.A.; Pourfallah, M. A comprehensive review of backfill materials and their effects on ground heat exchanger performance. Sustainability 2018, 10, 4486. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, H.; Lu, L.; Fang, Z. Investigation on influential factors of engineering design of geothermal heat exchangers. Appl. Therm. Eng. 2015, 84, 310–319. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.; Park, S.; Chung, W. Enhanced Detection Systems of Filling Rates Using Carbon Nanotube Cement Grout. Nanomaterials 2019, 10, 10. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H. Experimental investigation on the ice/snow melting performance of CNFP & MWCNT/cement-based deicing system. In Proceedings of the 6th International Workshop on Advanced Smart Materials and Smart Structures Technology, Dalian, China, 25–26 July 2011. [Google Scholar]
- Song, Y.S.; Youn, J. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon 2005, 43, 1378–1385. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Schadler, L.S.; Giannaris, C.; Rubio, A. Single-Walled Carbon Nanotube–Polymer Composites: Strength and Weakness. Adv. Mater. 2000, 12, 750–753. [Google Scholar] [CrossRef]
- Delaleux, F.; Py, X.; Olives, R.; Dominguez, A. Enhancement of geothermal borehole heat exchangers performances by improvement of bentonite grouts conductivity. Appl. Therm. Eng. 2012, 33–34, 92–99. [Google Scholar] [CrossRef]
- Lee, C.; Lee, K.; Choi, H.; Choi, H.P. Characteristics of thermally-enhanced bentonite grouts for geothermal heat exchanger in South Korea. Sci. China Technol. Sci. 2010, 53, 123–128. [Google Scholar] [CrossRef]
- Jobmann, M.; Buntebarth, G. Influence of graphite and quartz addition on the thermo physical properties of bentonite for sealing heat-generating radioactive waste. Appl. Clay Sci. 2009, 44, 206–210. [Google Scholar] [CrossRef]
- Peyvandi, A.; Soroushian, P.; Abdol, N.; Balachandra, A.M. Surface-modified graphite nanomaterials for improved reinforcement efficiency in cementitious paste. Carbon 2013, 63, 175–186. [Google Scholar] [CrossRef]
- Karaipekli, A.; Biçer, A.; Sarı, A.; Veer, V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, X. Study on paraffin/expanded graphite composite phase change thermal energy storage material. Energy Convers. Manag. 2006, 47, 303–310. [Google Scholar] [CrossRef]
- Bao, X.; Memon, S.A.; Yang, H.; Dong, Z.; Cui, H. Thermal properties of cement-based composites for geothermal energy applications. Materials 2017, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, Y.; Jin, X.; Lo, T.; Cui, H. Thermal and Mechanical Properties of Expanded Graphite/Paraffin Gypsum-Based Composite Material Reinforced by Carbon Fiber. Materials 2018, 11, 2205. [Google Scholar] [CrossRef]
- Luping, T.; Liu, J.; Wang, N.; Ye, L. Pre-Study of Graphene-Enhanced Cementitious Materials. Available online: https://pdfs.semanticscholar.org/0728/d6fb4819e59eef0c19653ab6785f4d851cfa.pdf (accessed on 5 May 2020).
- Wang, Q.; Li, S.Y.; Pan, S.; Guo, Z.W. Synthesis and properties of a silane and copolymer-modified graphene oxide for use as a water-reducing agent in cement pastes. New Carbon Mater. 2018, 33, 131–139. [Google Scholar] [CrossRef]
- Sharma, N.; Nasimul, S.; Chandra, B.; Yadav, S.; Biswas, K. Diamond & Related Materials Silica-graphene nanoplatelets and silica-MWCNT composites: Microstructure and mechanical properties. Diam. Relat. Mater. 2018, 87, 186–201. [Google Scholar]
- Aly, M.; Hashmi, M.S.J.; Olabi, A.G.; Messeiry, M.; Hussain, A.I. Effect of nano clay particles on mechanical, thermal and physical behaviours of waste-glass cement mortars. Mater. Sci. Eng. A 2011, 528, 7991–7998. [Google Scholar] [CrossRef]
- Aly, M.; Hashmi, M.S.J.; Olabi, A.G.; Messeiry, M.; Abadir, E.F.; Hussain, A.I. Effect of colloidal nano-silica on the mechanical and physical behaviour of waste-glass cement mortar. Mater. Des. 2012, 33, 127–135. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Birgisson, B.; Taylor, P.; Attoh Okine, N. Nanotechnology in Civil Infrastructure; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-16656-3. [Google Scholar]
- Shen, C.; Wang, H.; Zhang, T.; Zeng, Y. Silica Coating onto Graphene for Improving Thermal Conductivity and Electrical Insulation of Graphene/polydimethylsiloxane Nanocomposites. J. Mater. Sci. Technol. 2018, 35, 36–43. [Google Scholar] [CrossRef]
- Liu, K.; Shang, Y.; Yang, L.; Du, A. Silica/GO Hybrids Reinforced NR: Better Interface Interaction and Dynamic Behavior. J. Elastomers Plast. Available online: https://journals.sagepub.com/doi/10.1177/0095244319877671 (accessed on 12 May 2020).
- Sadiq, M.M. Reinforcement of Cement-Based Matrices with Graphite a Dissertation. (Civil Engineering–Doctor of Philosophy). Ph.D. Thesis, Michigan State University, Lansing, MI, USA, 2013. [Google Scholar]
- Cao, L.; Sinha, T.K.; Tao, L.; Li, H.; Zong, C.; Kim, J.K. Synergistic reinforcement of silanized silica-graphene oxide hybrid in natural rubber for tire-tread fabrication: A latex based facile approach. Compos. Part B Eng. 2019, 161, 667–676. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, D.; Yang, C.; Liu, Y.; Liu, Y. Effect of graphene oxide on the rheological properties of cement pastes. Constr. Build. Mater. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Berktas, I.; Ghafar, A.N.; Fontana, P.; Caputcu, A.; Menceloglu, Y.; Okan, B.S. Facile Synthesis of Graphene from Waste Tire/Silica Hybrid Additives and Optimization Study for the Fabrication of Thermally Enhanced Cement Grouts. Molecules 2020, 25, 886. [Google Scholar] [CrossRef] [PubMed]
- Mondal, T.; Bhowmick, A.K.; Krishnamoorti, R. Conducting instant adhesives by grafting of silane polymer onto expanded graphite. ACS Appl. Mater. Interfaces 2014, 6, 16097–16105. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, L.; Qiu, Y.; Yuan, Y. A new adsorbent of Pb(II) ions from aqueous solution synthesized by mechanochemical preparation of sulfonated expanded graphite. RSC Adv. 2017, 7, 38350–38359. [Google Scholar] [CrossRef]
- Desai, S.; Njuguna, J. Enhancement of thermal conductivity of materials using different forms of natural graphite. IOP Conf. Ser. Mater. Sci. Eng. 2012, 40, 012017. [Google Scholar] [CrossRef]
- Mengal, N.; Arbab, A.A.; Memon, A.A.; Sahito, I.A.; Jeong, S.H. A promising hybrid graphite counter electrode doped with fumed silica nano-spacers for efficient quasi-solid state dye sensitized solar cells. Electrochim. Acta 2018, 261, 246–255. [Google Scholar] [CrossRef]
- Rao, K.S.; Senthilnathan, J.; Liu, Y.-F.; Yoshimura, M. Role of Peroxide Ions in Formation of Graphene Nanosheets by Electrochemical Exfoliation of Graphite. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Shanmugharaj, A.; Kumar, K.T.; Sundari, G.S.; Kumar, E.S.; Ashwini, A.; Ramya, M.; Varsha, P.; Kalaivani, R.; Raghu, S.; Ryu, S. Study on the effect of silica–graphite filler on the rheometric, mechanical, and abrasion loss properties of styrene–butadiene rubber vulcanizates. J. Elastomers Plast. 2019, 51, 359–378. [Google Scholar] [CrossRef]
- Ederer, J.; Janoš, P.; Ecorchard, P.; Tolasz, J.; Štengl, V.; Beneš, H.; Perchacz, M.; Pop-Georgievski, O. Determination of amino groups on functionalized graphene oxide for polyurethane nanomaterials: XPS quantitation vs. functional speciation. RSC Adv. 2017, 7, 12464–12473. [Google Scholar] [CrossRef]
- Vinoda, B.; Vinuth, M.; Bodke, Y.; Manjanna, J. Photocatalytic Degradation of Toxic Methyl Red Dye Using Silica Nanoparticles Synthesized from Rice Husk Ash. J. Environ. Anal. Toxicol. 2015, 5, 2161-0525. [Google Scholar]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Pavoski, G.; Maraschin, T.; Fim, F.D.C.; Balzaretti, N.M.; Galland, G.B.; Moura, C.S.; Basso, N.R.D.S. Few layer reduced graphene oxide: Evaluation of the best experimental conditions for easy production. Mater. Res. 2017, 20, 53–61. [Google Scholar] [CrossRef]

| Constituent Type | D10 (μm) | D50 (μm) | D90 (μm) |
|---|---|---|---|
| Cement | 3.72 | 17.8 | 46.4 |
| Bentonite | 9.21 | 40.6 | 131 |
| Silica Sand AFS 30–35 | 401 | 621 | 912 |
| Silica Sand AFS 60–70 | 134 | 250 | 454 |
| Samples | Silica: APTES = 1:2(fSi) (g) | EG Amount (g) | Reaction Medium | Reaction Time (h) |
|---|---|---|---|---|
| H-EG-1 (fSi:EG = 1:1) | 1 | 1 | THF | 24 |
| H-EG-2 (fSi:EG = 1:5) | 1 | 5 | THF | 24 |
| Component | wt% |
|---|---|
| Cement | 33.94 |
| Silica Sand (30–35 AFS) | 32.845 |
| Silica Sand (60–65 AFS) | 32.845 |
| Bentonite | 0.37 |
| Sample Name | Carbon (at%) | Oxygen (at%) | Silicon (at%) | Nitrogen (at%) |
|---|---|---|---|---|
| Silica | 3.3 | 60 | 36.7 | - |
| Silica: APTES = 1:2 | 26 | 43 | 27 | 3.1 |
| EG | 98 | 2 | - | - |
| H-EG-1 | 50.5 | 30.3 | 18.2 | 1 |
| H-EG-2 | 88.2 | 6.7 | 3.2 | 1.7 |
| Sample Name | Crystallinity (%) | Amorphous (%) |
|---|---|---|
| Silica | 58.7 | 41.3 |
| Silica: APTES = 1:2 | 38.2 | 61.8 |
| EG | 89 | 11 |
| H-EG-1 | 70.6 | 29.4 |
| H-EG-2 | 68.7 | 31.3 |
| Sample Names | D Peak Intensity (a.u.) | G Peak Intensity (a.u.) | 2D Peak Intensity (a.u.) | ID/IG |
|---|---|---|---|---|
| EG | 1291.6 | 2108 | 1779 | 0.61 |
| H-EG-1 | 1585.8 | 2309.4 | 2222.6 | 0.68 |
| H-EG-2 | 2291.5 | 4364.1 | 3383.5 | 0.52 |
| Test no | Cement (g) | Silica Sand 30–35 AFS (g) | Silica Sand 60–70 AFS (g) | Bentonite (g) | Additive (g) | SP (g) | Water (g) | Marshcone (s) | Flowtable (cm) | Bleeding (%) | Density (g/cm3) | Thermal Conductivity (W/mK) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 930 | 900 | 900 | 10 | 0 Reference | 18.6 | 650 | 77 | 26 | 0.49 | 2.1 | 2.373 |
| 2 | 930 | 900 | 900 | 10 | 27.9 (fSi:EG = 1:1) H-EG-1 (3 wt%) | 18.6 | 750 | 90 | 25 | 0.22 | 2.01 | 2.175 |
| 3 | 930 | 900 | 900 | 10 | 27.9 (fSi:EG = 1:1) H-EG-1 (3 wt%) | 18.6 | 730 | 109 | 24 | 0.10 | 2.02 | 2.429 |
| 4 | 930 | 900 | 900 | 25 | 46.5 (fSi:EG = 1:5) H-EG-2 (5 wt%) | 18.6 | 735 | 103 | 30.3 | 1.7 | 1.95 | 2.656 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berktas, I.; Nejad Ghafar, A.; Fontana, P.; Caputcu, A.; Menceloglu, Y.; Saner Okan, B. Synergistic Effect of Expanded Graphite-Silane Functionalized Silica as a Hybrid Additive in Improving the Thermal Conductivity of Cementitious Grouts with Controllable Water Uptake. Energies 2020, 13, 3561. https://doi.org/10.3390/en13143561
Berktas I, Nejad Ghafar A, Fontana P, Caputcu A, Menceloglu Y, Saner Okan B. Synergistic Effect of Expanded Graphite-Silane Functionalized Silica as a Hybrid Additive in Improving the Thermal Conductivity of Cementitious Grouts with Controllable Water Uptake. Energies. 2020; 13(14):3561. https://doi.org/10.3390/en13143561
Chicago/Turabian StyleBerktas, Ilayda, Ali Nejad Ghafar, Patrick Fontana, Ayten Caputcu, Yusuf Menceloglu, and Burcu Saner Okan. 2020. "Synergistic Effect of Expanded Graphite-Silane Functionalized Silica as a Hybrid Additive in Improving the Thermal Conductivity of Cementitious Grouts with Controllable Water Uptake" Energies 13, no. 14: 3561. https://doi.org/10.3390/en13143561
APA StyleBerktas, I., Nejad Ghafar, A., Fontana, P., Caputcu, A., Menceloglu, Y., & Saner Okan, B. (2020). Synergistic Effect of Expanded Graphite-Silane Functionalized Silica as a Hybrid Additive in Improving the Thermal Conductivity of Cementitious Grouts with Controllable Water Uptake. Energies, 13(14), 3561. https://doi.org/10.3390/en13143561