Effect of Carbon Additives on the Electrochemical Performance of Li4Ti5O12/C Anodes
Abstract
1. Introduction
2. Materials and Methods
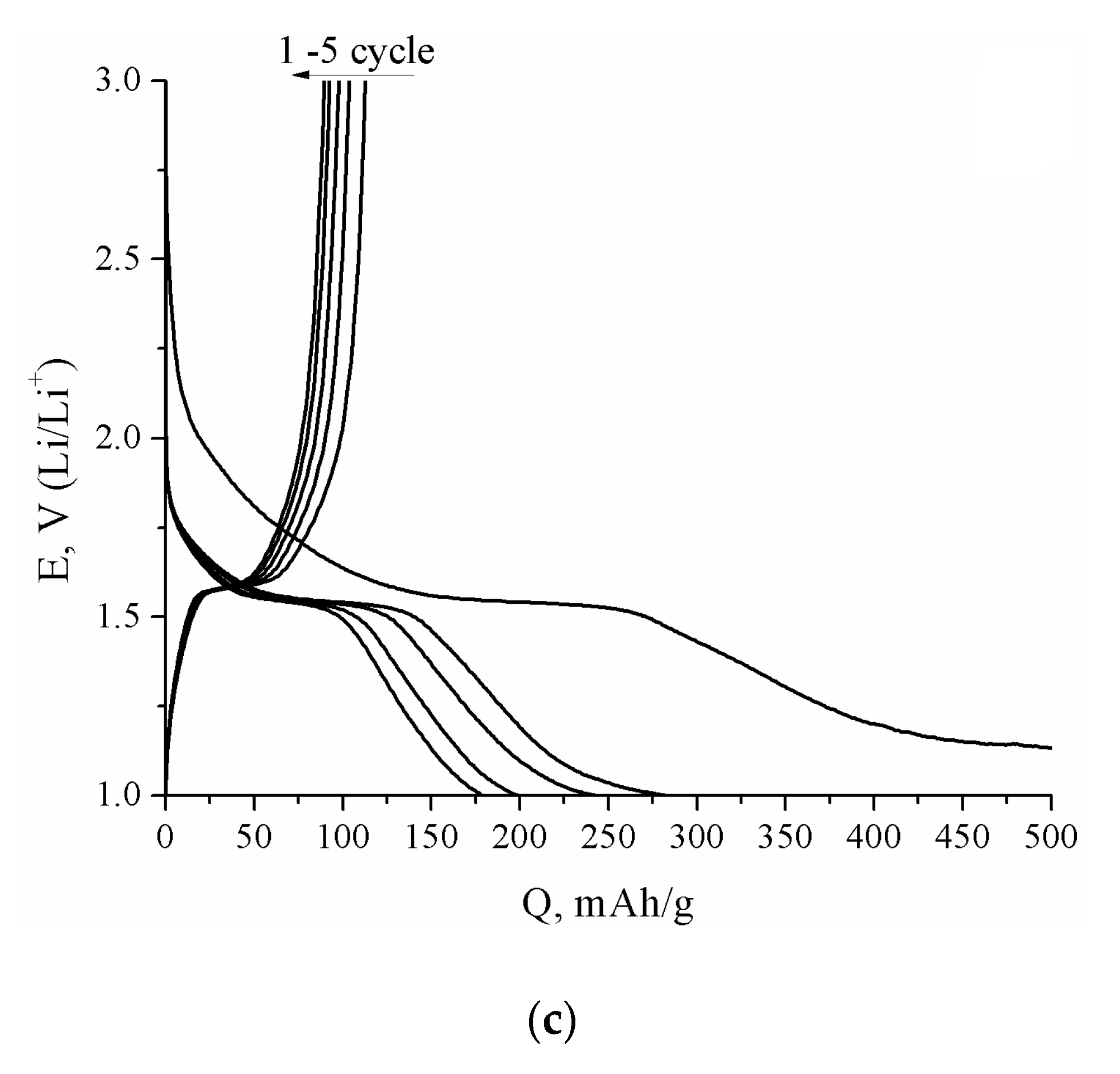
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ariyoshi, A.K.; Yamato, R.; Ohzuku, T. Zero-strain insertion mechanism of Li[Li1/3Ti5/3]O4 for advanced lithium-ion (shuttlecock) batteries. Electrochim. Acta 2005, 51, 1125–1129. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Liu, M.; Shao, Z. A comprehensive review of Li4Ti5O12-based electrodes for lithium-ion batteries: The latest advancements and future perspectives. Mater. Sci. Engineer. R 2015, 98, 1–71. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novák, P. Insertion Electrode Materials for Rechargeable Lithium Batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Kerisit, S.; Rosso, K.M.; Burton, S.D.; Sears, J.A.; Yang, Z.; Graff, G.L.; Liu, J.; Hu, J. Lithium diffusion in Li4Ti5O12 at high temperatures. J. Power Sources 2011, 196, 2211–2220. [Google Scholar] [CrossRef]
- Michalska, M.; Krajewski, M.; Ziolkowska, D.; Hamankiewicz, B.; Andrzejczuk, M.; Lipinska, L.; Korona, K.P.; Czerwinski, A. Influence of milling time in solid-state synthesis on structure, morphology and electrochemical properties of Li4Ti5O12 of spinel structure. Powder Technol. 2014, 266, 372–377. [Google Scholar] [CrossRef]
- Zhu, Y.-R.; Yin, L.-C.; Yi, T.-F.; Liu, H.; Xie, Y.; Zhu, R.-S. Electrochemical performance and lithium-ion intercalation kinetics of submicron-sized Li4Ti5O12 anode material. J. Alloys Compd. 2013, 547, 107–112. [Google Scholar] [CrossRef]
- Chauque, S.; Oliva, F.Y.; Visintin, A.; Barraco, D.; Leiva, E.P.M.; Cámara, O.R. Lithium titanate as anode material for lithium ion batteries: Synthesis, post-treatment and its electrochemical response. J. Electroanal. Chem. 2017, 799, 142–155. [Google Scholar] [CrossRef]
- Gu, Y.-J.; Guo, Z.; Liu, H.-Q. Structure and electrochemical properties of Li4Ti5O12 with Li excess as an anode electrode material for Li-ion batteries. Electrochim. Acta 2014, 123, 576–581. [Google Scholar] [CrossRef]
- Han, S.-W.; Ryu, J.H.; Jeong, J.; Yoon, D.-H. Solid-state synthesis of Li4Ti5O12 for high power lithium ion battery applications. J. Alloys Compd. 2013, 570, 144–149. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, A.; Dai, X.; Feng, L.; Li, J.; Li, J. Solid-state synthesis of submicron-sized Li4Ti5O12/Li2TiO3 composites with rich grain boundaries for lithium ion batteries. J. Power Sources 2014, 266, 114–120. [Google Scholar] [CrossRef]
- Zhang, E.; Zhang, H. Hydrothermal synthesis of Li4Ti5O12-TiO2 composites and Li4Ti5O12 and their applications in lithium-ion batteries. Ceram. Int. 2019, 45, 7419–7426. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Guan, Y.; Jin, Y.; Zhu, W.; Guo, X.; Qiu, X. Nano- Li4Ti5O12 with high rate performance synthesized by a glycerol assisted hydrothermal method. J. Power Sources 2013, 243, 661–667. [Google Scholar] [CrossRef]
- Yi, T.-F.; Wei, T.-T.; Li, Y.; He, Y.-B.; Wang, Z.-B. Efforts on enhancing the Li-ion diffusion coefficient and electronic conductivity of titanate-based anode materials for advanced Li-ion batteries. Energy Storage Mater. 2020, 26, 165–197. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, X.; Xu, B.; Lan, J.; Yu, Y.; Yang, X.; Lin, Y.; Nan, C. Li4Ti5O12 nanosheets assembled in tubular architecture for lithium storage. Chem. Eng. J. 2019, 361, 1371–1380. [Google Scholar] [CrossRef]
- Cao, N.; Song, Z.; Liang, Q.; Gao, X.; Qin, X. Hierarchical Li4Ti5O12/C composite for lithium-ion batteries with enhanced rate performance. Electrochim. Acta 2017, 235, 200–209. [Google Scholar] [CrossRef]
- Zou, S.; Wang, G.; Zhang, Y.; Xue, C.; Chen, H.; Yang, G.; Nan, H.; Wei, H.; Lin, H. Nano-structure and characterization of carbon composite with Al3+ and Mn4+ co-doped Li4Ti5O12 as anodes for Li-ion batteries. J. Alloy Compd. 2020, 816, 152609. [Google Scholar] [CrossRef]
- Chang-Jian, C.-W.; Ho, B.-C.; Chung, C.-K.; Chou, J.-A.; Chung, C.-L.; Huang, J.-H.; Huang, J.-H.; Hsiao, Y.-S. Doping and surface modification enhance the applicability of Li4Ti5O12 microspheres as high-rate anode materials for lithium ion batteries. Ceram. Int. 2018, 44, 23063–23072. [Google Scholar] [CrossRef]
- Stenina, I.A.; Sobolev, A.N.; Yaroslavtsev, S.A.; Rusakov, V.S.; Kulova, T.L.; Skundin, A.M.; Yaroslavtsev, A.B. Influence of iron doping on structure and electrochemical properties of Li4Ti5O12. Electrochim. Acta 2016, 219, 524–530. [Google Scholar] [CrossRef]
- Chen, Y.; Qian, C.; Zhang, P.; Zhao, R.; Lu, J.; Chen, M. Fluoride doping Li4Ti5O12 nanosheets as anode materials for enhanced rate performance of lithium-ion batteries. J. Electroanal. Chem. 2018, 815, 123–129. [Google Scholar] [CrossRef]
- Hsiao, K.C.; Liao, S.C.; Chen, J.M. Microstructure effect on the electrochemical property of Li4Ti5O12 as an anode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 7242–7247. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W. Micro/nano-structured Li4Ti5O12 as high rate anode material for lithium ion batteries. Solid State Ionics 2020, 349, 115297. [Google Scholar] [CrossRef]
- Wang, L.; Yue, S.; Zhang, Q.; Zhang, Y.; Li, Y.R.; Lewis, C.S.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S.; Wong, S.S. Morphological and Chemical Tuning of High-Energy-Density Metal Oxides for Lithium Ion Battery Electrode Applications. ACS Energy Lett. 2017, 2, 1465–1478. [Google Scholar] [CrossRef]
- Yue, J.; Badaczewski, F.M.; Voepel, P.; Leichtweiß, T.; Mollenhauer, D.; Zeier, W.G.; Smarsly, B.M. Critical Role of the Crystallite Size in Nanostructured Li4Ti5O12 Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 22580–22590. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Hao, T.; Osgood, H.; Zhang, B.; Chen, L.; Cui, L.; Song, X.-M.; Ogoke, O.; Wu, G. Advanced Mesoporous Spinel Li4Ti5O12/rGO Composites with Increased Surface Lithium Storage Capability for High-Power Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 9162–9169. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.; Chung, K.Y.; Kim, J. A Facile Supercritical Alcohol Route for Synthesizing Carbon Coated Hierarchically Mesoporous Li4Ti5O12 Microspheres. J. Phys. Chem. C 2014, 118, 183–193. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Peng, W.; Guo, H.; Li, X. Li4Ti5O12 co-modified with carbon nanotubes and pyrolytic carbon as an advanced anode material for lithium-ion batteries. Mater. Lett. 2014, 137, 413–416. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Nanomaterials for lithium-ion batteries and hydrogen energy. Pure Appl. Chem. 2017, 89, 1185–1194. [Google Scholar] [CrossRef]
- Ho, C.-K.; Li, C.-Y.V.; Deng, Z.; Chan, K.-Y.; Yung, H.; Yang, C. Hierarchical macropore-mesoporous shell carbon dispersed with Li4Ti5O12 for excellent high rate sub-freezing Li-ion battery performance. Carbon 2019, 145, 614–621. [Google Scholar] [CrossRef]
- Zhang, F.; Yi, F.; Meng, T.; Gao, A.; Shu, D.; Chen, H.; Cheng, H.; Zhou, X. In Situ Supramolecular Self-Assembly Assisted Synthesis of Li4Ti5O12–Carbon-Reduced Graphene Oxide Microspheres for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 916–924. [Google Scholar] [CrossRef]
- Yao, N.Y.; Liu, H.K.; Liang, X.; Sun, Y.; Feng, X.Y.; Chen, C.H.; Xiang, H.F. Li4Ti5O12 nanosheets embedded in three-dimensional amorphous carbon for superior-rate battery applications. J. Alloy Compd. 2019, 771, 755–761. [Google Scholar] [CrossRef]
- Kellerman, D.G.; Gorshkov, V.S.; Shalaeva, E.V.; Tsaryev, B.A.; Vovkotrub, E.G. Structure peculiarities of carbon-coated lithium titanate: Raman spectroscopy and electron microscopic study. Solid State Sci. 2012, 14, 72–79. [Google Scholar] [CrossRef]
- Wei, A.; Li, W.; Bai, X.; Zhang, L.; Liu, Z.; Wang, Y. A facile one-step solid-state synthesis of a Li4Ti5O12/graphene composite as an anode material for high-power lithium-ion batteries. Solid State Ionics 2019, 329, 110–118. [Google Scholar] [CrossRef]
- Yan, H.; Yao, W.; Fan, R.; Zhang, Y.; Luo, J.; Xu, J. Mesoporous Hierarchical Structure of Li4Ti5O12/Graphene with High Electrochemical Performance in Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 11360–11366. [Google Scholar] [CrossRef]
- Liu, J.; Wei, A.X.; Chen, M.; Xia, X. Rational synthesis of Li4Ti5O12/N-C nanotube arrays as advanced high-rate electrodes for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 3857–3863. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, M.; Shen, X.; Wu, Q.; Zhang, X.; Huan, L.; Diao, G. Preparation of Li4Ti5O12 nanosheets/carbon nanotubes composites and application of anode materials for lithium-ion batteries. Electrochim. Acta 2016, 204, 92–99. [Google Scholar] [CrossRef]
- Ni, H.F.; Fan, L.Z. Nano-Li4Ti5O12 anchored on carbon nanotubes by liquid phase deposition as anode material for high rate lithium-ion batteries. J. Power Sources 2012, 214, 195–199. [Google Scholar] [CrossRef]
- Lan, C.-K.; Bao, Q.; Huang, Y.-H.; Duh, J.-G. Embedding nano-Li4Ti5O12 in hierarchical porous carbon matrixes derived from water soluble polymers for ultra-fast lithium ion batteries anodic material. J. Alloy Compd. 2016, 673, 336–348. [Google Scholar] [CrossRef]
- Ni, D.; Sun, W.; Xie, L.; Fan, Q.; Wang, Z.; Sun, K. Bismuth oxyfluoride@CMK-3 nanocomposite as cathode for lithium ion batteries. J. Power Sources 2018, 374, 166–174. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Lu, P.; Sun, Y.; Xiang, H.F.; Liang, X.; Yu, Y. 3D amorphous carbon with controlled porous and disordered structures as a high-rate anode material for sodium ion batteries. Adv. Energy Mater. 2017, 8, 1702434. [Google Scholar] [CrossRef]
- Saikia, D.; Wang, T.-H.; Chou, C.-J.; Fang, J.; Tsai, L.-D.; Kao, H.-M. A comparative study of ordered mesoporous carbons with different pore structures as anode materials for lithium-ion batteries. RSC Adv. 2015, 5, 42922–42930. [Google Scholar] [CrossRef]
- Castro-Muñiz, A.; Lorenzo-Fierro, S.; Martínez-Alonso, A.; Tascón, J.M.D.; Fierro, V.; Suárez-García, F.; Paredes, J.I. Ordered mesoporous carbons obtained from low-value coal tar products for electrochemical energy storage and water remediation. Fuel Process. Technol. 2019, 196, 106152. [Google Scholar] [CrossRef]
- Dongil, A.B.; Bachiller-Baeza, B.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I.; Martínez-Alonso, A.; Tascón, J.M.D. Surface chemical modifications induced on high surface area graphite and carbon nanofibers using different oxidation and functionalization treatments. J. Colloid Interface Sci. 2011, 355, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Mironova, E.Y.; Ermilova, M.M.; Efimov, M.N.; Orekhova, N.V.; Yaroslavtsev, A.B. Detonation nanodiamonds as catalysts of steam reforming of ethanol. Russ. Chem. Bull. 2013, 62, 2317–2321. [Google Scholar] [CrossRef]
- Marciniak, M.; Goscianska, J.; Frankowski, M.; Pietrzak, R. Optimal synthesis of oxidized mesoporous carbons for the adsorption of heavy metal ions. J. Mol. Liq. 2019, 276, 630–637. [Google Scholar] [CrossRef]
- Moreno-Tovar, R.; Terrés, E.; Rangel-Mendez, R.J. Oxidation and EDX elemental mapping characterization of an ordered mesoporous carbon: Pb(II) and Cd(II) removal. Appl. Surf. Sci. 2014, 303, 373–380. [Google Scholar] [CrossRef]
- Solyanikova, A.S.; Chayka, M.Y.; Boryak, A.V.; Kravchenko, T.A.; Glotov, A.V.; Ponomarenko, I.V.; Kirik, S.D. Composite electrodes of electrochemical capacitors based on carbon materials with different structure. Russ. J. Electrochem. 2014, 50, 419–428. [Google Scholar] [CrossRef]
- Stenina, I.A.; Kulova, T.L.; Skundin, A.M.; Yaroslavtsev, A.B. Effects of carbon coating from sucrose and PVDF on electrochemical performance of Li4Ti5O12/C composites in different potential ranges. J. Solid State Electrochem. 2018, 22, 2631–2639. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Pereira-Ramo, J.-P. Raman Microspectrometry Applied to the Study of Electrode Materials for Lithium Batteries. Chem. Rev. 2010, 110, 1278–1319. [Google Scholar] [CrossRef] [PubMed]
- Leonidov, I.A.; Leonidova, O.N.; Perelyaeva, L.A.; Samigullina, R.F.; Kovyazina, S.A.; Patrakeev, M.V. Structure, Ionic Conduction, and Phase Transformations in Lithium Titanate Li4Ti5O12. Phys. Solid State 2003, 45, 2183–2188. [Google Scholar] [CrossRef]
- Stenina, I.A.; Sobolev, A.N.; Kuz’mina, A.A.; Kulova, T.L.; Skundin, A.M.; Tabachkova, N.Y.; Yaroslavtsev, A.B. Electrochemical properties of Li4Ti5O12/C and Li4Ti5O12/C/Ag nanomaterials. Inorg. Mater. 2017, 53, 1039–1045. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Kohl, S.; Drochner, A.; Vogel, H. Quantification of oxygen surface groups on carbon materials via diffuse reflectance FT-IR spectroscopy and temperature programmed desorption. Catal. Today 2010, 150, 67–70. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Ermilova, M.M.; Orekhova, N.V.; Muraviev, D.N.; Yaroslavtsev, A.B. Production of high purity hydrogen by ethanol steam reforming in membrane reactor. Catal. Today 2014, 236, 64–69. [Google Scholar] [CrossRef]
- Biniak, S.; Trykowski, G.; Walczyk, M.; Richert, M. Thermo-Chemical Modification of Low-Dimensional Carbons: An Infrared Study. J. Appl. Spectrosc. 2016, 83, 580–585. [Google Scholar] [CrossRef]
- Janus, P.; Janus, R.; Kuśtrowski, P.; Jarczewski, S.; Wach, A.; Silvestre-Albero, A.M.; Rodríguez-Reinoso, F. Chemically activated poly(furfuryl alcohol)-derived CMK-3 carbon catalysts for the oxidative dehydrogenation of ethylbenzene. Catal. Today 2014, 235, 201–209. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Ulus, A.; Yufit, V. Effect of carbon substrate on SEI composition and morphology. Electrochim. Acta 2004, 50, 391–395. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, Q.; Wang, Y.; Battaglia, V.S.; Huang, Y.; Zheng, H. The role of carbon bond types on the formation of solid electrolyte interphase on graphite surfaces. Carbon 2019, 148, 105–114. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Wang, Z.; Zhang, H. Electrochemical analysis graphite/electrolyte interface in lithium-ion batteries: p-Toluenesulfonyl isocyanate as electrolyte additive. Nano Energy 2017, 34, 131–140. [Google Scholar] [CrossRef]
- Xu, G.; Han, P.; Dong, S.; Liu, H.; Cui, G.; Chen, L. Li4Ti5O12-based energy conversion and storage systems: Status and prospects. Coord. Chem. Rev. 2017, 34315, 139–184. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Kanagaraj, A.B.; Al Nahyan, M.S.; Al Shibli, H.; Ashoor, A.A.; Fadaq, H.; Al Dahmani, S.; Choi, D.S. Electrical and electrochemical properties of carbon nanotube-based free standing LTO electrodes for current collector-free Li-ion batteries. Curr. Appl. Phys. 2019, 19, 1150–1155. [Google Scholar] [CrossRef]
- Zhun, J.; Duan, R.; Zhang, Y.; Zhu, J. A facial solvothermal reduction route for the production of Li4Ti5O12/graphene composites with enhanced electrochemical performance. Ceram. Int. 2016, 42, 334–340. [Google Scholar]
- Liu, J.; Shen, Y.; Chen, L.; Wang, Y.; Xia, Y. Carbon coated Li4Ti5O12 nanowire with high electrochemical performance under elevated temperature. Electrochim. Acta 2015, 156, 38–44. [Google Scholar] [CrossRef]
- Liu, K.; Cui, J.; Yin, J.; Man, J.; Cui, Y.; Wen, Z.; Sun, J. Ultra-long life core-shell structure Li4Ti5O12/C nanocomposite anode materials for lithium ion batteries. J. Alloy Compd. 2018, 765, 229–235. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Y.; Yao, J. Graphene oxide-confined synthesis of Li4Ti5O12 microspheres as high-performance anodes for lithium ion batteries. Electrochim. Acta 2015, 165, 422–429. [Google Scholar] [CrossRef]
- Zhu, Y.-R.; Wang, P.; Yi, T.-F.; Deng, L.; Xie, Y. Improved high-rate performance of Li4Ti5O12/carbon nanotube nanocomposite anode for lithium-ion batteries. Solid State Ionics 2015, 276, 84–89. [Google Scholar] [CrossRef]
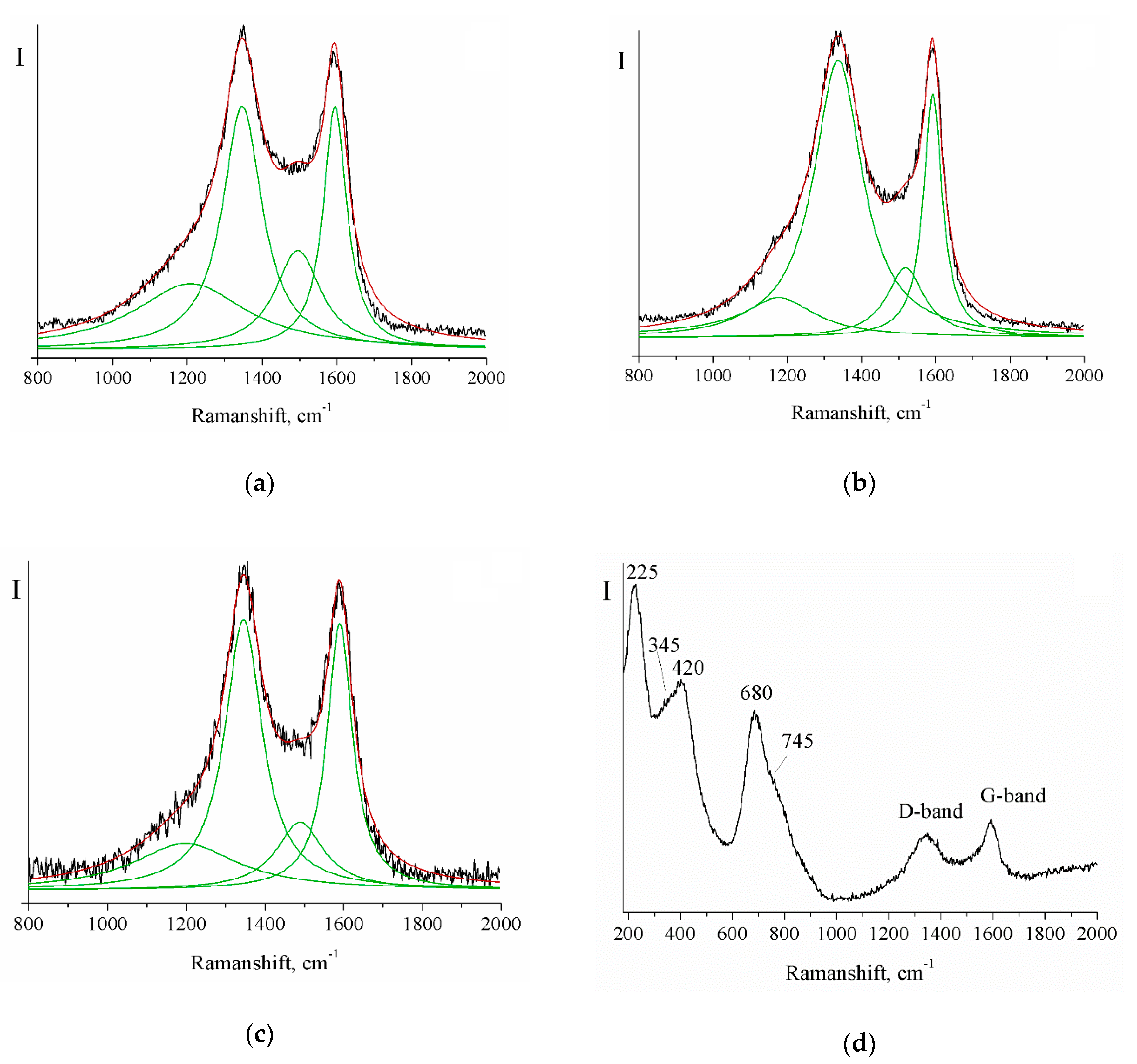
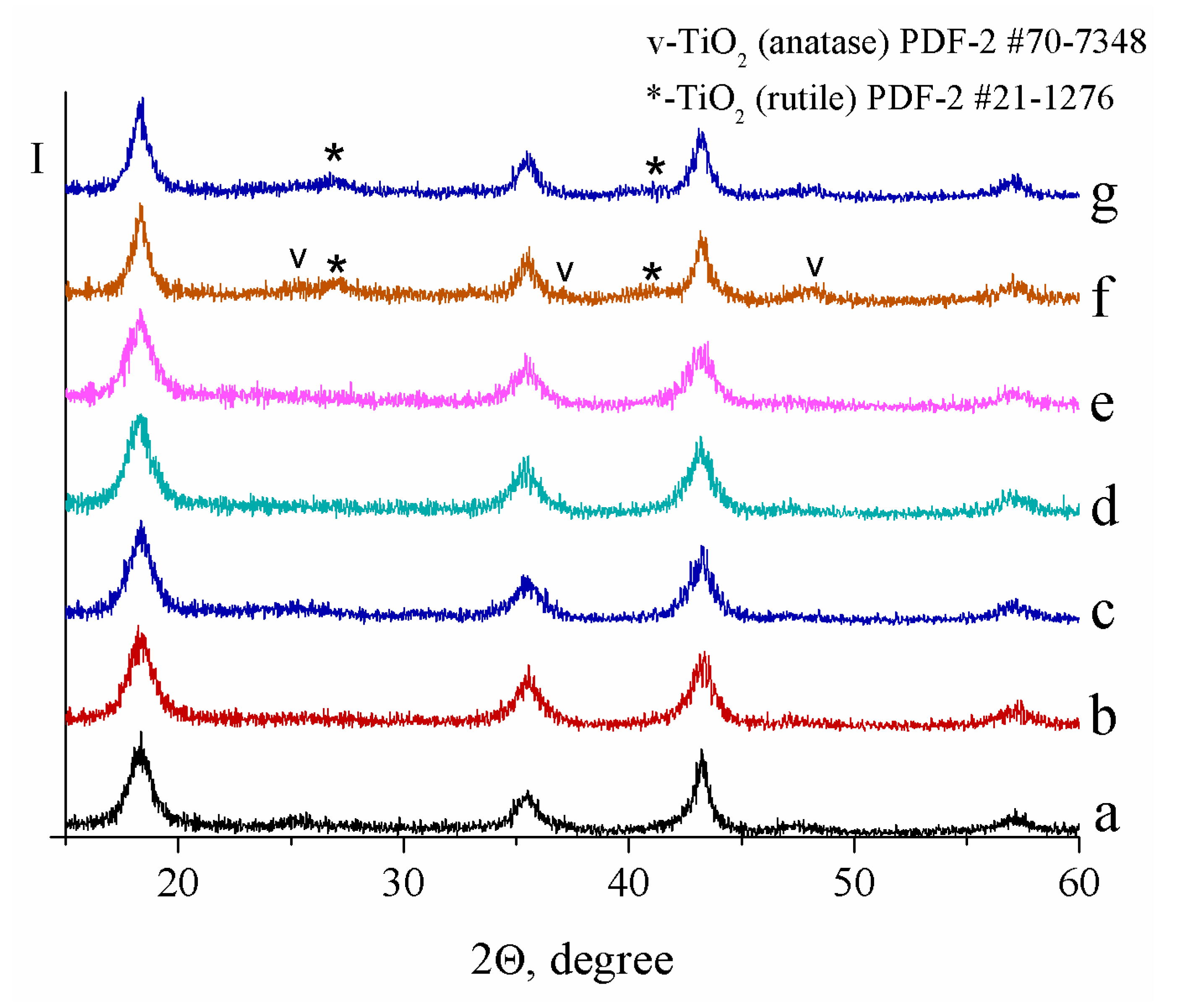

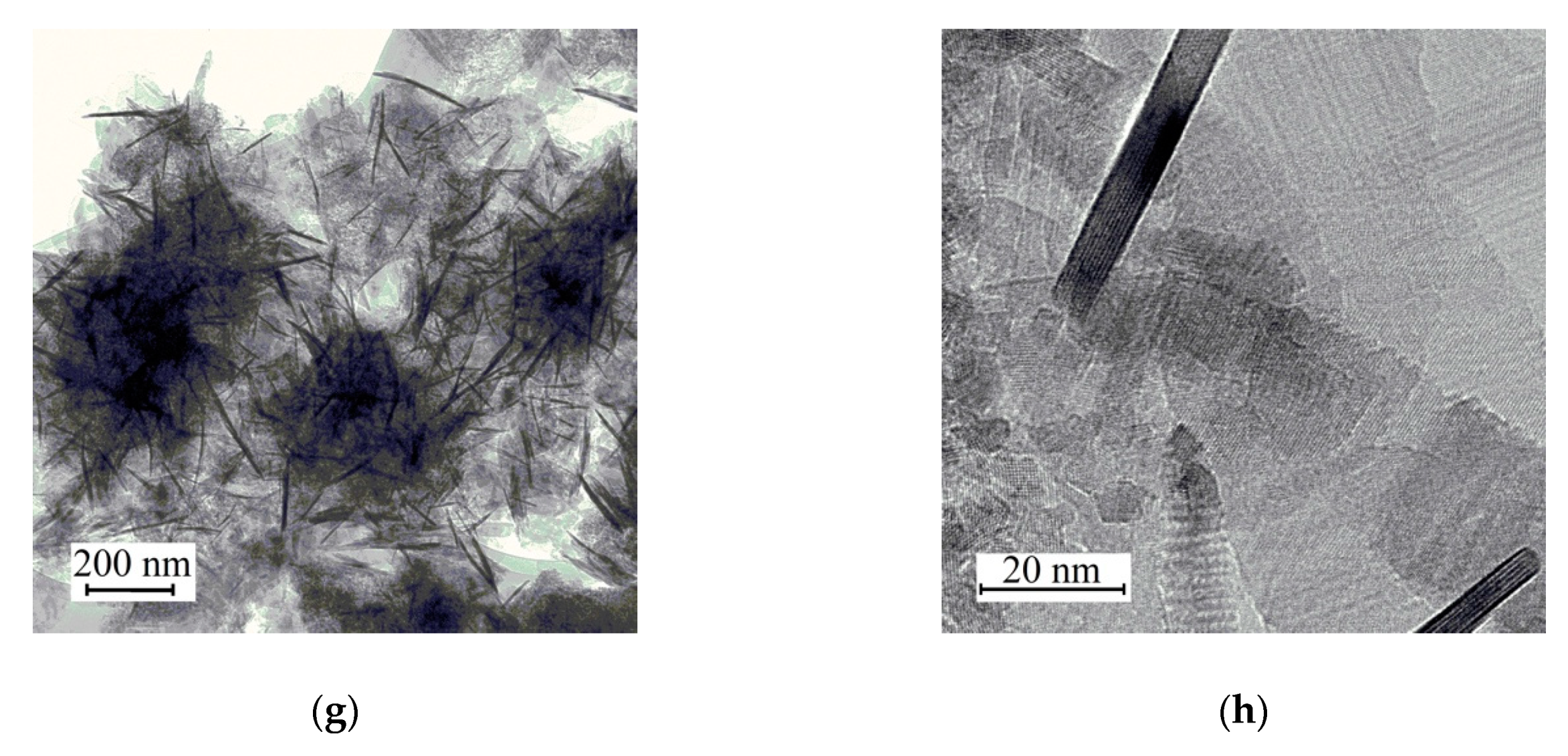
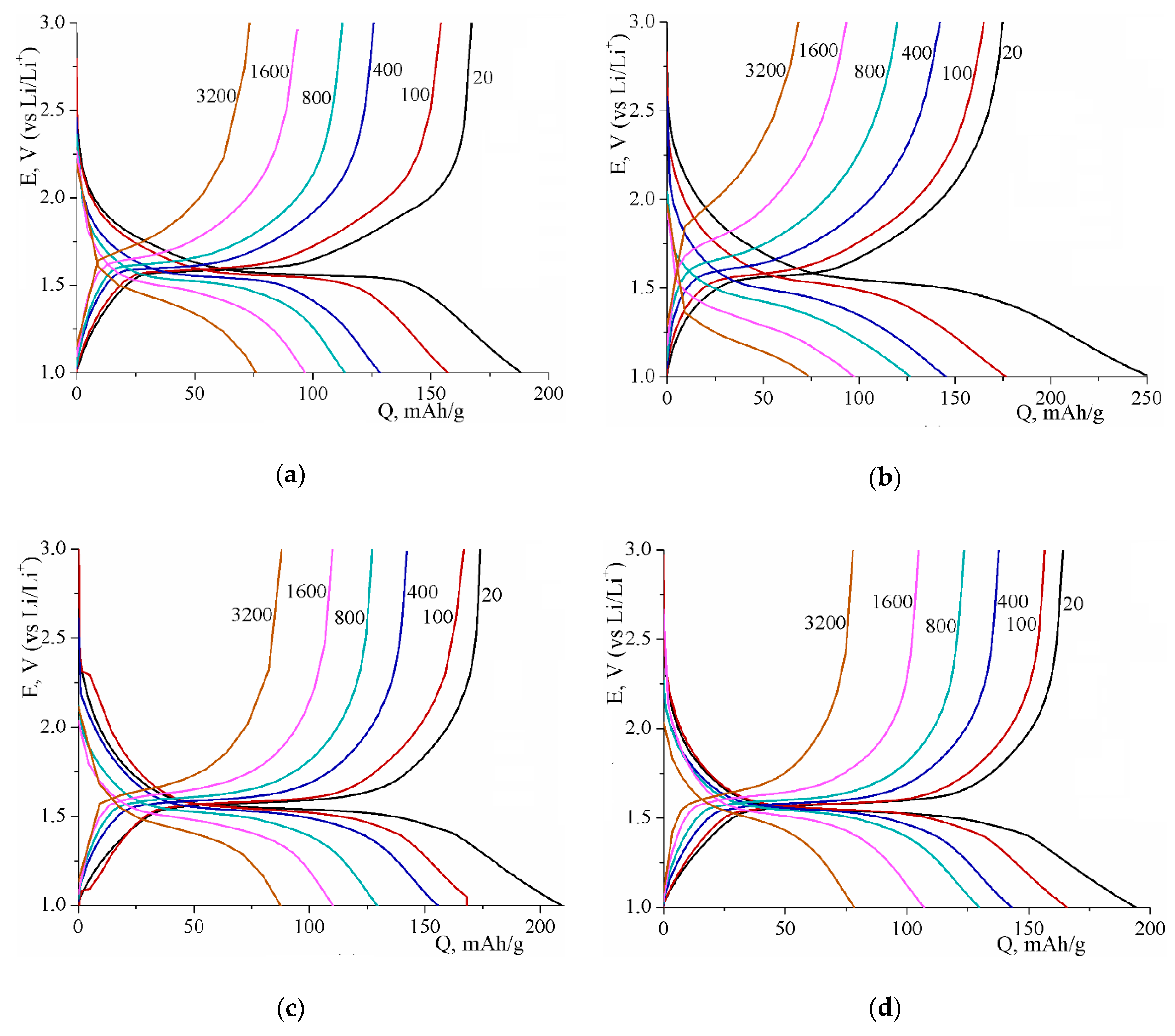
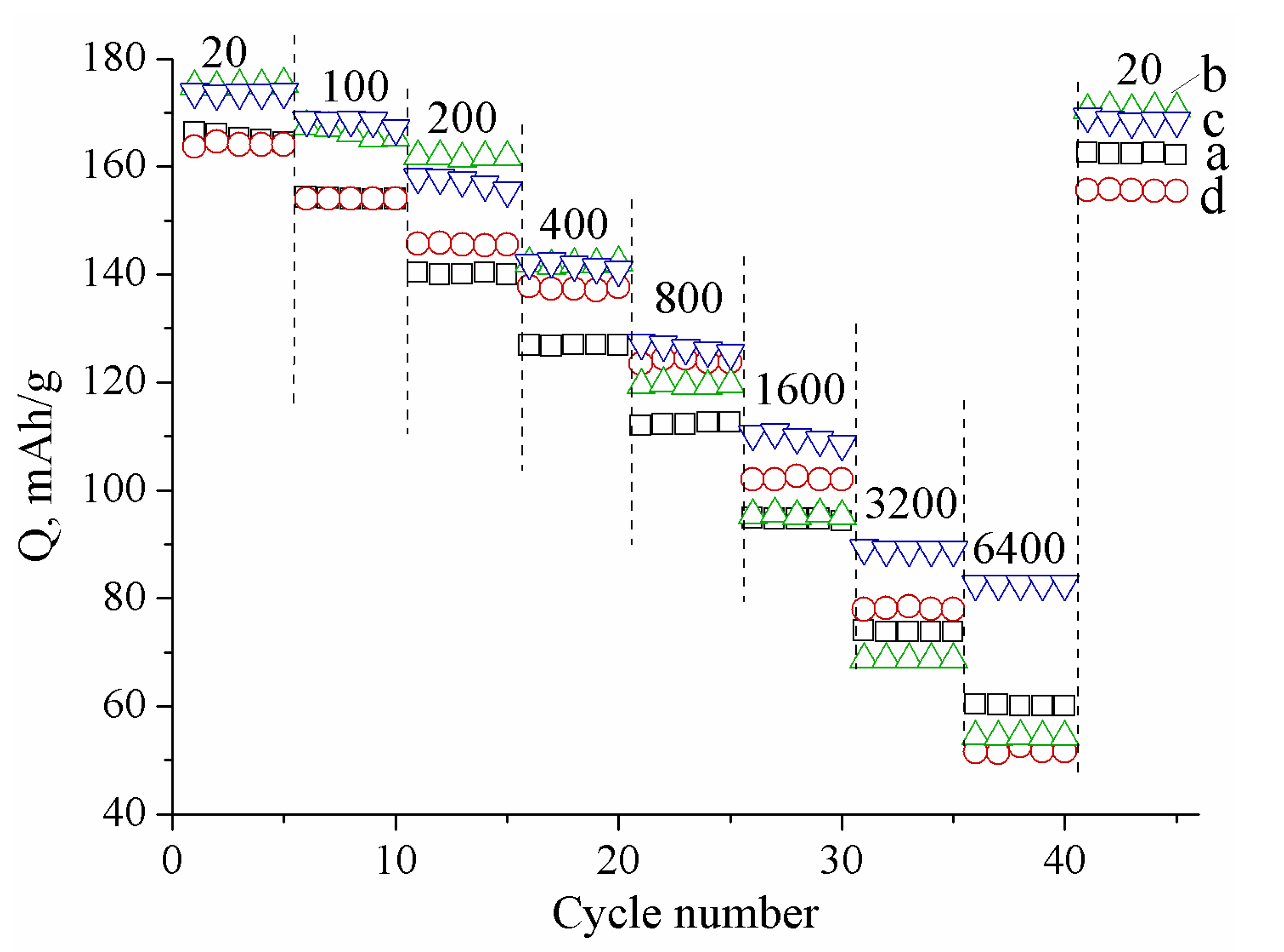
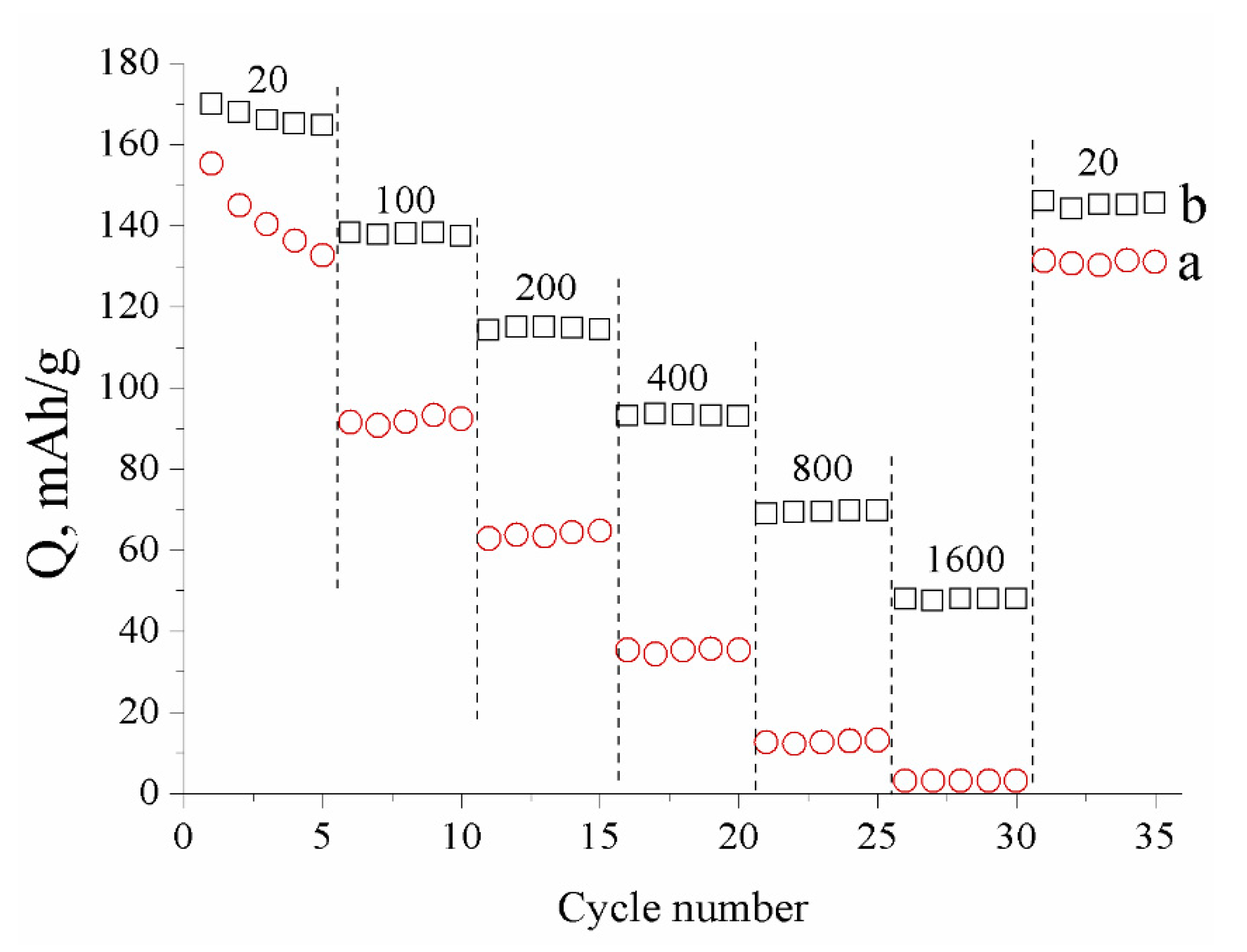
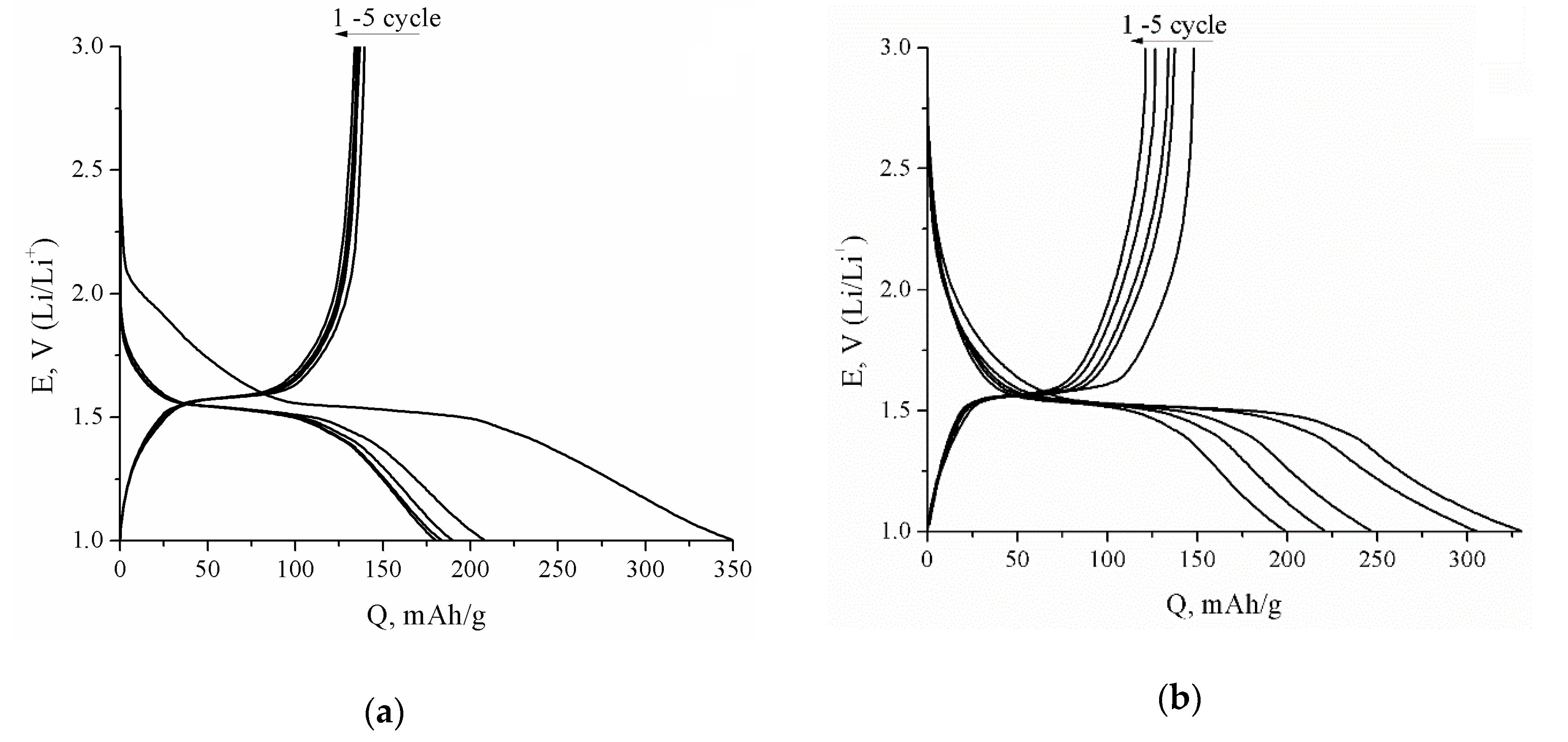
| Sample | Crystallite Size (nm) | Real Carbon Content (wt.%) |
|---|---|---|
| LTO | 8.6 | - |
| LTO/5%G_157M | 11.3 | 4.5 |
| LTO/20%G_157M | 10.8 | 18.9 |
| LTO/5%CR | 7.8 | 4.5 |
| LTO/20%CR | 7.6 | 19.2 |
| LTO/5%CB | 7.7 | 4.3 |
| LTO/20%CB | 7.9 | 19.0 |
| Sample | Mesoporous Carbon G_157M | Carbon Replicas | Carbon Black |
|---|---|---|---|
| BET surface area (m2/g) | 2225 | 662 | 60 |
| Average pore size (nm) | 1.84 | 1.95 | 1.91 |
| Pore volume (cm3/g) | 1.027 | 0.318 | 0.029 |
| Composite Material | Synthesis Method (Conditions) | Capacity, mAh/g (C-Rate) |
|---|---|---|
| Li4Ti5O12/carbon nanotubes/C 1 [28] | Solid state reaction (800 °C, 16 h, Ar) | 167 (0.1C), 151 (5C) |
| Li4Ti5O12/multi-walled carbon nanotubes [64] | Solid state reaction (850 °C, 26 h) | 166 (0.2C), 118 (5C) |
| Li4Ti5O12/graphene [65] | Li4Ti5O12: Hydrothermal (180 °C, 24 h) + calcination (700 °C, 8 h, Ar) Li4Ti5O12/graphene suspension: Hydrothermal (120 °C, 16 h) | 184 4 (0.1 C), 102 (5C) |
| Li4Ti5O12/carbon nanotubes [37] | Hydrothermal (120 °C, 16 h) + calcination (700 °C, 6 h, Ar) | 172 (0.1C), 157 (5C) |
| Li4Ti5O12/3D amorphous carbon [32] | Hydrothermal (180 °C, 18 h) + calcination (600 °C, 3 h, Ar) | 169 (0.2 C), 159 (5C) |
| Li4Ti5O12/C 2 [66] | Hydrothermal (100 °C, 24 h) + calcination (800 °C, 2 h, Ar) | 146 (0.1 C), 105 (5C) |
| Li4Ti5O12/C 3 [67] | Hydrothermal (200 °C, 36 h) + calcination (700 °C, 6 h, Ar) | 165 (0.2C), 112 (3.2C) |
| Li4Ti5O12/reduced graphene oxide [68] | Hydrothermal (180 °C, 36 h) + calcination (600 °C, 6 h, Ar) | 168 (0.5C), 138 (5C) |
| Li4Ti5O12/carbon nanotubes [69] | Hydrothermal (180 °C, 40 h) + calcination (600 °C, 6 h, N2) | 162 (0.5C), 128 (3C) |
| Li4Ti5O12/carbon black (this work) | Hydrothermal (130 °C, 12 h) + calcination (400 °C, 5 h, Ar) | 174 (0.1C), 127 (5C) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenina, I.; Shaydullin, R.; Kulova, T.; Kuz’mina, A.; Tabachkova, N.; Yaroslavtsev, A. Effect of Carbon Additives on the Electrochemical Performance of Li4Ti5O12/C Anodes. Energies 2020, 13, 3941. https://doi.org/10.3390/en13153941
Stenina I, Shaydullin R, Kulova T, Kuz’mina A, Tabachkova N, Yaroslavtsev A. Effect of Carbon Additives on the Electrochemical Performance of Li4Ti5O12/C Anodes. Energies. 2020; 13(15):3941. https://doi.org/10.3390/en13153941
Chicago/Turabian StyleStenina, Irina, Ruslan Shaydullin, Tatiana Kulova, Anna Kuz’mina, Nataliya Tabachkova, and Andrey Yaroslavtsev. 2020. "Effect of Carbon Additives on the Electrochemical Performance of Li4Ti5O12/C Anodes" Energies 13, no. 15: 3941. https://doi.org/10.3390/en13153941
APA StyleStenina, I., Shaydullin, R., Kulova, T., Kuz’mina, A., Tabachkova, N., & Yaroslavtsev, A. (2020). Effect of Carbon Additives on the Electrochemical Performance of Li4Ti5O12/C Anodes. Energies, 13(15), 3941. https://doi.org/10.3390/en13153941